Abstract
Acetylcholinesterase (AChE) at the neuromuscular junction (NMJ) is anchored to the synaptic basal lamina via a triple helical collagen Q (ColQ) in the form of asymmetric AChE (AChE/ColQ). The C-terminal domain of ColQ binds to MuSK, the muscle-specific receptor tyrosine kinase, that mediates a signal for acetylcholine receptor (AChR) clustering at the NMJ. ColQ also binds to heparan sulfate proteoglycans including perlecan.
Congenital defects of ColQ cause endplate AChE deficiency. A single intravenous administration of adeno-associated virus serotype 8 (AAV8)-COLQ to Colq−/− mice rescued motor functions, synaptic transmission, and the ultrastructure of NMJ. We also injected AAV1-COLQ-IRES-EGFP to the left tibialis anterior and observed colocalization of AChE/ColQ at all the examined NMJs of the non-injected limbs. Additionally, injection of purified recombinant AChE/ColQ protein complex into gluteus maximus accumulated AChE in non-injected forelimbs. These observations suggest that the tissue-targeting signal of ColQ can be exploited to specifically deliver the transgene product to the target tissue.
MuSK antibody-positive myasthenia gravis (MG) accounts for 5–15% of autoimmune MG. As AChR deficiency is typically mild and as cholinesterase inhibitors are generally ineffective or worsen myasthenic symptoms, we asked if the patient's MuSK-IgG interferes with binding of ColQ to MuSK. In vitro overlay of AChE/ColQ to muscle sections of Colq−/− mice revealed that MuSK-IgG blocks binding of ColQ to the NMJ. In vitro plate-binding of MuSK to ColQ disclosed that MuSK-IgG exerts a dose-dependent block of MuSK-ColQ interaction. In addition, passive transfer of MuSK-IgG to mice reduced the size and density of ColQ to ~10% of controls and had a lesser effect on the sizes and densities of AChR and MuSK. Elucidation of molecular mechanisms of specific binding of ColQ to the NMJ enabled us to ameliorate devastating myasthenic symptoms of Colq−/− mice and to reveal bases of anti-MuSK MG.
Keywords: Collagen Q, Acetylcholinesterase, Adeno-associated virus, Myasthenia gravis
1. Introduction
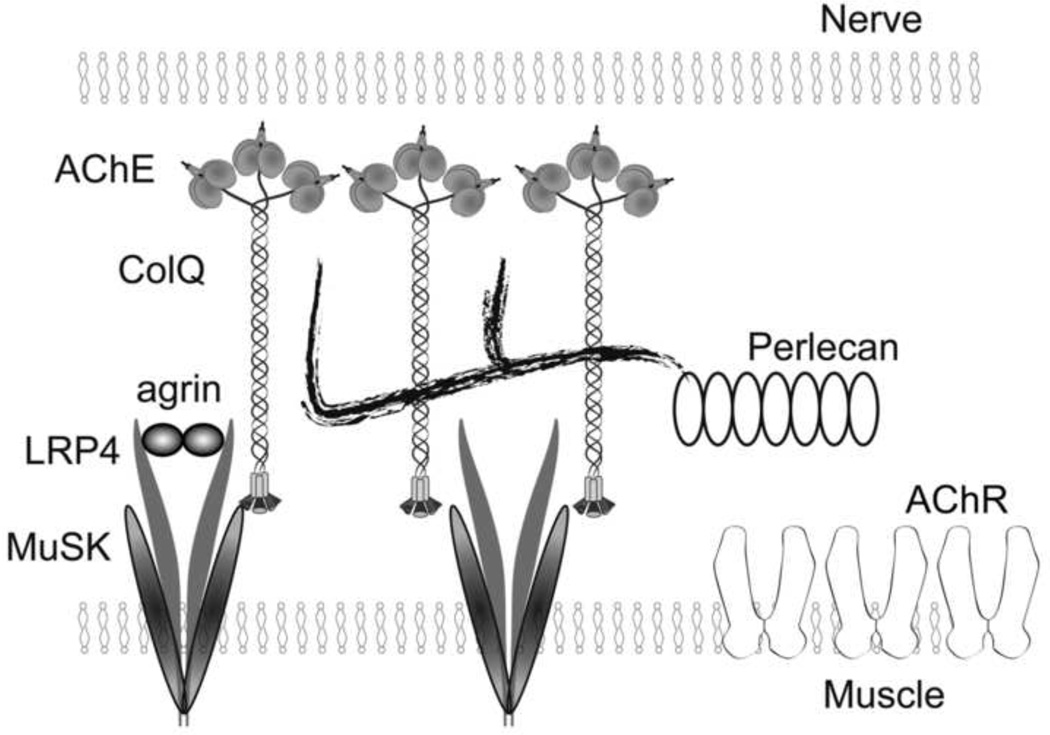
Acetylcholinesterase (AChE) at the neuromuscular junction (NMJ) is anchored to the synaptic basal lamina via a triple helical collagen Q (ColQ) in the form of asymmetric AChE (AChE/ColQ). The C-terminal domain of ColQ binds to MuSK. ColQ also binds to heparan sulfate proteoglycans including perlecan (Fig. 1) [1]. We exploited the specific binding properties of ColQ to synaptic and postsynaptic molecules to cure a congenital myasthenic syndrome associated with defective AChE [2] and to explore bases of myasthenia gravis (MG) associated with anti-MuSK antibody [3].
