Abstract
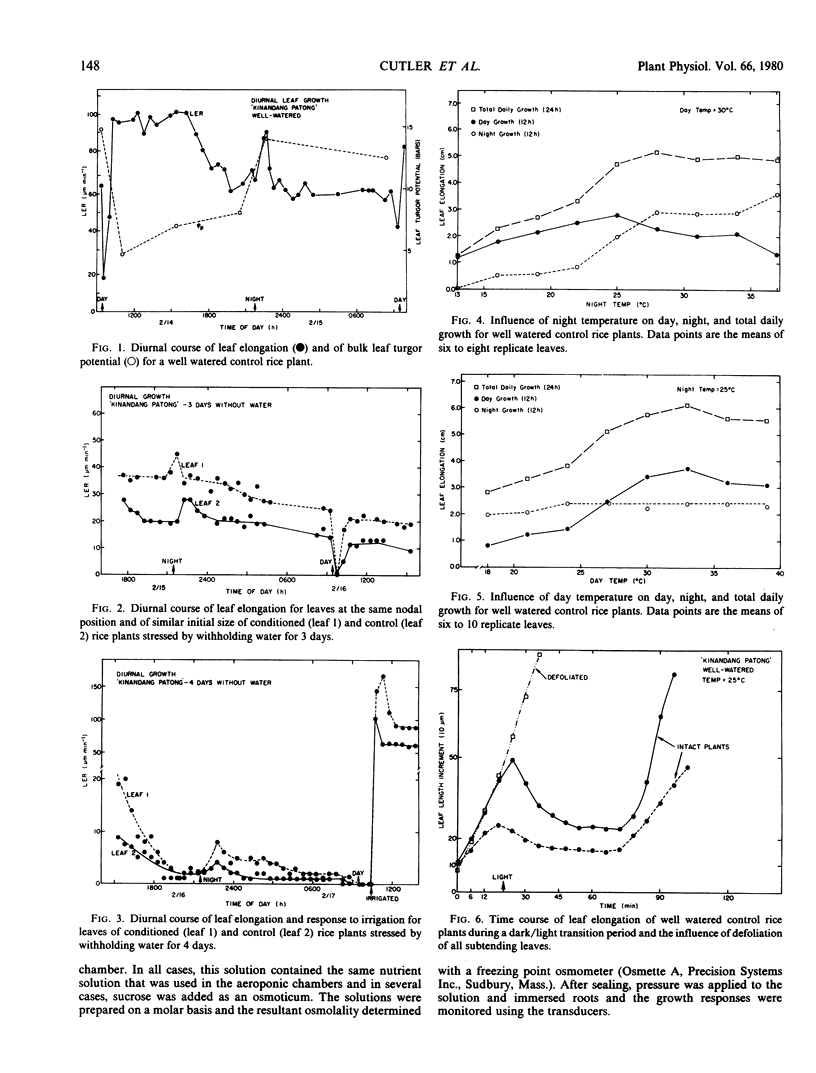
Some dynamic aspects of leaf elongation in rice were studied. Under both well watered and water-deficient conditions, leaf elongation rates were 15 to 30% greater during the day than during the night. Night temperatures below 27 C limited the rate of elongation at night but when night temperatures exceeded 27 C, night elongation rates exceeded rates during the day. The diurnal pattern of elongation was opposite to the pattern of bulk leaf turgor which was lower during the day than at night.
Superimposed on the general diurnal pattern of leaf elongation were perturbations associated with the light/dark transitions. The rate of leaf elongation declined within minutes after illumination and remained low for 15 to 60 minutes, after which rapid rates ensued. The rate of leaf elongation was transiently accelerated within minutes after transition to dark and then declined to steady night rates after 30 to 60 minutes. Removal or covering of all subtending leaves eliminated these perturbations. Irrigation during the light-induced inhibition period did not influence leaf elongation rates of well watered plants but in stressed plants, high rates of elongation resumed immediately after irrigation.
