Abstract
Lysosomal diseases are a family of over 50 disorders caused by defects in proteins critical for normal function of the endosomal/lysosomal system and characterized by complex pathogenic cascades involving progressive dysfunction of many organ systems, most notably brain. Evidence suggests that compromise in lysosomal function is highly varied and leads to changes in multiple substrate processing and endosomal signaling, in calcium homeostasis and ER stress, and in autophagocytosis and proteasome function. Neurons are highly vulnerable and show abnormalities in perikarya, dendrites, and axons, often in ways appearing unrelated to the primary lysosomal defect. A notable example is neuroaxonal dystrophy (NAD) which is characterized by formation of focal enlargements (spheroids) containing diverse organelles and other components consistent with compromise of retrograde axonal transport. While neurons may be universally susceptible to NAD, GABAergic neurons, particularly Purkinje cells, appear most vulnerable and ataxia and related features of cerebellar dysfunction are a common outcome. As NAD is found early in disease and thus may be a contributor to Purkinje cell dysfunction and death, understanding its link to lysosomal compromise could lead to therapies designed to prevent its occurrence and thereby ameliorate cerebellar dysfunction.
Keywords: lysosomal disease, Niemann-Pick type C, Purkinje cell, axoplasmic transport, axonal spheroid, autophagy
Introduction
Lysosomal diseases were first described clinically and pathologically over a hundred years ago and were subsequently defined and classified by biochemical analysis of stored materials, giving rise to a classification system that includes the mucopolysaccharidoses, glycosphingolipidoses, mucolipidoses, glycoproteinoses, and so forth [1]. Articulation of the lysosomal disease concept by H.G. Hers in 1965 [2] ushered in the discovery of individual lysosomal enzyme defects, followed subsequently by gene identification and mutation analysis [3]. This remarkable knowledge transition – from the era of clinical and pathological description to biochemical classification and to protein and gene discovery, has now turned toward emphasis of an entirely new research focus, appropriately named the Era of Pathogenesis [4]. That is, it is now clear that to truly understand (and particularly to treat) these and other inborn errors of metabolism it is necessary to know far more than the identity of the gene and protein defects. What must be understood are the details of the intricate series of consequences that occur following the loss of a particular protein – the pathogenic cascade. And importantly, this puzzle needs to be solved, not just generically, but rather for individual cell types since the specifics of a disease cascade likely will vary depending on the metabolic signature of the cell in question. Since most lysosomal diseases exhibit brain dysfunction, a cell of particular interest is the neuron. Yet even here, as described below, there is ample evidence that neuronal subtypes often display dramatic differences in their response to a defect in a given lysosomal protein.
The Pathogenic Cascade Affecting Neurons
At least two thirds of lysosomal diseases affect the brain and many types of cellular defects have been described here [5]. While some have major impact on oligodendroglia and myelination of axons leading to white matter lesions (the leukodystrophies such as Krabbe disease or metachromatic leukodystrophy), most lysosomal disorders exhibit the largest impact on neurons and gray matter [1]. In addition to intraneuronal “storage” impacting the endosomal/lysosomal system, other cellular changes include meganeurite formation, ectopic dendritogenesis, NAD and, ultimately, neurodegeneration. While in most diseases neuronal storage in brain appears universal, in others it may be restricted to highly select populations of neurons. In Fabry disease, for example, neuronal storage has been reported restricted primarily to select populations of neurons in nuclei of the amygdala, hypothalamus, brain stem and spinal cord [6]. Even in lysosomal diseases in which essentially all neurons show storage, some neuronal populations will exhibit specific vulnerabilities (e.g., ectopic dendritogenesis, axonal spheroids or early death) not evident in others. For any given lysosomal disease the wide array of pathological changes observed in brain can be viewed as an example of divergence of effect, that is, the outward manifestation of a complex and expanding disease cascade set in motion by the absence of function of a single protein. Importantly, in comparing different lysosomal diseases one also typically finds evidence of a convergence of effect, that is, the exact same cellular abnormalities often occur across many – though not necessarily all – lysosomal disorders [5]. Ectopic dendritogenesis, for example, has been documented principally in pyramidal neurons in primary ganglioside storage diseases, but also occurs in these same cells in some of the mucopolysaccharidoses, and in the glycoproteinosis known as α-mannosidosis. For each of these it appears linked in some manner to disturbances in the processing and resulting accumulation of glycosphingolipids [7,8]. Interestingly, however, this phenomenon has never been reported to occur in any other type of neurological disease. Axonal spheroid formation, or NAD, likewise, is found in these and some other lysosomal disorders but predominates in GABAergic neurons of the cerebral cortex rather than pyramidal neurons, and is particularly abundant on Purkinje cells of the cerebellum [9]. In this case, as detailed below, there is no apparent linkage to any specifically identified storage compounds in perikarya and thus the nature of the metabolic defect and pathogenic cascade that triggers the axonal compromise remains a mystery. Also, in contrast to ectopic dendritogenesis, there is evidence that spheroids very similar to those observed in lysosomal disease also occur in other neurological and genetic conditions, including Alzheimer’s disease [10].
