Abstract
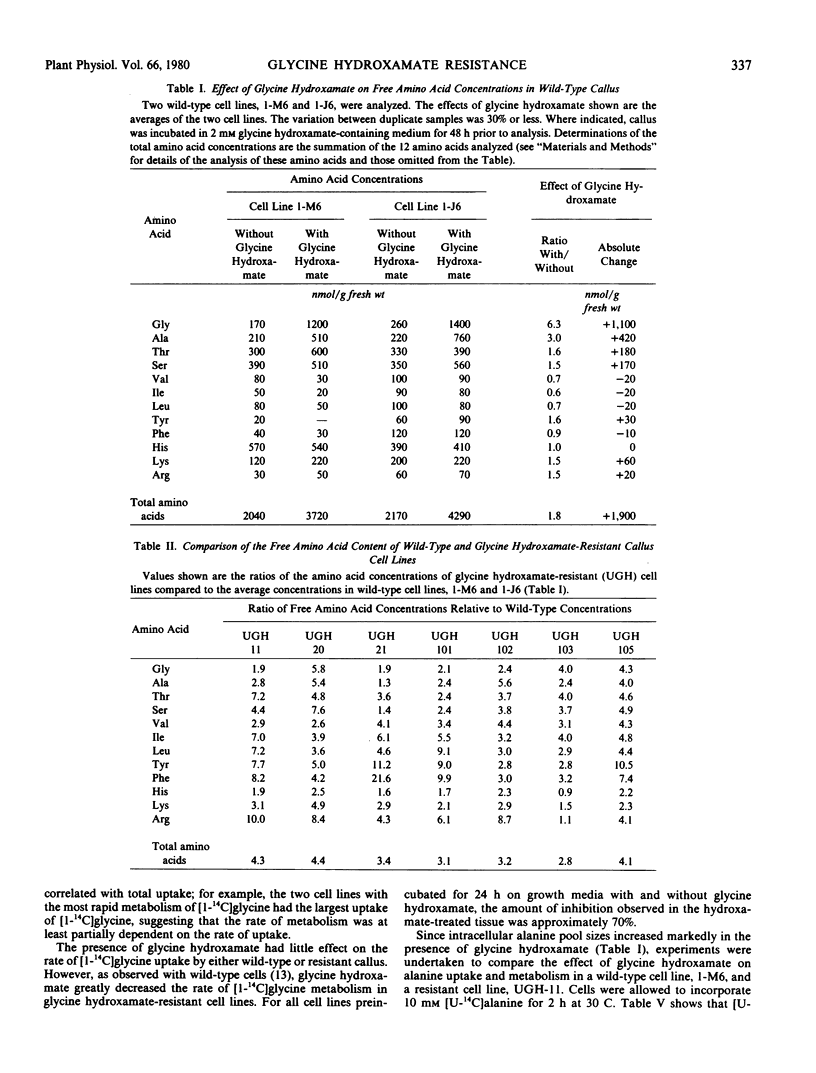
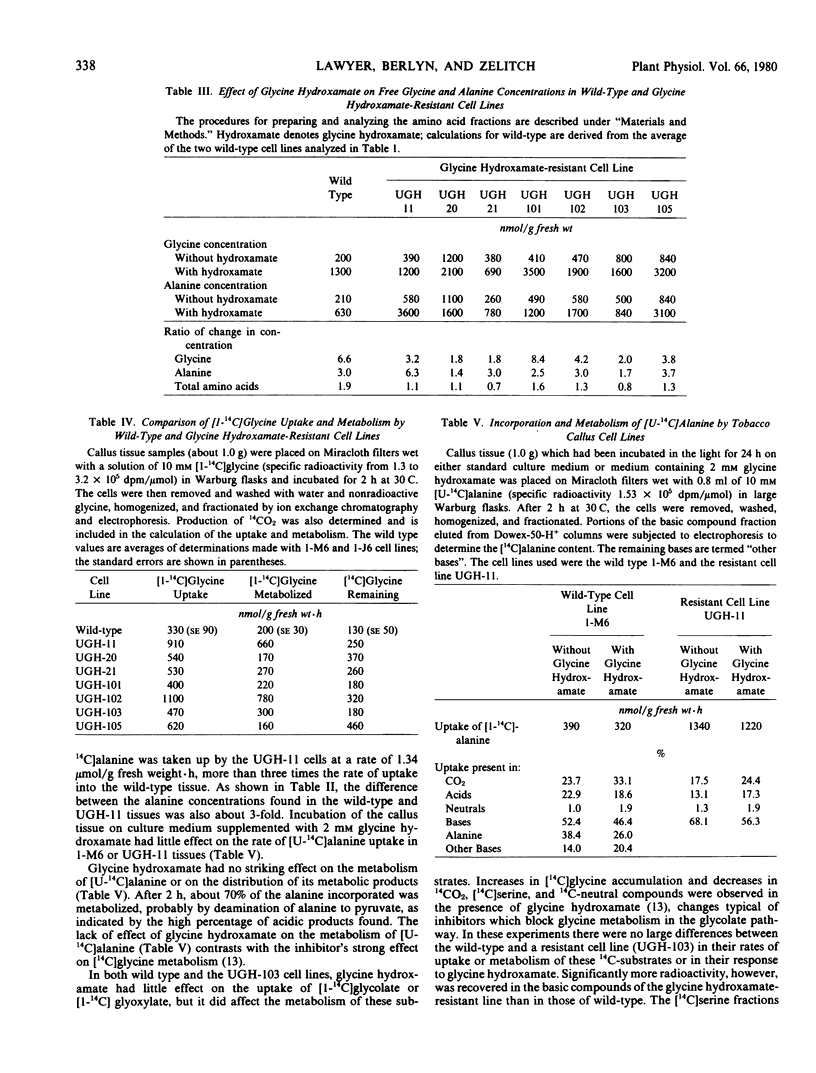
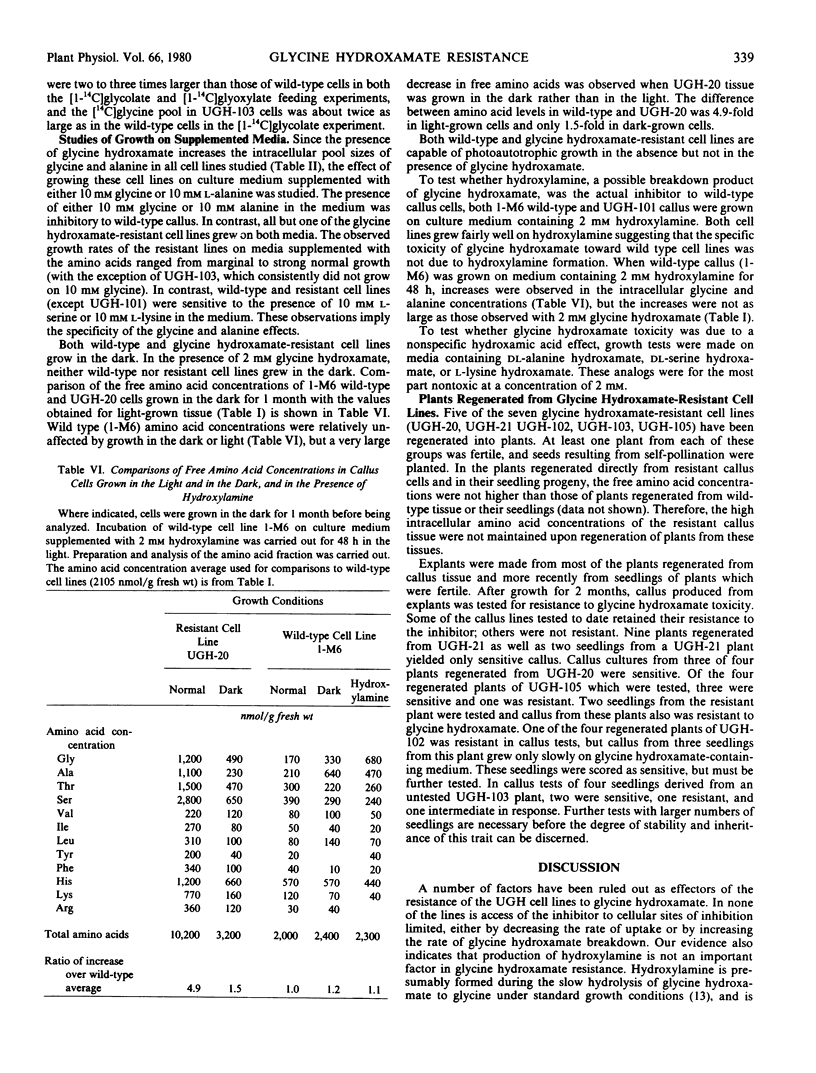
Seven lines of haploid Nicotiana tabacum tissue culture selected for resistance to normally toxic levels of the glycine analog glycine hydroxamate, a competitive inhibitor of the glycine decarboxylase reaction, were investigated. The presence of glycine hydroxamate greatly increased the intracellular concentration of both glycine and alanine in wild type and resistant cell lines, suggesting that the inhibitor blocks both glycine- and alanine-utilizing reactions. All the resistant cell lines, whether grown in the presence or absence of glycine hydroxamate, had high intracellular concentrations of the 12 free amino acids which were analyzed, including glycine and serine. (These lines averaged 3.6 times the total amino acid content of wild-type cells in the absence of the inhibitor). The resistant cell lines were indistinguishable from wild-type cell lines in their metabolism of radioactively labeled glycine hydroxamate and glycine. Comparison of the metabolism of radioactively labeled alanine, glycolate, and glyoxylate in wild-type and α resistant line also revealed no distinctive differences. Glycine decarboxylase activities were unaltered in the resistant cell lines. The cellular toxicity of glycine hydroxamate is considered in relation to (1) the competitive inhibition by