Abstract
Cubism was an influential early 20th century art movement characterized by angular, disjointed imagery. The two-dimensional appearance of Cubist figures and objects is created through juxtaposition of angles. The authors posit that the constrained perspectives found in Cubism may also be found in the clinical classification of brain disorders. Neurological disorders are often separated from psychiatric disorders as if they stem from different organ systems. Maintaining two isolated clinical disciplines fractionalizes the brain in the same way that Pablo Picasso fractionalized figures and objects in his Cubist art. This Neural Cubism perpetuates a clinical divide that does not reflect the scope and depth of neuroscience. All brain disorders are complex and multidimensional, with aberrant circuitry and resultant psychopharmacology manifesting as altered behavior, affect, mood or cognition. Trainees should receive a multidimensional education based on modern neuroscience, not a partial education based on clinical precedent. The authors briefly outline the rationale for increasing the integration of neurology and psychiatry and discuss a nested model with which clinical neuroscientists (neurologists and psychiatrists) can approach and treat brain disorders.
In his revolutionary 1907 painting Les Demoiselles d'Avignon, Pablo Picasso unveiled the fractionalized geometry that has become a hallmark of the art movement known as Cubism1. Picasso used juxtaposition of angles to create a sense of dissonance. To an observer, the visual presentation of figures lacks depth and fidelity because Picasso approached the scene through constrained perspectives. The forward-facing figures, for example, are bestowed with noses seen in profile.
We posit that the constrained perspectives found in Cubism may also be found in the clinical classification of brain disorders. To a neuroscientist, the clinical presentation of patients with brain disorders frequently lacks depth and fidelity because neurologists and psychiatrists approach the brain through constrained perspectives. Whereas neurologists normally approach neural circuits from a macroanatomical perspective, psychiatrists normally approach neural circuits from a cognitive, affective, or behavioral output perspective. These acts of Neural Cubism perpetuate the arbitrary classification of brain disorders as neurologic versus psychiatric and make it difficult for physicians and patients to conceptualize the multidimensional nature of brain disorders. Rather than fractionalizing the brain into clinical categories, undergraduate and graduate medical education should encourage a multidimensional approach based on neuroscience. In this Perspective, we briefly outline the rationale for expanding integrated training in neurology and psychiatry and discuss a nested model for clinical neuroscientists (neurologists and psychiatrists).
Historical Perspective
The pursuit of a unified perspective on brain function is not a new endeavor. Throughout history, renowned physicians like Oppenheim, Charcot, Babinski, Meynert, and others approached patients and their brain disorders from a neuropsychiatric vantage 2, 3. Even without modern neuroscience, these pioneers recognized that the Cartesian Dualism of the 17th century was archaic and inadequate. Johann Christian Reil, the physician who coined the term psychiatry in 1803, was also one of the founders of modern neurology. He argued fervently against the idea that brain disorders could be categorized as strictly mental, chemical, or physical4, 5. Despite its neuropsychiatric foundation, the field of psychiatry, heavily influenced by Freudian theories about the mind, gradually became associated with disorders for which there were no known brain-based etiologies2. By contrast, neurology became the discipline of demonstrable neuroanatomical pathology. This fractionalization of brain disorders into psychiatric versus neurological unfortunately reanimated the previously defunct spirit of Cartesian Dualism. The consequences of this fractionalization regrettably persist to the present day.
As neuroscience matured in the late 20th century, the schism between psychiatry and neurology began to regress. Although many lack traditional anatomical pathologies, psychiatric disorders like obsessive-compulsive disorder and depression are now associated with circuit pathology. The emergence of these dysfunctional circuits has refocused the field of psychiatry on brain-based etiologies for cognitive, affective and behavioral disturbances. Neuroscience is becoming increasingly important in psychiatric education6. Neurology has also benefited from neuroscience, further correlating clinical disease manifestations with aberrant neurophysiology and pharmacology. Once distinctive and clear, the criteria that distinguish neurological disorders from psychiatric disorders are becoming artificial and counterproductive. All brain disorders are complex and multidimensional; a truly neuroscientific approach would move beyond these historical categories.