Neuroaxonal Dystrophy
Just as certain types of neurons show the presence of renewed dendrite growth (ectopic dendritogenesis) following lysosomal system derangement, other neurons exhibit highly conspicuous axonal alterations consisting of bulbous swellings (so-called spheroids) along axons (Fig. 1). As introduced earlier, such changes are referred to as axonal spheroid formation or NAD, with the enlargements being identified in terminal synaptic arbors of affected neurons, in white matter and thus involving projection axons, and in proximal regions of axons, and axon collaterals, near the cell body (Fig. 2). Spheroids occur distal to the initial segment (IS) of axons and thus are clearly distinguishable from meganeurites which occur proximal to the IS [11]. Ultrastructural studies have shown that these focal enlargements contain collections of tubulovesicular profiles, mitochondria, possible autophagosomes, and dense and multivesicular-type bodies [12]. Occasionally there are also large numbers of neurofilaments, but in most cases the accumulation of organellar type materials predominate, hence the term, granular axonal spheroids. Interestingly, axonal spheroids characteristically do not contain the typical lysosomal inclusions found in neuronal perikarya which are themselves often representative of a particular disease type (e.g., membranous cytoplasmic bodies for primary gangliosidoses, compound bodies for MLIV disease, polymembranous cytoplasmic bodies for Niemann-Pick type C, etc.). Instead, axonal spheroids found across a wide variety of lysosomal diseases routinely resemble one another suggesting that their formation is due to a generic defect, possibly one involving axoplasmic transport [5,9,12,13].
Figure 1.
Confocal images of GFP-labeled Purkinje neurons and axons in a 4-week old transgenic Npc1−/− mouse with L7 promoter-driven GFP expression (L7-GFP Npc1−/−) A: Cerebellar cortex and white matter. Note the presence of numerous GFP+ swellings (arrows) affecting Purkinje cell axons as they project through the granule cell layer and into the underlying white matter (arrows). At 4 weeks of age, while such spheroids are common, Purkinje cells are abundant and reside in typical rows within the folium (arrowheads). Purkinje cell death is first observed at about 6 weeks of age. B: Deep cerebellar nucleus. Note the presence of numerous GFP+ swellings within terminal Purkinje cell axons (some highlighted by arrows) near the cell bodies of DCN neurons (some highlighted by asterisks). GFP+ Purkinje cells axon terminals create a dense meshwork in the deep cerebellar nuclei, whereas the projection neurons of the DCN are GFP−. Calibration bars in A and B equal 30 μm. Image in A represents maximum intensity projections of 35 μm thick confocal z-stacks, whereas B is a single plane confocal image.