Fig. 1.
Schematic diagram of anchoring of ColQ to NMJ. Twelve catalytic subunits of AChE are attached to ColQ to form ColQ-tailed AChE. Two heparan sulfate proteoglycan-binding domains of ColQ are bound to perlecan. C-terminal domain of ColQ is bound to MuSK.
Congenital defects of ColQ in humans cause endplate AChE deficiency (EAD) [4, 5]. EAD is an autosomal recessive disorder, which manifests as generalized muscle weakness, fatigue, amyotrophy, scoliosis, and minor facial abnormalities. There has been no rational therapy for EAD. We have developed a novel therapeutic strategy, the protein-anchoring therapy, in which the extracellular protein complex AChE/ColQ is delivered to the target NMJ using its specific binding affinity for MuSK and perlecan.
MG is an autoimmune disorder, in which autoantibody against AChR, MuSK, and LRP4 compromises the neuromuscular signal transmission. Five to 15 percent of MG patients carry antibodies against MuSK (MuSK-IgG) [6]. MuSK-MG patients usually do not respond to, or are even worsened by, cholinesterase inhibitors [7]. Anti-MuSK antibodies are largely IgG4 that do not activate complements [8]. However, the exact target of MuSK-IgG, remains elusive. We here examined an effect of MuSK-IgG on an interaction between ColQ and MuSK by in vitro overlay and plate-binding assays as well as by passive transfer of MuSK-IgG to wild-type mice, and found that MuSK-IgG blocks the ColQ-MuSK interaction.
2. Materials and Methods
2.1. Preparation of AAV carrying COLQ
Human COLQ cDNA was cloned into pAAV-MCS (AAV Helper-Free system, Stratagene) that carries the CMV promoter to obtain pAAV-COLQ. We also inserted internal ribosome entry site (IRES)-EGFP to make pAAV-COLQ-IRES-EGFP. The AAV8 or AAV1 particles were concentrated by CsCl gradient ultracentrifugation for 3 hrs and further purified with the quick dual ion-exchange procedures.
2.2. Administration of AAV carrying COLQ to Colq−/− mice
All animal studies were approved by the Animal Care and Use Committee of Nagoya University. AAV8-COLQ and AAV1-COLQ-IRES-EGFP were injected into the tail vein and the left anterior tibial muscle of four-week-old Colq−/− mice, respectively.
2.3. Purification and intramuscular injection of recombinant AChE/ColQ protein complex
pTargeT clones carrying human COLQ and human ACHE cDNAs were cotransfected into HEK293 cells. The protein extracts were loaded onto HiTrap Heparin HP columns (GE Healthcare). The concentration of purified recombinant AChE/ColQ was equivalent to ~4 μg/ml Torpedo AChE. We injected 50 μl of the purified AChE/ColQ protein complex in PBS daily into the gluteus maximus muscles of five-week-old Colq−/− mice for a week.
2.4. MuSK-MG patients
The studies were approved by the ethical review committees of Nagoya University Graduate School of Medicine and Mayo Clinic. An informed consent was obtained from each subject. We obtained serum from four MuSK-MG patients (Pts. 1–4) and a patient with limb-girdle muscular dystrophy as a control (Ct. 1). We also obtained expired fresh frozen plasma (Ct. 2) from Dr. Isao Takahashi at the Aichi Red Cross Blood Center with an institutional approval. The titers of anti-MuSK antibodies of Pts. 1–3 were 22.0 nM, 11.2 nM, 0.12 nM, respectively (normal: < 0.01 nM). Pt. 4 was positive for anti-MuSK antibody, but the titer was not determined. We purified IgG essentially as described elsewhere [9].
