Abstract
Tularemia is caused by a gram-negative, intracellular bacterial pathogen, Francisella tularensis (Ft). The history weaponization of Ft in the past has elevated concerns that it could be used as a bioweapon or an agent of bioterrorism. Since the discovery of Ft, three broad approaches adopted for tularemia vaccine development have included inactivated, live attenuated, or subunit vaccines. Shortcomings in each of these approaches have hampered the development of a suitable vaccine for prevention of tularemia. Recently, we reported an oxidant sensitive mutant of Ft LVS in putative EmrA1 (FTL_0687) secretion protein. The emrA1 mutant is highly sensitive to oxidants, attenuated for intramacrophage growth and virulence in mice. We reported that EmrA1 contributes to oxidant resistance by affecting the secretion of antioxidant enzymes SodB and KatG. This study investigated the vaccine potential of the emrA1 mutant in prevention of respiratory tularemia caused by Ft LVS and the virulent SchuS4 strain in C57BL/6 mice. We report that emrA1 mutant is safe and can be used at an intranasal (i. n.) immunization dose as high as 1x106 CFU without causing any adverse effects in immunized mice. The emrA1 mutant is cleared by vaccinated mice by day 14–21 post-immunization, induces minimal histopathological lesions in lungs, liver and spleen and a strong humoral immune response. The emrA1 mutant vaccinated mice are protected against 1000–10,000LD100 doses of i.n. Ft LVS challenge. Such a high degree of protection has not been reported earlier against respiratory challenge with Ft LVS using a single immunization dose with an attenuated mutant generated on Ft LVS background. The emrA1 mutant also provides partial protection against i.n. challenge with virulent Ft SchuS4 strain in vaccinated C57BL/6 mice. Collectively, our results further support the notion that antioxidants of Ft may serve as potential targets for development of effective vaccines for prevention of tularemia.
Introduction
Tularemia is a disease caused by a gram-negative, intracellular bacterial pathogen Francisella tularensis (Ft). The history of Ft weaponization has been documented by Japan, the former Soviet Union, and the United States [1,2]. This history has generated concerns regarding the potential use of Ft as a bioweapon or as an agent of bioterrorism [1,3,4,5]. Nonspecific symptoms of tularemia and the engineered antibiotic resistant strains undermine therapeutic options. In the last hundred years since the discovery of Ft, three broad approaches including inactivated, live attenuated, or subunit vaccines have been employed for tularemia vaccine development, but none of them have been successful [6,7,8,9,10,11,12,13]. Although, a Live Vaccine Strain (LVS) developed from the Russian strain Ft subspecies holarctica S15 is protective, it retains residual virulence in humans when immunized via aerosol or intranasal (i.n.) routes. Ft LVS is not approved by the US Food and Drug Administration for mass immunizations in the USA due to adverse reactions and residual virulence. Live attenuated mutants containing single gene deletions of the highly virulent category A Select Agent Ft SchuS4 strain have shown better protective efficacy than the Ft LVS [14]. However, the possibility of reversion of these mutants to fully virulent form is hampering their development into effective vaccines. Double or multiple gene deletion mutants of both the Ft LVS and SchuS4 strains which may not revert to virulent form are hyper-attenuated and fail to render any protection against i.n. challenge with Ft SchuS4. Inactivated Ft LVS or SchuS4 vaccines do not protect against virulent Ft [8,15,16] and subunit vaccines have been shown to possess limited protective ability due to the lack of a suitable platform for delivering multiple protective antigens simultaneously [7]. Collectively, these shortcomings have hampered the development of a suitable vaccine for prevention of tularemia.
Our previous work has demonstrated that the antioxidant mutant of Ft LVS carrying a point mutation in iron-containing superoxide dismutase gene (sodB) is partially attenuated for virulence in mice [17]. The sodB mutant when used as a vaccine protected 100% of BALB/c (unpublished data) and 40% of C57BL/6 mice against a lethal i.n. challenge with virulent Ft SchuS4 strain. The loss of SodB results in upregulation of several immunogenic proteins in the sodB mutant of Ft LVS [13]. Further, the catalase (KatG) of Ft LVS has been implicated in the suppression of host immune responses and evidence suggests that this antioxidant enzyme of Ft inhibits redox-sensitive signaling components to suppress innate immune responses of the host [18]. These findings indicate that antioxidant defenses of Ft LVS, specifically SodB and KatG may serve as potential targets for further vaccine development. Our observations are further supported by studies conducted using a modified BCG vaccine [19]. It has been reported that the BCG strain with diminished antioxidants was safer, persisted less than the parent BCG following vaccination, and provided greater protection against aerosolized challenge with Mycobacterium tuberculosis. The modified BCG deficient in antioxidant defenses when used as a vaccine, induces stronger immune response and enhanced recall response upon subsequent challenge with virulent M. tuberculosis [19].
Recently, we reported an oxidant-sensitive mutant of Ft LVS in putative EmrA1 (FTL_0687) secretion protein. We observed that the emrA1 mutant is highly sensitive to oxidants, and is attenuated for intramacrophage growth and virulence in mice [20]. Further investigations revealed that EmrA1 contributes to oxidant resistance by affecting secretion of antioxidant enzymes SodB and KatG. Further characterization of the emrA1 mutant revealed phenotypes characteristics of both the sodB and ΔkatG mutants of Ft LVS [20]. Based on the success of the sodB vaccine and the potential of KatG in the modulation of host’s immune response, in this study we investigated the vaccine potential of the emrA1 mutant of Ft LVS in prevention of experimental respiratory tularemia in C57BL/6 mice. We report that the emrA1 mutant is safe and can be used i.n. at an immunization dose as high as 1x106 CFU without causing any adverse effects in immunized mice, is cleared by the vaccinated mice by day 14–21 post-immunization, induces minimal histopathology in lungs, liver and spleen, elicits a strong humoral immune response, and protects against 1000–10,000LD100 doses of i.n. Ft LVS challenge. The emrA1 mutant also provides partial protection against i.n. challenge with virulent Ft SchuS4 strain in vaccinated C57BL/6 mice. Collectively, these findings further support the notion that antioxidants of Ft may serve as potential targets for development of effective vaccines for prevention of tularemia.
Materials and Methods
Ethics Statement
This study was carried out in strict accordance with the recommendations and guidelines of National Council for Research (NCR) for care and use of animals. All the animal experiments were conducted in the centralized Animal Resources Facilities of Albany Medical College and New York Medical College licensed by the USDA and the NYS Department of Health, Division of Laboratories and Research and accredited by the American Association for the Accreditation of Laboratory Care. The use of animals and protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Albany Medical College (Protocol Number 12–01001) and New York Medical College (Protocol Number 63-2-1213H). Mice were administered an anesthetic cocktail consisting of ketamine (5 mg/kg) and xylazine (4 mg/kg) and underwent experimental manipulation only after they failed to exhibit a toe pinch reflex. Mice exhibiting more than 20% weight loss, anorexia, dehydration and impairment of mobility were removed from the study and euthanized by approved means. Humane endpoints were also necessary for mice which survived at the conclusion of the experiment. Mice were administered an anesthetic cocktail of ketamine and xylazine intraperitoneally and then euthanized via cervical dislocation followed by cardiac puncture, a method that is consistent with Albany Medical College GLP Standard Operating Procedure on “Guidelines for Animal Euthanasia” (Document Number ARF-VC-004) and recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. In all experimental procedures, efforts were made to minimize pain and suffering.
Bacterial Strains
Ft LVS was obtained from BEI Resources (Manassas, VA). Ft SchuS4 used for challenge experiments was obtained from the U.S. Army Medical Research Institute for Infectious Diseases (USAMRIID, Frederick, MD). The emrA1 mutant (FTL_0687) as reported earlier was identified by screening transposon mutants of Ft LVS in the presence of H2O2 [20]. All the Francisella strains were cultured in Mueller Hinton Broth (MHB) or MH-chocolate agar plates as described previously [21,22]. All experiments involving Ft SchuS4 were conducted in CDC approved BSL3/Animal BSL3 facility of Albany Medical College, NY.
Mice
C57BL/6 mice (Charles River laboratories, NY) used in this study were maintained in a specific pathogen free environment in the Animal Facility of New York Medical College or Albany Medical College. All mice used in the experiment were six to eight weeks old of either sex. Prior to intranasal inoculations or experimental manipulations, mice were anesthetized via intraperitoneal injection of ketamine/xylazine cocktail. In all experimental procedures, efforts were made to minimize pain and suffering. All immunization and challenge experiments with Ft LVS were conducted in the Animal Facility of New York Medical College; while those with Ft SchuS4 were conducted in the Animal BSL3 facility of Albany Medical College. All the animal experiments were conducted according to the protocols approved by the IACUC at New York Medical College and Albany Medical College.
Immunizations
Mice were given a single i.n. immunization dose of ~1x106CFU of the emrA1 mutant in a 20μL (10 μL/nostril) suspension per mouse. The immunized mice were observed daily for clinical signs, morbidity, mortality, and weight loss. The immunized mice were challenged on day 42 post-primary immunizations. In experiments testing safety of the emrA1 mutant vaccine, equal numbers of C57BL/6 mice were inoculated i.n. with 1x106 CFU of Ft LVS as controls.