Updating the Educational Edifice
The most effective way to embrace clinical neuroscience is to change the way that medical students and residents are taught to approach brain disorders. Unlike the neuroscience curricula in graduate schools, the neuroscience curricula in most medical schools separate neurological disorders from psychiatric disorders. Graduate students interested in neuroscience, for example, can study neurological disorders like Alzheimer's disease and epilepsy just as easily as they can study psychiatric disorders like depression and post-traumatic stress disorder. These labels are of secondary importance to the aberrant circuitry being studied. Medical students interested in neuroscience, however, are typically required to choose between neurological disorders and psychiatric disorders as they prepare for residency. Even schools that integrate neurology and psychiatry in the basic science years fractionalize the brain during the clinical years. A 2006 American Academy of Neurology survey of clerkship directors reports that only 25% of medical schools feature a clinical clerkship that links or integrates elements of neurology and psychiatry. A larger survey in 2012 confirms that this percentage has not changed7.
Graduate medical education usually perpetuates the idea that neurology and psychiatry are separate and mutually exclusive. Neurology residents are only required to complete one month of psychiatry training according to the Accreditation Council for Graduate Medical Education (ACGME) website8. Psychiatry residents, by contrast, are required to complete two months of neurology training9. There are few opportunities for interdisciplinary or integrated training beyond these perfunctory rotations. According to the FREIDA Online database hosted by the American Medical Association10, the ACGME currently recognizes five combined specialty programs in neurology and psychiatry in the United States.
In 2009, the American Board of Psychiatry and Neurology (ABPN) initiated a moratorium on the creation of new combined specialty programs in neurology and psychiatry 11. Somewhat ironically, the ABPN has been certifying neurologists and psychiatrists since 1934 but has no current mechanism for approving new combined programs that reflect its own combined composition. . Thankfully, this issue is gradually being addressed. In 2012, the ABPN released a statement assuring current and future residents in currently established combined training programs that they would be eligible for dual boarding in neurology and psychiatry. This statement also described how the ABPN is working with the ACGME to establish a mechanism by which new neurology-psychiatry programs can be created and approved11.
In 2013, the ACGME launched their Next Accreditation System (NAS). They have also released details of their Milestones project, an initiative designed to create an ongoing competency evaluation throughout various levels of residency education12. Many of the Milestones for psychiatry, particularly those related to brain stimulation and neuroimaging, are congruent with the Milestones for neurology13. These new Milestones may enable a closer conversation between psychiatrists and neurologists, although the details of such a dialogue are just beginning to emerge. With converging ACGME Milestones in neurology and psychiatry, it seems to be an appropriate time to remove the moratorium and promote a modern training paradigm that teaches clinical neuroscientists to speak the languages of both neurology and psychiatry.
Education Before Clinical Integration
Ironically, many arguments against the clinical integration of neurology and psychiatry offer compelling insights into the reason why educational integration is so critical and timely (even for physicians who practice exclusively within the classical confines of psychiatry or neurology). In a 2005 editorial, Pies suggests that neuropsychiatry is merely a temporary overlap of two fields that should remain separate until a “meta-narrative” has emerged 14. He argues this position by invoking a philosophical construct in which an optician and an art critic are asked to describe a painting on a museum wall. The optician describes the technical details of the painting, summarizing color changes in terms of coordinates. The art critic describes the gestalt of the painting, summarizing color changes in terms of objects and figures. In this thought experiment, a third party can supposedly recreate the original work of art with each separate description but will fail if he or she tries to use both simultaneously. Pies extends this argument to neurology and psychiatry, explaining that these fields “cannot simply merge” because of their disparate discourses 14.
Although we agree with Pies's argument that an immediate clinical merger of neurology and psychiatry is far from simple, we disagree with his argument regarding language. The most accurate reproduction of the original work of art would indeed be produced by the third party who had access to descriptions from the optician and the art critic. To the point, a third party (or a first-hand observer) who had knowledge of both fields would be in an optimal position to recreate both the technical details of the painting as well as the gestalt of the painting. Critical aspects of the original are likely to be misunderstood or ignored if the third party only had access to one description. In our opinion, this philosophical construct highlights the fundamental problem with teaching students and residents that neurology and psychiatry are incompatible and mutually exclusive.