2.5. In vitro overlay and in vitro plate-binding assays of MuSK IgG
Human recombinant AChE/ColQ was overlaid on a mouse muscle section in the presence of the patient or control IgG, essentially as previously described [10]. For in vitro plate-binding assay, the extracellular domain (a.a. 1-393) of human MUSK cDNA and the extracellular domain (a.a. 1-1722) of human LRP4 cDNA were cloned into a mammalian expression vector to generate hMuSKect-myc and hLRP4N-FLAG, respectively. We coated the Maxi-Sorp Immuno Plate (Nunc) with 0.15 µg of purified hMuSKect-myc at 4 °C overnight, and added 1 pg to 100 µg of IgG at 4 °C for 6 hrs. We then added 0.12-Ellman units of AChE/ColQ, and quantified the bound AChE/ColQ by the Ellman method in the presence of 5×10−5 M ethopropazine [4].
2.6. Passive transfer of human IgG to mice
We intraperitoneally injected 40 mg IgG of Ct. 2 and Pt. 2 into six-week-old female C57BL/6J mice every day for 15 days. Signals for AChR, ColQ, and MuSK were quantified by the BZ-9000 microscope (Keyence).
3. Results – Protein-Anchoring Therapy
3.1. Intravenous administration of AAV8-COLQ normalizes motor functions of Colq−/− mice
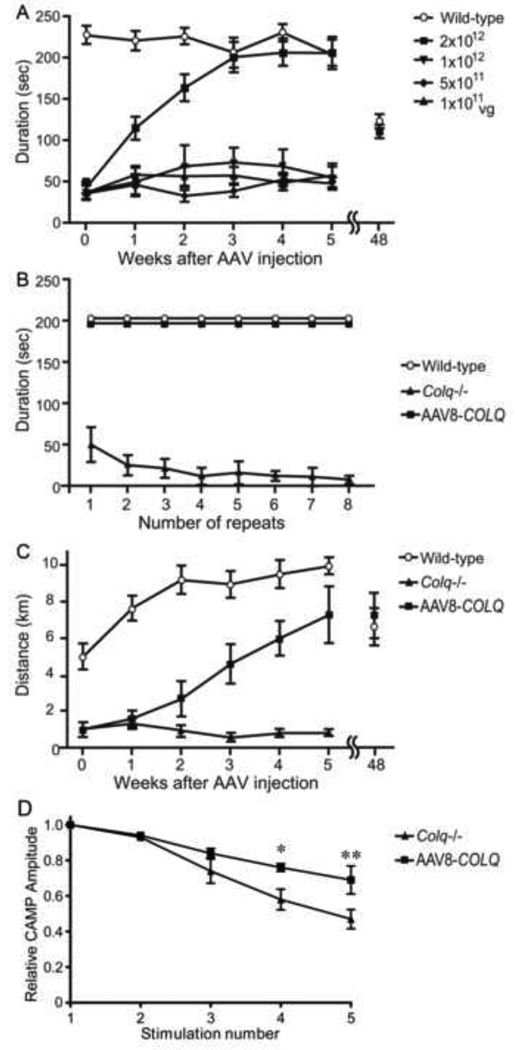
We intravenously administered 1×1011 to 2×1012 viral genome (vg) copies of AAV8-COLQ into four-week-old Colq−/− mice. These mice exhibit muscle weakness, myasthenia, tremor, kyphosis, involuntary vocalization, and a slower growth rate than their wild-type littermates [11]. A single injection of 2×1012 vg gradually improved their motor function, and ameliorated abnormal fatigability at six weeks after injection (Fig. 2). In longitudinal studies of three treated mice, all survived 18 to 20 months. Motor functions of the treated mice were declined at 48 weeks after injection but to the similar levels as those of wild-type (Fig. 2). Although the lifetime of the recombinant AChE/ColQ complex at the NMJ has not been studied, it is likely that AAV8-COLQ stayed in muscle cells episomally [12] and expressed ColQ for a long time, as has been observed skeletal muscle in other AAVs [13].
Fig. 2.
