Abstract
The acute and chronic effects of abused psychostimulants on monoamine transporters and associated neurobiology have encouraged development of candidate medications that target these transporters. Monoamine transporters in general, and dopamine transporters in particular, are critical molecular targets that mediate abuse-related effects of psychostimulants such as cocaine and amphetamine. Moreover, chronic administration of psychostimulants can cause enduring changes in neurobiology reflected in dysregulation of monoamine neurochemistry and behavior. The current review will evaluate evidence for the efficacy of monoamine transporter inhibitors and substrates to reduce abuse-related effects of stimulants in preclinical assays of stimulant self-administration, drug discrimination and reinstatement. In considering deployment of monoamine transport inhibitors and substrates as agonist-type medications to treat stimulant abuse, the safety and abuse liability of the medications are an obvious concern, and this will also be addressed. Future directions in drug discovery should identify novel medications that retain efficacy to decrease stimulant use but possess lower abuse liability, and evaluate the degree to which efficacious medications can attenuate or reverse neurobiological effects of chronic stimulant use.
Keywords: Dopamine, Serotonin, Norepinephrine, Monoamines, Cocaine, Amphetamine, Nonhuman Primates, Self-administration, Drug Discrimination, Neuroimaging
Introduction
Preclinical assessment of candidate medications to treat drug addiction relies on a two-step process that involves (1) detection of abuse-related effects produced by the target drug of abuse, followed by (2) evaluation of the efficacy and safety of candidate medications to reduce those abuse-related effects. Drug addiction is an operant behavior in which maladaptive patterns of drug use are maintained by drug delivery, and as a result, preclinical operant conditioning procedures have played a key role in medication development. This review will focus on use of preclinical operant procedures to evaluate inhibitors and substrates of dopamine, serotonin and norepinephrine transporters as candidate medications to treat psychostimulant addiction. The review will be divided into three sections. First, principles of operant behavior will be reviewed to illustrate their use in characterizing abuse-related effects of psychostimulants and evaluating efficacy of candidate medications to treat psychostimulant addiction. Second, we will discuss evidence from these and related procedures that implicates monoamine transporters as molecular targets of psychostimulants that mediate abuse liability and adapt to chronic stimulant exposure. Lastly, we will review evidence that regimens of treatment with monoamine transporter inhibitors and substrates can reduce abuse-related effects of psychostimulants in these preclinical procedures as well as in human laboratory studies and clinical trials. Taken together, the data to be discussed will show that monoamine transporter inhibitors and releasers can reduce abuse-related effects of psychostimulants and warrant further consideration as candidate medications for the treatment of psychostimulant addiction. Figure 1 illustrates categories of research on abuse-related behavior and neurobiological effects of abused stimulants.
Figure 1.
Categories of research on abuse-related behavioral and neurobiological effects of abused stimulants in the absence or presence of treatment with candidate medications. A large database now exists on behavioral and neurobiological effects of abused stimulants, and this work provides insight on mechanisms of stimulant addiction. This database also provides a foundation for research to examine effects of candidate medications on abuse-related behavioral and neurobiological effects of abused stimulants. At present, a substantial and growing body of data has examined effects of monoamine transporter inhibitors and substrates on abuse-related behavioral effects of stimulants, whereas a much smaller body of work has addressed effects of these compounds on abuse-related neurobiological effects of stimulants. Optimal candidate medications will reduce expression of abuse-related behavioral effects and reverse abuse-related neurobiological effects of abused stimulants.
Operant Behavior in Medications Development
Drug addiction is a maladaptive pattern of drug use leading to clinically significant impairment or distress (American_Psychiatric_Association, 2013). The development of medications to treat addiction is founded on the premise that addiction is caused in part by effects of the abused drug that can be blocked or reversed by medications. However, all drugs of abuse produce multiple effects, only some of which are likely to promote abuse. Cocaine, for example, produces local anesthetic, cardiovascular and neurobiological effects, but only a subset of neurobiological effects is thought to contribute to addiction (Biebuyck, 1990; Catterall & Mackie, 2005; Johanson & Fischman, 1989; O’Brien, 2006). One challenge in preclinical research on medications development is to identify those “abuse-related” effects that contribute to a drug’s abuse potential and that might serve as reasonable targets for intervention with medications.
One fruitful approach to this challenge has conceptualized drug addiction as an example of operant behavior, which can be defined as behavior shaped by its consequences in the framework of a 3-term contingency. This 3-term contingency can be diagrammed as follows:
where SD signifies a discriminative stimulus, R designates a response on the part of the organism, and SC designates a consequent stimulus (Ferster & Skinner, 1957; Skinner, 1938). The arrows specify the contingency that, in the presence of the discriminative stimulus SD, performance of the response R will result in delivery of the consequent stimulus SC. Consequent stimuli that increase responding leading to their delivery are operationally defined as reinforcers. The contingencies that relate discriminative stimuli, responses and consequent stimuli are defined by the schedule of reinforcement. For example, under a fixed-ratio (FR) schedule, a fixed number of responses in the presence of the SD is required to produce delivery of the SC (e.g. an FR 10 schedule would require 10 responses). Far more complex schedules of reinforcement are also possible. Drugs can function as either the discriminative or consequent stimulus in the 3-term contingency, and procedures that use drugs in these capacities play a key role in research to evaluate both the abuse liability of drugs and the efficacy of medications to treat drug abuse. Three commonly used families of procedures are briefly reviewed below.
Drug Self-Administration
In drug self-administration procedures, drug delivery serves as the consequent stimulus (Katz, 1989; Negus & Banks, 2011; Young & Herling, 1986). More specifically, conditions are established such that, in the presence of some discriminative stimulus (e.g. a stimulus light), emission of a response (e.g. pressing a response lever) produces delivery of a drug dose (e.g. i.v. delivery via a chronic i.v. catheter). A drug is considered to function as a reinforcer if some drug dose maintains higher response rates than vehicle, and many drugs of abuse including psychostimulants function as reinforcers in drug self-administration procedures. The high concordance between preclinical measures of drug reinforcement and clinical measures of abuse liability has encouraged use of drug self-administration for practical applications such as abuse liability assessment by regulatory agencies like the U.S. Drug Enforcement Agency (Ator & Griffiths, 2003; Carter & Griffiths, 2009). Moreover, drug self-administration by laboratory animals has clear parallels to human patterns of drug abuse that also involve sequences of behavior culminating in drug consumption. For all these reasons, drug reinforcement in assays of drug self-administration is often viewed as the most significant “abuse-related” effect amenable to preclinical study, and candidate medications can be evaluated for the degree to which they reduce self-administration of a target drug of abuse such as cocaine (Comer, Ashworth, Foltin, Johanson, Zacny & Walsh, 2008; Haney & Spealman, 2008; Mello & Negus, 1996).
Experiments to examine medication effects on drug self-administration can include many nuances (Mello et al., 1996). Three of those will be mentioned here. First, to be clinically efficacious, medication effects should be sustained rather than transient, because drug addiction is a chronic disorder that often demands chronic treatment. Assessment of the persistence of medication effects requires experimental designs that use chronic medication delivery. Second, to be clinically safe, medications should reduce consumption of the abused drug without producing undesirable effects. Although toxicology screens play a key role in safety assessment, useful insights to safety can also be provided by comparing medication effects on drug self-administration with effects on responding maintained by a non-drug reinforcer such as food. Some level of safety is implied by a profile of medication effects that includes selective reduction in drug self-administration with lesser effects on responding maintained by another reinforcer. Finally, medication effects on drug self-administration may vary as a function of the schedule of reinforcement used to maintain drug self-administration. This issue has been discussed in detail elsewhere (e.g. Negus and Banks, 2011) and is beyond the scope of this review. However, as a general rule, the strength of preclinical evidence for medication efficacy depends in part on the breadth of conditions across which a medication reduces drug self-administration.
Drug Discrimination
In drug discrimination procedures, the drug serves as the discriminative stimulus of the 3-term contingency (Colpaert, 1999; Glennon & Young, 2011). In a common example, subjects have access to two response levers, and responding on these levers produces food reinforcement contingent on the presence or absence of a training dose of a training drug. Specifically, responding on only one lever (the drug-appropriate lever) produces food reinforcement after drug delivery, and responding on only the other lever (the vehicle-appropriate lever) produces food after vehicle delivery. A drug is considered to function as a discriminative stimulus if subjects can be trained to respond differentially to its presence or absence. Both abused and non-abused drugs can produce discriminative stimulus effects, so the mere ability of a drug to function as a discriminative stimulus is not sufficient to signify abuse liability; however, all drugs of abuse can function as discriminative stimuli, and abuse liability of a test drug is indicated if it shares discriminative stimulus effects with a known drug of abuse (e.g. if the test drug produces drug-appropriate responding in a subject trained to discriminate a known drug of abuse such as cocaine) (Ator et al., 2003; Overton, 1987). In addition, discriminative stimulus effects of drugs in animals are homologous to subjective drug effects in humans, where discrimination is evidenced by different patterns of verbal behavior rather than by differential lever-pressing behavior (Carter et al., 2009; Schuster & Johanson, 1988). In view of these considerations, discriminative stimulus effects can also be considered as a category of abuse-related drug effects. Drug discrimination is used in medication development to assess the degree to which candidate medications mimic or modify discriminative stimulus effects of the target drug of abuse.
Reinstatement
Reinstatement procedures constitute a subtype of drug self-administration that adds a focus on the function of nondrug stimuli (Shaham, Shalev, Lu, De Wit & Stewart, 2003). In a common example, a nondrug stimulus (e.g. a stimulus light) functions as a discriminative stimulus in a drug self-administration procedure. Additional nondrug stimuli (e.g. additional stimulus lights or sounds) may also be paired with drug delivery such that, by classical conditioning mechanisms, they come to function as conditioned reinforcers. Once drug self-administration is established, some period of drug abstinence is imposed when self-administration sessions as a whole are omitted, or more commonly, when modified sessions are conducted during which the operant manipulandum is present but drug and some or all nondrug stimuli are omitted. This abstinence period produces not only withdrawal from the self-administered drug, but also a decline in rates of the operant behavior that produced drug, either because whole sessions are omitted and the operant behavior is not possible, or because drug and nondrug stimuli have been omitted and operant behavior extinguishes. At the conclusion of the abstinence period, test stimuli are introduced and rates of operant responding are reevaluated, usually with continued omission of drug reinforcement. In general, three types of test stimuli are used: (a) non-contingent treatments with the self-administered drug, (b) reintroduction of omitted nondrug discriminative stimuli and/or conditioned reinforcers (often collectively referred to as “cues”), and (c) stimuli such as foot shock intended to induce “stress.” Each of these drug, cue and stress stimuli can increase (or “reinstate”) rates of operant responding, and candidate medications can be evaluated for the degree to which they reduce stimulus-induced reinstatement.
One goal in the use of reinstatement procedures is to model the phenomenon of relapse in drug addiction, and by extension, medication effects on reinstatement are often interpreted as predictive of their utility to treat relapse (Epstein, Preston, Stewart & Shaham, 2006; Martin-Fardon & Weiss, 2013). However, the relationship between experimental reinstatement and clinical relapse is controversial (Katz & Higgins, 2003), and medication effects on reinstatement might be more profitably interpreted in terms of their effects on the stimulus functions of test stimuli. For example, drug-induced reinstatement likely involves multiple behavioral mechanisms that include discriminative stimulus effects (Gerber & Stretch, 1975). During a drug self-administration session, each self-administered drug dose can function not only as a reinforcing stimulus that increases the probability of preceding behaviors, but also as a discriminative stimulus associated with access to contingencies of drug availability. Consequently, non-contingent administration of drug can be expected to produce discriminative stimulus effects associated with drug availability and conducive to operant responding, and medication effects on drug reinstatement can be expected to resemble medication effects on discriminative stimulus effects of the drug under other circumstances (e.g. in conventional drug discrimination assays).
Monoamine Transporters as Molecular Targets for Medication Development
Monoamine Transporter Function
Monoamine transporters are transmembrane proteins located in plasma membranes of monoaminergic neurons, and include the dopamine transporter (DAT), serotonin transporter (SERT), and norepinephrine transporter (NET) (Amara & Kuhar, 1993; Langer & Galzin, 1988) (see (Lin, Canales, Bjorgvinsson, Thomsen, Qu, Liu et al., 2011)). Their main function is to terminate monoamine transmission by inward transport of released neurotransmitter. Within the cell, vesicular monoamine transporters (VMAT) concentrate neurotransmitter molecules in synaptic vesicles for additional cycles of release (Erickson, Eiden & Hoffman, 1992; Schuldiner, Shirvan & Linial, 1995). VMAT1 is preferentially expressed in neuroendocrine cells and VMAT2 is primarily expressed in the CNS (see (Wimalasena, 2011)).
Monoamines play important roles in normal brain function and are implicated in various neuropsychiatric disorders. Accordingly, the regulation of monoamines is critically important. Dopamine is implicated in many physiological processes such as movement, cognition, memory, and reward (Greengard, 2001). Cell bodies that produce dopamine are localized to the substantia nigra, the ventral tegmental area (VTA), and the hypothalamus. Dopamine neurons project to the caudate nucleus, putamen, nucleus accumbens, and prefrontal cortex (Westerink, 2006). Serotonin is also implicated in many functions, including mood, sleep, appetite, anxiety, fear, reward, and aggression (Barnes & Sharp, 1999; Hoyer, Hannon & Martin, 2002). Serotonin neurons are restricted to the raphe nuclei in the brainstem, and project to the cortex, thalamus, basal ganglia, hippocampus, and amygdala (Jacobs & Azmitia, 1992). Norepinephrine plays a critical role in arousal (Astier, Van Bockstaele, Aston-Jones & Pieribone, 1990), attention, memory, and mood (Grant, Aston-Jones & Redmond, 1988), and is synthesized primarily in the locus coeruleus and surrounding nuclei in mammals, including humans (Carpenter & Sutin, 1983). Anatomical studies have shown consistently that monoaminergic neurons each express a specific plasma membrane transporter (Hoffman, Hansson, Mezey & Palkovits, 1998). Alteration of cell surface expression of transporters is a critical mechanism for regulating monoamine transport. Trafficking of plasma membrane transporters has been shown to occur under basal conditions (Loder & Melikian, 2003) and can also be induced by transporter substrates (Chi & Reith, 2003; Furman, Chen, Guptaroy, Zhang, Holz & Gnegy, 2009), and inhibitors (Daws, Callaghan, Moron, Kahlig, Shippenberg, Javitch et al., 2002; Little, Elmer, Zhong, Scheys & Zhang, 2002; Rothman, Blough & Baumann, 2007).