For determination of the protective efficacy of the emrA1 mutant vaccine against the Ft SchuS4 challenge, various vaccination regimens were adopted. Mice were either immunized with 1x106 CFU of the emrA1 i.n. as a single dose; or an additional booster was given on day 21 of the primary immunization. Additionally, mice were first immunized i.n. and then boosted via intradermal (i.d.) route with 1x106 CFU of the emrA1 mutant on day 0 and day 21 respectively; or were first immunized via the i.d. route followed by a booster vaccination given by i.n. route. Based on observations that killed Ft LVS complexed with anti-Ft LPS monoclonal antibodies provides better protection against Ft SchuS4 than killed Ft LVS alone, we complexed 1x106 CFU of the emrA1 mutant with 5μg of anti-Ft LPS monoclonal antibodies (emrA1-mAb) following the protocol described earlier [23]. Mice were immunized either with a single dose of emrA1-mAb or boosted on day 21; or immunized alternatively using i.n. priming followed by i.d. booster on day 21 and vice-versa. Finally, mice were also immunized with a low dose of 1x103 CFU of the emrA1 mutant and boosted with this low dose on day 21 of the primary immunization using alternate i.n. or i.d. inoculations as described above before being challenged i.n. with Ft SchuS4.
Challenge
Mice were challenged i.n. with 1x107 or 1x108 CFU of Ft LVS after 42 days post-primary immunization. To determine the duration of immunity rendered by the emrA1 vaccine, the immunized mice were also challenged i.n. with 1x107 CFU of Ft LVS day 75 post-primary immunization. The protective efficacy of the emrA1 mutant vaccine against Ft SchuS4 was determined by challenging immunized mice with Ft SchuS4 on day 42 post-primary immunization. Mice were observed for morbidity and mortality for a period of 21 days. The survival data is represented as Kaplan-Meier survival curves and the statistical significance was determined by Log-Rank test. The immunization and challenge schedules are shown in Table 1.
Table 1. Immunization and challenge schedules used in this study.
| Vaccine | Primary Immunization | Booster Immunization** | Challenge Strain | Challenge*** | Time of challenge (Days)**** | |||
|---|---|---|---|---|---|---|---|---|
| Dose (CFU) | Route | Dose | Route | Dose (CFU) | Route | |||
| emrA1 Mutant | 1x106 | i.n. | - | - | Ft LVS | 1x107 | i.n. | 42 |
| 1x106 | i.n. | - | - | Ft LVS | 1x108 | i.n. | 42 | |
| 1x106 | i.n. | - | - | Ft LVS | 1x107 | i.n. | 75 | |
| 1x106 | i.n. | - | - | Ft SchuS4 # | 32 | i.n. | 21 | |
| 1x106 | i.n. | 1x106 | i.n. | Ft SchuS4 | 38 | i.n. | 42 | |
| 1x106 | i.n. | 1x106 | i.d. | Ft SchuS4 | 17 | i.n. | 42 | |
| 1x106 | i.d. | 1x106 | i.n. | Ft SchuS4 | 17 | i.n. | 42 | |
| 1x103 | i.n. | 1x103 | i.d. | Ft SchuS4 | 23 | i.n. | 42 | |
| 1x103 | i.d. | 1x103 | i.n. | Ft SchuS4 | 24 | i.n. | 42 | |
| emrA1 Mutant-mAb complex* | 1x106 | i.n. | - | - | Ft SchuS4 | 32 | i.n. | 21 |
| 1x106 | i.n. | 1x106 | i.d. | Ft SchuS4 | 17 | i.n. | 42 | |
| 1x106 | i.d. | 1x106 | i.n. | Ft SchuS4 | 17 | i.n. | 42 | |
*emrA1 mutant was complexed with 5μg/ml of anti-Ft LVS LPS monoclonal antibodies
**Booster vaccinations were administered on day 21 of the primary immunization
***Equal doses of Ft LVS or SchuS4 were administered i.n. to unvaccinated control mice
****Days post-primary immunization
# The target dose for all Ft SchuS4 challenge experiments was 25 CFU. The actual CFU administered are shown.
i.n. = Intranasal; i.d. = Intradermal; mAb = Monoclonal antibodies.
Post-immunization and post-challenge studies
Kinetics of bacterial clearance and histopathology
All mice following vaccination and challenge were weighed periodically to determine the progression of disease. Mice were bled by retro-orbital venipuncture to collect serum for determination of Ft specific antibodies. Mice were sacrificed on days 1, 5, 7, 14 and 21 post-immunization; and on days 5, 7 and 14 following challenge with Ft LVS. Lungs of the immunized and challenged mice were inflated with sterile PBS and excised aseptically. The liver and spleen were also removed aseptically. The right lobe of the lung and small portions of liver and spleen were stored in 10% formalin for histopathological evaluations. Lung, liver and spleen homogenates were prepared in a bead- beater using sterile zirconia beads, diluted ten-fold, and plated on chocolate agar plates for quantitation of bacterial numbers as described earlier [13]. The remaining lung and spleen homogenates were spun at 10,000 rpm for 10 minutes to remove the tissue debris. The clear supernatants were collected and used for quantitation of pro-inflammatory cytokines in lung and spleen. For histopathological evaluation, the organs were paraffin embedded, sectioned and stained with hematoxylene and eosin (H&E). The H&E stained sections were observed under light microscope and images were taken.
Pro-inflammatory cytokine analysis
Levels of pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), macrophage chemotactic protein (MCP-1), interleukin-12 (IL-12), interferon-gamma (IFN-γ) and interleukin-10 (IL-10) were measured in lung and spleen homogenates. Mouse inflammation kit (BD Biosciences) was used for the simultaneous quantitation of these pro-inflammatory cytokines as previously described [13].
Quantitation of Ft specific antibodies
Ft specific total IgG, IgG2a, IgG2b, IgG1 and IgA levels were quantitated in sera samples collected from immunized and challenged mice at various time intervals by ELISA. The plates were coated with Ft LVS lysates and the ELISA was performed using standard procedure.
Statistical analysis
All results were expressed as Means ± S.D or Mean ± SEM. Statistical comparisons between the groups were made using one-way ANOVA followed by Tukey-Kramer multiple comparison test. Survival results were plotted as Kaplan-Meier survival curves. Differences between the experimental groups were considered statistically significant at a P < 0.05 level. The data were statistically analyzed using GraphPad and InStat software.
Results
Mice immunized with the emrA1 mutant initially lose their body weight but rapidly regain it back
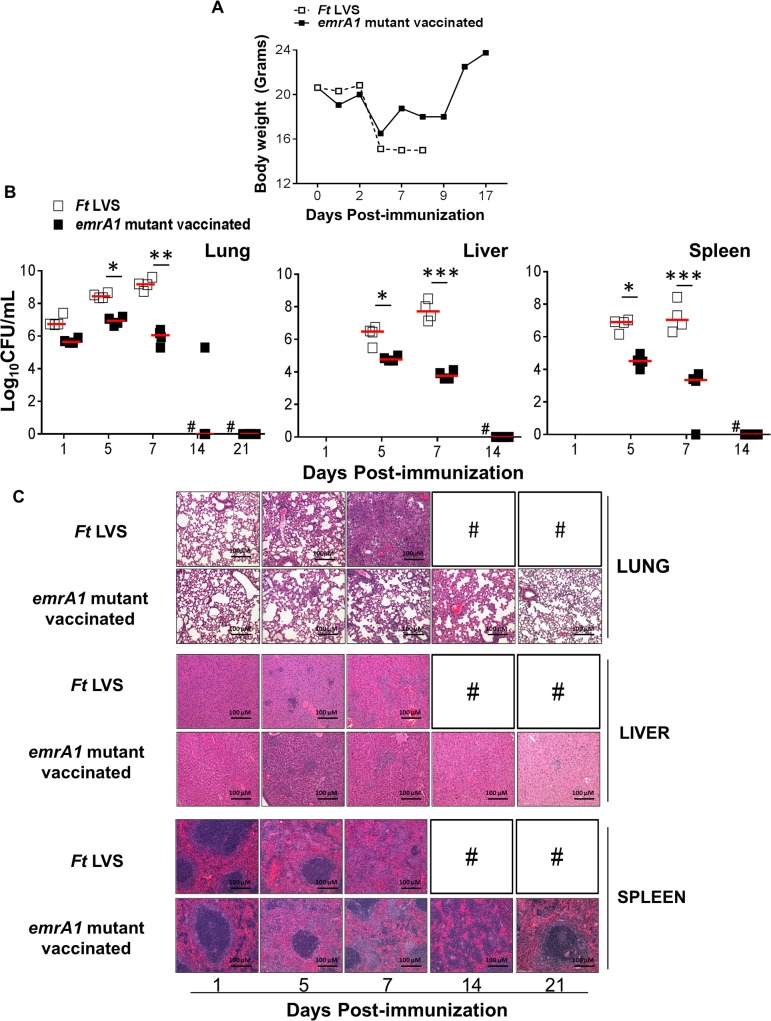
We have recently reported that the emrA1mutant of Ft LVS is highly attenuated for virulence and doses as high as 1x105 or 1x106 CFU administered i.n. do not cause any mortality in infected mice [20]. To determine the vaccine potential of the emrA1 mutant, we first investigated the safety of this mutant strain in C57BL/6 mice. Mice were immunized i.n. with 1x106 CFU of the emrA1 mutant and observed for any morbidity by determining the body weight, kinetics of bacterial clearance, and histopathological changes observed in lungs, liver and spleen. Mice infected with a similar dose of wild type Ft LVS were included as controls. It was observed that similar to mice infected with Ft LVS, those immunized with the emrA1 mutant did not show any changes in body weight for first three days, which was followed by a sudden weight loss by day 5 post-immunization. However, the emrA1 mutant immunized mice started to regain their body weights by day 7 and were over their initial body weights by day 17 post-immunization. Contrary to this, the Ft LVS infected mice did not regain their weights after day 5 post-infection and 100% mice succumbed to infection by day 8 post-infection (Fig 1A).
Fig 1. Immunization with the emrA1 mutant results in minimal weight loss, rapid bacterial clearance, and histopathological lesions in lung, liver and spleen.