Motor functions and compound muscle action potentials (CMAP) after intravenous injection of AAV8-COLQ to the tail vein of Colq−/− mice. (A) Durations on the rotarod that was linearly accelerated from 0 to 40 rpm in 240 sec. Six mice were studied for each group. (B) Fatigue test using the rotarod was performed on six mice in each group. (C) Voluntary movements were quantified by a counter-equipped running wheel for 24 h on 6 mice in each group. (D) CMAP amplitudes of quadriceps muscles after 2-Hz stimulation of the femoral nerve. Treatment ameliorated CMAP decrements in 3 mice in each group (P < 0.05 by two-way ANOVA). *P < 0.05 and **P < 0.01 by Bonferroni post-hoc test. Mean and SE are indicated in each panel.
3.2. AAV8-COLQ normalizes the neuromuscular synaptic transmission
Electrophysiological studies revealed that AAV8-COLQ reduced decrements of the compound muscle action potentials in response to repetitive nerve stimulation at 2 Hz, reduced amplitudes of miniature endplate potentials, shortened the MEPP decay time constants, and acquired responses to neostigmine.
3.3. Human AChE/ColQ is anchored to the mouse NMJ
Histological studies revealed that ColQ and AChE were colocalized to AChR at the NMJ. The NMJ ultrastructures of treated mice were also improved. Quantitative analysis of soleus slow-twitch muscle demonstrated that invagination of Schwann cells was mitigated, which increased the nerve terminal length. Postsynaptic area and postsynaptic membrane length were also increased in treated mice.
3.4. AAV8-COLQ restores AChE/ColQ to 89.3% of wild-type
Sucrose density gradient fractionations of AChE exhibited A4, A8, and A12 peaks of AChE/ColQ species that were indistinguishable from those of wild-type. AAV8-COLQ restored the AChE/ColQ protein complex to 89.3 ± 9.6% (mean ± SD, n = 4) of wild-type at 6 weeks after treatment. The amount of AChE/ColQ at 48 weeks was still 81.8 ± 21.6% (mean ± SD, n = 2) of the age-matched wild-type mice (n = 3).
3.5. Intramuscular injection of AAV1-COLQ-IRES-EGFP expresses AChE/ColQ at NMJs of non-injected limbs
We packed COLQ cDNA into the AAV serotype 1 (AAV1) that locally infects the injected muscle fibers [14]. In addition, we fused COLQ and IRES-EGFP to express EGFP in infected cells. We injected 2×1011 vg of AAV1-COLQ-IRES-EGFP into the left anterior tibial muscle of Colq−/− mice. We observed colocalization of ColQ and AChE at all the examined NMJs of the injected and non-injected limb muscles. In contrast, expression of intracellular EGFP was not observed in non-injected limb muscles.
3.6. AChE/ColQ protein complex binds to remote NMJs
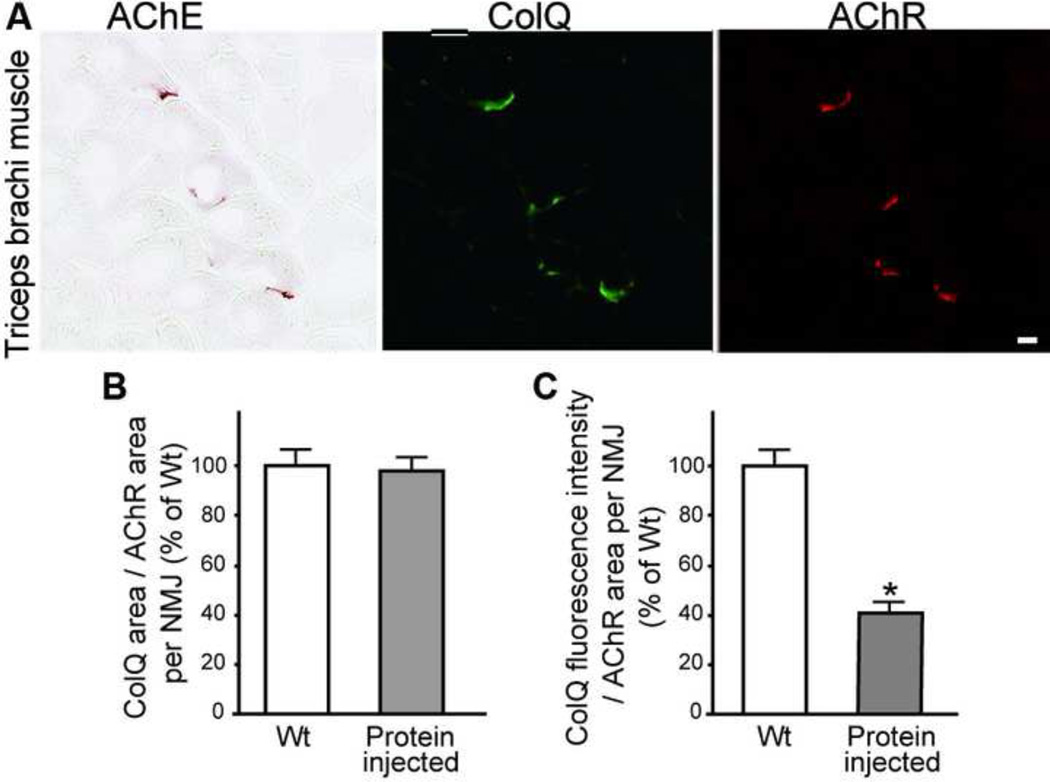
To directly test that AChE/ColQ protein complex moved in the mouse body, the gluteus maximus muscles of 5-week-old Colq−/− mice (n = 4) were injected daily with 0.2 µg of purified recombinant human AChE/ColQ for seven days. ColQ and AChE were detected in all the examined NMJs from triceps muscles (Fig. 3A). Quantitative analysis of ColQ signal intensities of non-injected triceps revealed that the ColQ-positive areas became similar to that of wild-type (Fig. 3B), and that the ColQ signal intensity reached ~41.6% of wild-type (Fig 3C).
