Abstract
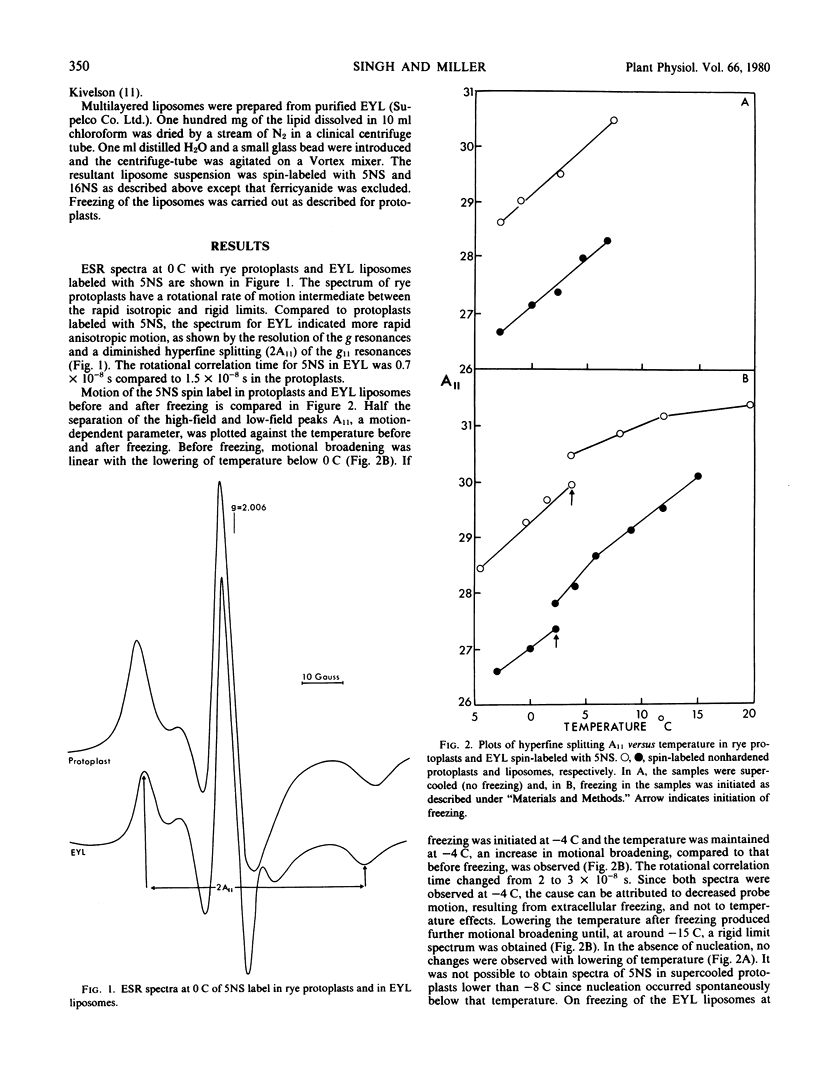
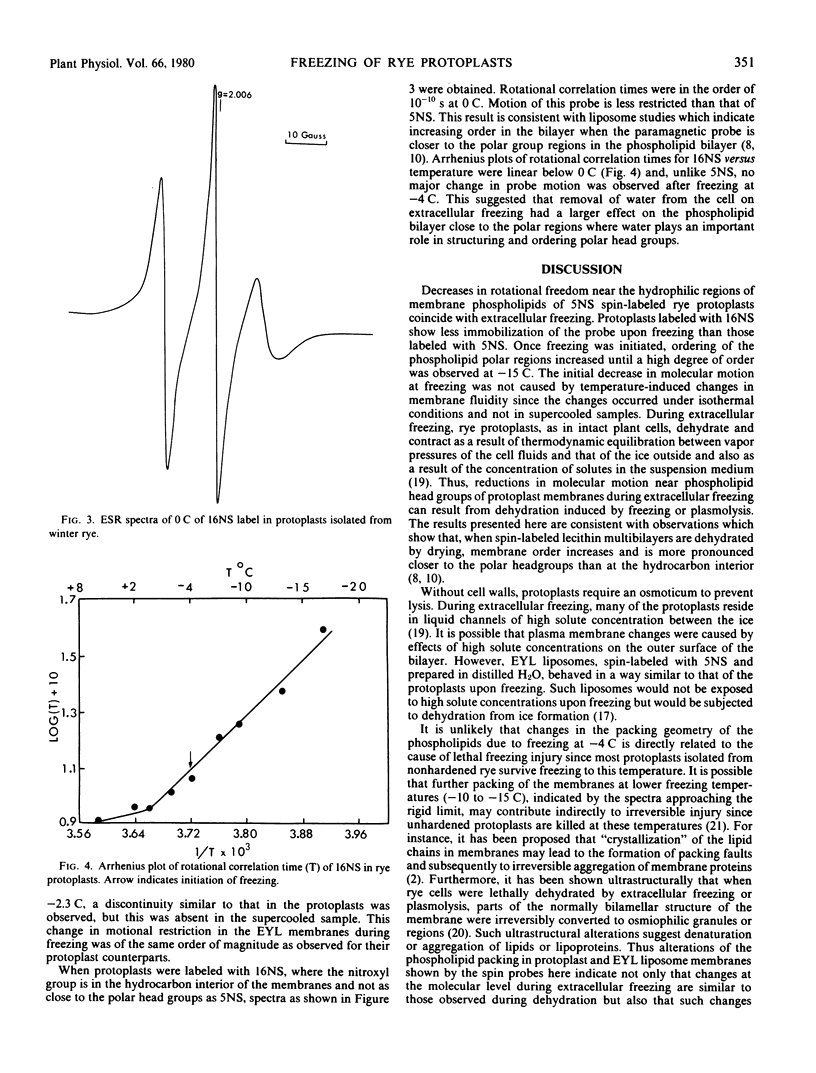
Protoplasts isolated from epicotyls of nonhardened winter rye seedlings were spin-labeled with the N-oxyl-4-4-dimethyloxazolidine derivatives of 5-ketostearic (5NS) and 16-ketostearic (16NS) acids. Spectra of the membrane-bound labels showed motional broadening with a rotational correlation time of 1.5 × 10−8 second for 5NS and 1.5 × 10−10 second for 16NS at 0 C. A procedure was developed to follow membrane changes in these protoplasts during extracellular freezing. With freezing, molecular motion of 5NS, but not of 16NS, spin probes was restricted. The increase in molecular order near the hydrated end of the membrane did not result from lowered temperatures inasmuch as no such change was observed in supercooled samples. These changes are probably due to dehydration of protoplast membranes during extracellular freezing. Similar results were obtained with multilayered egg yolk lecithin and are consistent with previous observations of changes in lecithin multibilayers during dehydration. Such alterations in membrane order might lead to irreversible membrane damage during extracellular freezing of plant cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannon B., Polnaszek C. F., Butler K. W., Eriksson L. E., Smith I. C. The fluidity and organization of mitochondrial membrane lipids of the brown adipose tissue of cold-adapted rats and hamsters as determined by nitroxide spin probes. Arch Biochem Biophys. 1975 Apr;167(2):505–518. doi: 10.1016/0003-9861(75)90493-2. [DOI] [PubMed] [Google Scholar]
- Chapman D., Gómez-Fernández J. C., Goñi F. M. Intrinsic protein--lipid interactions. Physical and biochemical evidence. FEBS Lett. 1979 Feb 15;98(2):211–223. doi: 10.1016/0014-5793(79)80186-6. [DOI] [PubMed] [Google Scholar]
- Garber M. P., Steponkus P. L. Alterations in Chloroplast Thylakoids during an in Vitro Freeze-Thaw Cycle. Plant Physiol. 1976 May;57(5):673–680. doi: 10.1104/pp.57.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. H., Dehlinger P. J., Van S. P. Shape of the hydrophobic barrier of phospholipid bilayers (evidence for water penetration in biological membranes). J Membr Biol. 1974;15(2):159–192. doi: 10.1007/BF01870086. [DOI] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Loss of Adenosine Triphosphate Synthesis Caused by Freezing and Its Relationship to Frost Hardiness Problems. Plant Physiol. 