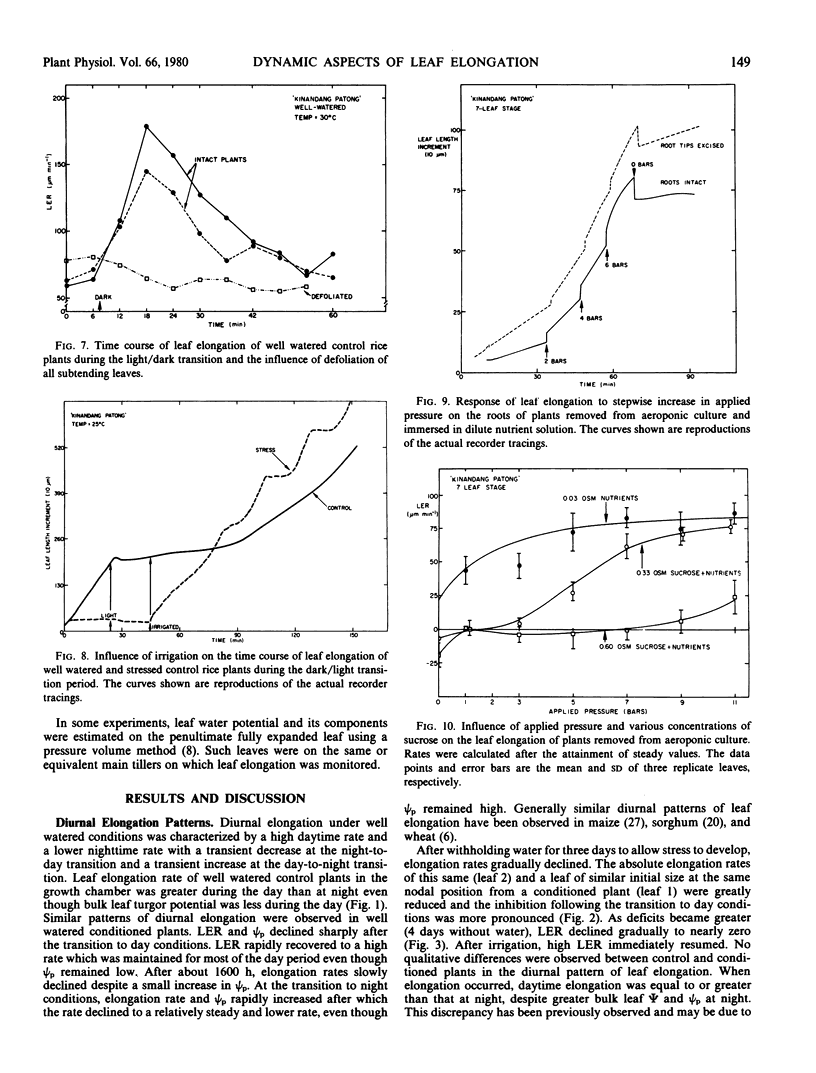
The rate of elongation was accelerated by hydrostatic pressure applied to roots of intact plants. The rate of leaf elongation increased with increasing pressure to about 5 bars and then showed no further increase with increasing pressure. This suggests that the rate of water uptake normally limits the rate of leaf elongation. The response to pressure could be altered by addition of an osmoticum to the root medium and elongation occurred only when the gradient of total water potential between the substrate and elongating leaf allowed water absorption. A model of leaf expansion based on water potential gradients is proposed to explain these observations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acevedo E., Hsiao T. C., Henderson D. W. Immediate and subsequent growth responses of maize leaves to changes in water status. Plant Physiol. 1971 Nov;48(5):631–636. doi: 10.1104/pp.48.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg J. E., Turner N. C. Water potential gradients in field tobacco. Plant Physiol. 1970 Aug;46(2):343–346. doi: 10.1104/pp.46.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. B., Cummins W. R. Growth rate and turgor pressure: auxin effect studies with an automated apparatus for single coleoptiles. Plant Physiol. 1974 Dec;54(6):863–869. doi: 10.1104/pp.54.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. B. Growth Physics in Nitella: a Method for Continuous in Vivo Analysis of Extensibility Based on a Micro-manometer Technique for Turgor Pressure. Plant Physiol. 1968 Aug;43(8):1169–1184. doi: 10.1104/pp.43.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenetz P. S., List A., Jr A model for predicting growth responses in plants to changes in external water potential: Zea mays primary roots. J Theor Biol. 1973 Apr;39(1):29–45. doi: 10.1016/0022-5193(73)90203-8. [DOI] [PubMed] [Google Scholar]
- Molz F. J. Growth-induced Water Potentials in Plant Cells and Tissues. Plant Physiol. 1978 Sep;62(3):423–429. doi: 10.1104/pp.62.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel R. W., Del Tredici P., Torrey J. G. Method for growing plants aeroponically. Plant Physiol. 1976 Mar;57(3):344–346. doi: 10.1104/pp.57.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]