Fig. 3.
(A) Daily injection of the purified recombinant human AChE/ColQ protein complex into the gluteus maximus muscles of Colq−/− mice induces expression of AChE/ColQ in the non-injected triceps muscle. Scale bar = 10 μm. (B) The size of ColQ-positive area is normalized for the size of AChR-positive area at the NMJs of non-injected triceps. (C) Signal intensities of ColQ at the NMJs of non-injected triceps. Mean and SE are indicated in (B) and (C). *P < 0.001.
4. Results – Anti-MuSK antibody
4.1. In vitro overlay assay discloses that MuSK-IgG blocks binding of AChE/ColQ to the NMJ of a muscle section of Colq−/− mouse
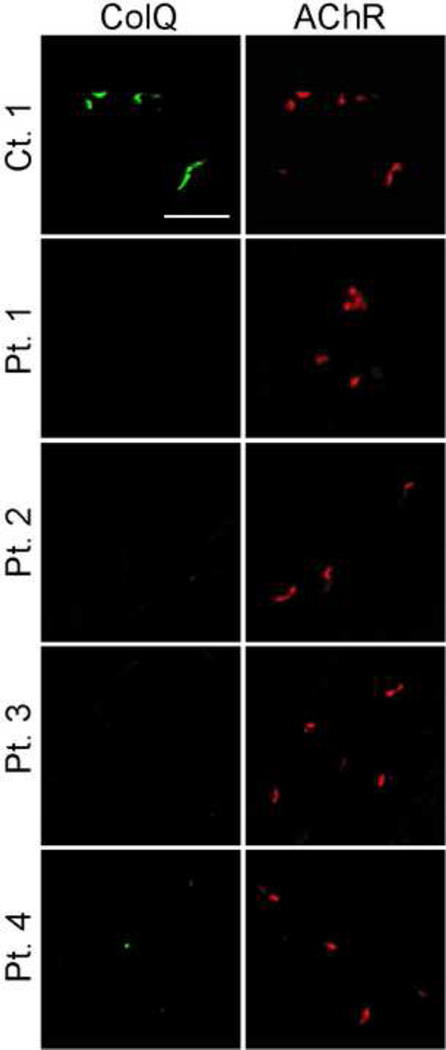
We previously demonstrated that the purified recombinant human AChE/ColQ could bind to the frog NMJ in vitro [10]. The in vitro overlay assay using Colq−/− mice showed that MuSK-IgG blocks anchoring of AChE/ColQ to the mouse NMJs (Fig. 4).
Fig. 4.
In vitro overlay assays. Purified recombinant AChE/ColQ was overlaid on a 10-µm quadriceps muscle section of Colq−/− mice in the presence of purified IgG of a control or patients. ColQ is stained with anti-ColQ antibody and AChR with Alexa594-labeled α-bungarotoxin. Scale bar = 50 µm.