1964 Sep;39(5):712–719. doi: 10.1104/pp.39.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P., Libertini L. J., Hebert V. C., Griffith O. H. Lipid spin labels in lecithin multilayers. A study of motion along fatty acid chains. J Mol Biol. 1971 Jul 14;59(1):77–98. doi: 10.1016/0022-2836(71)90414-1. [DOI] [PubMed] [Google Scholar]
- Scarth G. W. DEHYDRATION INJURY AND RESISTANCE. Plant Physiol. 1941 Jan;16(1):171–179. doi: 10.1104/pp.16.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch D., Chapman D. Liposome bilayer model systems of freezing living cells. FEBS Lett. 1971 Aug 15;16(3):207–212. doi: 10.1016/0014-5793(71)80135-7. [DOI] [PubMed] [Google Scholar]
- Siminovitch D., De la Roche I. A. Freezing behavior of free protoplasts of winter rye. Cryobiology. 1978 Apr;15(2):205–213. doi: 10.1016/0011-2240(78)90025-1. [DOI] [PubMed] [Google Scholar]
- Siminovitch D., Singh J., de la Roche I. A. Studies on membranes in plant cells resistant to extreme freezing. I. Augmentation of phospholipids and membrane substance without changes in unsaturation of fatty acids during hardening of black locust bark. Cryobiology. 1975 Apr;12(2):144–153. doi: 10.1016/s0011-2240(75)80006-x. [DOI] [PubMed] [Google Scholar]
- Singh J., de La Roche I. A., Siminovitch D. Differential scanning calorimeter analyses of membrane lipids isolated from hardened and unhardened black locust bark and from winter rye seedlings. Cryobiology. 1977 Oct;14(5):620–624. doi: 10.1016/0011-2240(77)90173-0. [DOI] [PubMed] [Google Scholar]
- Singh J., de la Roche A. I., Siminovitch D. Relative insensitivity of mitochondria in hardened and nonhardened rye coleoptile cells to freezing in situ. Plant Physiol. 1977 Nov;60(5):713–715. doi: 10.1104/pp.60.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest S. C., Steponkus P. L. Freeze-thaw injury to isolated spinach protoplasts and its simulation at above freezing temperatures. Plant Physiol. 1978 Nov;62(5):699–705. doi: 10.1104/pp.62.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche I. A., Pomeroy M. K., Andrews C. J. Changes in fatty acid composition in wheat cultivars of contrasting hardiness. Cryobiology. 1975 Oct;12(5):506–512. doi: 10.1016/0011-2240(75)90032-2. [DOI] [PubMed] [Google Scholar]