Diversification, Not Dilution
Some authors who support the idea of interdisciplinary training have previously cautioned that it might dilute the rigor of training in each composite discipline 15- 17. From this perspective, neurology's “rigorous clinical examination skills, its empiricism, and its objectivity” might suffer if a trainee is also asked to demonstrate the “well-developed interviewing skills, understanding of multiple causations of behavioral disturbance, appreciation of individual variation, ability to deal with ambiguity, interpersonal context, and the combination of biological with psychological and behavioral therapies” of psychiatry 17. Taken together, these opinions seem to suggest that interdisciplinary training may only be appropriate for physicians who treat disorders that fall into the “clinical territory” of neuropsychiatry 17.
From a neuroscience vantage, the designation of brain disorders as neurological versus psychiatric is a clinical exercise that is often arbitrary and counterproductive 3, 18, 19. The vast complexity of the brain demands that those who study and interface with it clinically possess a multidimensional knowledge base and skill set. Yudofsky and Hales18 expertly outline the damage done by such simplistic labeling schemes. Reducing multidimensional disorders to one-dimensional disorders (e.g. Parkinson's as exclusively a movement disorder) also reduces the likelihood that patients will receive the comprehensive care that they require and deserve. Moreover, artificial labels may also contribute to stigmatization of “mental illness” in terms of public perception and insurance reimbursement 18.
Increasing interdisciplinary training in neurology and psychiatry will enable clinical neuroscientists to use all available tools and knowledge to diagnose and treat brain disorders. These resources should not be restricted to one discipline versus another. In some ways, the benefits of interdisciplinary training parallel the benefits of research training for clinicians. Rather than diluting clinical skills, research training enables physicians to improve and diversify their skill set. This diversification enables them to derive insights in one discipline based on their training in another. A similar argument can be made for interdisciplinary training for neurologists and psychiatrists. With a comprehensive education, clinical neuroscientists can address the cognitive, affective, and behavioral manifestations of brain diseases. In the following section, we outline a nested hierarchy model that summarizes how the complementary discourses in neurology and psychiatry can be used to achieve a greater depth of clinical reasoning.
Nested Hierarchies
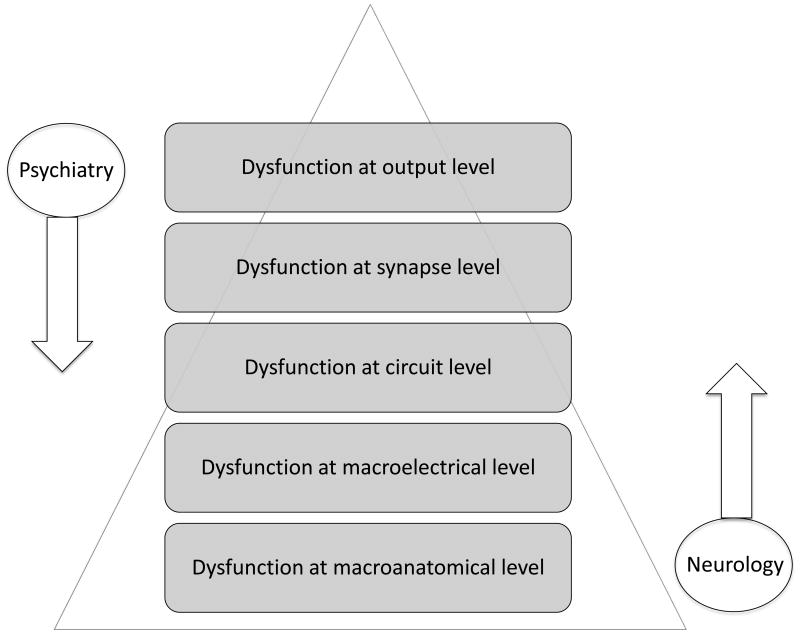
Neuroscience has begun to frame brain disorders in terms of circuit-level dynamics. As a result, the top-down approach of neurology has met the bottom-up approach of psychiatry (see Figure 1). Despite this convergence, little has been done to integrate the complementary discourses of neurology and psychiatry. In the late 1980s, for example, DeLong and Georgopoulus mapped the thalamocortical loops implicated in many brain diseases20. These circuits are prevalent in neuroscience research yet have not been fully integrated into the clinical discourse21. Thankfully, the discrepancy between basic and clinical neuroscience is slowly being reconciled.