4.2. In vitro plate-binding assay shows that MuSK-IgG blocks binding of AChE/ColQ to MuSK
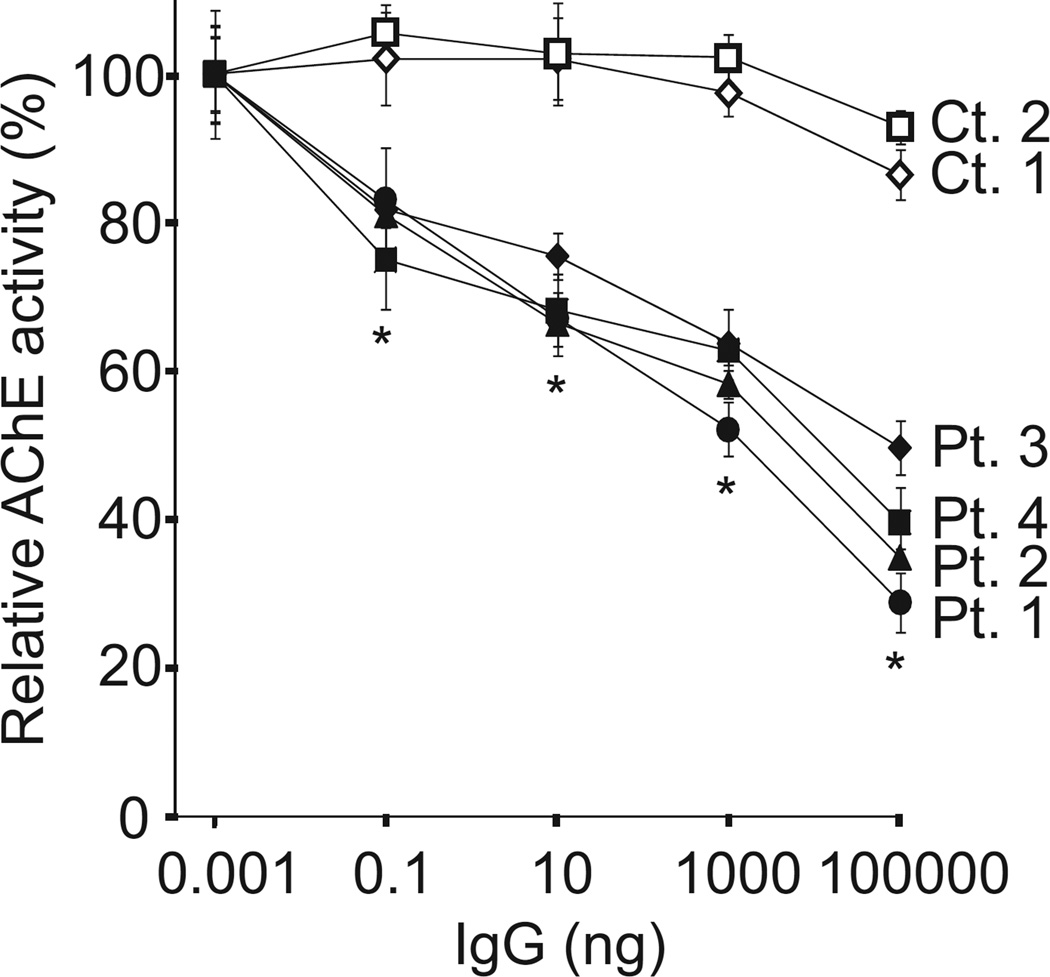
We next quantified an effect of MuSK-IgG on an interaction of human ColQ and human MuSK by an in vitro plate-binding assay. In two controls, AChE/ColQ remained bound to MuSK even in the presence of 100 µg of IgG, whereas in four MuSK-MG patients the amounts of bound AChE/ColQ were proportionally decreased with increasing amounts of the patient’s IgG (Fig. 5).
Fig. 5.
In vitro plate-binding assays. Increasing amounts of MuSK-IgG block binding of the purified recombinant AChE/ColQ to the extracellular domain of human MuSK that is coated on a 96-well plate. Mean and SE of 3 experiments are plotted. *P < 0.01 between controls and patients.