Psychostimulants enhance monoaminergic signaling by interfering with transporter function. However, psychostimulants differ in their relative affinity for DAT, SERT and NET. For example, cocaine has approximately equal affinity for these three transporters, whereas amphetamine, methamphetamine and methylphenidate all have relatively lower affinity for SERT compared to their affinity for DAT and NET (see (Howell & Kimmel, 2008)). In addition, psychostimulants differ in their actions as reuptake inhibitors versus substrate-type releasers (Fleckenstein, Gibb & Hanson, 2000; Rothman et al., 2007). Transporter inhibitors, including cocaine, interfere with transporter function but are not transported into the nerve terminal. In contrast, substrate-type releasers, including amphetamine and methamphetamine, are transported into the cytoplasm of the nerve terminal. Transporter substrates elevate extracellular monoamine levels by reversing the process of transporter-mediated exchange. They also increase cytoplasmic levels of monoamines by interfering with vesicular storage (Rudnick, 1997; Rudnick & Clark, 1993). Typically, substrates are more effective than inhibitors in increasing extracellular monoamines because the former increase the pool of neurotransmitters available for release by transporter-mediated exchange. Moreover, the effectiveness of substrates in increasing extracellular monoamines is not dependent upon the basal rate of neurotransmitter release. In contrast, the effectiveness of inhibitors is impulse-dependent and, therefore, limited by the tone of presynaptic activity.
Role of Monoamine Transporters in Acute Behavioral Effects of Psychostimulants
Abundant evidence implicates monoamine transporters in general, and dopamine transporters in particular, as molecular targets that mediate abuse-related effects of psychostimulants such as cocaine and amphetamine (Koob, 1992; Lile & Nader, 2003; Natarajan & Yamamoto, 2011; Rothman & Glowa, 1995; Woolverton & Johnson, 1992). For example, the potency and efficacy of cocaine and a series of related drugs to maintain drug self-administration correlated with their potency to inhibit uptake of dopamine but not serotonin or norepinephrine (Ritz, Lamb, Goldberg & Kuhar, 1987). Similarly, cocaine-like reinforcing effects (Bergman, Madras, Johnson & Spealman, 1989; Hiranita, Soto, Newman & Katz, 2009; Howell & Byrd, 1995; Woolverton, 1987) and discriminative stimulus effects (Katz, Izenwasser & Terry, 2000; Kleven, Anthony & Woolverton, 1990a; Schama, Howell & Byrd, 1997; Spealman, 1993, 1995) are produced by dopamine-selective uptake inhibitors, but generally not by serotonin- or norepinephrine-selective uptake inhibitors. With amphetamine and other monoamine releasers, it has been more difficult to identify compounds that dissociate effects mediated by dopamine and norepinephrine release, and abused releasers like amphetamine generally display similar to slightly higher (~3-fold) potency to promote release of norepinephrine vs. dopamine in in vitro assays (Rothman, Baumann, Dersch, Romero, Rice, Carroll et al., 2001). However, potency and efficacy of a series of releasers to maintain self-administration or produce cocaine-like discriminative stimulus effects was correlated with potency to release dopamine/norepinephrine (Negus, Mello, Blough, Baumann & Rothman, 2007; Wee, Anderson, Baumann, Rothman, Blough & Woolverton, 2005). Lastly, disruption of dopaminergic signaling disrupts expression of abuse-related effects by abused psychostimulants. For example, the reinforcing and/or discriminative stimulus effects of cocaine can be blocked by lesions to the mesolimbic dopamine system (Caine & Koob, 1994), by genetic modification of dopamine transporters (Thomsen, Hall, Uhl & Caine, 2009a; Thomsen, Han, Gu & Caine, 2009b), or by pharmacologic antagonists of dopamine receptors (Bergman, Kamien & Spealman, 1990; Caine et al., 1994; Caine, Negus, Mello & Bergman, 2000; Negus, Mello, Lamas & Mendelson, 1996).
The dopaminergic system is clearly an important site of action for abused stimulants, but preclinical studies have also indicated that the serotonergic system can effectively modulate the behavioral effects of cocaine and amphetamine. Although compounds that selectively increase serotonin neurotransmission lack behavioral-stimulant effects and do not reliably maintain self-administration behavior (Howell et al., 1995; Vanover, Nader & Woolverton, 1992), a negative relationship was observed between the potencies of several cocaine- and amphetamine-like drugs in self-administration studies and their binding affinities for serotonin uptake sites (Ritz & Kuhar, 1989; Ritz et al., 1987). Co-administration of agents that induce robust increases in both dopamine and serotonin produces minimal behavioral-stimulant effects (Bauer, Banks, Blough & Negus, 2013; Baumann, Ayestas, Dersch, Brockington, Rice & Rothman, 2000) and does not maintain self-administration behavior (Glatz, Ehrlich, Bae, Clarke, Quinlan, Brown et al., 2002) in rodents. Similarly, monoamine-releasing agents have decreased reinforcing efficacy in rhesus monkeys when serotonin releasing potency is increased relative to dopamine (Negus et al., 2007; Wee et al., 2005). The behavioral and neurochemical profile of DAT inhibitors is also influenced by their actions at multiple monoamine transporters in squirrel monkeys (Ginsburg, Kimmel, Carroll, Goodman & Howell, 2005).
Studies in nonhuman primates also support a significant but subordinate role for norepinephrine uptake in the discriminative-stimulus effects of cocaine (Spealman, 1995). More recent studies in squirrel monkeys have also documented that NET inhibition can play a significant role in cocaine-induced reinstatement (Platt, Rowlett & Spealman, 2007). There is also a significant positive correlation between potency of drug-induced norepinephrine release and the drug dose that produces stimulant-like subjective effects in humans following oral administration (Rothman et al., 2001). However, it should be noted that there is little evidence that norepinephrine plays a primary role in the reinforcing properties of psychomotor stimulants in rodents (Tella, 1995) or nonhuman primates (Kleven & Woolverton, 1990c; Mello, Lukas, Bree & Mendelson, 1990; Woolverton, 1987).
This evidence implicating monoamine transporters, and especially dopamine transporters, as molecular targets of abused psychostimulants provides a sound rationale for development of transporter inhibitors and substrates as medications that also target monoamine transporters. A second line of evidence derives from studies showing that chronic exposure to abused psychostimulants can modulate monoamine transporters, associated monoaminergic systems and indices of cortical function.
Neurobiological Effects of Chronic Psychostimulant Administration
Chronic administration of psychostimulants can cause enduring changes in neurobiology and corresponding changes in sensitivity to acute drug effects on neurochemistry and behavior. Both sensitization and tolerance have been reported to develop during repeated administration of stimulants in animal studies (Woolverton & Weiss, 1998). However, the outcome can depend upon a variety of procedural variables including the drug effect under investigation, the dosing regimen, the environmental context associated with drug administration and the animal species. The vast majority of studies have focused on sensitization to locomotor-stimulant effects in rodent models. Stimulants including cocaine and amphetamines can produce robust sensitization in rodents, usually identified as a progressive increase in locomotor activity or stereotyped behavior with drug dosing (Robinson & Berridge, 2000). There is substantial evidence that the mesocorticolimbic dopamine system and its excitatory glutamatergic inputs are critical for the development of sensitization to the behavioral effects of psychostimulants in rodents (Carlezon & Nestler, 2002; Wolf, Sun, Mangiavacchi & Chao, 2004). In contrast, tolerance to the neurochemical effects of cocaine has been reported in nonhuman primates. Rhesus monkeys trained to self-administer cocaine showed a significant increase in striatal extracellular dopamine following the first injection of a session, but the response to cocaine was attenuated following the second injection, indicative of acute tolerance (Bradberry, 2000). In a related study initiated in drug-naïve rhesus monkeys, subjects were trained to self-administer cocaine under limited-access conditions (1 hour/day) for 10 weeks, followed by extended-access conditions (4 hour/day) for 10 weeks, and microdialysis studies were conducted at the end of each phase (Kirkland Henry, Davis & Howell, 2009). Under both self-administration conditions, cocaine-induced increases in extracelluar dopamine were blunted compared to drug-naïve conditions, indicating that cocaine self-administration resulted in a hypofunctional dopamine system.
Efforts to define the long-term neurobiological consequences of psychostimulant administration in rodents have focused primarily on the dopaminergic system and have yielded inconsistent results. For example, cocaine exposure has been reported to increase, decrease or have no effect on DAT density in rodents (Boulay, Duterte-Boucher, Leroux-Nicollet, Naudon & Costentin, 1996; Claye, Akunne, Davis, DeMattos & Soliman, 1995; Letchworth, Daunais, Hedgecock & Porrino, 1997; Letchworth, Sexton, Childers, Vrana, Vaughan, Davies et al., 1999; Pilotte, Sharpe & Kuhar, 1994; Tella, Ladenheim, Andrews, Goldberg & Cadet, 1996; Wilson, Nobrega, Corrigall, Coen, Shannak & Kish, 1994). Similarly, chronic cocaine administration in rodents has been reported to increase, decrease or have no effect on dopamine D1- or D2-receptor density (Dwoskin, Peris, Yasuda, Philpott & Zahniser, 1988; Goeders & Kuhar, 1987; Kleven, Perry, Woolverton & Seiden, 1990b; Kuhar & Pilotte, 1996). The equivocal results likely reflect different dosing regimens and withdrawal periods, as well as the use of non-contingent drug administration protocols that do not model voluntary drug use. Active drug self-administration protocols and periods of drug abstinence can have profound influences on the neurobiology of dopamine systems (Mateo, Lack, Morgan, Roberts & Jones, 2005). Accordingly, a more consistent picture has emerged from nonhuman primate studies of cocaine self-administration (Table 1). For example, in rhesus monkeys trained to self-administer i.v. cocaine, initial exposure led to moderate decreases in DAT density in the striatum as determined postmortem with quantitative autoradiography (Letchworth, Nader, Smith, Friedman & Porrino, 2001). However, longer exposure resulted in increased striatal DAT density that was most pronounced in the ventral striatum at the level of the nucleus accumbens. Importantly, the increases in DAT binding observed after long-term cocaine self-administration in nonhuman primates corresponded closely to increases observed in post-mortem tissue of human cocaine addicts (Little, Kirkman, Carroll, Clark & Duncan, 1993; Staley, Hearn, Ruttenber, Wetli & Mash, 1994). In related studies, rhesus monkeys trained to self-administer cocaine on a daily basis over 18–22 months showed lower dopamine D1 binding density as determined post-mortem with quantitative autoradiography (Moore, Vinsant, Nader, Porrino & Friedman, 1998a; Nader, Daunais, Moore, Nader, Moore, Smith et al., 2002). In parallel studies using the same self-administration schedule and quantitative autoradiography, dopamine D2 binding density was lower in all regions of the striatum rostral to the anterior commissure (Moore, Vinsant, Nader, Porrino & Friedman, 1998b; Nader et al., 2002). Collectively, these drug-induced changes provide additional evidence of a hypofunctional dopamine system that may contribute to the development of dependence associated with long-term psychostimulant use.
Table 1.
Neurobiological Effects of Chronic Cocaine Administration in Nonhuman Primates and Humans
| Effect | Nonhuman Primates | Humans |
|---|---|---|
| Dopamine Transporter | ↑ Letchworth, et al., 2001 = Czoty, et al., 2007 |
↑ Little, et al., 1993 ↑ Staley, et al., 1994 |
| Dopamine D1 Receptor | ↓ Moore, et al., 1998a ↓ Nader, et al., 2002 |
= Martinez, et al., 2009 |
| Dopamine D2 Receptor | ↓ Moore, et al., 1998b ↓ Nader, et al., 2002 ↓ Czoty, et al., 2004 |
↓ Volkow and Fowler, 2000 ↓ Volkow, et al., 2001a |
| Dopamine Release | ↓ Kirkland Henry, et al., 2009 | ↓ Martinez, et al., 2007 ↓ Volkow, et al., 1997 |
| Serotonin Transporter | ↑ Banks, et al., 2008 ↑ Gould, et al., 2011 |
↑ Jacobsen, et al., 2000 ↑ Mash, et al., 2000 |
| Serotonin 5HT2A Receptor | ↑ Sawyer, et al., 2012 | |
| Norepinephrine Transporter | ↑ Ding, et al., 2010 | |
| Distribution of Cerebral Activation |
↑ Henry, et al., 2010 | |
| Cerebral Perfusion | ↓ Volkow, et al., 1998 | |
| Cerebral Metabolism | ↓ Reivich, et al., 1985 ↓ Volkow, et al., 2001a |
Chronic exposure to cocaine also induces changes in the serotonin system. Increases in SERT density following chronic cocaine exposure have been reported in cells (Kittler, Lau & Schloss, 2010), rodents (Cunningham, Paris & Goeders, 1992), nonhuman primates (Banks, Czoty, Gage, Bounds, Garg, Garg et al., 2008; Gould, Gage, Banks, Blaylock, Czoty & Nader, 2011), and humans (Jacobsen, Staley, Malison, Zoghbi, Seibyl, Kosten et al., 2000; Mash, Staley, Izenwasser, Basile & Ruttenber, 2000). Cocaine exposure has also been reported to affect density and/or function of post-synaptic 5HT receptors, ranging from decreases in 5HT3 receptors in the nucleus accumbens shell of rats sensitized to cocaine (Ricci, Stellar & Todtenkopf, 2004) to reduced sensitivity of 5HT1A receptors (Baumann & Rothman, 1998). Cocaine exposure and withdrawal also have been reported to affect 5HT2A receptor expression and function, although no consensus has been reached as to the exact nature of these effects (Carrasco & Battaglia, 2007; Carrasco, Van de Kar, Sullivan, Landry, Garcia, Muma et al., 2006; Huang, Liang, Lee, Wu & Hsu, 2009). Overall, chronic cocaine clearly results in an altered state of the serotonin system, which could contribute to the development of cocaine dependence.