glycine hydroxamate of the glycine- and alanine-utilizing enzymes and (2) the resultant imbalances caused by high intracellular concentrations of these amino acids. The significance of elevation of total free amino acid concentration in effecting resistance to the inhibitor is discussed.
Plants were regenerated from 5 of these lines and callus cultures of explants were tested for glycine hydroxamate resistance. Plants from seedlings of two lines which retained the resistant characteristic in explanted callus did not have high amino acid levels in leaves.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATFIELD G. N., MORRIS C. J. Analytical separations by highvoltage paper electrophoresis. Amino acids in protein hydrolysates. Biochem J. 1961 Dec;81:606–614. doi: 10.1042/bj0810606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrend J., Mateles R. I. Nitrogen metabolism in plant cell suspension cultures: I. Effect of amino acids on growth. Plant Physiol. 1975 Nov;56(5):584–589. doi: 10.1104/pp.56.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyn M. B., Zelitch I., Beaudette P. D. Photosynthetic characteristics of photoautotrophically grown tobacco callus cells. Plant Physiol. 1978 Apr;61(4):606–610. doi: 10.1104/pp.61.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyn M. B., Zelitch I. Photoautotrophic growth and photosynthesis in tobacco callus cells. Plant Physiol. 1975 Dec;56(6):752–756. doi: 10.1104/pp.56.