C57BL/6 mice were immunized i.n. with 1×106 CFU of the emrA1 mutant. Mice infected with equal numbers of wild type Ft LVS were used as controls. (A) The immunized mice were weighed at the indicated times post-immunization to track the progress of infection. (B) On days 1, 5, 7, 14 and 21 post-immunization, mice (n = 4 per group/time point) were euthanized and bacterial burdens were quantified in their lung, liver and spleen. Bacterial counts in organs are expressed as Log10CFU/mL. The P values were determined using one way ANOVA. *P<0.05; **P<0.01; ***P<0.001. (C) Excised lungs, livers and spleens were preserved in 10% formalin, paraffin embedded, sliced into 5 μM thin sections and stained with Hematoxylene & Eosin. Stained sections were observed for histopathological lesions under a light microscope (Magnification 100×). # = Ft LVS infected mice succumbed to infection.
Mice immunized with the emrA1 mutant clear bacteria by day 14–21 post-immunization
We determined the kinetics of bacterial clearance in mice immunized with 1x106 CFU of the emrA1 mutant by quantitating the bacterial load in lung, liver and spleen on days 1, 5, 7, 14 and 21 post-immunization. It was observed that the numbers of emrA1 mutant went up nearly fivefold by day 5 as compared to day 1 post-immunization and then gradually reduced and were completely cleared from the lungs by day 21 post-immunization. Contrary to this, the bacterial numbers went up exponentially in the lungs of mice infected with 1x106 CFU of Ft LVS and peaked at day 7 post-infection following which 100% of these mice died. The emrA1 mutant disseminated to liver and spleen; however, significantly lower bacterial numbers were observed on days 5 and 7 post-immunization as compared to those observed in Ft LVS infected mice. The emrA1 mutant bacteria were completely cleared from liver and spleen by day 14 post-infection whereas in Ft LVS infected mice, the bacterial numbers increased from days 5 to 7 post-infection (Fig 1B). These results indicate that the emrA1 mutant is cleared by mice despite a high immunization dose by day 14 from liver and spleen, and by day 21 from lungs. These results also indicate that the emrA1 mutant, although attenuated for virulence, survives in host for a length of time that may be sufficient to induce an effective immune response [21].
Mice immunized with the emrA1 mutant induce minimal histopathological lesions in lung, liver and spleen
We next examined the histopathological lesions in lung, liver, and spleen of mice immunized with 1x106 CFU of the emrA1 mutant and compared with those observed in mice infected with a similar dose of Ft LVS. The lesions in the lungs of Ft LVS infected mice progressed from patchy inflammatory foci to severe inflammation (day 5 post-infection), consolidation of lungs, and necrotizing pneumonia (day 7 post-infection). However, no such lesions were observed in the lungs of the emrA1 mutant immunized mice and lung sections appeared normal except for focal inflammatory lesions on day 5 and 7 post-infection which were resolved completely by day 21 post-immunization. The livers of Ft LVS mice revealed progressive development of granulomas from day 5 to day 7 post-infection which eventually collapsed and became necrotic by day 7 post-infection. Granulomas were also observed in livers from the emrA1 mutant immunized mice on day 5 post-immunization. However, these granulomas started to resolve by day 7 and were not observed by day 14–21 post-immunization. Similar to lung and liver, severe histopathological lesions were also observed in the spleen of Ft LVS infected mice. These lesions were far less severe in the emrA1 mutant immunized mice and were associated with proliferation of germinal centers by day 7 and started to resolve by day 14 and reverted back to normal architecture by day 21 post-immunization (Fig 1C).
Collectively, these results demonstrate that mice can tolerate 1x106 immunization dose of the emrA1 mutant and quickly regain their body weights. The emrA1 mutant is cleared by day 14–21 post-immunization without causing extensive pathological changes in lung, liver and spleen indicating that this mutant is safe as a vaccine.
Mice immunized with the emrA1 mutant induce regulated production of pro-inflammatory cytokines and a potent antibody response
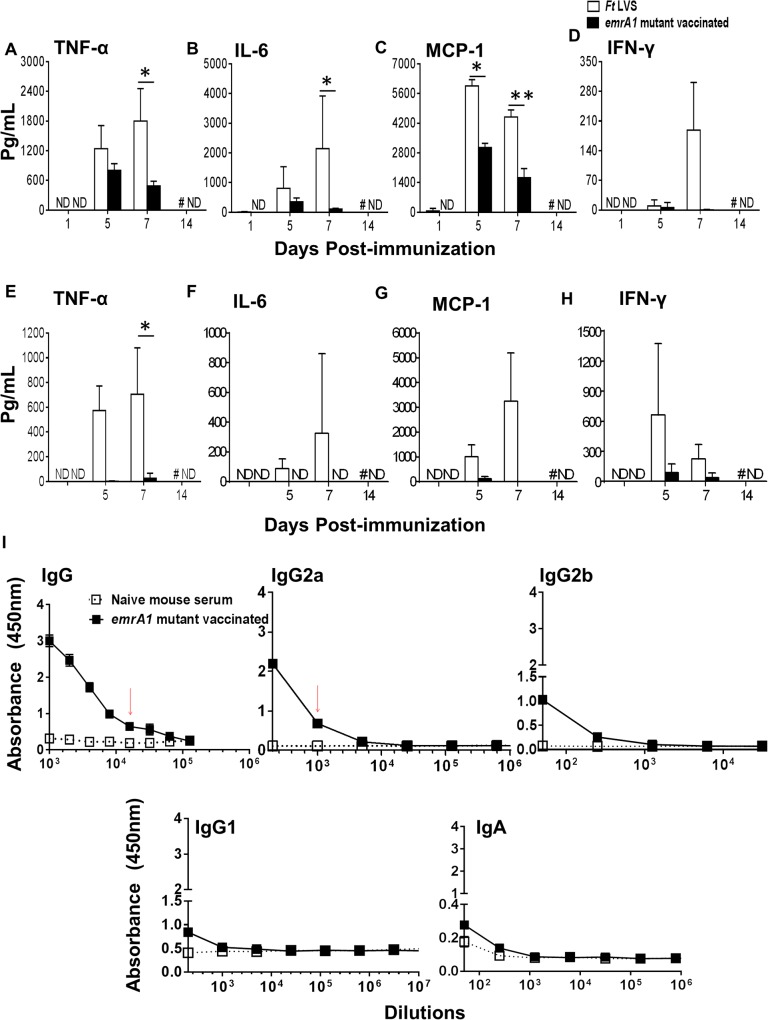
We determined the levels of pro-inflammatory cytokines TNF-α, IL-6, MCP-1, and IFN-γ in mice immunized with 1x106 CFU of the emrA1 mutant and compared with those observed in mice infected with an equal dose of Ft LVS on days 1, 5, 7, and 14 post-immunization. None of the four cytokines were detected on day 1 post-immunization in the lung homogenates of either the emrA1 mutant or Ft LVS infected mice. The levels of all four cytokines increased gradually thereafter and were significantly elevated in Ft LVS infected mice on day 7 post-infection. Significantly elevated levels of MCP-1 were also observed in Ft LVS infected mice on day 5 post-infection. These results indicate that infection of mice with 1x106 CFU of Ft LVS which is 100 times the LD100 dose result in a cytokine storm which is usually followed by death. Contrary to this, in the lung homogenates of the emrA1 mutant immunized mice, the levels of these pro-inflammatory cytokines peaked at day 5 and gradually reduced by day 7 post-immunization before returning to the baseline values (Fig 2A–2D). The pattern of pro-inflammatory cytokines in spleens of Ft LVS infected mice was similar to that observed in lungs with peak levels observed at day 7 post-infection. However, very low to undetectable levels of TNF-α, IL-6, MCP-1 and IFN-γ were observed in spleens of the emrA1 mutant immunized mice (Fig 2E–2H). Furthermore, no detectable levels of anti-inflammatory cytokine IL-10 were observed in lung or spleen homogenates of mice immunized with the emrA1 mutant. These results indicate that mice immunized with the emrA1 mutant induce regulated production of pro-inflammatory cytokines which may be associated with reducing bacterial load (Fig 1B). Further, lower levels of these cytokines also inflict less pathology observed in the lungs and spleens of the emrA1 immunized mice as compared to the Ft LVS infected mice (Fig 1C).
Fig 2. Mice immunized with the emrA1 mutant induce regulated production of pro-inflammatory cytokines and a potent antibody response.
C57BL/6 mice were immunized i.n. with 1×106 CFU of the emrA1 mutant or Ft LVS. On days 1, 5, 7 and 14 post-immunization, mice (n = 4 per group/time point) were euthanized and their excised lungs and spleens were homogenized. Clear lung (A-D) and spleen (E-H) homogenates were used for quantification of indicated pro-inflammatory cytokines using flow cytometric analysis. The data are represented as Mean ± S.D. The P values were determined using one way ANOVA. *P<0.05; **P<0.01. (I) On day 42 post-immunization, mice (n = 3 per group/ time point) were anesthetized and bled retroorbitally to obtain serum. Ft specific total IgG, IgG2a, IgG2b, IgG1 and IgA levels in serum samples were determined by ELISA. The data are represented as Mean ± S.D. of absorbance values measured at 450 nm. Red arrows indicate antibody titers. # = Ft LVS immunized mice succumbed to infection; ND = Not detected.
We also determined the levels of Ft LVS specific total IgG, IgG2a, IgG2b, IgG1 and IgA levels in sera collected from the emrA1 immunized mice on day 42 post-immunization, the time at which the immunized mice were challenged. A potent antibody response comprising of Ft specific total IgG and IgG2a antibodies was observed in mice immunized with the emrA1 mutant. On the other hand, very low levels of IgG2b, IgG1 and IgA antibodies were observed in the immunized mice (Fig 2I).