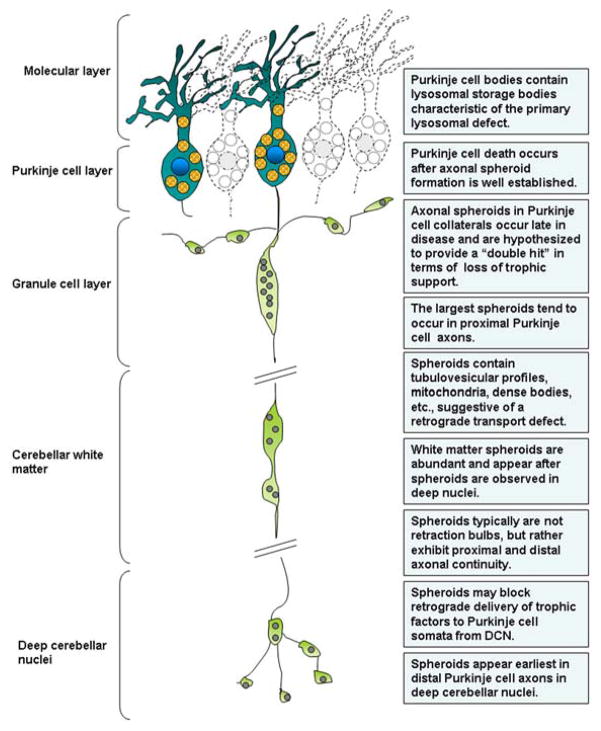
Figure 2.
Schematic illustration showing the likely distribution of spheroids within a Purkinje cell axon in late Niemann-Pick type C disease and summarizing the characteristics of this phenomenon as explored in cat and mouse models [15,16].
Spheroids have been studied extensively in animal models of lysosomal disorders where they are found to be abundant in primary ganglioside storage diseases (GM1 and GM2 gangliosidosis), in MLIV disease and the Niemann-Pick disorders (types A and C), in the glycoproteinosis, α-mannosidosis, and so forth [9,12–16]. Their incidence and distribution correlate closely with the onset and progression of neurological signs displayed by affected animals, suggesting that they are major players in generating neuronal dysfunction [9]. Spheroids are most readily visualized in neurons using immunocytochemistry to label components trafficked in axons, including enzymes (glutamic acid decarboxylase) as well as calcium binding proteins (parvalbumin, calbindin) [9,12–16]. The use of immunostaining techniques to localize these cell selective markers in brain tissue from lysosomal diseases has shown that axonal spheroids occur most commonly on neurons that synthesize GABA as a neurotransmitter, including Purkinje cells, intrinsic neurons of the cerebral cortex (basket cells and other inhibitory neurons) and select GABAergic neuronal populations in basal ganglia and elsewhere in brain. Spheroids are also abundant in numerous areas of the CNS in human lysosomal diseases like NPC disease (17; Sikora and Elleder, unpublished data). In cats with NPC disease axonal spheroids occur in massive numbers in inhibitory circuits of the cerebral cortex, in basal ganglia, and in cerebellum [15]. Mouse models show somewhat less involvement of the cerebral cortex and basal ganglia, but Purkinje cells are often severely affected [16,18].
The similarity between spheroids of lysosomal storage diseases and those found distal to nerve crush or ligation experiments in normal animals [19], has suggested that a defect in retrograde transport may be contributing to their formation. Studies, for example in NPC disease, have also reported that spheroids are initially found in distal portions of axons and subsequently in more proximal areas [15], suggesting a slow, distal to proximal migration of spheroid components over time. That distal axonal spheroids tend to be smaller and proximal ones larger in cerebellar Purkinje cells is consistent with this view (Fig. 2). The prominence of spheroids in axons of Purkinje cells and the finding that these cells are also highly prone to die early (but after spheroid formation) in many storage diseases suggests that the two events are linked, with spheroids in some manner compromising Purkinje cell viability. A similar relationship may exist for cerebral cortex, e.g., in human NPC disease, in that spheroids in axons of GABAergic neurons may lead to functional compromise of inhibitory circuits, a view supported by the occurrence of intractable seizures in late disease [20].