Functional neuroimaging permits longitudinal evaluation of drug effects on neurochemistry using designs that involve repeated measures over extended periods of time. This approach has been used effectively in nonhuman primates to characterize both transient and long-lasting changes in brain chemistry that are associated with drug history. For example, PET imaging studies conducted in socially housed cynomolgus monkeys characterized the effects of chronic cocaine exposure in dominant and subordinate subjects. Although dominant male monkeys initially exhibited higher D2 receptor availability and lower rates of cocaine self-administration (Morgan, Grant, Gage, Mach, Kaplan, Prioleau et al., 2002), chronic exposure to self-administered cocaine resulted in reductions in D2 receptor availability and enhanced cocaine self-administration to levels comparable with subordinate monkeys (Czoty, Morgan, Shannon, Gage & Nader, 2004). A subsequent study examined D2 receptor availability during extended cocaine abstinence (Nader, Morgan, Gage, Nader, Calhoun, Buchheimer et al., 2006). In subjects with short-term exposure over 1 week, D2 receptor availability returned to pre-drug levels within 3 weeks. In subjects with long-term exposure over 1 year, some showed complete recovery within 3 months, whereas others did not recover after 1 year of abstinence. It is interesting to note that individual differences in the rate of recovery of D2 receptor availability have also been observed following drug-induced increases by the D2 receptor antagonist raclopride (Czoty, Gage & Nader, 2005). Baseline DAT availability as determined by PET imaging was negatively correlated with sensitivity to cocaine reinforcement in female cynomolgus monkeys (Nader, Nader, Czoty, Riddick, Gage, Gould et al., 2012). However, self-administration of a low dose of cocaine over 9 weeks did not significantly affect DAT availability in any brain region (Czoty, Reboussin, Calhoun, Nader & Nader, 2007). Likewise, rhesus monkeys employed in a within-subject, longitudinal design showed increased 5HT2A receptor availability following a 3-month period of cocaine self-administration (Sawyer, Mun, Nye, Kimmel, Voll, Stehouwer et al., 2012). Collectively, these studies demonstrate substantial but yet to be fully elucidated plasticity of monoamine systems in response to exposure to stimulants.
Human studies that have used functional imaging to characterize the effects of psychostimulant use on neurobiology have focused primarily on long-term changes in individuals with a complex history of multidrug use. Similar to nonhuman primates, chronic exposure to stimulant drugs in humans may also lead to significant changes in neuronal markers of dopaminergic function. PET studies characterizing dopamine D2 receptors have reliably documented long-lasting decreases in D2 receptor density in stimulant abusers (Volkow & Fowler, 2000). The reduction in D2 receptor function may further decrease the sensitivity of reward circuits to stimulation by natural rewards and increase the risk for drug taking (Volkow, Fowler, Wang & Swanson, 2004). Interestingly, no difference in D1 receptor density was observed between cocaine-dependent subjects and matched controls (Martinez, Slifstein, Narendran, Foltin, Broft, Hwang et al., 2009). In cocaine abusers, DAT density appears to be elevated shortly after cocaine abstinence but then to normalize with long-term detoxification (Malison, Best, van Dyck, McCance, Wallace, Laruelle et al., 1998). Methamphetamine also induces changes in the density of brain dopamine markers in human users (Johanson, Frey, Lundahl, Keenan, Lockhart, Roll et al., 2006; McCann, Szabo, Scheffel, Dannals & Ricaurte, 1998; Sekine, Iyo, Ouchi, Matsunaga, Tsukada, Okada et al., 2001; Volkow, Chang, Wang, Fowler, Ding, Sedler et al., 2001a; Volkow, Chang, Wang, Fowler, Franceschi, Sedler et al., 2001b; Volkow, Chang, Wang, Fowler, Franceschi, Sedler et al., 2001c). Interestingly, reduced DAT availability correlated with the duration of drug use, and impaired memory function was associated with a reduction in DAT availability (Volkow, Chang, Wang, Fowler, Leonido-Yee, Franceschi et al., 2001d). PET imaging to quantify DAT availability identified partial recovery of DAT binding in methamphetamine abusers during protracted abstinence (Volkow et al., 2001b). A subsequent study found that memory deficits in abstinent methamphetamine users were associated with decreases in striatal DAT binding potentials (McCann, Kuwabara, Kumar, Palermo, Abbey, Brasic et al., 2008). Lastly, NET availability in humans (Ding, Singhal, Planeta-Wilson, Gallezot, Nabulsi, Labaree et al., 2010) was greater in subjects with a cocaine self-administration history compared to control groups.
PET imaging measures of protein binding in vivo are complemented by studies that have documented cocaine-induced changes in brain metabolic activity as a function of cocaine self-administration history (Henry, Murnane, Votaw & Howell, 2010). Experimentally naive rhesus monkeys were given increasing access to cocaine self-administration, and PET imaging with FDG was used to measure acute cocaine-induced changes in brain metabolism in the cocaine-naive state and during limited- and extended-access conditions. In the cocaine-naive state, cocaine-induced increases in brain metabolism were restricted to the anterior cingulate and medial prefrontal cortex, consistent with other studies reporting acute activation of the anterior cingulate by cocaine (Howell, Votaw, Goodman & Lindsey, 2010; Murnane & Howell, 2010). Others have reported deficits in basal brain metabolic activity determined with PET imaging that were closely linked to cocaine-induced cognitive impairments in rhesus monkeys (Gould, Duke & Nader, 2013; Gould, Gage & Nader, 2012). Interestingly, increased cocaine exposure from limited through extended access, reported by (Henry et al., 2010), recruited cocaine-induced metabolic effects in frontal cortical areas and within the striatum. In apparent contrast, tolerance to cocaine- and amphetamine-induced synaptic release of dopamine in the striatum was observed in these same animals under both access conditions (Kirkland Henry et al., 2009). It is noteworthy that blunting of dopamine release has also been recorded in cocaine-dependent humans, in experiments using PET imaging (Martinez, Narendran, Foltin, Slifstein, Hwang, Broft et al., 2007). Accordingly, further investigation of the relationship between drug self-administration, tolerance to the dopaminergic effects of cocaine, and recruitment of cortical activation may be highly relevant toward efforts to develop treatments for cocaine addiction.
PET imaging has documented drug-induced brain metabolic effects in chronic cocaine users (Volkow, Mullani, Gould, Adler & Krajewski, 1988). Measures of brain glucose metabolism with FDG in chronic users documented transient increases in metabolic activity in dopamine-associated brain regions during cocaine withdrawal (Volkow, Fowler, Wolf, Hitzemann, Dewey, Bendriem et al., 1991), whereas decreases in frontal brain metabolism persisted after months of detoxification. The same pattern of decreased glucose metabolism (Reivich, Alavi, Wolf, Fowler, Russell, Arnett et al., 1985) and perfusion deficits (Volkow et al., 1988) was observed in the prefrontal cortex of cocaine users who were imaged on multiple occasions. Moreover, detoxified cocaine abusers had a marked decrease in dopamine release as measured by PET imaging (Volkow, Wang, Fischman, Foltin, Fowler, Abumrad et al., 1997; Volkow, Wang, Fowler, Logan, Angrist, Hitzemann et al., 1997). Self-reports of “high” induced by methylphenidate were also less intense in cocaine abusers. A recent study using fMRI during a working memory task in cocaine-dependent subjects showed impaired activation in frontal, striatal, and thalamic brain regions (Moeller, Steinberg, Schmitz, Ma, Liu, Kjome et al., 2010). Importantly, thalamic activation significantly correlated with response to cognitive behavioral therapy in combination with pharmacotherapy that included amphetamine and modafinil. Lastly, regional brain glucose metabolism has been characterized in conjunction with dopamine D2 receptor availability (Volkow et al., 2001a). Reductions in striatal D2 receptors were associated with decreased metabolic activity in the orbital frontal cortex and anterior cingulate cortex in detoxified individuals. In contrast, the orbital frontal cortex was hypermetabolic in active cocaine abusers (Volkow et al., 1991). In addition, chronic methamphetamine users showed reduced striatal D2 receptors, the loss of which was related to the function of the orbitofrontal cortex (Volkow et al., 2001a), a region important for executive functions. Collectively, these findings observed in stimulant abusers document the significant dysregulation of dopamine systems that are reflected in brain metabolic changes in areas involved in reward circuitry.
Evaluation of Monoamine Transporter Inhibitors and Substrates on Abuse-Related Effects of Psychostimulants
The acute and chronic effects of abused psychostimulants on monoamine transporters and associated neurobiology have encouraged development of candidate medications that target these transporters. It is theoretically possible to develop medications that might block psychostimulant effects at monoamine transporters without affecting normal transporter function to mediate uptake of endogenous monoamines. For example, dopamine transporter mutants have been identified that have low affinity for cocaine but transport dopamine normally (Thomsen et al., 2009b), and one theme in medications development has focused on compounds that might function as stimulant antagonists with lesser effects on dopamine transport (Meltzer, Liu, Blanchette, Blundell & Madras, 2002; Rothman, Becketts, Radesca, de Costa, Rice, Carroll et al., 1993; Rothman, Dersch, Ananthan & Partilla, 2009). To date, though, these efforts have not yielded promising compounds, and research has focused instead on compounds that function as monoamine transporter inhibitors or substrates. In the broader context of medications development for drug abuse, this constitutes an example of an “agonist” approach to drug abuse treatment (Grabowski, Shearer, Merrill & Negus, 2004; Lile et al., 2003; Rothman, Blough & Baumann, 2002a; Rothman et al., 2007). Agonist approaches are typified by the use of opioid agonists like methadone or buprenorphine to treat opioid dependence or the use of nicotine formulations to treat tobacco dependence. In both cases, treatment is accomplished by chronic delivery of a medication that shares pharmacodynamic effects with the abused drug, and utility as an anti-addiction medication is associated with four attributes. First, agonist medications can be expected to produce some level of reinforcing effect similar to that produced by the abused drug, and this reinforcing effect can be leveraged by clinicians to promote medication compliance and reinforce therapeutically desirable behaviors. Second, agonist medications also mitigate withdrawal from the abused drug by replacing effects of the abused drug at the molecular target. Third, ideal agonist medications have a relatively slow onset of action and long duration of action relative to the abused drug. The slow onset of action is thought to reduce abuse liability, and the long duration of action facilitates maintenance of stable drug levels to minimize cycling of drug effects and associated biological adaptations. In a last and related point, agonist medications are administered via routes of administration (e.g. oral, sublingual or transdermal) that contribute to slow onset and long duration while also being safer than intravenous or smoked routes of administration common in addiction.
The remainder of this review will focus on evidence for the efficacy of chronic treatment with monoamine transporter inhibitors and substrates to reduce abuse-related effects of stimulants in preclinical assays of stimulant self-administration, drug discrimination and reinstatement. Most of this work has evaluated modulation of abuse-related effects of cocaine, but, where available, data on modulation of abuse-related effects of other stimulants will also be considered. Effects of monoamine transporter inhibitors will be discussed first, followed by review of data with transporter substrates. In considering deployment of monoamine transporter inhibitors and substrates as agonist-type medications to treat stimulant abuse, the safety and abuse liability of the medications are an obvious concern, and this will also be addressed.
Effects of monoamine transporter inhibitors
Preclinical studies in nonhuman primates provide convincing evidence that selective inhibitors of dopamine uptake may be useful pharmacotherapies in the treatment of cocaine abuse. Several cocaine analogs and other DAT inhibitors have been developed and characterized for their ability to reduce cocaine self-administration. Perhaps the largest class of compounds studied is the 3-phenyltropane analogs (Carroll, Howell & Kuhar, 1999). The phenyltropane analog RTI-113 effectively decreased cocaine self-administration in squirrel monkeys (Howell, Czoty, Kuhar & Carrol, 2000) and rhesus monkeys (Negus, Mello, Kimmel, Howell & Carroll, 2009b) trained under second-order schedules of i.v. cocaine delivery. Moreover, RTI-113 maintained its effectiveness when the unit dose of cocaine was increased from 0.1 to 0.3 mg/kg/injection, indicating that the ability of RTI-113 to suppress cocaine self-administration could not be surmounted by a higher dose of cocaine (Howell et al., 2000). However, doses of RTI-113 that suppressed cocaine self-administration also caused a general disruption of operant behavior maintained by a comparable schedule of stimulus-termination (Howell et al., 2000) or food delivery (Negus, Baumann, Rothman, Mello & Blough, 2009a). Similar results have been obtained with the cocaine analog PTT in rhesus monkeys trained under a fixed-interval schedule of i.v. cocaine delivery (Nader, Grant, Davies, Mach & Childers, 1997). Pre-session administration of PTT decreased response rates and total session intake at multiple unit doses of cocaine (0.03 and 0.1 mg/kg/injection). The effectiveness of selective DAT inhibitors to decrease cocaine self-administration extends to phenylpiperazine derivatives. GBR 12909 dose dependently decreased cocaine self-administration in rhesus monkeys trained under multiple fixed-ratio schedules of i.v. cocaine and food delivery (Glowa, Wojnicki, Matecka & Bacher, 1995). Although GBR 12909 decreased rates of responding maintained by cocaine and food, large decreases in cocaine-maintained responding could be obtained at doses of GBR 12909 that had little effect on food-maintained responding. Hence, there was evidence for a selective decrease in cocaine-maintained responding at a low unit dose of cocaine (0.01 mg/kg/injection). However, the selectivity was not evident at a higher unit dose of cocaine (0.056 mg/kg/injection). When GBR 12909 was administered chronically as a decanoate derivative, selective reductions in cocaine self-administration were sustained over a four-week period (Glowa, Fantegrossi, Lewis, Matecka, Rice & Rothman, 1996).