Mice immunized with the emrA1 mutant are protected against 1000LD100–10,000LD100 challenge dose of Ft LVS
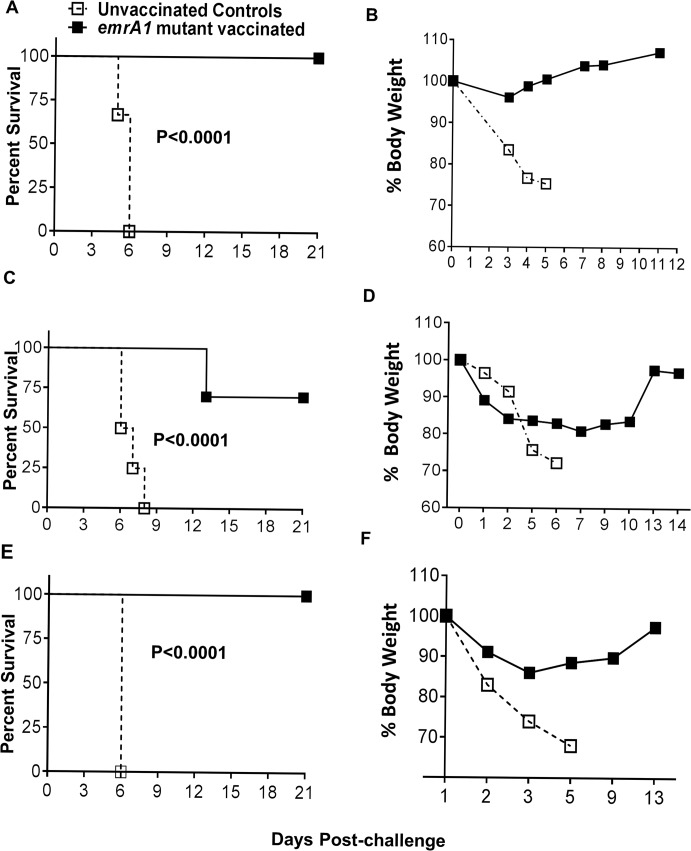
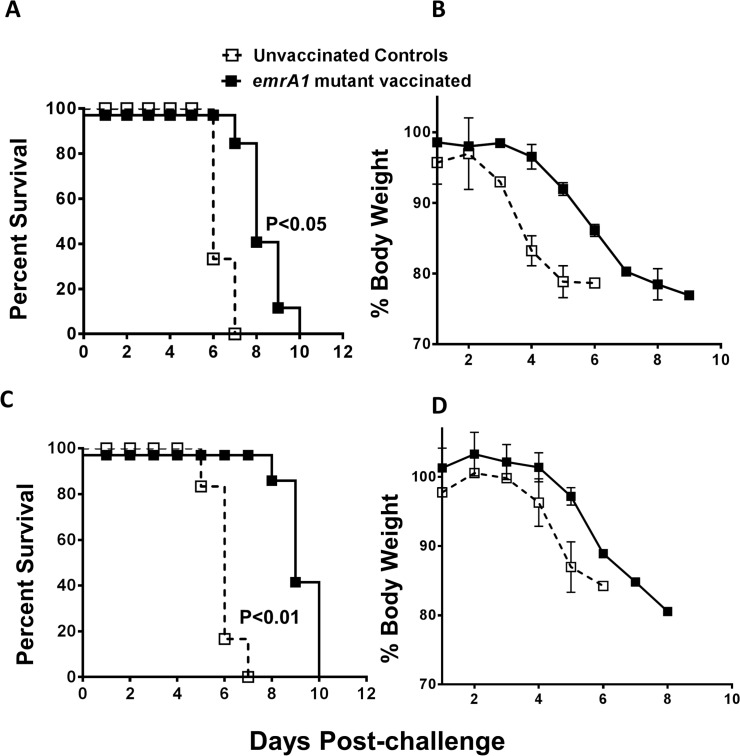
The LD100 dose of Ft LVS in our hands is 1x104 CFU by the i.n. route. To determine the protective efficacy of the emrA1 mutant vaccine, we challenged the emrA1 mutant immunized mice with 1x107 (1000LD100) or 1x108 (10,000LD100) CFU of wild type Ft LVS by i.n. route. It was observed that 100% of C57BL/6 mice immunized with the emrA1 mutant and challenged with 1x107 CFU of Ft LVS survived until day 21 post-challenge at which time the experiment was terminated. All the unvaccinated control mice that received similar challenge dose died by day 6 post-challenge. The emrA1 mutant immunized mice exhibited minimal loss of body weight following the challenge whereas majority of unvaccinated controls lost more than 20% of their body weight by day 5 post-challenge were removed from the study and euthanized (Fig 3A and 3B). When the challenge dose was increased to 1x108 CFU of Ft LVS, 70% (7/10) mice immunized with the emrA1 mutant survived the challenge. The emrA1 mutant immunized mice receiving the higher challenge dose showed reduction in body weight for first two days post-challenge which remained unchanged till day 10 after which these mice regained their starting body weights. The unvaccinated controls lost their body weights quickly and either succumbed to the challenge dose or removed from the study if the weight loss was more than 20% of their starting body weight (Fig 3C and 3D).
Fig 3. Mice immunized with the emrA1 mutant are protected against 1000LD100–10,000LD100 challenge dose of Ft LVS.
C57BL/6 mice (n = 5–10 per group) were immunized i.n. with 1×106 CFU of the emrA1 mutant. (A, B) On day 42 of the primary immunization mice were challenged i.n. with 1×107 CFU of wild type Ft LVS. Age matched unvaccinated mice challenged with a similar dose of Ft LVS were kept as controls. (C, D) On day 42 of the primary immunization mice were challenged i.n. with 1×108 CFU of wild type Ft LVS. Age matched unvaccinated mice challenged with a similar dose of Ft LVS were kept as controls. (E, F) On day 75 of the primary immunization mice were challenged i.n. with 1×107 CFU of wild type Ft LVS. Age matched unvaccinated mice challenged with a similar dose of Ft LVS were kept as controls. The Challenged mice were observed for morbidity and mortality for a period of 21 days post-challenge (A, C, E). The mice were weighed at the indicated times post-challenge to monitor the progression of infection (B, D, F). The survival results are expressed as Kaplan-Meier survival curves and P values were determined by Log-rank test. Body weights of mice are expressed percent body weights.
To determine the duration of immune protection, mice immunized with 1x106 CFU of the emrA1 mutant were challenged with 1x107 CFU of Ft LVS on day 75 of the primary immunization. One hundred percent of mice that received this challenge dose survived and showed a weight loss pattern similar to that observed for mice challenged with a similar dose on day 42 post-primary immunization (Fig 3E and 3F).
Collectively, these data demonstrate that emrA1 mutant is not only safe but also provides protection against extremely high challenge doses of Ft LVS. In addition, the protective immune response is maintained against a 1000LD100 challenge dose until day 75 after a single dose immunization indicating a prolonged period of immune protection.
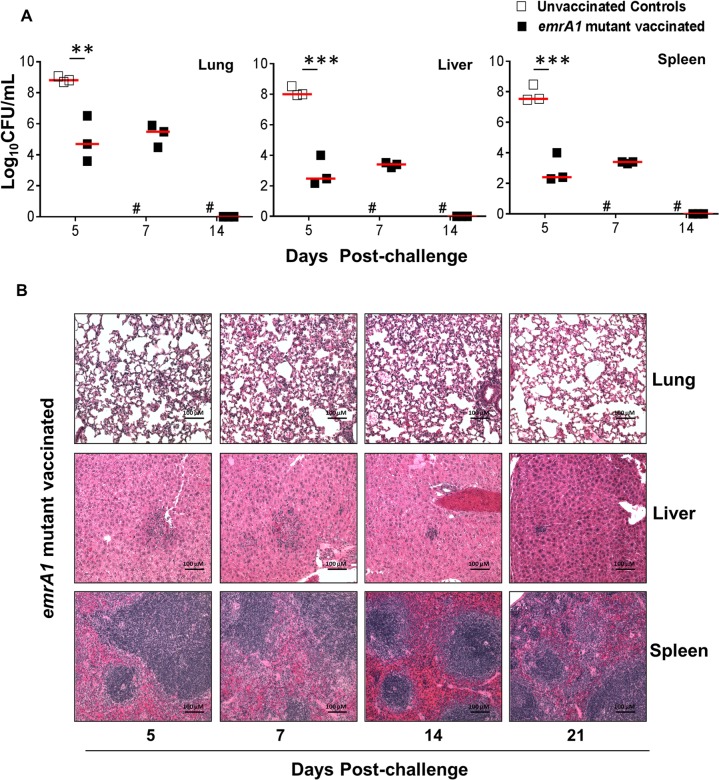
The emrA1 mutant vaccinated mice clear bacteria rapidly and exhibit minimal histopathological lesions in lung, liver and spleen following lethal Ft LVS challenge
We observed that 100% of mice immunized with the emrA1 mutant and challenged i.n. with 1x107 CFU of Ft LVS survived the infection. We next investigated the kinetics of bacterial clearance in vaccinated mice on days 5, 7 and 14 following the challenge. The bacterial numbers recovered from lungs of the vaccinated mice were nearly 20-fold lower than the challenge dose on day 5 post-challenge. On the contrary, these numbers were nearly 20-fold higher in the lungs of unvaccinated controls. On day 7 post-challenge, the bacterial numbers in the lungs of emrA1 mutant vaccinated mice remained similar to those observed for day 5 and were cleared completely and became undetectable by day 14 post-challenge. Similar patterns were also observed for bacterial numbers recovered from livers and spleens of the emrA1 mutant vaccinated and challenged mice. However, the bacterial numbers recovered from liver and spleens were much lower than those observed in the lungs of the vaccinated mice (Fig 4A). These results indicate that the emrA1 mutant vaccinated mice clear Ft very rapidly upon an extremely lethal challenge.