Spheroids may cause the early demise of affected neurons like Purkinje cells as a consequence of a block in retrograde transport of a critical component, e.g., a growth factor, essential for cell survival. The importance of retrograde transport of growth factors for sustained survival of neurons has recently received greater attention for a spectrum of neurodegenerative conditions [21–24]. Similarly, spheroids may compromise the output of affected neurons by blocking or otherwise interfering with action potential propagation [see 9] or possibly with synaptic vesicle trafficking. Such alternations in the signaling dynamics of neural circuits may in turn increase energy demands on individual neurons, further exacerbating the metabolic stress of storage. Overall, the occurrence of spheroids is poised within the pathogenic cascade to be a major player in generating many of the clinical features of storage diseases, from ataxia, tremors and related movement disorders to seizures. Importantly, the potential role of NAD in a host of other neurodegenerative diseases – from Alzheimer’s to Huntington’s, has also recently drawn attention [10, 25–27].
Links between the presence of axonal spheroids and neuron death in lysosomal diseases (or indeed, other conditions) have not been systematically explored. While Purkinje cells are known for their exquisite sensitivity to lysosomal compromise and early death, normal Purkinje cells are in fact unique in one feature compared to most neurons: They are remarkably resistant to axotomy [28–30]. That is, undercutting or lesioning the white matter of the cerebellum does not in itself lead to a chromatolytic reaction in Purkinje cell somata as it would in most other neuron types. Indeed, Purkinje neurons with severed axons survive for long periods of time following complete axotomy whereas most neurons die [28,29]. This has been interpreted to suggest that they draw growth factor support locally from the region of the Purkinje cell layer (via axon collaterals) rather than exclusively from their projection nuclei (the deep cerebellar nuclei [DCN] and vestibular nuclei [VN]) [see 30]. This possibility is reinforced by the discovery that local Purkinje cell axon collaterals become more robust following axotomy and loss of the parent axon. Thus, Purkinje cells lacking their parent axons remain viable for extended periods of time as they apparently convert their status as projection neurons to that of local circuit neurons [30]. Such transformation, however, does not compensate for the functional deficits following the loss of their projection input to DCN. The notable resilience of normal Purkinje cells following axotomy makes the prominence of early Purkinje cell death in lysosomal diseases even more intriguing, indeed paradoxical, and raises the question of whether the persistence of a spheroid-possessing long axon and its physiologic/metabolic consequences provide a significant burden on survival (Fig. 2). Importantly, however, in diseases like NPC where Purkinje cell loss is so prominent, spheroids occur earliest in parent axons projecting to DCN, but before death of these cells spheroids also form in their collateral branches [15]. This finding is consistent with Purkinje cells receiving a “double hit” in terms of compromise or loss of retrograde trophic factor support prior to their demise – coming first from the parent axon and subsequently from its collaterals.
Interestingly, while early onset and prominent Purkinje cell death is characteristic of many lysosomal diseases, not all Purkinje cells are equally vulnerable to early death. In NPC disease, for example, studies in the mouse model have shown a complex pattern of Purkinje cell loss with zebrin II-negative cells dying first, followed only later by zebrin II-positive Purkinje cells [31]. Additionally, ectopic expression of the small heat shock protein, HSP25, occurs preferentially in surviving Purkinje cells. The last Purkinje cells to die in NPC disease and other mouse models of lysosomal disorders are those in lobule X [18,31,32]. These Purkinje cells, as resident projection neurons of the archicerebellum (vestibulocerebellum) are known to project directly to VN rather than to DCN. Whether such connectivity differences are in some manner directly linked to this longer survival is unknown. Interestingly, other genetic conditions not directly related to lysosomal dysfunction, e.g., the Purkinje cell degeneration (pcd) mouse, follow an identical pattern of Purkinje cell loss, complete with spheroids, longer survival of cells in lobule X, and changes in specific projection nuclei (VN and DCN) [33,34]. For pcd, the gene defect is now known to lead to a deficiency of carboxypeptidase 1 (CCP/Nna1), a cytosolic protease believed to act downstream of proteasome function [35]. Absence of this enzyme in mice leads to elevations in non-degraded peptides derived from cytosolic and mitochondrial proteins, alterations in autophagy, and in neuron death. Interestingly, possible downstream alterations in proteasome function have gained attention in lysosomal disease as a component of the pathogenic cascade [1,4], in part driven by evidence implicating a role for autophagy in these diseases [36,37] and by studies showing that autophagy, the proteasome and the endosomal/lysosomal system are intimately coupled in maintaining cell homeostasis [38].