Figure 1.
A diagram that illustrates how the complementary discourses of neurology and psychiatry converge at the level of circuits. Psychiatrists and neurologists may currently begin their diagnostic approaches at different ends of the neuroscientific spectrum, but ultimately each specialist has to address the top-down and bottom-up manifestations of brain diseases in order to provide efficient and effective care to patients.
A timely series of federal and non-profit initiatives illustrates how clinical neuroscience is changing the way that brain disorders are studied, classified, and approached. First, the National Institute of Mental Health (NIMH) launched its Research Domain Criteria (RDoC) project that is intended to develop circuit- and biomarker-based classification systems for disorders typically treated by psychiatrists. Second, the ACGME launched its Psychiatry Milestone Project that is intended to enrich psychiatric education with clinical neuroscience. Third, and most recently, the federal government has launched multimillion-dollar programs like the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) initiative and the Defense Advanced Research Projects Agency (DARPA) Systems-Based Neurotechnology and Understanding for the Treatment of Neuropsychological Illness initiative. One primary goal of these complex neuroscience projects is to develop invasive and non-invasive neuromodulation technologies for brain disorders. These initiatives emphasize how neuroscience should take precedence over the historical categories of neurology and psychiatry.
As the NIMH reframes its classification system and academic medicine shifts its evaluations toward milestones and competencies 22, 23, the fields of neurology and psychiatry have a timely opportunity to reframe the clinical approach to brain disorders. Excellent pilot programs that were previously launched to encourage the integration of neurology and psychiatry need to be further developed and expanded at the level of undergraduate and graduate medical education24, 25. We propose a nested hierarchy model with which brain disorders can be classified based on their multidimensional manifestations26. In the following paragraphs, we describe each nested level (Level 1—Macroanatomical, Level 2—Macroelectrical, Level 3—Circuit, Level 4—Synapse, Level 5—Output) and explain the utility of this interdisciplinary approach for clinical neuroscientists.
The first nested level is the macroanatomical level. A stroke affecting the corticospinal tract, for example, can be classified as a macroanatomical lesion (Level 1) 27. The effects of a stroke, however, are not restricted to this “neurological” level. Stroke patients tend to exhibit resultant EEG slowing (Level 2) 28 that reflects damage to underlying motoric circuitry (Level 3) 29. This pathology typically results in a hyperglutamatergic state (Level 4)30 and the lesion and its multilevel effects prevent the patient from executing intended movements or speech (Level 5)31. If the stroke had occurred in the prefrontal cortex instead of the corticospinal tract, then the effects of the macroanatomical lesion could have been traced through this model to downstream effects of altered affect, mood, or personality32, 33.
The second nested level is the macroelectrical level. A primary generalized seizure, for example, can be classified as a macroelectrical disturbance (Level 2) that has significant downstream effects on motor circuitry (Level 3). Seizures are typically associated with a GABA-glutamate imbalance (Level 4) that can manifest as resultant motoric dysfunction and/or altered consciousness (Level 5)34. The triggers for such seizures have been identified at various model levels, from emotional stress (Level 5) to missed medications (Level 4 and 3) and sleep deprivation (Level 2) 35. Seizures at the macroelectrical level can also be induced for therapeutic reasons (e.g. electroconvulsive therapy for refractory depression or catatonia), primarily because they have downstream effects on circuits 36 (Level 3), synapses 37 (Level 4), and brain output (Level 5).
The third nested level is the circuit level. Dystonia, some types of seizure disorders, and most “axis 1” psychopathology can be understood as circuit-level disturbances 38. Circuit disorders tend to have a combination of “neurological” and “psychiatric” symptoms and are thus sometimes co-managed by psychiatrists and neurologists. Major depressive disorder and Parkinson's disease, for example, may respectively present with psychomotor retardation and various neurobehavioral syndromes 39, 40. Circuit-level dynamics can be influenced by medications like ketamine that work at multiple synapses (Level 4) within a circuit (Level 3)41. Circuits can also be intervened upon with invasive and non-invasive brain stimulation. This burgeoning field offers tremendous potential for mapping and modulating dysfunctional circuits in neurology and psychiatry. Circuit physiology can be altered with direct nodal stimulation (e.g. deep brain stimulation)42 or with non-invasive stimulation at a cortical component of the dysfunctional circuit (e.g. transcranial magnetic stimulation, transcranial direct-current stimulation)43, 44. These interventions affect lower nested levels by influencing neurotransmitter release (Level 4) 45, 46 and brain outputs like affect, mood, pain, and movement (Level 5).