Fig 4. The emrA1 mutant vaccinated mice clear bacteria rapidly and exhibit minimal histopathological lesions in lung, liver and spleen following lethal Ft LVS challenge.
C57BL/6 mice immunized i.n. with 1×106 CFU of the emrA1 mutant or the unvaccinated control mice were challenged i.n. with 1×107 CFU of Ft LVS 42 days post-immunization. (A) On days 5, 7, and 14 post-challenge, mice (n = 3 per group/time point) were euthanized and bacterial burdens were quantified in their lung, liver and spleen. Bacterial counts in organs are expressed as Log10 CFU/mL. The P values were determined using one way ANOVA. **P<0.01; ***P<0.001. (B) Lungs, livers and spleens collected at the indicated times post-challenge were preserved in 10% formalin, embedded in paraffin blocks, sliced into 5 μM thin sections and stained with Hematoxylene & Eosin. Stained sections were observed for histopathological lesions under a light microscope (Magnification 100×). # = Unvaccinated mice succumbed to infection.
We also evaluated histopathological lesions in lung, liver and spleen of emrA1 mutant vaccinated mice challenged with 1x107 CFU of Ft LVS on days 5, 7, 14 and 21 post-challenge. As observed upon immunization, the emrA1 mutant vaccinated mice exhibited minimal pathological lesions consisting of mild inflammatory foci in lungs on days 5 and 7 post-challenge. No lesions were observed in emrA1 vaccinated and Ft LVS challenged mice on days 14 and 21 post-challenge. Liver showed formation of granulomas on day 5 post-challenge. These granulomas gradually reduced in size and very small granulomas were observed on day 21 post-challenge even when bacteria were completely cleared from liver. The splenic architecture of the emrA1 mutant vaccinated mice remained unaltered on day 5 and 7 post-challenge; however, proliferation of germinal centers were observed on days 14 and 21 post-challenge again at a time when bacteria were not recovered from the spleens (Fig 4B). Collectively, these results demonstrate that mice immunized with the emrA1 mutant clear subsequent Ft challenge very efficiently and do not exhibit extensive pathological lesions in lung, liver or spleen.
The emrA1 mutant vaccinated mice induce sustained production of pro-inflammatory cytokines and a potent antibody response following lethal Ft LVS challenge
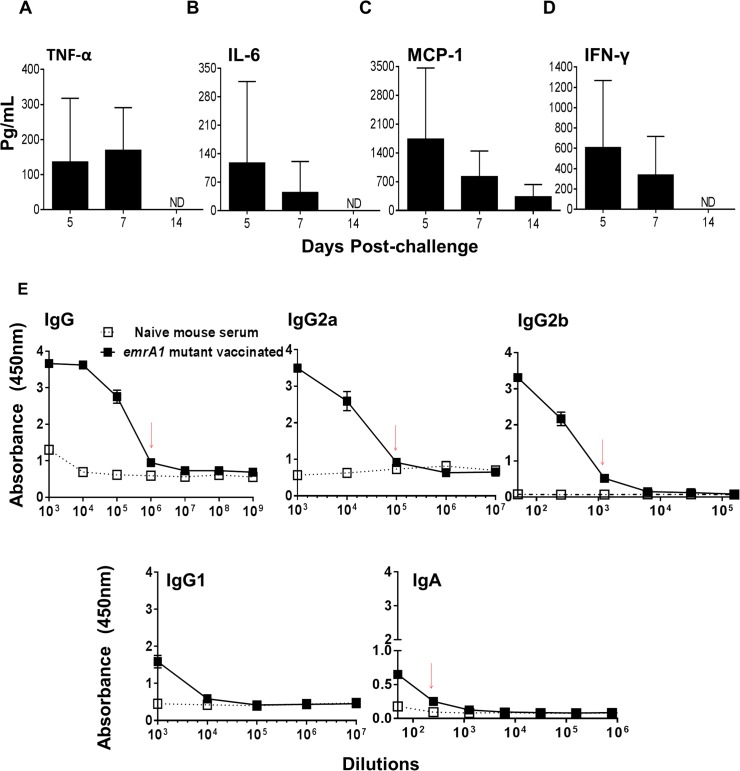
We next investigated the levels of pro-inflammatory cytokines in mice vaccinated with the emrA1 mutant and challenged with 1x107 CFU of Ft LVS on days 5, 7 and 14 post-challenge. Unlike the pro-inflammatory cytokine profiles observed following vaccination with the emrA1 mutant, the challenged mice showed sustained production of TNF-α and MCP-1 on days 5 and 7 post-challenge. Further, the MCP-1 levels were detected until day 14 post-challenge. The IFN-γ, which was undetectable in lung homogenates of mice following vaccination with the emrA1 mutant, was found in high levels in lung homogenates of mice challenged with Ft LVS on days 5 and 7 post-challenge (Fig 5A–5D). Collectively, these results indicate that the emrA1 mutant vaccinated mice induce a sustained production and elevated levels of TNF-α, MCP-1 and IFN-γ following lethal Ft LVS challenge.
Fig 5. The emrA1 mutant vaccinated mice induce sustained production of pro-inflammatory cytokines and a potent antibody response following lethal Ft LVS challenge.
C57BL/6 mice immunized i.n. with 1×106 CFU of the emrA1 mutant were challenged i.n. with 1×107 CFU of wild type Ft LVS 42 days post-immunization. (A-D) On days 5, 7 and 14 post-challenge, mice (n = 3 per group/time point) were euthanized and their excised lungs were homogenized. Clear lung homogenates were used for quantification of indicated pro-inflammatory cytokines using flow cytometric analysis. The data are represented as Mean ± S.D. (E) On day 21 post-challenge, mice (n = 3 per group) were anesthetized and bled retroorbitally to obtain serum. Ft specific total IgG, IgG2a, IgG2b, IgG1 and IgA levels in serum samples were determined by ELISA. The data are represented as Mean ± S.D. of absorbance values measured at 450 nm. Red arrows indicate antibody titers. ND = Not detected.
We also determined the levels of Ft specific total IgG, IgG2a, IgG2b, IgG1 and IgA levels in mice challenged i.n. with Ft LVS. It was observed that Ft specific total IgG, IgG2a and IgG2b antibody titers were elevated by 10–100 folds by day 21 post-challenge as compared to those observed following immunization. Additionally, similar to those observed following immunization, low levels of Ft specific IgG1 and IgA antibodies were observed in challenged mice (Fig 5E). These results indicate that development of a potent IFN-γ-mediated innate immune response and rapid elevation of Ft specific total IgG, IgG2a and IgG2b levels in the emrA1 vaccinated mice following challenge may play an important role in rapid resolution of infection.
The emrA1 mutant vaccinated mice are partially protected against Ft SchuS4 challenge
Having observed a solid protection against i.n. challenge with 1000 to 10,000LD100 doses of Ft LVS, we next investigated if the emrA1 mutant immunized mice are protected against an i.n. challenge with highly virulent and category A select agent Ft SchuS4. C57BL/6 mice were immunized with a single dose of 1x106 CFU of the emrA1 mutant and challenged i.n. with 32 CFU of Ft SchuS4. All the control mice died by day 7 with a median time to death (MTD) of 6 days. The emrA1 mutant vaccinated mice survived longer but 100% of vaccinated mice died by day 10 with a significantly higher MTD of 8 days. The pattern of body weight loss of the control and the emrA1 mutant vaccinated mice did not differ except that the sudden drop in weight of vaccinated mice was observed by day 6 as opposed to day 3 post-challenge observed for control mice (Fig 6A and 6B). In order to see if protection against SchuS4 challenge could be improved further, following a primary immunization with 1x106 CFU of the emrA1 mutant, we also gave a booster with a similar vaccination dose i.n. on day 21 of the primary immunization. The vaccinated mice were challenged with 38 CFU of Ft SchuS4 on day 42 of the primary immunization. However, with this vaccination regimen too, all the vaccinated mice succumbed to Ft SchuS4 challenge with an MTD of 9 days which was significantly higher than the control mice (MTD = 6 days) (Fig 6C and 6D). We further modified our vaccination strategies by immunizing a group of mice with 1x106 CFU of the emrA1 mutant i.n. followed by a booster on day 21 with a similar dose i.d. Conversely, another group was immunized first i.d. and then boosted by i.n. route. These mice were challenged with 17 CFU of Ft SchuS4 on day 42 of the primary immunization. All control mice died by days 6–8 post-challenge. Both the groups of vaccinated mice succumbed to Ft SchuS4 challenge by day 13 post-challenge with an MTD of 10 days indicating a slight improvement over the previous vaccination regimen (Fig 7A and 7B).
Fig 6. The emrA1 mutant vaccinated mice are partially protected against Ft SchuS4 challenge.
C57BL/6 mice (n = 10 per group) were immunized i.n. with 1×106 CFU of the emrA1 mutant. (A) On day 21 of the primary immunization, mice were challenged i.n. with 32 CFU of Ft SchuS4. Age matched unvaccinated mice challenged with a similar dose of Ft SchuS4 served as controls. (B) The mice were weighed at the indicated times post-challenge to monitor the progression of infection. (C) C57BL/6 mice (n = 10 per group) were immunized i.n. with 1×106 CFU of the emrA1 mutant and boosted with a similar dose on day 21. On day 42 of the primary immunization mice were challenged i.n. with 38 CFU of Ft SchuS4. Age matched unvaccinated mice challenged with a similar dose of Ft SchuS4 served as controls. (D) The mice were weighed at the indicated times post-challenge to monitor the progression of infection. The survival results are expressed as Kaplan-Meier survival curves and P values were determined by Log-rank test. Body weights of mice are expressed as percent body weight and represented as Mean ± S.D of percent body weight.