As mentioned earlier, axonal spheroids do not appear to contain lysosomal debris or storage bodies characteristic of the lysosomal disease in question. Indeed, the extent to which lysosomal components are normally found within axons remains controversial and most models favor the retrograde transport of materials in endosomal vesicles (retrosomes or signaling endosomes, autophagosomes) for subsequent fusion with the lysosomal system in perikarya after retrograde transport. A fundamental question involving spheroids is therefore why a disturbance in function of the lysosomal system, presumably confined to neuronal perikarya, would be accompanied by such dramatic cellular pathology in distant parts of the axon. Several possibilities can be cited. One is that the lysosomal system defect within perikarya, when sufficiently severe, may restrict the axonal transport of molecular motors such as dynein or related proteins critical for retrograde transport. In support of this hypothesis is the finding that mice lacking such transport proteins (e.g., retrolinkin or BPAG1n4) have been shown to exhibit similar axonal pathology [39,40]. Secondly, since axonal transport is an energy requiring activity for the neuron, inadequacy of mitochondrial function or distribution either secondary to transport defects or to increased energy needs secondary to altered firing within the Purkinje cell neural network, could over time significantly compromise Purkinje cell viability. Thirdly, since many of the vesicular components present in spheroids resemble autophagosomes, and knockout of the autophagy gene Atg7 leads to axonal spheroid formation in the absence of autophagosomes [41], autophagy dysfunction may underlie spheroid pathology in lysosomal diseases. Finally, autophagy may be induced to occur in exaggerated fashion in some lysosomal diseases as an aberrant salvage mechanism [e.g., see 36] and as a result lead to an overproduction of autophagosomes that in turn cannot be readily cleared from axons. Interestingly, none of these hypotheses have been rigorously tested in lysosomal diseases.
One issue that has become clear in recent years is that the demise of Purkinje cells in NPC disease is cell autonomous. The study of chimeric mice with nervous systems comprised of neurons with, and neurons without, a functional NPC1 protein revealed that normal neurons could not rescue those lacking NPC1, and that the presence of mutant Purkinje cells did not affect the fate of neighboring normal neurons [42]. Similar results were recently found in mice lacking NPC1 exclusively in Purkinje cells. That is, the presence of normal cerebellar neurons (basket and stellate cells, granule cells, etc) and glia (including Bergmann glia and oligodendroglia supporting Purkinje cell axons) had no influence over the cell death fate of Purkinje cells lacking NPC1 [43]. Together, these studies provide a convincing view that Purkinje cell death in NPC disease is fully cell autonomous. The unresolved question therefore is the identification of exactly what it is about the disease cascade in these disorders that makes Purkinje cells so vulnerable to early death. As summarized above, one candidate mechanism surely is the added metabolic burden placed on these cells by a progressively dysfunctional axon.
Rescuing Purkinje Cells
A key goal of research on lysosomal diseases is to use knowledge gained in terms of disease cascade analysis to better address the development of new therapies. Studies using an inducible model of a lysosomal disease has revealed that while intraneuronal storage in Purkinje cells (and other neuron types) can be completely reversed under appropriate circumstances, elimination of subsequent cellular changes are not so easily eliminated [44]. Thus, while meganeurites on cortical pyramidal neurons readily disappear after storage elimination, many ectopic dendrites do not [44]. This may be due to stabilization of the aberrant dendrites new synapse formation. For axonal spheroids, many of these too remain as a persisting component in brain even long after the elimination of neuronal storage, and indeed, may even continue to contribute to cerebellar dysfunction [44]. Thus better understanding mechanisms underlying NAD and the nature of its subsequent burden on Purkinje cell viability remain important questions relevant to successful therapy development for this disease.