4.3. Passive transfer of MuSK-IgG to wild-type mice exhibits reduced ColQ signals at the NMJs
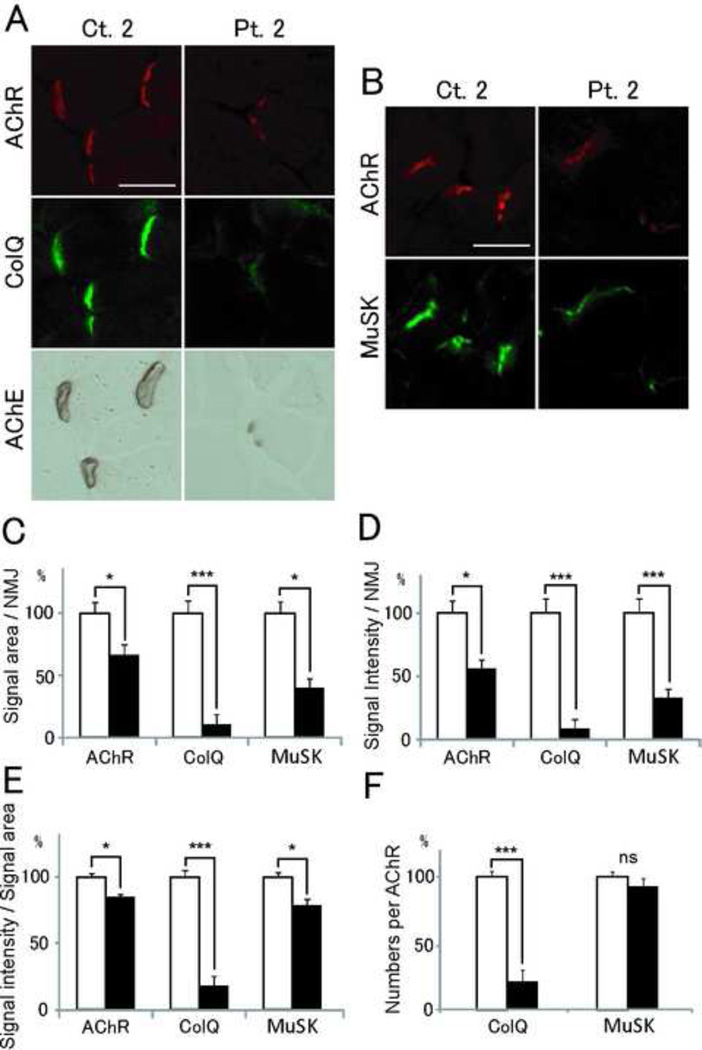
We injected IgG of Ct. 2 and Pt. 2 for 15 days to C57BL/6J female mice. Quantitative analysis revealed that signal areas, intensities, and densities of ColQ in mice injected with patient IgG were significantly reduced (Fig. 6). On the other hand, those of AChR and MuSK were only moderately reduced. Thus, MuSK-IgG compromised anchoring of AChE/ColQ to the NMJs and had a less prominent effect on the expression of MuSK and AChR.
Fig. 6.
Passive transfer of MuSK-IgG of Ct. 2 and Pt. 2 to C57BL/6J mice. (A and B) Quadriceps muscle sections of mice injected with IgG of Ct. 2 or Pt. 2 are stained for AChR by Alexa594-labeled α-bungarotoxin, ColQ and MuSK by immunostaining, AChE by cytochemical staining. Scale bar = 40 µm. Signal areas (C), intensities (D), and densities (intensity/area) (E) of the indicted molecules per NMJ are shown in mean and SE. (F) Densities of ColQ and MuSK are normalized for the density of AChR to estimate the number of ColQ and MuSK per AChR. Open and closed bars represent Ct. 2 and Pt. 2, respectively. *P < 0.05; ***P < 0.001; ns, not significant.
5. Discussion
5.1. Specific binding of ColQ to NMJ
We exploited the specific binding properties of ColQ to MuSK and perlecan to develop the protein-anchoring therapy for EAD [2] and to prove that MuSK-IgG blocks binding of ColQ to MuSK [3].
5.2. Protein-anchoring therapy
A single intravenously administration of muscle-tropic AAV8-COLQ led to body-wide recovery of AChE at the NMJ, and exhibited marked and life-long effects on motor deficits in Colq−/− mice. The long-distance delivery of AChE/ColQ was proven by intramuscular injection of AAV1-COLQ-IRES-EGFP, as well as of the AChE/ColQ protein complex, although efficiencies of anchoring of AChE/ColQ were lower than that of AAV8-COLQ. Another disadvantage of recombinant AChE/ColQ complex is that it is difficult to make a large amount of recombinant AChE/ColQ complex that is pure enough to be used for patients. The present study opens a new therapeutic avenue for treating many inherited defects of extracellular matrix proteins.