A subsequent series of studies was conducted in nonhuman primates that evaluated the effectiveness of DAT inhibitors in reducing cocaine self-administration, and PET neuroimaging quantified DAT occupancy at behaviorally relevant doses. Selective DAT inhibitors were effective in reducing cocaine self-administration but only at high levels of DAT occupancy. For example, effective doses of the DAT-selective inhibitor RTI-113, which dose-dependently reduced cocaine-maintained responding, produced DAT occupancies between 72 and 84% (Wilcox, Lindsey, Votaw, Goodman, Martarello, Carroll et al., 2002). Similar results were observed with other DAT-selective inhibitors, including the phenyltropane RTI-177 and the phenylpiperazine GBR 12909 (Lindsey, Wilcox, Votaw, Goodman, Plisson, Carroll et al., 2004). At doses that decreased rates of cocaine self-administration by 50%, DAT occupancy was approximately 70% for both compounds. Clearly, DAT inhibitors can be effective in reducing cocaine self-administration. However, high levels of DAT occupancy may be required.
A possible limitation to the use of selective DAT inhibitors as medications for treatment of cocaine addiction is their potential for abuse, given their documented reinforcing effects (Howell & Wilcox, 2001). Selective DAT inhibitors, including phenyltropanes (Howell, Carroll, Votaw, Goodman & Kimmel, 2007; Howell et al., 2000; Lindsey et al., 2004; Wilcox et al., 2002) and phenylpiperazines (Bergman et al., 1989; Howell & Byrd, 1991; Lindsey et al., 2004), reliably maintain self-administration behavior in nonhuman primates. However, several selective DAT inhibitors maintained lower rates of responding compared with cocaine across a broad range of doses, even though DAT occupancy was equal to or greater than that observed for cocaine (Howell et al., 2007; Lindsey et al., 2004; Wilcox et al., 2002). In behavioral studies in rodents and nonhuman primates, these compounds had a slower onset and a longer duration of action compared with cocaine (Howell et al., 2000; Kimmel, Joyce, Carroll & Kuhar, 2001). Moreover, in PET neuroimaging studies which characterized the time-course of drug uptake in brain there was a clear trend toward an inverse relationship between the time to peak uptake of drugs in striatum and the peak number of infusions received under a progressive-ratio schedule of i.v. self-administration, such that the faster-onset drugs produced greater levels of responding relative to the slower-onset drugs (Kimmel, Negus, Wilcox, Ewing, Stehouwer, Goodman et al., 2008). There also was a close correspondence between the time course of drug uptake in brain and drug-induced increases in extracellular dopamine in striatum (Ginsburg et al., 2005; Kimmel et al., 2008; Kimmel, O’Connor, Carroll & Howell, 2007). Hence, the reinforcing effects and pattern of drug self-administration is likely influenced by pharmacokinetics in addition to steady-state levels of DAT occupancy. Accordingly, pharmacokinetic considerations should play an important role in medication development.
A number of clinical studies suggest that the wake-promoting drug modafinil may improve the clinical outcomes for treatment of cocaine dependence (Anderson, Reid, Li, Holmes, Shemanski, Slee et al., 2009; Dackis, Kampman, Lynch, Pettinati & O’Brien, 2005; Dackis, Lynch, Yu, Samaha, Kampman, Cornish et al., 2003; Hart, Haney, Vosburg, Rubin & Foltin, 2008), although a recent study did not find a significant main effect of modafinil on the rate or duration of cocaine use in cocaine-dependent patients (Dackis, Kampman, Lynch, Plebani, Pettinati, Sparkman et al., 2012). The potential therapeutic effects of modafinil for the treatment of cocaine dependence may involve a DAT-mediated mechanism (Volkow, Fowler, Logan, Alexoff, Zhu, Telang et al., 2009; Zolkowska, Jain, Rothman, Partilla, Roth, Setola et al., 2009). To this end, recent studies in rhesus monkeys demonstrated that the in vivo effects of modafinil at the DAT are similar to other stimulants, such as cocaine (Andersen, Kessler, Murnane, McClung, Tufik & Howell, 2010; Newman, Negus, Lozama, Prisinzano & Mello, 2010). Modafinil induced nocturnal locomotor-stimulant effects, reinstated extinguished responding previously maintained by cocaine, and exhibited cocaine-like discriminative stimulus effects. An effective dose of modafinil resulted in approximately 60% DAT occupancy in the striatum and significantly increased extracellular dopamine levels, comparable to effects observed following cocaine doses that reliably maintain self-administration (Votaw, Howell, Martarello, Hoffman, Kilts, Lindsey et al., 2002; Wilcox, Kimmel, Lindsey, Votaw, Goodman & Howell, 2005). Importantly, chronic treatment with modafinil selectively reduced cocaine self-administration compared to food-maintained behavior (Newman et al., 2010). Collectively, these results document low-potency DAT-related effects in nonhuman primates that may be relevant for its therapeutic effectiveness in humans. Regarding its abuse potential, low potency at the DAT appears to limit modafinil self-administration in nonhuman primates (Gold & Balster, 1996) and its abuse liability in humans (Jasinski, 2000; Vosburg, Hart, Haney, Rubin & Foltin, 2010).
Preclinical studies have clearly indicated that serotonin plays an important role in the behavioral effects of cocaine. Acute administration of SERT inhibitors attenuate the behavioral-stimulant effects of cocaine in squirrel monkeys (Howell et al., 1995; Howell, Czoty & Byrd, 1997) and decrease cocaine self-administration in rodents (Carroll, Lac, Asencio & Kragh, 1990) and nonhuman primates (Czoty, Ginsburg & Howell, 2002; Kleven & Woolverton, 1993). The SERT inhibitor alaproclate also attenuated cocaine-induced increases in extracellular dopamine in squirrel monkeys (Czoty et al., 2002) and cocaine-induced activation of prefrontal cortex in rhesus monkeys (Howell, Hoffman, Votaw, Landrum, Wilcox & Lindsey, 2002). Moreover, the SERT inhibitor fluoxetine attenuated cue-induced reinstatement of cocaine self-administration in rodents (Baker, Tran-Nguyen, Fuchs & Neisewander, 2001; Burmeister, Lungren & Neisewander, 2003) and decreased ratings of cocaine’s positive subjective effects in a human laboratory setting (Walsh, Preston, Sullivan, Fromme & Bigelow, 1994). Consistent with these findings, acute administration of the SERT inhibitors fluoxetine and citalopram attenuated cocaine-induced reinstatement in rhesus monkeys (Figure 2; unpublished data). Fluoxetine also attenuated reinstatement by the psychostimulants MDMA and benzylpiperazine (McClung, Fantegrossi & Howell, 2010). Despite the overwhelming evidence suggesting that SERT inhibitors can reduce abuse-related effects of cocaine, Fluoxetine has typically failed to show reductions in cocaine abuse in clinical trials (Grabowski, Rhoades, Elk, Schmitz, Davis, Creson et al., 1995; Lima, Reisser, Soares & Farrell, 2003; Schmitz, Averill, Stotts, Moeller, Rhoades & Grabowski, 2001; Winstanley, Bigelow, Silverman, Johnson & Strain, 2011). This may be due in part to the different dosing regimens employed by the preclinical and clinical studies; preclinical studies generally employ single dose treatments whereas clinical studies administer the drug chronically. However, clinical studies with more selective SERT inhibitors have shown more promise. For example, citalopram reduced cocaine use in cocaine-dependent patients (Moeller, Schmitz, Steinberg, Green, Reist, Lai et al., 2007) and sertraline delayed relapse in recently abstinent cocaine-dependent patients (Oliveto, Poling, Mancino, Williams, Thostenson, Pruzinsky et al., 2012).
Figure 2.
Effects of acute IM pretreatments with the SERT inhibitors fluoxetine or citalopram on cocaine-induced reinstatement of extinguished cocaine self-administration in rhesus monkeys (N=3). Subjects were trained to self-administer cocaine under a second-order schedule and could take a maximum of 0.5 mg/kg per session 5 days per week. Subsequently, saline was substituted for cocaine, and once extinction criteria were met (response rates <20% of cocaine-maintained rates), response-independent cocaine (0.1mg/kg) was administered IV prior to extinction sessions. Both fluoxetine and citalopram significantly attenuated cocaine-induced reinstatement (* p <0.05 with respect to control).
A recent study was the first to examine the effects of chronic fluoxetine treatment at clinically relevant concentrations in a nonhuman primate model of cocaine abuse (Sawyer et al., 2012). Chronic fluoxetine treatment attenuated cocaine-induced reinstatement and dopamine overflow. Furthermore, these effects persist up to 6 weeks after the conclusion of fluoxetine treatment. It is also noteworthy that RTI-112, a mixed-action inhibitor of DAT and SERT, significantly reduced cocaine self-administration by rhesus monkeys at doses producing levels of DAT occupancy below the limit of detection (Lindsey et al., 2004). Furthermore, co-administration of the selective SERT inhibitors fluoxetine or citalopram and the selective DAT inhibitor RTI-336 produced more robust reductions in cocaine self-administration than RTI-336 alone, even at comparable levels of DAT occupancy by RTI- 336 (Howell et al., 2007). Collectively, there is convincing evidence that SERT inhibition can enhance suppression of cocaine self-administration by DAT inhibitors, indicating that duel DAT/SERT inhibitors warrant consideration as viable medications for cocaine addiction.
NET inhibitors appear less promising as therapeutics for psychostimulant abuse. Studies in squirrel monkeys indicate that NET inhibition may contribute to the discriminative-stimulus effects of cocaine (Spealman, 1995) and cocaine-induced reinstatement (Platt et al., 2007). However, pretreatment with desipramine in rhesus monkeys trained under a second-order schedule of i.v. cocaine delivery had inconsistent effects and actually increased cocaine self-administration in some animals (Mello et al., 1990). In addition, food-maintained behavior was affected by pretreatment doses that influenced drug self-administration, demonstrating a lack of selectivity. In another study, pretreatment with desipramine in rhesus monkeys trained under multiple fixed-ratio schedules of i.v. cocaine and food delivery had no effect on cocaine self-administration (Kleven et al., 1990c). Desipramine was one of the first medications reported to be effective in an out-patient, controlled clinical trial. An initial meta-analysis found desipramine to be effective in reducing relapse to cocaine use (Levin & Lehman, 1991) but subsequent clinical trials did not confirm its effectiveness (Arndt, Dorozynsky, Woody, McLellan & O’Brien, 1992; Campbell, Thomas, Gabrielli, Liskow & Powell, 1994). A recent study with atomoxetine, aNET inhibitor used in the treatment of ADHD, provided no support for its utility in the treatment of cocaine dependence (Walsh, Middleton, Wong, Nuzzo, Campbell, Rush et al., 2013).
A recent study in rhesus monkeys evaluated the effects of chronic methylphenidate, a DAT and NET inhibitor used in the treatment of ADHD, and found that methylphenidate treatment either disrupted food-maintained behavior or increased cocaine self-administration (Czoty, Martelle, Gould & Nader, 2013). Indatraline is an example of a nonselective monoamine transporter inhibitor with similar potencies at DAT, SERT and NET. Pretreatment with indatraline in rhesus monkeys trained under alternating daily sessions of cocaine and food availability produced dose-dependent decreases in cocaine self-administration over a broad range of cocaine doses (Negus, Brandt & Mello, 1999). Moreover, reductions in cocaine self-administration were sustained during 7 consecutive days of indatraline pretreatment. When substituted for cocaine in self-administration sessions, indatraline maintained lower rates of responding compared with cocaine. However, indatraline had undesirable side effects, including behavioral stereotypies and trends toward weight loss that limit its clinical utility. In clinical studies, mazindol, a DAT and NET inhibitor used in the treatment of obesity, did not alter the subjective effects of cocaine in a human laboratory study (Preston, Sullivan, Berger & Bigelow, 1993). Moreover, in a 6- week, placebo-controlled study in cocaine dependent subjects, mazindol did not differ from placebo in reducing cocaine use and mazindol treatment was not well tolerated (Stine, Krystal, Kosten & Charney, 1995).