Fig 7. Immunization with emrA1 mutant using a prime-boost vaccination regimen improves the extent of protection against Ft SchuS4 challenge.
(A) C57BL/6 mice (n = 10 per group) were immunized i.n. with 1×106 CFU of the emrA1 mutant and boosted i.d. on day 21 with a similar dose. On day 42 of the primary immunization mice were challenged i.n. with 17 CFU of Ft SchuS4. Age matched unvaccinated mice challenged with a similar dose of Ft SchuS4 served as controls. (B) C57BL/6 mice (n = 10 per group) were immunized i.d. with 1×106 CFU of the emrA1 mutant and boosted i.n. on day 21 with a similar dose. On day 42 of the primary immunization mice were challenged i.n. with 17 CFU of Ft SchuS4. Age matched unvaccinated mice challenged with a similar dose of Ft SchuS4 served as controls. The survival results are expressed as Kaplan-Meier survival curves and P values were determined by Log-rank test.
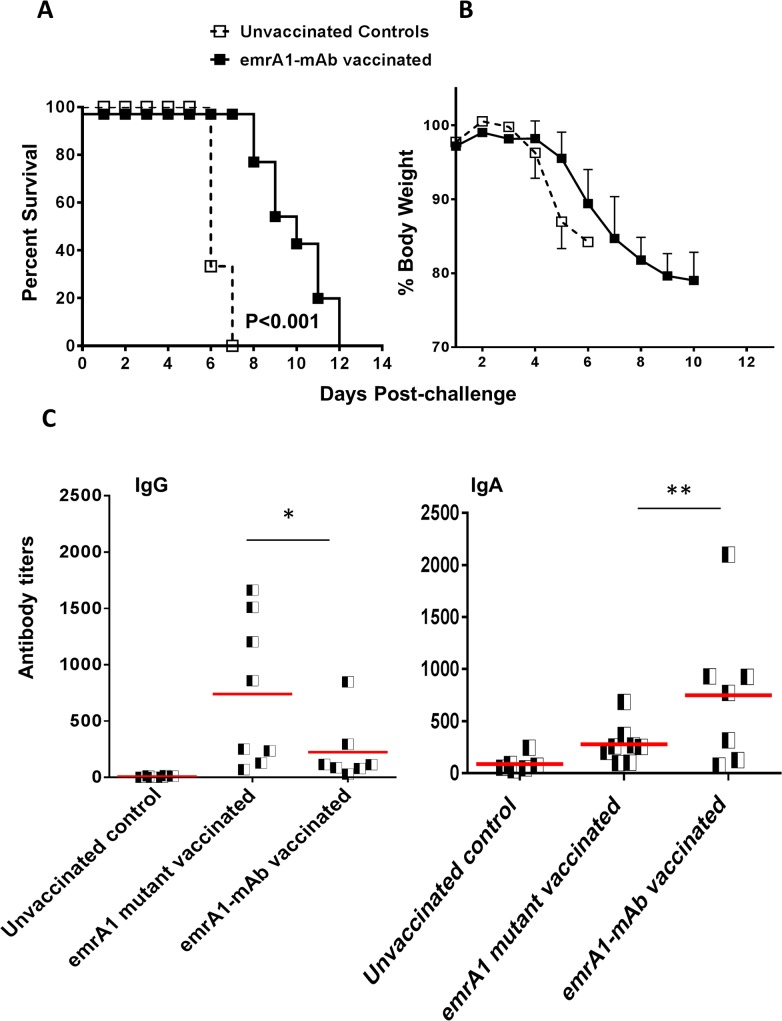
It has been shown that killed Ft LVS, when complexed with anti-Ft LPS monoclonal antibodies and used as a vaccine, enhances the protective efficacy against Ft SchuS4 challenge as compared to the killed Ft alone [23]. Based on this observation, we investigated next if immunizing mice with emrA1 mutant immune complexed with the anti-Ft LVS LPS monoclonal antibodies improves protection against Ft SchuS4 challenge. A single dose i.n. immunization with 1x106 CFU of the emrA1 mutant complexed with 5μg/ml of anti-Ft LPS monoclonal antibodies (emrA1-mAb) significantly enhanced the survival of mice challenged with 32 CFU of Ft SchuS4. The vaccinated mice survived as long as day 12 post-challenge with an MTD significantly higher than unvaccinated controls (Fig 8A and 8B). We next investigated if the difference in the duration of protection offered by emrA1-mAb is due to differences in profile of antibody responses. Indeed, the emrA1-mAb mice induced significantly elevated IgA levels as early as day 14 post-immunization as compared to those immunized with the emrA1 mutant alone (Fig 8C). On the contrary, mice vaccinated with the emrA1 mutant alone induced higher levels of total IgG antibodies (Fig 8C). Results from experiments conducted using alternate i.n. priming and i.d. boost or vice-versa with emrA1-mAb allowed mice to survive as long as day 15 and day 17 post-challenge with 17 CFU of Ft SchuS4, respectively (Fig 9A and 9B). Collectively, these results demonstrate that vaccination with the emrA1 mutant provides partial protection against Ft SchuS4 challenge.
Fig 8. Immunization with emrA1-mAb complexes improves the extent of protection against Ft SchuS4 challenge.
(A) C57BL/6 mice (n = 10 per group) immunized i.n. with 1×106 CFU of the emrA1 mutant-mAb immune complexes. On day 42 of the primary immunization mice were challenged i.n. with 32 CFU of Ft SchuS4. Age matched unvaccinated mice challenged with a similar dose of Ft SchuS4 served as controls. The survival results are expressed as Kaplan-Meier survival curves and P values were determined by Log-rank test. (B) The mice were weighed at the indicated times post-challenge to monitor the progression of infection. (C) The indicated Ft specific antibodies were determined in serum from immunized mice on day 14 post-immunization. The results are expressed as antibody titers.
Fig 9. Immunization with emrA1-mAb complexes using a prime-boost vaccination regimen or a low dose immunization improves the extent of protection against Ft SchuS4 challenge.
(A) C57BL/6 mice (n = 10 per group) were immunized i.d. with 1×106 CFU of the emrA1 mutant-mAb immune complex and boosted i.n. on day 21 with a similar dose. On day 42 of the primary immunization mice were challenged i.n. with 17 CFU of Ft SchuS4. Age matched unvaccinated mice challenged with a similar dose of Ft SchuS4 served as controls. (B) C57BL/6 mice (n = 10 per group) immunized i.n. with 1×106 CFU of the emrA1mutant-mAb immune complex and boosted i.d. on day 21 with a similar dose. On day 42 of the primary immunization mice were challenged i.n. with 17 CFU of Ft SchuS4. Age matched unvaccinated mice challenged with a similar dose of Ft SchuS4 served as controls. (C) C57BL/6 mice (n = 10 per group) immunized i.n. with 1×103 CFU of the emrA1 mutant and boosted i.d. on day 21 with a similar dose. On day 42 of the primary immunization mice were challenged i.n. with 17 CFU of Ft SchuS4. Age matched unvaccinated mice challenged with a similar dose of Ft SchuS4 served as controls. (D) C57BL/6 mice (n = 10 per group) immunized i.d. with 1×103 CFU of the emrA1 mutant and boosted i.n. on day 21 with a similar dose. On day 42 of the primary immunization mice were challenged i.n. with 17 CFU of Ft SchuS4. Age matched unvaccinated mice challenged with a similar dose of Ft SchuS4 served as controls. The survival results are expressed as Kaplan-Meier survival curves and P values were determined by Log-rank test.
Finally, we hypothesized that primary immunization and boosting with 1x106 CFU of the emrA1 mutant may result in anergy due to an antigen overload. So, we reduced the immunization dose of the emrA1 mutant in subsequent vaccination studies. Mice were vaccinated i.n. with 1x103 CFU of the emrA1 mutant and boosted with a similar dose i.d. on day 21. Mice were challenged with 23 CFU of Ft SchuS4 via i.n. route. It was observed that not only mice had an extended MTD as compared to the control mice, 20% (2/10) mice survived the challenge (Fig 9C and 9D). These results indicate that use of alternate vaccination strategies may further improve the level of protection using emrA1 mutant as a vaccine.
Discussion
Several vaccination strategies have been employed for the development of an effective vaccine against tularemia since the isolation of Ft over a century ago. Of all the vaccination strategies, use of live Ft LVS or the mutants generated on Ft LVS, Ft SchuS4, or F. novicida backgrounds have been successful in providing protection against respiratory challenge with Ft SchuS4 [24,25,14,26,27,28]. Studies have shown that both antibodies and T-cell responses are required for effective protection against virulent Ft strains [29]. This explained why killed or subunit vaccines failed to provide adequate protection against Ft SchuS4. The Ft LVS was developed in the 1950s by multiple passages of the Ft holarctica (type B strain) on peptone cysteine agar. These passages led to spontaneous mutations in the genome that made it relatively avirulent to humans while retaining its virulence in mice [30]. However, due to the lack of genetic analysis tools at the time, the exact nature of these spontaneous mutation(s) could not be determined. Modern sequencing technology has revealed that repeat-mediated deletions occurred in two genes namely pilA encoding a putative type IV pilin and FTT0918 encoding a putative outer membrane protein [31]. These mutations collectively led to the attenuation seen in Ft LVS. Reintroduction of these deleted foci into Ft LVS restores virulence back to levels of parent type B strain [32]. Ft LVS, although attenuated for virulence in humans has yet to be approved as a vaccine for mass immunization in the USA due to various reasons such as possibility of reversion, as well as cases of vaccine induced tularemia [33]. Furthermore, it confers poor immunity against high dose aerosol challenges with the virulent SchuS4 strain [34] and possesses potent immunosuppressive properties [35,22]. Currently, Ft LVS is used only for immunization of personnel at a high risk of infection [36]. Thus a vaccine generated on Ft LVS background lacking the traits that undermine its vaccine potential may prove useful in prevention of tularemia.