5.3. MuSK-IgG blocks binding of ColQ and MuSK
We showed that MuSK-IgG blocks binding of ColQ and MuSK by in vitro overlay and plate-binding assays as well as by passive transfer of MuSK-IgG to wild-type mice. Our observations are consistent with all the previous reports that cholinesterase inhibitors have no effect; MEPP decay time constants are prolonged in passive transfer model mice; and AChE are deficient in passive transfer and active immunization model animals. Our observations, however, are challenged by the lack of AChE or AChR defects at the NMJs of biopsied intercostal muscles of MuSK-MG patients. Further studies are required to elucidate the discordance between animal models and MuSK-MG patients.
Highlights.
The C-terminal domain of collagen Q binds to MuSK and perlecan.
Congenital defects of collagen Q cause endplate acetylcholinesterase deficiency.
Protein-anchoring therapy for collagen Q is proposed and proven in Colq−/− mice.
MuSK autoantibody accounts for 5–15% of myasthenia gravis.
MuSK-IgG blocks binding of MuSK to collagen Q.
Acknowledgements
The current studies were supported by Grants-in-Aid from the MEXT and MHLW of Japan, Grant from ANR maladies rares of France, and Grants from the NIH and the Muscular Dystrophy of the United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peng HB, Xie H, Rossi SG, Rotundo RL. Acetylcholinesterase clustering at the neuromuscular junction involves perlecan and dystroglycan. J. Cell Biol. 1999;145:911–921. doi: 10.1083/jcb.145.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito M, Suzuki Y, Okada T, Fukudome T, Yoshimura T, Masuda A, Takeda S, Krejci E, Ohno K. Protein-anchoring Strategy for Delivering Acetylcholinesterase to the Neuromuscular Junction. Mol Ther. 2012;20:1384–1392. doi: 10.1038/mt.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawakami Y, Ito M, Hirayama M, Sahashi K, Ohkawara B, Masuda A, Nishida H, Mabuchi N, Engel AG, Ohno K. Anti-MuSK autoantibodies block binding of collagen Q to MuSK. Neurology. 2011;77:1819–1826. doi: 10.1212/WNL.0b013e318237f660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohno K, Brengman J, Tsujino A, Engel AG. Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9654–9659. doi: 10.1073/pnas.95.16.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donger C, Krejci E, Pou Serradell A, Eymard B, Bon S, Nicole S, Chateau D, Gary F, Fardeau M, J M, Guicheney P. Mutation in the human acetylcholinesterase-associated collagen gene, COLQ, is responsible for congenital myasthenic syndrome with end-plate acetylcholinesterase deficiency (Type Ic) Am. J. Hum. Genet. 1998;63:967–975. doi: 10.1086/302059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrugia ME, Robson MD, Clover L, Anslow P, Newsom-Davis J, Kennett R, Hilton-Jones D, Matthews PM, Vincent A. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain. 2006;129:1481–1492. doi: 10.1093/brain/awl095. [DOI] [PubMed] [Google Scholar]
- 7.Evoli A, Tonali PA, Padua L, Monaco ML, Scuderi F, Batocchi AP, Marino M, Bartoccioni E. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. 2003;126:2304–2311. doi: 10.1093/brain/awg223. [DOI] [PubMed] [Google Scholar]
- 8.McConville J, Farrugia ME, Beeson D, Kishore U, Metcalfe R, Newsom-Davis J, Vincent A. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann. Neurol. 2004;55:580–584. doi: 10.1002/ana.20061. [DOI] [PubMed] [Google Scholar]
- 9.Horejsi J, Smetana R. The isolation of gamma globulin from blood-serum by rivanol. Acta Med. Scand. 1956;155:65–70. doi: 10.1111/j.0954-6820.1956.tb14351.x. [DOI] [PubMed] [Google Scholar]
- 10.Kimbell LM, Ohno K, Engel AG, Rotundo RL. C-terminal and heparinbinding domains of collagenic tail subunit are both essential for anchoring acetylcholinesterase at the synapse. J. Biol. Chem. 2004;279:10997–11005. doi: 10.1074/jbc.M305462200. [DOI] [PubMed] [Google Scholar]
- 11.Feng G, Krejci E, Molgo J, Cunningham JM, Massoulie J, Sanes JR. Genetic analysis of collagen Q: roles in acetylcholinesterase and butyrylcholinesterase assembly and in synaptic structure and function. J. Cell Biol. 1999;144:1349–1360. doi: 10.1083/jcb.144.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay MA. AAV vectors and tumorigenicity. Nat. Biotechnol. 2007;25:1111–1113. doi: 10.1038/nbt1007-1111. [DOI] [PubMed] [Google Scholar]
- 13.Riviere C, Danos O, Douar AM. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]