Effects of monoamine transporter substrates
Studies with amphetamine have provided the most compelling evidence to date for efficacy of monoamine transporter substrates to treat cocaine addiction. Clinical trials (Grabowski, Rhoades, Schmitz, Stotts, Daruzska, Creson et al., 2001; Mariani, Pavlicova, Bisaga, Nunes, Brooks & Levin, 2012) and drug self-administration studies in humans (Greenwald, Lundahl & Steinmiller, 2010; Rush, Stoops, Sevak & Hays, 2010), nonhuman primates (Czoty, Martelle, Garrett & Nader, 2008; Negus, 2003; Negus & Mello, 2003a, b) and rats (Chiodo, Lack & Roberts, 2008; Thomsen, Barrett, Negus & Caine, 2013) agree in showing that amphetamine maintenance reduces cocaine-taking behavior. An example of this effect is shown in Figure 3 (Negus et al., 2003b). In this study, rhesus monkeys were equipped with chronic double-lumen i.v. catheters and trained to respond for food or cocaine during alternating daily components of food and cocaine availability. Self-administered cocaine injections (0.001-0.1 mg/kg/injection) were delivered through one lumen of the double lumen catheter and the second lumen was used to chronically infuse saline or various amphetamine doses (0.01-0.1 mg/kg/hr; 23hr/day) for periods of 7 to 28 consecutive days. Amphetamine produced a dose-dependent decrease in both cocaine- and food-maintained responding; however, tolerance developed to amphetamine effects on food-maintained responding, and for most of the treatment period, monkeys responded at saline control levels for food pellets. Moreover, all monkeys maintained their body weights during amphetamine treatment. Conversely, cocaine self-administration was nearly eliminated by the fifth day of treatment, remained suppressed for the remainder of treatment, and gradually recovered only after termination of treatment.
Figure 3.
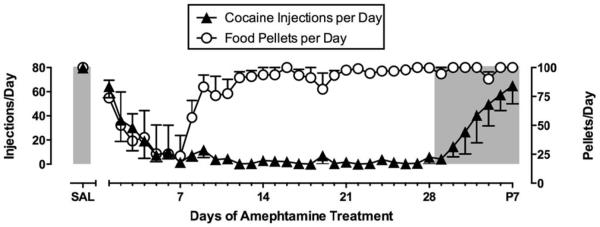
Effects of continuous treatment with d-amphetamine (0.1 mg/kg/hr, IV) for 28 days on responding maintained by cocaine (0.01 mg/kg/injection, IV) and food pellets (1 gram banana-flavored pellets) in rhesus monkeys (N=4). Monkeys could earn a maximum of 80 cocaine injections and 100 food pellets each during alternating components of cocaine and food availability. Points above “Sal” show rates of cocaine- and food-maintained responding during saline treatment. Amphetamine treatment produced an initial decrease in cocaine- and food-maintained responding, but tolerance developed to effects on food-maintained responding while cocaine self-administration was depressed throughout the 28-day treatment period. Adapted from Negus and Mello, 2003b.
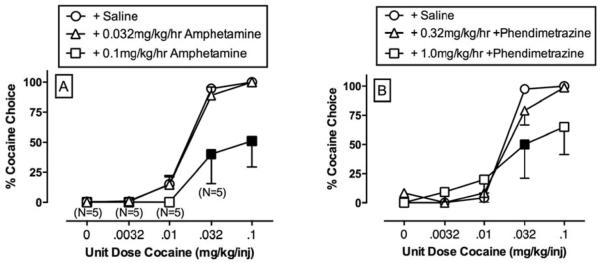
Five additional points also warrant mention. First, the experiment in Figure 3 shows similar amphetamine-induced reductions in cocaine- and food-maintained responding during the first 7 days of treatment, and behavioral selectivity of amphetamine effects did not emerge until after the first week. However, other experiments with amphetamine have shown that, even at these early time points, amphetamine can selectively decrease cocaine self-administration more than food-maintained responding, and this effect is apparent across a broad range of self-administered cocaine doses (Negus et al., 2003b). Second, amphetamine reduced cocaine self-administration at daily amphetamine doses of 0.74-2.3 mg/kg/day (0.032-0.1 mg/kg/hr × 23 hr/day). This dose range overlaps with the range of amphetamine doses that decreased cocaine use in clinical trials (Grabowski et al., 2004; Mariani et al., 2012) and with recommended amphetamine doses for treating disorders such as narcolepsy and attention deficit hyperactivity disorder. Third, amphetamine maintenance also reduced cocaine self-administration under other schedules of reinforcement, including progressive-ratio schedules (Chiodo et al., 2008; Czoty et al., 2008; Negus et al., 2003a) and concurrent schedules of cocaine vs. food choice (Banks, Blough & Negus, 2013b; Negus et al., 2003b; Thomsen et al., 2013). Fourth, selective reduction in cocaine self-administration by amphetamine or related medications depends on sustained maintenance of treatment and is not apparent with acute treatment. Indeed, acute treatment with amphetamine or related medications can increase self-administration of low cocaine doses or of saline (Thomsen et al., 2013), a phenomenon that may be related to cocaine-like discriminative stimulus effects of these medications (see below). Lastly, the profile of amphetamine effects on cocaine- and food-maintained responding under this procedure is unusual relative to effects of other candidate medications. For example, dopamine receptor antagonists or opioid receptors agonists/antagonists may also decrease cocaine self-administration in this procedure, but those effects are often transient, and rates of food-maintained responding are often reduced as much or more than rates of cocaine self-administration (Do Carmo, Mello, Rice, Folk & Negus, 2006; Negus et al., 2011; Negus & Mello, 2002, 2004; Negus et al., 1996; Negus, Mello, Portoghese & Lin, 1997).
Amphetamine has also been evaluated in assays of cocaine discrimination and reinstatement of cocaine self-administration. When administered acutely, amphetamine substitutes fully for the discriminative stimulus effects of cocaine (D’Mello & Stolerman, 1977; Negus et al., 2009a), which is consistent with its related mechanism of action, its cocaine-like subjective effects in humans (Fischman, Schuster, Resnekov, Shick, Krasnegor, Fennell et al., 1976), and its putative potential to function of as an agonist medication for treatment of cocaine addiction. As might be expected from its similar discriminative stimulus effects, acute amphetamine also reinstates extinguished cocaine self-administration (Gerber et al., 1975; Schenk & Partridge, 1999). However, chronic amphetamine administration produces cross tolerance to both the discriminative stimulus effects and reinstating effects of cocaine (Norman, Norman, Hall & Tsibulsky, 1999; Peltier, Li, Lytle, Taylor & Emmett-Oglesby, 1996). Taken as a whole, these findings converge in suggesting that amphetamine maintenance reduces expression of the abuse-related effects of cocaine.
In contrast to the growing literature describing effects of amphetamine maintenance on abuse-related reinforcing, discriminative stimulus and reinstating effects of cocaine, far fewer studies have examined effects of amphetamine maintenance on abuse-related effects of other stimulants. Amphetamine treatment produces tolerance to its own discriminative stimulus effects (Barrett, Caul & Smith, 2005) and cross-tolerance to the amphetamine-like discriminative stimulus effects of the weak transporter substrate cathine (Schechter, 1990). However, it has been suggested that transporter substrates may function more effectively as medications to treat abuse of transporter inhibitor stimulants like cocaine, and conversely, transporter inhibitors may function as more effective medications for treatment of addiction to transporter substrate stimulants like amphetamine (Stoops & Rush, 2013).
Many transporter substrates other than amphetamine have been identified, and one important dimension along which they vary is relative selectivity to promote release of dopamine/norepinephrine vs. serotonin. Amphetamine has relatively high selectivity as a substrate for DAT/NET vs. SERT, and it occupies one end of this spectrum, whereas the serotonin-selective releaser fenfluramine occupies the other end of this spectrum (higher relative potency as a substrate for SERT vs. DAT/NET). Other compounds have been identified with DAT/NET vs. SERT selectivities intermediate between those of amphetamine and fenfluramine (Baumann, Ayestas, Partilla, Sink, Shulgin, Daley et al., 2012; Rothman, Clark, Partilla & Baumann, 2003; Rothman, Katsnelson, Vu, Partilla, Dersch, Blough et al., 2002b). Preclinical metrics of abuse liability correlate positively with DAT/NET selectivity, such that DAT/NET-selective substrates like amphetamine reliably maintain drug self-administration and produce other abuse-related effects such as cocaine-like discriminative stimulus effects; however, expression of these effects declines as DAT/NET vs. SERT selectivity declines (Bauer et al., 2013; Wee et al., 2005). Consequently, manipulation of DAT/NET vs. SERT selectivity has offered one potential strategy to reduce abuse-liability of transporter substrates as candidate medications. Moreover, as noted above, chronic stimulant exposure associated with abuse can disrupt both dopaminergic and serotonergic signaling, and this has led to the hypothesis that stimulant abuse may produce a “dual-deficit” in dopaminergic and serotonergic systems that can be mitigated by medications with dual action at DAT and SERT (Rothman, Blough & Baumann, 2008).
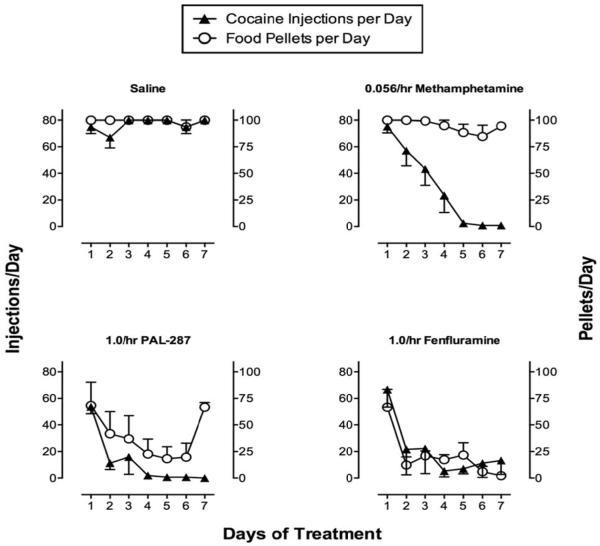
In view of these considerations, recent studies have compared changes in cocaine self-administration produced by a series of transporter substrates that varied in their selectivity for DAT/NET vs. SERT (Banks, Blough & Negus, 2011; Kohut, Fivel, Blough, Rothman & Mello, 2013; Negus et al., 2009a; Negus et al., 2007; Rothman, Blough, Woolverton, Anderson, Negus, Mello et al., 2005). Illustrative results are shown in Figure 4 from an experimental procedure similar to that shown above with amphetamine (Negus et al., 2007). Specifically, responding maintained in rhesus monkeys by food and cocaine (0.01 mg/kg/injection) was evaluated during continuous infusion for seven consecutive days with saline or with various compounds including methamphetamine (DAT/NET>SERT), PAL-287 (DAT/NET=SERT) and fenfluramine (DAT/NET<SERT). These three transporter substrates all produced dose-dependent and sustained decreases in cocaine self-administration, but they differed in their relative effects on food-maintained responding. Like amphetamine, methamphetamine selectively decreased cocaine self-administration at a dose that produced little effect on food-maintained responding, and in this experiment, the selective reduction in cocaine self-administration was evident during the first days of treatment. Similar results have been obtained in this or similar procedures with other transporter substrates selective for DAT/NET vs. SERT including phenmetrazine and phentermine (Negus et al., 2009a; Negus et al., 2007; Wojnicki, Rothman, Rice & Glowa, 1999), and these results with methamphetamine also agree with a clinical trial that showed efficacy of methamphetamine maintenance to decrease cocaine use by cocaine-dependent patients (Mooney, Herin, Schmitz, Moukaddam, Green & Grabowski, 2009). Conversely, the doses of PAL-287 and fenfluramine that reduced cocaine self-administration also produced concurrent decreases in food-maintained responding. Similar results were also found with these and other compounds in a different procedure that assessed concurrent choice between cocaine and food in rhesus monkeys (Banks et al., 2011). Specifically, chronic infusion with DAT/NET-selective compounds reduced cocaine choice and promoted reallocation of behavior to food choice, but non-selective or SERT-selective compounds only reduced overall responding without affecting cocaine vs. food choice. Taken together, these results suggest that decreasing pharmacological selectivity for DAT/NET vs. SERT correlated with decreased behavioral selectivity to reduce cocaine- vs. food-maintained responding. The implication for medications development is that SERT activity may reduce abuse liability of transporter substrates, but it is also associated with recruitment of other undesirable effects likely to impede use of these compounds as medications.
Figure 4.
Effects of 7-day treatment with saline or transporter substrates on responding maintained by cocaine (0.01 mg/kg/injection) or food pellets by rhesus monkeys. Monkeys could earn a maximum of 80 cocaine injections and 100 food pellets each day during alternating components of cocaine and food availability. Treatment consisted of continuous infusion with saline (upper left) or optimal treatment doses of the DAT/NET>SERT-selective substrate methamphetamine (0.056 mg/kg/hr), the nonselective DAT/NET/SERT substrate PAL-287 (1.0 mg/kg/hr) or the SER>DAT/NET-selective substrate fenfluramine (1.0 mg/kg/hr). All substrates produced sustained decreases in cocaine self-administration, and behavioral selectivity to decrease cocaine-maintained responding corresponded to pharmacological selectivity for DAT/NET vs. SERT. Adapted from Negus et al. 2007.
Selectivity for DAT/NET vs. SERT also influences the discriminative stimulus and reinstating effects of transporter substrates. For example, the cocaine-like discriminative stimulus effects (Negus et al., 2007) and reinstatement effects (Burmeister et al., 2003; Schenk, Hely, Gittings, Lake & Daniela, 2008; Spealman, Barrett-Larimore, Rowlett, Platt & Khroyan, 1999) of transporter substrates declines as selectivity for DAT/NET vs. SERT declines. Acute fenfluramine treatment attenuated cue-induced reinstatement and partially reduced cocaine-induced reinstatement of cocaine self-administration (Burmeister et al., 2003); however, effects of chronic treatment with transporter substrates other than amphetamine have not been examined in studies of cocaine discrimination or cocaine reinstatement.
Little work has been done to examine effects of transporter substrates other than amphetamine on abuse-related effects of stimulants other than cocaine. In one study, acute pretreatment with the DAT/NET>SERT substrate phentermine decreased self-administration of the DAT-selective uptake inhibitor GBR12909, suggesting that substrates may be effective to reduce abuse liability of transporter inhibitors other than cocaine (Wojnicki et al., 1999). Alternatively, a regimen of chronic methamphetamine administration increased subsequent self-administration of methamphetamine (Woolverton, Cervo & Johanson, 1984), a finding potentially consistent with the hypothesis that transporter inhibitors will more effective than substrates for treating abuse of substrates (Stoops et al., 2013). However, this study used high methamphetamine doses (up to 40 mg/kg/day) relative to doses that effectively decreased cocaine self-administration, and methamphetamine self-administration was evaluated 1-1.5 months after methamphetamine treatment rather than during treatment.