EmrA1 is a transmembrane component of the multidrug efflux pumps belonging to Major Facilitator Superfamily (MFS) of transporters. We have reported that the emrA1 mutant of Ft LVS is sensitive to several oxidants and antimicrobial agents. The emrA1 mutant exhibits diminished intramacrophage growth that can be restored to the wild type Ft LVS levels either by inhibition of reactive oxygen species by chemical inhibitors or infection in NADPH oxidase deficient macrophages [20]. We have further shown that oxidant sensitivity of the emrA1 mutant is due to the failure to secrete antioxidant enzymes SodB and KatG. Further characterization of the emrA1 mutant revealed that it exhibits oxidant sensitive phenotypes of both the sodB and ΔkatG mutants [20]. Based on these properties of the emrA1 mutant, we hypothesized that the emrA1 mutant will provide superior protection against respiratory tularemia caused by Ft. We tested the vaccine potential of the emrA1 mutant in prevention of respiratory tularemia caused by Ft in this study.
Attenuated mutants of Ft LVS when used as vaccines have been shown to offer protection in mice against i.d. challenge with Ft SchuS4 strain [37,38,25]. Similarly, attenuated mutants of Type A Ft strains when used as vaccines protect against a homologous SchuS4 challenge [28,39] [40] [41]. A low dose immunization with the attenuated mutants of SchuS4 either by i.n. or i.d. routes have been shown to protect mice from a low dose (10 CFU) i.n. challenge. Contrarily, a high dose i.d. immunization with these mutants do not protect immunized mice against a higher (200 CFU) i.n. challenge dose [14]. In majority of these studies, protection against i.n. challenge with F. tularensis SchuS4 strain was achieved only in BALB/c mice and the mutants used in these studies were either not tested or failed to provide protection in C57BL/6 mice. Furthermore, these studies did not use prime-boost vaccination strategies probably due to the residual virulence of the mutants and/or potential for adverse effects due to repeated immunizations. The results from our present study demonstrate that emrA1 mutant is sufficiently attenuated and repeated high dose booster vaccinations can be administered to improve the level of protection against i.n. challenge with Ft SchuS4 strain without causing any adverse reaction in the immunized mice.
Our results show that emrA1 mutant is safe and does not cause any adverse effects in mice even when administered at a dose as high as 1x106 CFU by i.n. route. Mice exhibited weight loss for a brief period but regained it quickly, showed minimal-to-none histopathological lesions in lung, liver and spleen and cleared the bacteria by day 14 post-immunization. The immunized mice were solidly protected against an i.n. challenge dose as high as 1000 to 10,000 LD100 of Ft LVS. Such a high degree of protection has not been reported earlier against respiratory challenge with Ft LVS using a single immunization dose with attenuated mutant generated on Ft LVS background. A single immunization with lower doses (1x104 and 1x105 CFU i.n.) of emrA1 mutant reduced the efficacy of protection against 1x107 CFU challenge dose but did provide protection against lower challenge doses (not shown). Further, the fact that challenged mice clear the bacteria efficiently without exhibiting a significant weight loss or histopathological lesions are indicative of robust protective immunity. Additionally, 100% protection observed in mice challenged with 1x107 CFU of Ft LVS after 75 days of a single dose immunization indicates that emrA1 vaccination induces a long-lasting immunity.
The correlates of immune protection that can provide various quantifiable markers for a successful vaccination have not been very well established for tularemia [42,43]. Although antibodies are required for protection, they have been judged as a poor correlate of immune protection. However, levels of TNF-α, IFN-γ, MCP-1 and IL-17 that are upregulated on activation of T cells have been shown to be the markers of a successful vaccination [44,21]. A sustained pro-inflammatory cytokine response consisting of high levels of TNF-α, IFN-γ and MCP-1 in the emrA1 mutant vaccinated mice following i.n challenge with Ft LVS is indicative of a strong recall response. An enhancement of antibody mediated immune response immediately after challenge also indicates that a concerted action of both the humoral and cell mediated arms of adaptive immune response causes rapid resolution of infection. However, despite the solid protection observed against high challenge doses of Ft LVS, the emrA1 mutant vaccinated mice were only partially protected against the virulent Ft SchuS4 challenge. Single and prime boost strategies using alternate i.n. and i.d. vaccination schedules significantly increased the MTD against Ft SchuS4 challenge, but eventually all the vaccinated mice succumbed to infection. Surprisingly, priming and boosting with a low dose of emrA1 mutant not only increased MTD but also protected 20% of the vaccinated mice against lethal Ft SchuS4 challenge. Moreover, when these surviving mice were sacrificed and their lung homogenates plated at the termination of the experiment, they were found to carry Ft SchuS4 in lungs indicating that these mice did receive the challenge dose and were able to keep the infection under control. These results are intriguing and are being investigated further.
The unexpected lack of protection observed in the emrA1 mutant vaccinated mice against Ft SchuS4 is surprising and reaffirms the previous observations that immune components required for protection against Ft LVS differ from those required for Ft SchuS4. The choice of C57BL/6 mouse model in vaccination studies to demonstrate protection against Ft SchuS4 challenge is based on the assumption that any vaccine candidate that can protect this hard-to-protect model will be an indicator of its protective efficacy in humans. However, this assumption is proven wrong by a recent study. It has been reported that iglD mutant of F. novicida fails to provide any protection in mice against a pulmonary challenge with Ft SchuS4 [45]. However, this mutant when used as a vaccine protected Fisher 344 rats and non-human primates against Ft SchuS4 challenge. Additionally, in this study when Ft LVS was compared in parallel with iglD mutant, it provided protection in both these animal models against Ft SchuS4 challenge. It is significant to mention that similar to iglD mutant, immunization with Ft LVS does not protect C57BL/6 mice against Ft SchuS4 challenge [45]. Thus, based on these observations, we speculate that the emrA1 mutant when used as a vaccine in Fisher rats or non-human primate models may render 100% protection against Ft SchuS4 challenge. Future studies are directed at testing the vaccine potential of the emrA1 mutant in these two animal models.
Our previous studies have shown that a mutant of Ft LVS deficient in iron-containing superoxide dismutase B (SodB) is superior to Ft LVS in protecting C57BL/6 mice against a respiratory Ft SchuS4 challenge [13]. We demonstrated that superior protection offered by the sodB mutant is due to its attenuated virulence as compared to wild type Ft LVS and upregulation of several immunogenic proteins resulting from oxidative stress [13]. Further, we have demonstrated that the antioxidant enzyme KatG of Ft LVS is involved in the suppression of host’s innate immune response by inhibiting the redox-sensitive signaling components [18]. Moreover, a mutant of Mycobacterium bovis BCG in iron-containing superoxide dismutase (SodA) strain has shown to confer better protection in immunized mice than the parent BCG strain against virulent M. tuberculosis challenge [19]. A modified BCG containing a mutation in sodA gene and sigma factor SigH which regulates the expression of several genes required for resistance to oxidative stress, induced stronger immune responses than the parent BCG during primary vaccination as well as an enhanced recall response upon subsequent challenges resulting in superior protection against M. tuberculosis challenge [46,47]. These observations indicate that antioxidant enzymes may be targeted to develop effective vaccines against bacterial pathogens. In the context of tularemia prevention, antioxidant defenses of Ft may specifically be targeted to improve the protective efficacy and reduce the immunosuppressive properties of parent Ft LVS strain. Moreover, we speculate that given a 100% identity of nucleotide and amino acid sequences of the emrA1 gene and protein between Ft LVS and SchuS4, the emrA1mutant of Ft SchuS4 may have a phenotype similar to that observed for the emrA1 mutant of Ft LVS. However, a more rigorous assessment of the vaccine potential would require generation of emrA1 mutant in the Ft SchuS4 background. To conclude, results from the present study indicate that Ft antioxidant mutants may be developed into effective vaccines for tularemia prophylaxis.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported in whole or in part, by National Institutes of Health Grants P01AI056320 (CSB, EJG), R56AI101109-01 (CSB), and R15AI107698-01 (MM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Broussard LA. Biological agents: weapons of warfare and bioterrorism. Mol Diagn. 2001;6: 323–333. [DOI] [PubMed] [Google Scholar]
- 2. Vogel G. Infectious diseases. An obscure weapon of the cold war edges into the limelight. Science. 2003;302: 222–223. [DOI] [PubMed] [Google Scholar]
- 3. Gallagher-Smith M, Kim J, Al-Bawardy R, Josko D. Francisella tularensis: possible agent in bioterrorism. Clin Lab Sci. 2004;17: 35–39. [PubMed] [Google Scholar]
- 4. Lamps LW, Havens JM, Sjostedt A, Page DL, Scott MA. Histologic and molecular diagnosis of tularemia: a potential bioterrorism agent endemic to North America. Mod Pathol. 2004;17: 489–495. [DOI] [PubMed] [Google Scholar]
- 5. Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2: 967–978. [DOI] [PubMed] [Google Scholar]
- 6. Anthony LS, Kongshavn PA. Experimental murine tularemia caused by Francisella tularensis, live vaccine strain: a model of acquired cellular resistance. Microb Pathog. 1987;2: 3–14. [DOI] [PubMed] [Google Scholar]