The fourth nested level is the cellular level, with a focus on synapses. Neurologists and psychiatrists regularly use medications that act directly at this level of the model. Pharmacotherapies for depression47 for example, alter synaptic physiology (Level 4) and thus induce downstream effects on brain outputs like affect, mood, and behavior (Level 5). Most of the pharmacotherapies used in clinical medicine have “neurological” and “psychiatric” indications and effects. Altering glutamate levels, for example, not only prevents seizures48 and headaches49 but also affects anxiety50 and mood51.
The fifth and final nested level is the brain output level. Although movement disorders typically fall into the realm of neurology, most of the disorders that fit within this brain output level fall into the realm of psychiatry. At the opposite end of the model from the macroanatomical lesions of neurology, the disorders in this realm are sometimes mischaracterized as character flaws or as ethereal phenomena unrelated to brain activity. In reality, however, pathologies in this nested level either result from or affect higher order levels in the model. A patient experiencing a traumatic event, for example, can exhibit a mood disorder (Level 5) that manifests as a bottom-up disturbance affecting cells and synapses 52 (Level 4), neuronal circuits 53 (Level 3), macroelectrical function54 (Level 2), and even macroanatomical changes on gray and white matter 55-57 (Level 1).
The purpose of discussing this nested model is to highlight the multidimensional manifestations of brain disorders. Psychiatrists and neurologists may currently begin their diagnostic approaches at different ends of the neuroscientific spectrum, but ultimately each specialist has to address the top-down and bottom-up manifestations of brain diseases in order to provide the most efficient and effective care to patients. Every patient, from those with altered mental status in an Emergency Department to those with Parkinson's or Alzheimer's in a continuity clinic, would benefit from a physician who has a knowledge base rooted in neuroscience. Integrated training based on neuroscience will enable neurologists and psychiatrists to approach the brain from a unified neuropsychiatric perspective and thus to achieve greater depths of clinical reasoning.
Conclusion
After Picasso's death in 1973, the individuals in charge of his estate released a series of preliminary sketches for Les Demoiselles d'Avignon. These posthumous documents reveal that the original scene included a young medical student cradling a skull in his hands 1. In the context of art history, a Cubist medical student holding a skull can be viewed as a memento mori58. In the context of this Perspective, however, a Cubist medical student holding a skull can be viewed as an ironic symbol of the Neural Cubism that is entrenched in the educational system. As the NIMH restructures its classification systems and academic medicine shifts toward milestones and competencies 22, 23, neurologists and psychiatrists have a timely opportunity to reform our educational system and increase the depth of clinical reasoning. Integrating the artificially divided fields of neurology and psychiatry at the level of undergraduate and graduate medical education will help to create clinical neuroscientists who are able to address the multidimensional manifestations of brain disorders. It is time to move beyond Neural Cubism.
Acknowledgments
Funding/Support: Mr. Taylor is funded by the National Institute on Drug Abuse (F30DA033748) and Dr. Williams is funded by the National Institute of Mental Health (R25 DA020537).
Footnotes
Editor's note: A commentary by S. Benjamin appears on pages XXX-XXX.
Other Disclosures: None reported.
Ethical Approval: Reported as not applicable.
Contributor Information
Mr. Joseph J. Taylor, Medical University of South Carolina, Charleston, South Carolina.
Dr. Nolan R. Williams, Department of Psychiatry and Behavioral Science, Stanford University, Stanford, California.
Dr. Mark S. George, Medical University of South Carolina, Charleston, South Carolina; Ralph H. Johnson VA Medical Center; Brain Stimulation: Basic, Translational and Clinical Research in Neuromodulation.