Numerous studies are underway to correct the metabolic defects present in lysosomal diseases, with treatments such as enzyme replacement therapy showing promise for non-brain correction of those disorders caused by lysosomal enzyme deficiency. For storage diseases with severe brain involvement and particularly for those caused by defects in transmembrane proteins of late endosomes and lysosomes (e.g., NPC1 and MLIV diseases), correction of the metabolic defect in brain has proved very difficult and has spurred attention toward small molecule therapies that have the ability to cross the blood brain barrier and impact the metabolic defect and storage. Miglustat (N-butyldeoxynojirimycin) a glycosphingolipid synthesis inhibitor, has shown promise in delaying onset and prolonging life in NPC disease and in animal models has shown particular benefit in slowing the loss of Purkinje cells [45; Davidson and Walkley, unpublished]. Likewise, the more recently discovered substrate reducer for NPC disease, hydroxypropyl-β-cyclodextrin, also ameliorates storage in Purkinje neurons and slows their loss over time leading to significant clinical benefit [46; Davidson and Walkley, et al., unpublished]. In neither case, however, is it presently understood exactly how these drugs provide benefit. In terms of miglustat, for example, a reduction in cholesterol storage in Purkinje neurons following treatment is not believed to occur [45], suggesting that it may be benefiting these cells through some other mechanism.
Conclusions
Most lysosomal diseases affect the brain and cause complex disease cascades leading to neuronal dysfunction, and in some cases, to neurodegeneration. The formation of axonal spheroids, e.g., in Purkinje cells, is a prominent example of such a cascade event – one that may cause disrupted function of neural circuits for months or years, and later followed by neuron death and additional neurological compromise. Understanding the basis of such cascade events, their relationship to the original gene/protein defect, to the lysosomal system, and to the overall integrated state of the neuron and its long term viability, are critical steps toward designing fully effective therapies for these disorders. Insight here, too, can be expected to provide clues for therapy for other neurodegenerative diseases in which axonal compromise has been documented.
Acknowledgments
We gratefully acknowledge the NIH [HD045561 and NS053677], the Ara Parseghian Medical Research Foundation and Dana’s Angel’s Research Trust for grant funding (SUW). Support for JS was provided as a research project [0021620806] from the Ministry of Education, Youth and Sports of the Czech Republic.
Abbreviations used
- GABA
γ-amino butryric acid
- NAD
neuroaxonal dystrophy
- IS
initial segment
- MLIV
mucolipidosis type IV
- DCN
deep cerebellar nuclei
- VN
vestibular nuclei
- NPC
Niemann-Pick type C
References
- 1.Walkley SU. Lysosomal Disorders of the Nervous System. In: Gilman S, editor. Neurobiology of Disease. Academic Press; New York: 2006. pp. 1–18. [Google Scholar]
- 2.Hers HG. Progress in Gastroenterology: Inborn lysosomal diseases. Gastroenterology. 1965;48:625–633. [PubMed] [Google Scholar]
- 3.Neufeld E. Lysosomal storage diseases. Ann Rev Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- 4.Walkley SU. Lysosomal diseases – Why so complex? J Inherit Met Dis. 2009;32:181–189. doi: 10.1007/s10545-008-1040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walkley SU. Pathogenic Cascades and Brain Dysfunction. In: Platt F, Walkley SU, editors. Lysosomal Disorders of the Brain. Oxford University Press; Oxford: 2004. pp. 290–324. [Google Scholar]
- 6.deVeber GA, Schwarting GA, Kolodny EH, Kowall NW. Fabry disease: immunocytochemical characterization of neuronal involvement. Ann Neurol. 1992;31:409–415. doi: 10.1002/ana.410310410. [DOI] [PubMed] [Google Scholar]
- 7.Walkley SU, Zervas M, Wiseman S. Gangliosides as modulators of dendritogenesis in storage disease-affected and normal pyramidal neurons. Cerebral Cortex. 2000;10:1028–1037. doi: 10.1093/cercor/10.10.1028. [DOI] [PubMed] [Google Scholar]