Although increases in SERT activity reduce abuse liability of transporter substrates, increased SERT activity is also associated with other undesirable effects that are likely to limit clinical utility. Consequently, other approaches are also being explored to reduce abuse liability of substrate medications while retaining both DAT/NET selectivity and behavioral selectivity to reduce cocaine-maintained responding. One alternative approach is to use prodrugs that generate DAT/NET-selective substrates as active metabolites. Use of prodrugs has the potential to reduce abuse liability by delaying onset of drug action after drug administration (Schindler, Panlilio & Thorndike, 2009). As one example, lisdexamfetamine is a Schedule II drug approved for treatment of attention deficit hyperactivity disorder, and it is a prodrug for amphetamine that has a slower onset of action and functions as a less reliable reinforcer than amphetamine (Heal, Buckley, Gosden, Slater, France & Hackett, 2013). As another example, phendimetrazine is a weak transporter inhibitor that also functions as a prodrug for the DAT/NET-selective substrate phenmetrazine (Banks, Blough, Fennel, Snyder & Negus, 2013a; Banks et al., 2013b; Rothman et al., 2002b). Phendimetrazine is a clinically available Schedule III anorectic agent, and preclinical studies suggest it has lower abuse liability than its metabolite phenmetrazine or other DAT/NET-selective substrates like amphetamine (Corwin, Woolverton, Schuster & Johanson, 1987). Despite being a weak transporter inhibitor with low abuse liability, Figure 5 shows that maintenance on phendimetrazine produces a reduction in cocaine vs. food choice by rhesus monkeys that is similar to the effect produced by amphetamine (Banks et al., 2013b).
Figure 5.
Effects of 14-day treatment with either amphetamine or phendimetrazine on choice between cocaine and food by rhesus monkeys. Cocaine injections and food pellets were available simultaneously during daily experimental sessions consisting of five sequential components, with the available cocaine dose increasing from 0 (no injection) to 0.1 mg/kg/injection across components. Continuous infusion with amphetamine (N=6) or phendimetrazine (N=4) produced rightward shifts in the cocaine-choice dose-effect curve and promoted reallocation of behavior away from cocaine choice and toward food choice. Filled points indicate a significant effect of amphetamine or phendimetrazine vs. saline as determined by two-way analysis of variance followed by the LSD multiple comparisons post hoc test. Notations of “N=5” in the left panel indicate that five of six monkeys contributed to the point during treatment with 0.1 mg/kg/hr amphetamine, and one monkey failed to respond for either food or cocaine. Adapted from Banks et al., 2013.
Safety
Studies cited above provide evidence to suggest that medications functioning as dopamine transporter inhibitors or substrates may have efficacy to decrease abuse-related effects of cocaine in animals and to decrease stimulant use by addicted humans. However, the clinical utility of these medications will depend not only on their efficacy, but also on their safety. This issue has been addressed in detail by others (Grabowski et al., 2004; Mariani et al., 2012), but three points will be highlighted here. First, many dopamine transporter inhibitors and substrates are already available for treatment of other disorders including obesity, narcolepsy, and attention deficit hyperactivity disorder, and particularly for the last of these indications, treatment involves chronic administration to a wide range of subjects including children. Consequently, there is already substantial clinical experience in the use of these medications that could guide strategies for their use to treat addiction. Second, the constellation of undesirable effects associated with these medications is well established and in particular includes cardiovascular effects and abuse liability. Although these effects would remain a clear concern given the risk of diversion as well as in patients being treated for addiction, existing data suggest that histories of stimulant abuse sufficient to warrant pharmacotherapy are likely to render patients tolerant to the cardiovascular effects and abuse liability of these compounds. For example, one recent study found that subjects with a history of “crack” cocaine use were tolerant to abuse-related subjective effects of amphetamine (Comer, Mogali, Saccone, Askalsky, Martinez, Walker et al., 2013). More generally, it will be important to weigh risks associated with treatment against risks associated with continued use of the abused stimulant. Lastly, data from both preclinical and clinical studies cited above suggest that subjects can be safely maintained on dopamine transporter inhibitors or substrates despite continued access to cocaine or other abuse stimulants. In particular, preclinical studies that show selective and sustained decreases in cocaine self-administration with lesser or no effect on food-maintained responding provide compelling evidence to suggest that reductions in cocaine self-administration can be achieved without general disruption of other adaptive behaviors (Mello et al., 1996). Moreover, data from choice studies suggest that these medications may not only reduce cocaine use but also promote reallocation of behavior toward responding maintained by non-drug alternative reinforcers (Banks & Negus, 2012; Haney et al., 2008; Vocci & Appel, 2007).
Conclusions and Future Directions
Monoamine transporters in general, and dopamine transporters in particular, are critical molecular targets that mediate abuse-related effects of psychostimulants such as cocaine and amphetamine. Moreover, chronic administration of psychostimulants can cause enduring changes in neurobiology reflected in dysregulation of monoamine neurochemistry and behavior. Findings observed in stimulant abusers document significant dysregulation of dopamine systems that are associated with brain metabolic changes in areas involved in reward circuitry and cognition. The current review has evaluated abundant evidence for the efficacy of monoamine transporter inhibitors and releasers to reduce abuse-related effects of stimulants in preclinical assays of stimulant self-administration, drug discrimination and reinstatement, providing a strong rationale for developing monoamine transporter inhibitors and releasers as medications for the treatment of stimulant addiction. Amphetamine maintenance has shown strong translational evidence of efficacy and safety to reduce cocaine self-administration and warrants further consideration as a candidate agonist medication for treatment of cocaine addiction. In general, inhibitors produce less selective decreases than substrates in cocaine- vs. food-maintained responding, and inhibitors have been less effective than releasers in human laboratory studies and clinical trials of cocaine dependence. However, it remains to be determined whether transporter inhibitors are more effective in the treatment of dependence to substrate-type stimulants such as amphetamine and methamphetamine. A possible limitation to the use of DAT inhibitors and substrates as medications for treatment of cocaine addiction is their potential for abuse, given their documented reinforcing effects. Previous approaches to mitigate this concern have included pharmacokinetic considerations of medications with slow onset and extended duration of action, and mixed action compounds that target DAT and SERT. Future directions in drug discovery should identify novel medications that retain efficacy to decrease cocaine use but possess lower abuse liability, and evaluate the degree to which efficacious medications can attenuate or reverse neurobiological effects of chronic cocaine use.
Acknowledgments
The authors would like to thank Susan Marshall for technical assistance in preparation of the manuscript. Supported by Office of Research Infrastructure Programs/OD P51OD11132 (LLH), DA 031246 (LLH), and DA026946 (SSN)
Non-standard Abbreviations
- DAT
dopamine transporter
- SERT
serotonin transporter
- NET
norepinephrine transporter
- PET
positron emission tomography
- FDG
fluorodeoxyglucose
- ADHD
attention deficit hyperactivity disorder
- PAL-287
1-napthyl-2-aminopropane
- RTI-113
phenyl 3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
- RTI-177
5-[(1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-azabicyclo [3.2.1]octan-2-yl]-3-(4-methylphenyl)-1,2-oxazole
- GBR 12909
1-[2-[bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine
- RTI-112
(1R,2S,3S)-methyl 3-(4-chloro-3-methylphenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
- RTI-336
3β-(4-chlorophenyl)-2β-[3-(4β- methylphenyl)isoxazol-5-yl]tropane hydrochloride
- PTT
2β-propanoyl-3β-(4-tolyl)-tropane
Footnotes
Conflict of Interest Statement The authors have no conflicts of interest to declare.
References
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- American_Psychiatric_Association . Diagnostic and Statistical Manual of Mental Disorders. 5th Edition American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology (Berl) 2010;210:439–448. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt IO, Dorozynsky L, Woody GE, McLellan AT, O’Brien CP. Desipramine treatment of cocaine dependence in methadone-maintained patients. Arch Gen Psychiatry. 1992;49:888–893. doi: 10.1001/archpsyc.1992.01820110052008. [DOI] [PubMed] [Google Scholar]
- Astier B, Van Bockstaele EJ, Aston-Jones G, Pieribone VA. Anatomical evidence for multiple pathways leading from the rostral ventrolateral medulla (nucleus paragigantocellularis) to the locus coeruleus in rat. Neurosci Lett. 1990;118:141–146. doi: 10.1016/0304-3940(90)90612-d. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2001;155:18–26. doi: 10.1007/s002130000676. [DOI] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennel TR, Snyder RW, Negus SS. Chronic phendimetrazine treatment reduces both cocaine choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology. 2013a doi: 10.1038/npp.2013.180. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol. 2011;22:824–836. doi: 10.1097/FBP.0b013e32834d63ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of 14-day treatment with the schedule III anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2013b;131:204–213. doi: 10.1016/j.drugalcdep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Gage HD, Bounds MC, Garg PK, Garg S, Nader MA. Effects of cocaine and MDMA self-administration on serotonin transporter availability in monkeys. Neuropsychopharmacology. 2008;33:219–225. doi: 10.1038/sj.npp.1301420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical Determinants of Drug Choice under Concurrent Schedules of Drug Self-Administration. Adv Pharmacol Sci. 2012;2012 doi: 10.1155/2012/281768. 281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Barrett RJ, Caul WF, Smith R. Withdrawal, tolerance, and sensitization to dopamine mediated interoceptive cues in rats trained on a three-lever drug-discrimination task. Pharmacol Biochem Behav. 2005;81:1–8. doi: 10.1016/j.pbb.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse. 2000;36:102–113. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr., Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Alterations in serotonergic responsiveness during cocaine withdrawal in rats: similarities to major depression in humans. Biol Psychiatry. 1998;44:578–591. doi: 10.1016/s0006-3223(98)00123-1. [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Biebuyck J. Pharmacology and therapeutic applications of cocaine. Anesthesiology. 1990;73:518–531. doi: 10.1097/00000542-199009000-00024. [DOI] [PubMed] [Google Scholar]
- Boulay D, Duterte-Boucher D, Leroux-Nicollet I, Naudon L, Costentin J. Locomotor sensitization and decrease in [3H]mazindol binding to the dopamine transporter in the nucleus accumbens are delayed after chronic treatments by GBR12783 or cocaine. J Pharmacol Exp Ther. 1996;278:330–337. [PubMed] [Google Scholar]
- Bradberry CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–218. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D1-like and D2-like agonists in rats trained to discriminate cocaine from saline: influence of experimental history. Exp Clin Psychopharmacol. 2000;8:404–414. doi: 10.1037//1064-1297.8.3.404. [DOI] [PubMed] [Google Scholar]
- Campbell JL, Thomas HM, Gabrielli W, Liskow BI, Powell BJ. Impact of desipramine or carbamazepine on patient retention in outpatient cocaine treatment: preliminary findings. J Addict Dis. 1994;13:191–199. doi: 10.1300/j069v13n04_07. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Carpenter MB, Sutin J. Human Neuroanatomy. Williams & Wilkins; Baltimore: 1983. [Google Scholar]
- Carrasco GA, Battaglia G. Withdrawal from a single exposure to cocaine increases 5-HT2A receptor and G protein function. Neuroreport. 2007;18:51–55. doi: 10.1097/01.wnr.0000246324.43567.55. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD, Sullivan NR, Landry M, Garcia F, Muma NA, Battaglia G. Cocaine-mediated supersensitivity of 5-HT2A receptors in hypothalamic paraventricular nucleus is a withdrawal-induced phenomenon. Neuroscience. 2006;143:7–13. doi: 10.1016/j.neuroscience.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Howell LL, Kuhar MJ. Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem. 1999;42:2721–2736. doi: 10.1021/jm9706729. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1990;35:237–244. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;105(Suppl 1):S14–25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Mackie K. Local anesthetics. In: Hardman JG, Limbird LE, editors. The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2005. pp. 369–388. [Google Scholar]
- Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther. 2003;307:729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Lack CM, Roberts DC. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology (Berl) 2008;200:465–473. doi: 10.1007/s00213-008-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claye LH, Akunne HC, Davis MD, DeMattos S, Soliman KF. Behavioral and neurochemical changes in the dopaminergic system after repeated cocaine administration. Mol Neurobiol. 1995;11:55–66. doi: 10.1007/BF02740684. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Mogali S, Saccone PA, Askalsky P, Martinez D, Walker EA, Jones JD, Vosburg SK, Cooper ZD, Roux P, Sullivan MA, Manubay JM, Rubin E, Pines A, Berkower EL, Haney M, Foltin RW. Effects of Acute Oral Naltrexone on the Subjective and Physiological Effects of Oral D-Amphetamine and Smoked Cocaine in Cocaine Abusers. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Woolverton WL, Schuster CR, Johanson CE. Anorectics: effects on food intake and self-administration in rhesus monkeys. Alcohol Drug Res. 1987;7:351–361. [PubMed] [Google Scholar]
- Cunningham KA, Paris JM, Goeders NE. Chronic cocaine enhances serotonin autoregulation and serotonin uptake binding. Synapse. 1992;11:112–123. doi: 10.1002/syn.890110204. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. PET imaging of striatal dopamine D2 receptors in nonhuman primates: increases in availability produced by chronic raclopride treatment. Synapse. 2005;58:215–219. doi: 10.1002/syn.20200. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Garrett BE, Nader MA. Chronic d-amphetamine alters food-reinforced responding and cocaine self-administration under a progressive-ratio schedule in rhesus monkeys. FASEB Journal. 2008;22:713–714. [Google Scholar]