References
- 1.Hunter S, Jacobus J, Wheeler D. Modern Art, Revised and Updated. 3rd. New York: Pearson; 2004. [Google Scholar]
- 2.Price BH, Adams RD, Coyle JT. Neurology and psychiatry: closing the great divide. Neurology. 2000 Jan 11;54(1):8–14. doi: 10.1212/wnl.54.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Lee TS, Ng BY, Lee WL. Neuropsychiatry--an emerging field. Annals of the Academy of Medicine, Singapore. 2008 Jul;37(7):601–605. [PubMed] [Google Scholar]
- 4.Marneros A. Psychiatry's 200th birthday. Br J Psychiatry. 2008 Jul;193(1):1–3. doi: 10.1192/bjp.bp.108.051367. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan RM. Johann Christian Reil and the naming of our specialty. Australasian psychiatry : bulletin of Royal Australian and New Zealand College of Psychiatrists. 2012 Apr;20(2):157–158. doi: 10.1177/1039856211432463. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin S. Educating psychiatry residents in neuropsychiatry and neuroscience. International review of psychiatry. 2013 Jun;25(3):265–275. doi: 10.3109/09540261.2013.786689. [DOI] [PubMed] [Google Scholar]
- 7.Poechmann N. Manager, Education & Travel, American Academy of Neurology. Personal communication with J Taylor. 2013 Dec 3; [Google Scholar]
- 8.Accreditation Council of Graduate Medical Education. Psychiatry in Neurology Requirement Clarification. [Accessed August 19, 2014]; http://www.acgme.org/acgmeweb/tabid/325/ProgramandInstitutionalAccreditation/MedicalSpecialties/Neurology/PsychiatryinNeurologyRequirementClarification.aspx.
- 9.Accreditation Council of Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Psychiatry. [Accessed August 19, 2014]; http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/400_psychiatry_0701.
- 10.Rowley BD. AMA--Fellowship and Residency Electronic Interactive Database Access (AMA-FREIDA): a computerized residency selection tool. JAMA : the journal of the American Medical Association. 1988 Aug 26;260(8):1059. [PubMed] [Google Scholar]
- 11.Faulker L, Summers R. Update for Psychiatry GME Programs on Combined Training Program Accreditation/Approval February 2012. [Accessed August 19, 2014]; https://www.umassmed.edu/uploadedFiles/Combined%20program%20letter.pdf.
- 12.Nasca TJ, Philibert I, Brigham T, Flynn TC. The next GME accreditation system--rationale and benefits. N Engl J Med. 2012 Mar 15;366(11):1051–1056. doi: 10.1056/NEJMsr1200117. [DOI] [PubMed] [Google Scholar]
- 13.Accreditation Council of Graduate Medical Education. The Psychiatry Milestone Project. [Accessed August 19, 2014]; doi: 10.4300/JGME-06-01s1-11. http://acgme.org/acgmeweb/Portals/0/PDFs/Milestones/PsychiatryMilestones.pdf. [DOI] [PMC free article] [PubMed]
- 14.Pies R. Why psychiatry and neurology cannot simply merge. J Neuropsychiatry Clin Neurosci. 2005 Summer;17(3):304–309. doi: 10.1176/jnp.17.3.304. [DOI] [PubMed] [Google Scholar]
- 15.Sachdev PS. Whither neuropsychiatry? J Neuropsychiatry Clin Neurosci. 2005 Spring;17(2):140–144. doi: 10.1176/jnp.17.2.140. [DOI] [PubMed] [Google Scholar]
- 16.Sachdev PS. Neuropsychiatry - a discipline for the future. Journal of psychosomatic research. 2002;53:625–627. [Google Scholar]
- 17.Sachdev PS. An agenda for neuropsychiatry as a 21st century discipline. Acta neuropsychiatrica. 2007;19:2–5. doi: 10.1111/j.1601-5215.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 18.Yudofsky SC, Hales RE. Neuropsychiatry and the future of psychiatry and neurology. Am J Psychiatry. 2002 Aug;159(8):1261–1264. doi: 10.1176/appi.ajp.159.8.1261. [DOI] [PubMed] [Google Scholar]
- 19.Martin JB. The integration of neurology, psychiatry, and neuroscience in the 21st century. Am J Psychiatry. 2002 May;159(5):695–704. doi: 10.1176/appi.ajp.159.5.695. [DOI] [PubMed] [Google Scholar]
- 20.DeLong MR, Georgopoulus AP. Motor Functions of the Basal Ganglia. Comprehensive Physiology. 2011:1017–1061. [Google Scholar]
