Abstract
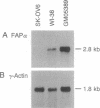
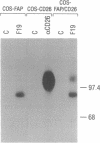
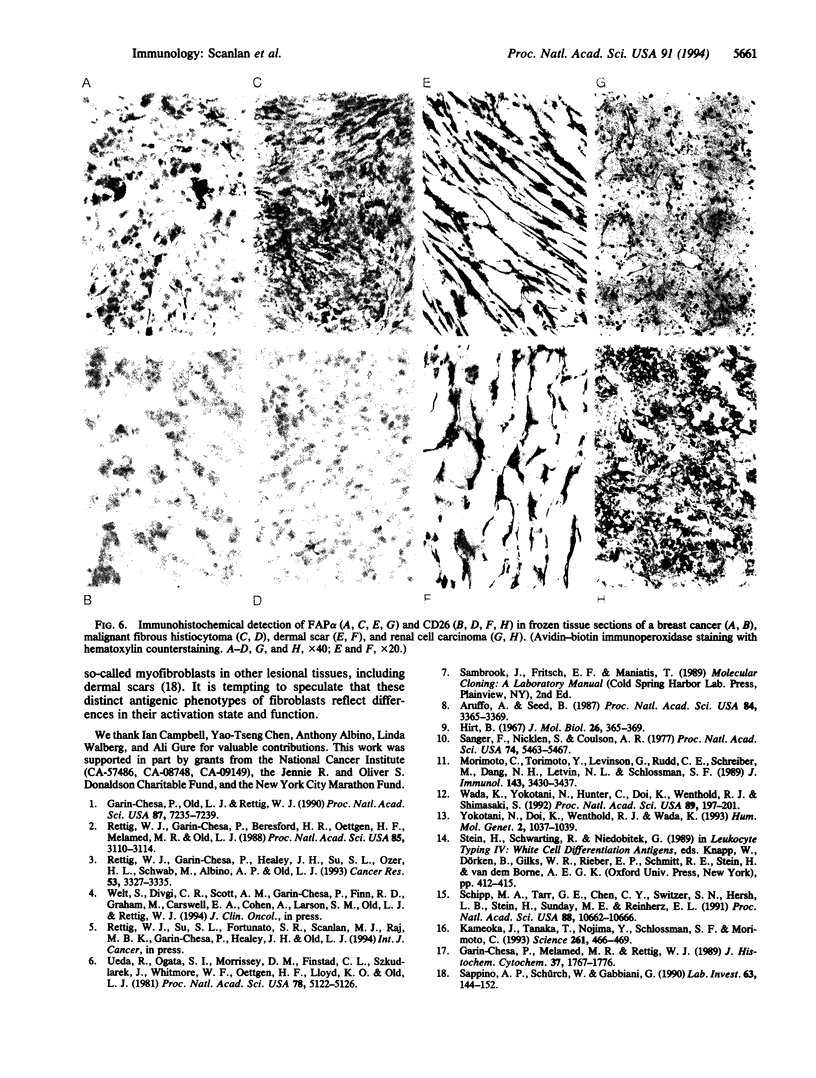
The human fibroblast activation protein alpha (FAP alpha) is a M(r) 95,000 cell surface antigen selectively expressed in reactive stromal fibroblasts of epithelial cancers, granulation tissue of healing wounds, and malignant cells of bone and soft tissue sarcomas. Normal adult tissues are generally FAP alpha-, but some fetal mesenchymal tissues transiently express the molecule. Because of its restricted normal tissue distribution and abundant expression in the stroma of over 90% of breast, colorectal, and lung carcinomas, FAP alpha is under clinical evaluation as a target for immunodetection and immunotherapy of epithelial cancers. In the present study, we have isolated a full-length cDNA for FAP alpha through expression cloning in COS-1 cells. The FAP alpha cDNA codes for a type II integral membrane protein with a large extracellular domain, transmembrane segment, and short cytoplasmic tail. FAP alpha shows 48% amino acid sequence identity to the T-cell activation antigen CD26, a membrane-bound protein with dipeptidyl peptidase IV (DPPIV) activity; however, unlike FAP alpha, CD26 is widely expressed in normal tissues. Three catalytic domains shared by DPPIV homologues in different species and by other serine proteases are conserved in FAP alpha. Immunochemical analysis of COS-1 cells coexpressing FAP alpha and CD26 revealed that the two molecules form heteromeric cell surface complexes, suggesting that a previously identified FAP alpha-associated M(r) 105,000 protein of cultured fibroblasts and growth factor-stimulated melanocytes, FAP beta, is identical to CD26. In vivo coexpression of FAP alpha and CD26 is found in reactive fibroblasts of healing wounds but not in tumor stromal fibroblasts or sarcomas (FAP alpha +/CD26-). The putative serine protease activity of FAP alpha and its in vivo induction pattern may indicate a role for this molecule in the control of fibroblast growth or epithelial-mesenchymal interactions during development, tissue repair, and epithelial carcinogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Garin-Chesa P., Melamed M. R., Rettig W. J. Immunohistochemical analysis of human neuronectin expression in normal, reactive, and neoplastic tissues. J Histochem Cytochem. 1989 Dec;37(12):1767–1776. doi: 10.1177/37.12.2685107. [DOI] [PubMed] [Google Scholar]
- Garin-Chesa P., Old L. J., Rettig W. J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kameoka J., Tanaka T., Nojima Y., Schlossman S. F., Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993 Jul 23;261(5120):466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Torimoto Y., Levinson G., Rudd C. E., Schrieber M., Dang N. H., Letvin N. L., Schlossman S. F. 1F7, a novel cell surface molecule, involved in helper function of CD4 cells. J Immunol. 1989 Dec 1;143(11):3430–3439. [PubMed] [Google Scholar]
- Rettig W. J., Garin-Chesa P., Beresford H. R., Oettgen H. F., Melamed M. R., Old L. J. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci U S A. 1988 May;85(9):3110–3114. doi: 10.1073/pnas.85.9.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig W. J., Garin-Chesa P., Healey J. H., Su S. L., Ozer H. L., Schwab M., Albino A. P., Old L. J. Regulation and heteromeric structure of the fibroblast activation protein in normal and transformed cells of mesenchymal and neuroectodermal origin. Cancer Res. 1993 Jul 15;53(14):3327–3335. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino A. P., Schürch W., Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990 Aug;63(2):144–161. [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp M. A., Tarr G. E., Chen C. Y., Switzer S. N., Hersh L. B., Stein H., Sunday M. E., Reinherz E. L. CD10/neutral endopeptidase 24.11 hydrolyzes bombesin-like peptides and regulates the growth of small cell carcinomas of the lung. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10662–10666. doi: 10.1073/pnas.88.23.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda R., Ogata S., Morrissey D. M., Finstad C. L., Szkudlarek J., Whitmore W. F., Oettgen H. F., Lloyd K. O., Old L. J. Cell surface antigens of human renal cancer defined by mouse monoclonal antibodies: identification of tissue-specific kidney glycoproteins. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5122–5126. doi: 10.1073/pnas.78.8.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K., Yokotani N., Hunter C., Doi K., Wenthold R. J., Shimasaki S. Differential expression of two distinct forms of mRNA encoding members of a dipeptidyl aminopeptidase family. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):197–201. doi: 10.1073/pnas.89.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani N., Doi K., Wenthold R. J., Wada K. Non-conservation of a catalytic residue in a dipeptidyl aminopeptidase IV-related protein encoded by a gene on human chromosome 7. Hum Mol Genet. 1993 Jul;2(7):1037–1039. doi: 10.1093/hmg/2.7.1037. [DOI] [PubMed] [Google Scholar]