- Czoty PW, Martelle SE, Gould RW, Nader MA. Effects of chronic methylphenidate on cocaine self-administration under a progressive-ratio schedule of reinforcement in rhesus monkeys. J Pharmacol Exp Ther. 2013;345:374–382. doi: 10.1124/jpet.113.204321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology (Berl) 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Reboussin BA, Calhoun TL, Nader SH, Nader MA. Long-term cocaine self-administration under fixed-ratio and second-order schedules in monkeys. Psychopharmacology (Berl) 2007;191:287–295. doi: 10.1007/s00213-006-0665-z. [DOI] [PubMed] [Google Scholar]
- D’Mello GD, Stolerman IP. Comparison of the discriminative stimulus properties of cocaine and amphetamine in rats. Br J Pharmacol. 1977;61:415–422. doi: 10.1111/j.1476-5381.1977.tb08434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat. 2012;43:303–312. doi: 10.1016/j.jsat.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O’Brien CP. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, Galli A. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290:1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- Ding YS, Singhal T, Planeta-Wilson B, Gallezot JD, Nabulsi N, Labaree D, Ropchan J, Henry S, Williams W, Carson RE, Neumeister A, Malison RT. PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S,S)-[(11)C]O-methylreboxetine and HRRT. Synapse. 2010;64:30–38. doi: 10.1002/syn.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Carmo GP, Mello NK, Rice KC, Folk JE, Negus SS. Effects of the selective delta opioid agonist SNC80 on cocaine- and food-maintained responding in rhesus monkeys. Eur J Pharmacol. 2006;547:92–100. doi: 10.1016/j.ejphar.2006.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Peris J, Yasuda RP, Philpott K, Zahniser NR. Repeated cocaine administration results in supersensitivity of striatal D-2 dopamine autoreceptors to pergolide. Life Sci. 1988;42:255–262. doi: 10.1016/0024-3205(88)90634-0. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JD, Eiden LE, Hoffman BJ. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1992;89:10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF. Schedules of Reinforcement. Appleton-Century-Crofts; New York: 1957. [Google Scholar]
- Fischman MW, Schuster CR, Resnekov L, Shick JF, Krasnegor NA, Fennell W, Freedman DX. Cardiovascular and subjective effects of intravenous cocaine administration in humans. Arch Gen Psychiatry. 1976;33:983–989. doi: 10.1001/archpsyc.1976.01770080101010. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW, Gnegy M. Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J Neurosci. 2009;29:3328–3336. doi: 10.1523/JNEUROSCI.5386-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3:1055–1061. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, Howell LL. Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys. Pharmacol Biochem Behav. 2005;80:481–491. doi: 10.1016/j.pbb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Glatz AC, Ehrlich M, Bae RS, Clarke MJ, Quinlan PA, Brown EC, Rada P, Hoebel BG. Inhibition of cocaine self-administration by fluoxetine or D-fenfluramine combined with phentermine. Pharmacol Biochem Behav. 2002;71:197–204. doi: 10.1016/s0091-3057(01)00657-8. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. John Wiley and Sons; Hoboken, NJ: 2011. [Google Scholar]
- Glowa JR, Fantegrossi WE, Lewis DB, Matecka D, Rice KC, Rothman RB. Sustained decrease in cocaine-maintained responding in rhesus monkeys with 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-hydroxy-3-phenylpropyl) piperazinyl decanoate, a long-acting ester derivative of GBR 12909. J Med Chem. 1996;39:4689–4691. doi: 10.1021/jm960551t. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Wojnicki FH, Matecka D, Bacher JD. Effects of dopamine reuptake inhibitors on food- and cocaine-maintained responding: I. Dependence on unit dose of cocaine. Exp Clin Psychopharmacol. 1995;3:219–231. [Google Scholar]
- Goeders NE, Kuhar MJ. Chronic cocaine administration induces opposite changes in dopamine receptors in the striatum and nucleus accumbens. Alcohol Drug Res. 1987;7:207–216. [PubMed] [Google Scholar]
- Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology (Berl) 1996;126:286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- Gould RW, Duke AN, Nader MA. PET studies in nonhuman primate models of cocaine abuse: Translational research related to vulnerability and neuroadaptations. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Gage HD, Banks ML, Blaylock BL, Czoty PW, Nader MA. Differential effects of cocaine and MDMA self-administration on cortical serotonin transporter availability in monkeys. Neuropharmacology. 2011;61:245–251. doi: 10.1016/j.neuropharm.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Gage HD, Nader MA. Effects of chronic cocaine self-administration on cognition and cerebral glucose utilization in Rhesus monkeys. Biol Psychiatry. 2012;72:856–863. doi: 10.1016/j.biopsych.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Elk R, Schmitz J, Davis C, Creson D, Kirby K. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled double-blind trials. J Clin Psychopharmacol. 1995;15:163–174. doi: 10.1097/00004714-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Grant SJ, Aston-Jones G, Redmond DE., Jr. Responses of primate locus coeruleus neurons to simple and complex sensory stimuli. Brain Res Bull. 1988;21:401–410. doi: 10.1016/0361-9230(88)90152-9. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of dopamine signaling. Biosci Rep. 2001;21:247–269. doi: 10.1023/a:1013205230142. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Sustained release d-amphetamine reduces cocaine but not ‘speedball’-seeking in buprenorphine-maintained volunteers: a test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacology. 2010;35:2624–2637. doi: 10.1038/npp.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Buckley NW, Gosden J, Slater N, France CP, Hackett D. A preclinical evaluation of the discriminative and reinforcing properties of lisdexamfetamine in comparison to d-amfetamine, methylphenidate and modafinil. Neuropharmacology. 2013;73C:348–358. doi: 10.1016/j.neuropharm.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Henry PK, Murnane KS, Votaw JR, Howell LL. Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging Behav. 2010;4:212–219. doi: 10.1007/s11682-010-9100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther. 2009;329:677–686. doi: 10.1124/jpet.108.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol. 1998;19:187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Characterization of the effects of cocaine and GBR 12909, a dopamine uptake inhibitor, on behavior in the squirrel monkey. J Pharmacol Exp Ther. 1991;258:178–185. [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther. 1995;275:1551–1559. [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Howell LL, Czoty PW, Byrd LD. Pharmacological interactions between serotonin and dopamine on behavior in the squirrel monkey. Psychopharmacology (Berl) 1997;131:40–48. doi: 10.1007/s002130050263. [DOI] [PubMed] [Google Scholar]
- Howell LL, Czoty PW, Kuhar MJ, Carrol FI. Comparative behavioral pharmacology of cocaine and the selective dopamine uptake inhibitor RTI-113 in the squirrel monkey. J Pharmacol Exp Ther. 2000;292:521–529. [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Wilcox KM, Lindsey KP. Cocaine-induced brain activation determined by positron emission tomography neuroimaging in conscious rhesus monkeys. Psychopharmacology (Berl) 2002;159:154–160. doi: 10.1007/s002130100911. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Howell LL, Votaw JR, Goodman MM, Lindsey KP. Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology (Berl) 2010;208:191–199. doi: 10.1007/s00213-009-1720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates. J Pharmacol Exp Ther. 2001;298:1–6. [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Lee CC, Wu MY, Hsu KS. Repeated cocaine administration decreases 5-HT(2A) receptor-mediated serotonergic enhancement of synaptic activity in rat medial prefrontal cortex. Neuropsychopharmacology. 2009;34:1979–1992. doi: 10.1038/npp.2009.10. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Malison RT, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB. Elevated central serotonin transporter binding availability in acutely abstinent cocaine-dependent patients. Am J Psychiatry. 2000;157:1134–1140. doi: 10.1176/appi.ajp.157.7.1134. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Katz JL. Drugs as reinforcers: pharmacological and behavioural factors. In: Liebman JM, Cooper SJ, editors. The Neuropharmacological Basis of Reward. Clarendon Press; Oxford: 1989. pp. 164–213. [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Terry P. Relationships among dopamine transporter affinities and cocaine-like discriminative-stimulus effects. Psychopharmacology (Berl) 2000;148:90–98. doi: 10.1007/s002130050029. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Joyce AR, Carroll FI, Kuhar MJ. Dopamine D1 and D2 receptors influence dopamine transporter synthesis and degradation in the rat. J Pharmacol Exp Ther. 2001;298:129–140. [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav. 2008;90:453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, O’Connor JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86:45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland Henry P, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology (Berl) 2009;205:237–247. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler K, Lau T, Schloss P. Antagonists and substrates differentially regulate serotonin transporter cell surface expression in serotonergic neurons. Eur J Pharmacol. 2010;629:63–67. doi: 10.1016/j.ejphar.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Anthony EW, Woolverton WL. Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1990a;254:312–317. [PubMed] [Google Scholar]
- Kleven MS, Perry BD, Woolverton WL, Seiden LS. Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res. 1990b;532:265–270. doi: 10.1016/0006-8993(90)91768-c. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of bromocriptine and desipramine on behavior maintained by cocaine or food presentation in rhesus monkeys. Psychopharmacology (Berl) 1990c;101:208–213. doi: 10.1007/BF02244128. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of three monoamine uptake inhibitors on behavior maintained by cocaine or food presentation in rhesus monkeys. Drug Alcohol Depend. 1993;31:149–158. doi: 10.1016/0376-8716(93)90067-z. [DOI] [PubMed] [Google Scholar]
- Kohut SJ, Fivel PA, Blough BE, Rothman RB, Mello NK. Effects of methcathinone and 3-Cl-methcathinone (PAL-434) in cocaine discrimination or self-administration in rhesus monkeys. Int J Neuropsychopharmacol. 2013 doi: 10.1017/S146114571300059X. (In press) [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Pilotte NS. Neurochemical changes in cocaine withdrawal. Trends Pharmacol Sci. 1996;17:260–264. doi: 10.1016/0165-6147(96)10024-9. [DOI] [PubMed] [Google Scholar]
- Langer SZ, Galzin AM. Studies on the serotonin transporter in platelets. Experientia. 1988;44:127–130. doi: 10.1007/BF01952194. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Daunais JB, Hedgecock AA, Porrino LJ. Effects of chronic cocaine administration on dopamine transporter mRNA and protein in the rat. Brain Res. 1997;750:214–222. doi: 10.1016/s0006-8993(96)01384-4. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth SR, Sexton T, Childers SR, Vrana KE, Vaughan RA, Davies HM, Porrino LJ. Regulation of rat dopamine transporter mRNA and protein by chronic cocaine administration. J Neurochem. 1999;73:1982–1989. [PubMed] [Google Scholar]
- Levin FR, Lehman AF. Meta-analysis of desipramine as an adjunct in the treatment of cocaine addiction. J Clin Psychopharmacol. 1991;11:374–378. [PubMed] [Google Scholar]
- Lile J, Nader M. The abuse liability and therapeutic potential of drugs evaluated for cocaine addiction as predicted by animal models. Curr Neuropharmacol. 2003:21–46. [Google Scholar]
- Lima MS, Reisser AA, Soares BG, Farrell M. Antidepressants for cocaine dependence. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD002950. CD002950. [DOI] [PubMed] [Google Scholar]
- Lin Z, Canales JJ, Bjorgvinsson T, Thomsen M, Qu H, Liu QR, Torres GE, Caine SB. Monoamine transporters: vulnerable and vital doorkeepers. Prog Mol Biol Transl Sci. 2011;98:1–46. doi: 10.1016/B978-0-12-385506-0.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, Rice KC, Howell LL. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309:959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol Pharmacol. 2002;61:436–445. doi: 10.1124/mol.61.2.436. [DOI] [PubMed] [Google Scholar]
- Little KY, Kirkman JA, Carroll FI, Clark TB, Duncan GE. Cocaine use increases [3H]WIN 35428 binding sites in human striatum. Brain Res. 1993;628:17–25. doi: 10.1016/0006-8993(93)90932-d. [DOI] [PubMed] [Google Scholar]
- Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malison RT, Best SE, van Dyck CH, McCance EF, Wallace EA, Laruelle M, Baldwin RM, Seibyl JP, Price LH, Kosten TR, Innis RB. Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I] beta-CIT SPECT. Am J Psychiatry. 1998;155:832–834. doi: 10.1176/ajp.155.6.832. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Pavlicova M, Bisaga A, Nunes EV, Brooks DJ, Levin FR. Extended-release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biol Psychiatry. 2012;72:950–956. doi: 10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. Modeling relapse in animals. Curr Top Behav Neurosci. 2013;13:403–432. doi: 10.1007/7854_2012_202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR, Perez A, Abi-Dargham A, Fischman MW, Kleber HD, Laruelle M. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology. 2009;34:1774–1782. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Staley JK, Izenwasser S, Basile M, Ruttenber AJ. Serotonin transporters upregulate with chronic cocaine use. J Chem Neuroanat. 2000;20:271–280. doi: 10.1016/s0891-0618(00)00102-2. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet. 1998;352:1433–1437. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- McClung J, Fantegrossi W, Howell LL. Reinstatement of extinguished amphetamine self-administration by 3,4-methylenedioxymethamphetamine (MDMA) and its enantiomers in rhesus monkeys. Psychopharmacology (Berl) 2010;210:75–83. doi: 10.1007/s00213-010-1818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Bree MP, Mendelson JH. Desipramine effects on cocaine self-administration by rhesus monkeys. Drug Alcohol Depend. 1990;26:103–116. doi: 10.1016/0376-8716(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Meltzer PC, Liu S, Blanchette H, Blundell P, Madras BK. Design and synthesis of an irreversible dopamine-sparing cocaine antagonist. Bioorg Med Chem. 2002;10:3583–3591. doi: 10.1016/s0968-0896(02)00244-4. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33:367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, Rathnayaka N, Kramer LA, Narayana PA. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse. 1998a;30:88–96. doi: 10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on striatal dopamine D1 receptors in rhesus monkeys. Synapse. 