- 21.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. Journal of psychosomatic research. 2002 Aug;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 22.Heist K, Post J, Meade L, Brandenburg S. Milestones: do learners and teachers agree? The American journal of medicine. 2013 Mar;126(3):270–274. doi: 10.1016/j.amjmed.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Carraccio C, Wolfsthal SD, Englander R, Ferentz K, Martin C. Shifting paradigms: from Flexner to competencies. Academic medicine : journal of the Association of American Medical Colleges. 2002 May;77(5):361–367. doi: 10.1097/00001888-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Lacy T, Hughes JD. A neural systems-based neurobiology and neuropsychiatry course: integrating biology, psychodynamics, and psychology in the psychiatric curriculum. Acad Psychiatry. 2006 Sep-Oct;30(5):410–415. doi: 10.1176/appi.ap.30.5.410. [DOI] [PubMed] [Google Scholar]
- 25.Posner J, Stewart J, Rieder R. Neurobiological formulations: integrating clinical and biological psychiatry. Acad Psychiatry. 2007 Nov-Dec;31(6):479–484. doi: 10.1176/appi.ap.31.6.479. [DOI] [PubMed] [Google Scholar]
- 26.Biel DM, Griffiths TL, Jordan M, Tenenbaum JB. Hierarchical topic models and the nested Chinese restaurant process. Advances in Neural Information Processing Systems. 2004;16:106–114. [Google Scholar]
- 27.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain : a journal of neurology. 2007 Jan;130(Pt 1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 28.Doherty MJ, Walting PJ, Morita DC, et al. Do nonspecific focal EEG slowing and epileptiform abnormalities favor one hemisphere? Epilepsia. 2002 Dec;43(12):1593–1595. doi: 10.1046/j.1528-1157.2002.24002.x. [DOI] [PubMed] [Google Scholar]
- 29.Chang WH, Kim YH, Yoo WK, et al. rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restorative neurology and neuroscience. 2012;30(3):179–189. doi: 10.3233/RNN-2012-110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davalos A, Castillo J, Serena J, Noya M. Duration of glutamate release after acute ischemic stroke. Stroke; a journal of cerebral circulation. 1997 Apr;28(4):708–710. doi: 10.1161/01.str.28.4.708. [DOI] [PubMed] [Google Scholar]
- 31.Lotze M, Cohen LG. Volition and imagery in neurorehabilitation. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2006 Sep;19(3):135–140. doi: 10.1097/01.wnn.0000209875.56060.06. [DOI] [PubMed] [Google Scholar]
- 32.Turner MA, Andrewes DG. Assessing the cognitive regulation of emotion in depressed stroke patients. International journal of rehabilitation research. Internationale Zeitschrift fur Rehabilitationsforschung. Revue internationale de recherches de readaptation. 2010 Jun;33(2):180–182. doi: 10.1097/MRR.0b013e32832e6c57. [DOI] [PubMed] [Google Scholar]
- 33.Vataja R, Leppavuori A, Pohjasvaara T, et al. Poststroke depression and lesion location revisited. The Journal of neuropsychiatry and clinical neurosciences. 2004 Spring;16(2):156–162. doi: 10.1176/jnp.16.2.156. [DOI] [PubMed] [Google Scholar]
- 34.Beydoun A, D'Souza J. Treatment of idiopathic generalized epilepsy - a review of the evidence. Expert opinion on pharmacotherapy. 2012 Jun;13(9):1283–1298. doi: 10.1517/14656566.2012.685162. [DOI] [PubMed] [Google Scholar]
- 35.Balamurugan E, Aggarwal M, Lamba A, Dang N, Tripathi M. Perceived trigger factors of seizures in persons with epilepsy. Seizure. 2013 Jun 24; doi: 10.1016/j.seizure.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Abbott CC, Lemke NT, Gopal S, et al. Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Frontiers in psychiatry / Frontiers Research Foundation. 2013;4:10. doi: 10.3389/fpsyt.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landau AM, Chakravarty MM, Clark CM, Zis AP, Doudet DJ. Electroconvulsive therapy alters dopamine signaling in the striatum of non-human primates. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011 Jan;36(2):511–518. doi: 10.1038/npp.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tenney JR, Fujiwara H, Horn PS, Jacobson SE, Glauser TA, Rose DF. Focal corticothalamic sources during generalized absence seizures: A MEG study. Epilepsy research. 