- 7. Ashtekar AR, Katz J, Xu Q, Michalek SM. A mucosal subunit vaccine protects against lethal respiratory infection with Francisella tularensis LVS. PLoS ONE. 2012;7: e50460 10.1371/journal.pone.0050460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baron SD, Singh R, Metzger DW. Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect Immun. 2007;75: 2152–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belyi YF, Petrosov VV, Tartakovskii IS, Suchkov YG. Live tularemia vaccine but not proteins purified from Francisella tularensis can confer protection against lethal Listeria infection in mice. APMIS. 1995;103: 107–112. [PubMed] [Google Scholar]
- 10. Burke DS. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977;135: 55–60. [DOI] [PubMed] [Google Scholar]
- 11.Conlan JW, Oyston P. Vaccines against Francisella tularensis. Ann N Y Acad Sci. 2007; [DOI] [PubMed]
- 12. Fulop M, Manchee R, Titball R. Role of two outer membrane antigens in the induction of protective immunity against Francisella tularensis strains of different virulence. FEMS Immunol Med Microbiol. 1996;13: 245–247. [DOI] [PubMed] [Google Scholar]
- 13. Bakshi CS, Malik M, Mahawar M, Kirimanjeswara GS, Hazlett KR, Palmer LE, et al. An improved vaccine for prevention of respiratory tularemia caused by Francisella tularensis SchuS4 strain. Vaccine. 2008;26: 5276–5288. 10.1016/j.vaccine.2008.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rockx-Brouwer D, Chong A, Wehrly TD, Child R, Crane DD, Celli J, et al. Low dose vaccination with attenuated Francisella tularensis strain SchuS4 mutants protects against tularemia independent of the route of vaccination. PLoS ONE. 2012;7: e37752 10.1371/journal.pone.0037752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eyles JE, Hartley MG, Laws TR, Oyston PC, Griffin KF, Titball RW. Protection afforded against aerosol challenge by systemic immunisation with inactivated Francisella tularensis live vaccine strain (LVS). Microb Pathog. 2008;44: 164–168. [DOI] [PubMed] [Google Scholar]
- 16. Lavine CL, Clinton SR, ngelova-Fischer I, Marion TN, Bina XR, Bina JE, et al. Immunization with heat-killed Francisella tularensis LVS elicits protective antibody-mediated immunity. Eur J Immunol. 2007;37: 3007–3020. [DOI] [PubMed] [Google Scholar]
- 17. Bakshi CS, Malik M, Regan K, Melendez JA, Metzger DW, Pavlov VM, et al. Superoxide dismutase B gene (sodB)-deficient mutants of Francisella tularensis demonstrate hypersensitivity to oxidative stress and attenuated virulence. J Bacteriol. 2006;188: 6443–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melillo AA, Bakshi CS, Melendez JA. Francisella tularensis antioxidants harness reactive oxygen species to restrict macrophage signaling and cytokine production. J Biol Chem. 2010;285: 27553–27560. 10.1074/jbc.M110.144394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sadagopal S, Braunstein M, Hager CC, Wei J, Daniel AK, Bochan MR, et al. Reducing the activity and secretion of microbial antioxidants enhances the immunogenicity of BCG. PLoS ONE. 2009;4: e5531- 10.1371/journal.pone.0005531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma Z, Banik S, Rane H, Mora VT, Rabadi SM, Doyle CR, et al. EmrA1 membrane fusion protein of Francisella tularensis LVS is required for resistance to oxidative stress, intramacrophage survival and virulence in mice. Mol Microbiol. 2014;91: 976–995. 10.1111/mmi.12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahawar M, Rabadi SM, Banik S, Catlett SV, Metzger DW, Malik M, et al. Identification of a live attenuated vaccine candidate for tularemia prophylaxis. PLoS ONE. 2013;8: e61539- 10.1371/journal.pone.0061539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahawar M, Atianand MK, Dotson RJ, Mora V, Rabadi SM, Metzger DW, et al. Identification of a novel Francisella tularensis factor required for intramacrophage survival and subversion of innate immune response. J Biol Chem. 2012;287: 25216–25229. 10.1074/jbc.M112.367672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol. 2008;180: 5548–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conlan JW, Shen H, Golovliov I, Zingmark C, Oyston PC, Chen W, et al. Differential ability of novel attenuated targeted deletion mutants of Francisella tularensis subspecies tularensis strain SCHU S4 to protect mice against aerosol challenge with virulent bacteria: effects of host background and route of immunization. Vaccine. 2010;28: 1824–1831. 10.1016/j.vaccine.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J, Ryder C, Mandal M, Ahmed F, Azadi P, Snyder DS, et al. Attenuation and protective efficacy of an O-antigen-deficient mutant of Francisella tularensis LVS. Microbiology. 2007;153: 3141–3153. [DOI] [PubMed] [Google Scholar]
- 26. Sammons-Jackson WL, McClelland K, Manch-Citron JN, Metzger DW, Bakshi CS, Garcia E, et al. Generation and characterization of an attenuated mutant in a response regulator gene of Francisella tularensis live vaccine strain (LVS). DNA Cell Biol. 2008;27: 387–403. 10.1089/dna.2007.0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tempel R, Lai XH, Crosa L, Kozlowicz B, Heffron F. Attenuated Francisella novicida transposon mutants protect mice against wild-type challenge. Infect Immun. 2006;74: 5095–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Twine S, Bystrom M, Chen W, Forsman M, Golovliov I, Johansson A, et al. A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect Immun. 2005;73: 8345–8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59: 2922–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev. 2002;15: 631–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Forslund AL, Kuoppa K, Svensson K, Salomonsson E, Johansson A, Bystrom M, et al. Direct repeat-mediated deletion of a type IV pilin gene results in major virulence attenuation of Francisella tularensis. Mol Microbiol. 2006;59: 1818–1830. [DOI] [PubMed] [Google Scholar]
- 32. Salomonsson E, Kuoppa K, Forslund AL, Zingmark C, Golovliov I, Sjostedt A, et al. Reintroduction of two deleted virulence loci restores full virulence to the live vaccine strain of Francisella tularensis. Infect Immun. 2009;77: 3424–3431. 10.1128/IAI.00196-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hornick RB, Eigelsbach HT. Aerogenic immunization of man with live Tularemia vaccine. Bacteriol Rev. 1966;30: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107: 702–714. [DOI] [PubMed] [Google Scholar]
- 35. Dotson RJ, Rabadi SM, Westcott EL, Bradley S, Catlett SV, Banik S, et al. Repression of Inflammasome by Francisella tularensis during Early Stages of Infection. J Biol Chem. 2013;288: 23844–23857. 10.1074/jbc.M113.490086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hornick R. Tularemia revisited. N Engl J Med. 2001;345: 1637–1639. [DOI] [PubMed] [Google Scholar]
- 37. Sammons-Jackson WL, McClelland K, Manch-Citron JN, Metzger DW, Bakshi CS, Garcia E, et al. Generation and characterization of an attenuated mutant in a response regulator gene of Francisella tularensis live vaccine strain (LVS). DNA Cell Biol. 2008;27: 387–403. 10.1089/dna.2007.0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pechous R, Celli J, Penoske R, Hayes SF, Frank DW, Zahrt TC. Construction and characterization of an attenuated purine auxotroph in a Francisella tularensis live vaccine strain. Infect Immun. 2006;74: 4452–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pechous RD, McCarthy TR, Mohapatra NP, Soni S, Penoske RM, Salzman NH, et al. A Francisella tularensis Schu S4 purine auxotroph is highly attenuated in mice but offers limited protection against homologous intranasal challenge. PLoS ONE. 2008;3: e2487 10.1371/journal.pone.0002487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qin A, Scott DW, Thompson JA, Mann BJ. Identification of an essential Francisella tularensis subsp. tularensis virulence factor. Infect Immun. 2009;77: 152–161. 10.1128/IAI.01113-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Andersson H, Hartmanova B, Kuolee R, Ryden P, Conlan W, Chen W, et al. Transcriptional profiling of host responses in mouse lungs following aerosol infection with type A Francisella tularensis. J Med Microbiol. 2006;55: 263–271. [DOI] [PubMed] [Google Scholar]
- 42. De PR, Chou AY, Bosio CM, Huang CY, Follmann DA, Elkins KL. Development of functional and molecular correlates of vaccine-induced protection for a model intracellular pathogen, F. tularensis LVS. PLoS Pathog. 2012;8: e1002494- 10.1371/journal.ppat.1002494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ryden P, Twine S, Shen H, Harris G, Chen W, Sjostedt A, et al. Correlates of protection following vaccination of mice with gene deletion mutants of Francisella tularensis subspecies tularensis strain, SCHU S4 that elicit varying degrees of immunity to systemic and respiratory challenge with wild-type bacteria. Mol Immunol. 2013;54: 58–67. 10.1016/j.molimm.2012.10.043 [DOI] [PubMed] [Google Scholar]
- 44. Eneslatt K, Normark M, Bjork R, Rietz C, Zingmark C, Wolfraim LA, et al. Signatures of T cells as correlates of immunity to Francisella tularensis. PLoS ONE. 2012;7: e32367- 10.1371/journal.pone.0032367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chu P, Cunningham AL, Yu JJ, Nguyen JQ, Barker JR, Lyons CR, et al. Live attenuated Francisella novicida vaccine protects against Francisella tularensis pulmonary challenge in rats and non-human primates. PLoS Pathog. 2014;10: e1004439- 10.1371/journal.ppat.1004439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kernodle DS. Decrease in the effectiveness of Bacille Calmette-Guerin vaccine against pulmonary tuberculosis: a consequence of increased immune suppression by microbial antioxidants, not overattenuation. Clin Infect Dis. 2010;51: 177–184. 10.1086/653533 [DOI] [PubMed] [Google Scholar]
- 47. Edwards KM, Cynamon MH, Voladri RK, Hager CC, DeStefano MS, Tham KT, et al. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2001;164: 2213–2219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.