1998b;28:1–9. doi: 10.1002/(SICI)1098-2396(199801)28:1<1::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Howell LL. Development of an apparatus and methodology for conducting functional magnetic resonance imaging (fMRI) with pharmacological stimuli in conscious rhesus monkeys. J Neurosci Methods. 2010;191:11–20. doi: 10.1016/j.jneumeth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Grant KA, Davies HM, Mach RH, Childers SR. The reinforcing and discriminative stimulus effects of the novel cocaine analog 2beta-propanoyl-3beta-(4-tolyl)-tropane in rhesus monkeys. J Pharmacol Exp Ther. 1997;280:541–550. [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, Blaylock BL, Kaplan JR, Garg PK, Davies HM, Morton D, Garg S, Reboussin BA. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol Psychiatry. 2012;72:414–421. doi: 10.1016/j.biopsych.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan R, Yamamoto BK. The Basal Ganglia as a Substrate for the Multiple Actions of Amphetamines. Basal Ganglia. 2011;1:49–57. doi: 10.1016/j.baga.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Banks ML. Making the right choice: Lessons from drug discrimination for research on drug reinforcement and drug self-administration. In: Glennon R, Young R, editors. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. John Wiley and Sons; Hoboken, NJ: 2011. pp. 361–388. [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective suppression of cocaine- versus food-maintained responding by monoamine releasers in rhesus monkeys: benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine. J Pharmacol Exp Ther. 2009a;329:272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Brandt MR, Mello NK. Effects of the long-acting monoamine reuptake inhibitor indatraline on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 1999;291:60–69. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of mu-opioid agonists on cocaine- and food-maintained responding and cocaine discrimination in rhesus monkeys: role of muagonist efficacy. J Pharmacol Exp Ther. 2002;300:1111–1121. doi: 10.1124/jpet.300.3.1111. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 2003a;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic methadone treatment on cocaine- and food-maintained responding under second-order, progressive-ratio and concurrent-choice schedules in rhesus monkeys. Drug Alcohol Depend. 2004;74:297–309. doi: 10.1016/j.drugalcdep.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Kimmel HL, Howell LL, Carroll FI. Effects of the monoamine uptake inhibitors RTI-112 and RTI-113 on cocaine- and food-maintained responding in rhesus monkeys. Pharmacol Biochem Behav. 2009b;91:333–338. doi: 10.1016/j.pbb.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Lamas X, Mendelson JH. Acute and chronic effects of flupenthixol on the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1996;278:879–890. [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- Newman JL, Negus SS, Lozama A, Prisinzano TE, Mello NK. Behavioral evaluation of modafinil and the abuse-related effects of cocaine in rhesus monkeys. Exp Clin Psychopharmacol. 2010;18:395–408. doi: 10.1037/a0021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AB, Norman MK, Hall JF, Tsibulsky VL. Priming threshold: a novel quantitative measure of the reinstatement of cocaine self-administration. Brain Res. 1999;831:165–174. doi: 10.1016/s0006-8993(99)01423-7. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Drug addiction and drug abuse. In: Brunton L, Lazo J, Parker K, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11th edition McGraw-Hill; New York: 2006. pp. 607–628. [Google Scholar]
- Oliveto A, Poling J, Mancino MJ, Williams DK, Thostenson J, Pruzinsky R, Gonsai K, Sofuoglu M, Gonzalez G, Tripathi S, Kosten TR. Sertraline delays relapse in recently abstinent cocaine-dependent patients with depressive symptoms. Addiction. 2012;107:131–141. doi: 10.1111/j.1360-0443.2011.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton DA. Applications and limitations of the drug discrimination method for the study of drug abuse. In: Bozarth MJ, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer Verlag; New York: 1987. pp. 291–340. [Google Scholar]
- Peltier RL, Li DH, Lytle D, Taylor CM, Emmett-Oglesby MW. Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther. 1996;277:212–218. [PubMed] [Google Scholar]
- Pilotte NS, Sharpe LG, Kuhar MJ. Withdrawal of repeated intravenous infusions of cocaine persistently reduces binding to dopamine transporters in the nucleus accumbens of Lewis rats. J Pharmacol Exp Ther. 1994;269:963–969. [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Noradrenergic mechanisms in cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2007;322:894–902. doi: 10.1124/jpet.107.121806. [DOI] [PubMed] [Google Scholar]
- Preston KL, Sullivan JT, Berger P, Bigelow GE. Effects of cocaine alone and in combination with mazindol in human cocaine abusers. J Pharmacol Exp Ther. 1993;267:296–307. [PubMed] [Google Scholar]
- Reivich M, Alavi A, Wolf A, Fowler J, Russell J, Arnett C, MacGregor RR, Shiue CY, Atkins H, Anand A, et al. Glucose metabolic rate kinetic model parameter determination in humans: the lumped constants and rate constants for [18F]fluorodeoxyglucose and [11C]deoxyglucose. J Cereb Blood Flow Metab. 1985;5:179–192. doi: 10.1038/jcbfm.1985.24. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Stellar JR, Todtenkopf MS. Subregion-specific down-regulation of 5-HT3 immunoreactivity in the nucleus accumbens shell during the induction of cocaine sensitization. Pharmacol Biochem Behav. 2004;77:415–422. doi: 10.1016/j.pbb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther. 1989;248:1010–1017. [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Becketts KM, Radesca LR, de Costa BR, Rice KC, Carroll FI, Dersch CM. Studies of the biogenic amine transporters. II. A brief study on the use of [3H]DA-uptake-inhibition to transporter-binding-inhibition ratios for the in vitro evaluation of putative cocaine antagonists. Life Sci. 1993;53:PL267–272. doi: 10.1016/0024-3205(93)90602-y. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Ann N Y Acad Sci. 2002a;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. AAPS J. 2007;9:E1–10. doi: 10.1208/aapsj0901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers: potential treatment agents for stimulant addiction. Exp Clin Psychopharmacol. 2008;16:458–474. doi: 10.1037/a0014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, Baumann MH. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther. 2005;313:1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Clark RD, Partilla JS, Baumann MH. (+)-Fenfluramine and its major metabolite, (+)-norfenfluramine, are potent substrates for norepinephrine transporters. J Pharmacol Exp Ther. 2003;305:1191–1199. doi: 10.1124/jpet.103.049684. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Dersch CM, Ananthan S, Partilla JS. Studies of the biogenic amine transporters. 13. Identification of “agonist” and “antagonist” allosteric modulators of amphetamine-induced dopamine release. J Pharmacol Exp Ther. 2009;329:718–728. doi: 10.1124/jpet.108.149088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Glowa JR. A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development. Focus on GBR 12909. Mol Neurobiol. 1995;11:1–19. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Katsnelson M, Vu N, Partilla JS, Dersch CM, Blough BE, Baumann MH. Interaction of the anorectic medication, phendimetrazine, and its metabolites with monoamine transporters in rat brain. Eur J Pharmacol. 2002b;447:51–57. doi: 10.1016/s0014-2999(02)01830-7. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Mechanisms of biogenic amine transporters. In: Reith M, editor. Neurotransmitter transporters: structure, function, and regulation. Humana Press; Totowa, NY: 1997. pp. 73–100. [Google Scholar]
- Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during D-amphetamine maintenance. J Clin Psychopharmacol. 2010;30:152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Sawyer EK, Mun J, Nye JA, Kimmel HL, Voll RJ, Stehouwer JS, Rice KC, Goodman MM, Howell LL. Neurobiological changes mediating the effects of chronic fluoxetine on cocaine use. Neuropsychopharmacology. 2012;37:1816–1824. doi: 10.1038/npp.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schama KF, Howell LL, Byrd LD. Serotonergic modulation of the discriminative-stimulus effects of cocaine in squirrel monkeys. Psychopharmacology (Berl) 1997;132:27–34. doi: 10.1007/s002130050316. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Rats become acutely tolerant to cathine after amphetamine or cathinone administration. Psychopharmacology (Berl) 1990;101:126–131. doi: 10.1007/BF02253729. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hely L, Gittings D, Lake B, Daniela E. Effects of priming injections of MDMA and cocaine on reinstatement of MDMA- and cocaine-seeking in rats. Drug Alcohol Depend. 2008;96:249–255. doi: 10.1016/j.drugalcdep.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology (Berl) 1999;147:285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Thorndike EB. Effect of rate of delivery of intravenous cocaine on self-administration in rats. Pharmacol Biochem Behav. 2009;93:375–381. doi: 10.1016/j.pbb.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Averill P, Stotts AL, Moeller FG, Rhoades HM, Grabowski J. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63:207–214. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol Rev. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser. 1988;4:161–175. doi: 10.1007/978-3-642-73223-2_13. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Skinner BF. The behavior of organisms. Appleton-Century-Crofts; New York: 1938. [Google Scholar]
- Spealman RD. Modification of behavioral effects of cocaine by selective serotonin and dopamine uptake inhibitors in squirrel monkeys. Psychopharmacology (Berl) 1993;112:93–99. doi: 10.1007/BF02247368. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1995;275:53–62. [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Staley JK, Hearn WL, Ruttenber AJ, Wetli CV, Mash DC. High affinity cocaine recognition sites on the dopamine transporter are elevated in fatal cocaine overdose victims. J Pharmacol Exp Ther. 1994;271:1678–1685. [PubMed] [Google Scholar]
- Stine SM, Krystal JH, Kosten TR, Charney DS. Mazindol treatment for cocaine dependence. Drug Alcohol Depend. 1995;39:245–252. doi: 10.1016/0376-8716(95)01174-4. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Agonist Replacement for Stimulant Dependence: A Review of Clinical Research. Curr Pharm Des. 2013 doi: 10.2174/138161281940131209142843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella SR. Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol Biochem Behav. 1995;51:687–692. doi: 10.1016/0091-3057(94)00438-o. [DOI] [PubMed] [Google Scholar]
- Tella SR, Ladenheim B, Andrews AM, Goldberg SR, Cadet JL. Differential reinforcing effects of cocaine and GBR-12909: biochemical evidence for divergent neuroadaptive changes in the mesolimbic dopaminergic system. J Neurosci. 1996;16:7416–7427. doi: 10.1523/JNEUROSCI.16-23-07416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav. 2013;99:211–233. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009a;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009b;331:204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Nader MA, Woolverton WL. Evaluation of the discriminative stimulus and reinforcing effects of sertraline in rhesus monkeys. Pharmacol Biochem Behav. 1992;41:789–793. doi: 10.1016/0091-3057(92)90228-8. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102(Suppl 1):96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001c;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001d;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Angrist B, Hitzemann R, Lieberman J, Pappas N. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154:50–55. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend. 2010;106:233–236. doi: 10.1016/j.drugalcdep.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw JR, Howell LL, Martarello L, Hoffman JM, Kilts CD, Lindsey KP, Goodman MM. Measurement of dopamine transporter occupancy for multiple injections of cocaine using a single injection of [F-18]FECNT. Synapse. 2002;44:203–210. doi: 10.1002/syn.10068. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Middleton LS, Wong CJ, Nuzzo PA, Campbell CL, Rush CR, Lofwall MR. Atomoxetine does not alter cocaine use in cocaine dependent individuals: double blind randomized trial. Drug Alcohol Depend. 2013;130:150–157. doi: 10.1016/j.drugalcdep.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Sullivan JT, Fromme R, Bigelow GE. Fluoxetine alters the effects of intravenous cocaine in humans. J Clin Psychopharmacol. 1994;14:396–407. [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Westerink RH. Targeting exocytosis: ins and outs of the modulation of quantal dopamine release. CNS Neurol Disord Drug Targets. 2006;5:57–77. doi: 10.2174/187152706784111597. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse. 2005;58:220–228. doi: 10.1002/syn.20199. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, Howell LL. Self-administration of cocaine and the cocaine analog RTI-113: relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43:78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Nobrega JN, Corrigall WA, Coen KM, Shannak K, Kish SJ. Amygdala dopamine levels are markedly elevated after self- but not passive-administration of cocaine. Brain Res. 1994;668:39–45. doi: 10.1016/0006-8993(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Wimalasena K. Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry. Med Res Rev. 2011;31:483–519. doi: 10.1002/med.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley EL, Bigelow GE, Silverman K, Johnson RE, Strain EC. A randomized controlled trial of fluoxetine in the treatment of cocaine dependence among methadone-maintained patients. J Subst Abuse Treat. 2011;40:255–264. doi: 10.1016/j.jsat.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FH, Rothman RB, Rice KC, Glowa JR. Effects of phentermine on responding maintained under multiple fixed-ratio schedules of food and cocaine presentation in the rhesus monkey. J Pharmacol Exp Ther. 1999;288:550–560. [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(Suppl 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Evaluation of the role of norepinephrine in the reinforcing effects of psychomotor stimulants in rhesus monkeys. Pharmacol Biochem Behav. 1987;26:835–839. doi: 10.1016/0091-3057(87)90618-6. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Cervo L, Johanson CE. Effects of repeated methamphetamine administration on methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1984;21:737–741. doi: 10.1016/s0091-3057(84)80012-x. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends Pharmacol Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Weiss SRB. Tolerance and sensitization to cocaine: an integrated view. In: Higgens S, Katz J, editors. Cocaine abuse: behavior, pharmacology, and clinical applications. Academic Press; San Diego: 1998. pp. 107–134. [Google Scholar]
- Young AM, Herling S. Drugs as reinforcers: studies in laboratory animals. In: Goldberg SR, Stolerman IP, editors. Behavioral Analysis of Drug Dependence. Academic Press; Orlando, FL: 1986. pp. 9–67. [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]