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson P. S. Methionine sulfoximine--resistant mutants of tobacco. Science. 1973 Jun 29;180(4093):1366–1368. doi: 10.1126/science.180.4093.1366. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L. The effects of amino acids and ammonium on the growth of plant cells in suspension culture. Plant Physiol. 1970 Apr;45(4):372–375. doi: 10.1104/pp.45.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer A. L., Zelitch I. Inhibition of glutamate:glyoxylate aminotransferase activity in tobacco leaves and callus by glycidate, an inhibitor of photorespiration. Plant Physiol. 1978 Feb;61(2):242–247. doi: 10.1104/pp.61.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer A. L., Zelitch I. Inhibition of glycine decarboxylation and serine formation in tobacco by glycine hydroxamate and its effect on photorespiratory carbon flow. Plant Physiol. 1979 Nov;64(5):706–711. doi: 10.1104/pp.64.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. E., Widholm J. Characterization of Carrot and Tobacco Cell Cultures Resistant to p-Fluorophenylalanine. Plant Physiol. 1975 Aug;56(2):233–238. doi: 10.1104/pp.56.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Biochemical bases for the antimetabolite action of L-serine hydroxamate. J Bacteriol. 1971 Jun;106(3):972–982. doi: 10.1128/jb.106.3.972-982.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