2013 Jun 10; doi: 10.1016/j.eplepsyres.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Buyukdura JS, McClintock SM, Croarkin PE. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2011 Mar 30;35(2):395–409. doi: 10.1016/j.pnpbp.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weintraub D, Burn DJ. Parkinson's disease: the quintessential neuropsychiatric disorder. Mov Disord. 2011 May;26(6):1022–1031. doi: 10.1002/mds.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheidegger M, Walter M, Lehmann M, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PloS one. 2012;7(9):e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006 Nov;31(11):2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 43.Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009 Jan;34(2):522–534. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 44.Le K, Liu L, Sun M, Hu L, Xiao N. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2013 Feb;20(2):257–262. doi: 10.1016/j.jocn.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn J, Janouschek H, Raptis M, et al. In vivo evidence of deep brain stimulation-induced dopaminergic modulation in Tourette's syndrome. Biol Psychiatry. 2012 Mar 1;71(5):e11–13. doi: 10.1016/j.biopsych.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 46.Taylor JJ, Borckardt JJ, Canterberry M, et al. Naloxone-Reversible Modulation of Pain Circuitry by Left Prefrontal rTMS. Neuropsychopharmacology. 2013 Jan 11; doi: 10.1038/npp.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guaiana G, Barbui C, Hotopf M. Amitriptyline versus other types of pharmacotherapy for depression. Cochrane database of systematic reviews. 2003;(2):CD004186. doi: 10.1002/14651858.CD004186. [DOI] [PubMed] [Google Scholar]
- 48.Schlumberger E, Chavez F, Palacios L, Rey E, Pajot N, Dulac O. Lamotrigine in treatment of 120 children with epilepsy. Epilepsia. 1994 Mar-Apr;35(2):359–367. doi: 10.1111/j.1528-1157.1994.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 49.Silberstein SD, Lipton RB, Dodick DW, et al. Efficacy and safety of topiramate for the treatment of chronic migraine: a randomized, double-blind, placebo-controlled trial. Headache. 2007 Feb;47(2):170–180. doi: 10.1111/j.1526-4610.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 50.Bruno A, Mico U, Pandolfo G, et al. Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. Journal of psychopharmacology. 2012 Nov;26(11):1456–1462. doi: 10.1177/0269881111431751. [DOI] [PubMed] [Google Scholar]
- 51.Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. The British journal of psychiatry : the journal of mental science. 2009 Jan;194(1):4–9. doi: 10.1192/bjp.bp.107.048504. [DOI] [PubMed] [Google Scholar]
- 52.LaFrance WC, Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010 Sep 28;75(13):1166–1173. doi: 10.1212/WNL.0b013e3181f4d5a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcin B, Roze E, Mesrati F, et al. Transcranial magnetic stimulation as an efficient treatment for psychogenic movement disorders. J Nerol Neurosurg Psychiatry. 2013;84:1043–1046. doi: 10.1136/jnnp-2012-304062. [DOI] [PubMed] [Google Scholar]
- 54.Lagopoulos J, Xu J, Rasmussen I, et al. Increased theta and alpha EEG activity during nondirective meditation. Journal of alternative and complementary medicine. 2009 Nov;15(11):1187–1192. doi: 10.1089/acm.2009.0113. [DOI] [PubMed] [Google Scholar]
- 55.Holzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry research. 2011 Jan 30;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004 Nov 17;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozel FA, Johnson KA, Nahas Z, et al. Fractional anisotropy changes after several weeks of daily left high-frequency repetitive transcranial magnetic stimulation of the prefrontal cortex to treat major depression. J ECT. 2011 Mar;27(1):5–10. doi: 10.1097/YCT.0b013e3181e6317d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.San Juan R. The Turn of the Skull: Andreas Vesalius and the Early Modern Memento Mori. Art History. 2012;35:958–975. [Google Scholar]