- 8.Walkley SU, Baker HJ, Rattazzi MC, Haskins ME, Wu JY. Neuroaxonal dystrophy in neuronal storage disorders: Evidence for major GABAergic neuron involvement. Journal of the Neurological Sciences. 1991;104:1–8. doi: 10.1016/0022-510x(91)90208-o. [DOI] [PubMed] [Google Scholar]
- 9.Siegel DA, Walkley SU. Growth of ectopic dendrites on cortical pyramidal neurons in neuronal storage diseases correlates with abnormal accumulation of GM2 ganglioside. J Neurochemistry. 1994;62:1852–1862. doi: 10.1046/j.1471-4159.1994.62051852.x. [DOI] [PubMed] [Google Scholar]
- 10.Stokin GB, Lillo C, Falzone TL, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 11.Walkley SU, Pierok AP. Ferric ion-ferrocyanide staining in ganglioside storage disease establishes that meganeurites are of axon hillock origin and distinct from axonal spheroids. Brain Research. 1986;382:379–386. doi: 10.1016/0006-8993(86)91348-x. [DOI] [PubMed] [Google Scholar]
- 12.Walkley SU. Cellular pathology of lysosomal storage diseases. Brain Pathol. 1998;8:175–193. doi: 10.1111/j.1750-3639.1998.tb00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walkley SU. Pathobiology of neuronal storage disease. International Review of Neurobiology. 1988;9:191–244. doi: 10.1016/s0074-7742(08)60087-2. [DOI] [PubMed] [Google Scholar]
- 14.Micsenyi MC, Dobrenis K, Stephney G, Pickel J, Vanier MT, Slaugenhaupt SA, Walkley SU. Neuropathology of the Mcoln1−/− knockout mouse model of Mucolipidosis IV. J Neuropath Exp Neurol. 2009;68:125–135. doi: 10.1097/NEN.0b013e3181942cf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.March PA, Thrall MA, Wurzelmann S, Brown D, Walkley SU. Dendritic and axonal abnormalities in feline Niemann-Pick disease type C. Acta Neuropathol. 1997;94:164–172. doi: 10.1007/s004010050689. [DOI] [PubMed] [Google Scholar]
- 16.Zervas M, Dobrenis K, Walkley SU. Neurons lacking functional NPC1 accumulate gangliosides and cholesterol and undergo dendritic and axonal alterations. J Neuropathol Experimental Neurol. 2001;60:49–64. doi: 10.1093/jnen/60.1.49. [DOI] [PubMed] [Google Scholar]
- 17.Elleder M, Jirasek A, Smid F, Ledvinova J, Besley GT. Niemann-Pick disease type C. Study on the nature of the cerebral storage process. Acta Neuropathol. 1985;66:325–336. doi: 10.1007/BF00690966. [DOI] [PubMed] [Google Scholar]
- 18.Sleat DE, Wiseman JA, El-Banna M, Price SM, Verot L, Shen MM, Tint GS, Vanier MT, Walkley SU, Lobel P. Genetic evidence for non-redundant functional cooperativity between NPC1 and NPC2 in lipid transport. PNAS. 2004;101:5886–5891. doi: 10.1073/pnas.0308456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukita S, Ishikawa H. The movement of membranous organelles in axons: Electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol. 1980;84:513–530. doi: 10.1083/jcb.84.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson MC, Vanier MT, Suzuki K, et al. Niemann-Pick disease Type C: A lipid trafficking disorder. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic & Molecular Bases of Inherited Disease. 8. Vol. 3. McGraw-Hill; New York: 2001. pp. 3611–3633. [Google Scholar]
- 21.Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling molecules. Current Opinion in Neurobiol. 2008;18:270–275. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibáñez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends in Cell Biol. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Cui B, He L, Chen L, Mobley WC. The coming of age of axonal neurotrophin signaling endosomes. J Proteomics. 2009;72:46–55. doi: 10.1016/j.jprot.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bronfman FC, Escudero CA, Weis J, Kruttgen A. Endosomal transport of neurotrophins: roles in signaling and neurodegenerative disease. Develop Neurobiol. 2007;67:1183–1203. doi: 10.1002/dneu.20513. [DOI] [PubMed] [Google Scholar]
- 25.Chevalier-Larsen E, Holzbaur ELF. Axonal transport and neurodegenerative disease. Biochem Biophys Acta. 2006;1762:1094–1108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Roy S, Zhang B, Lee V, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 2005;109:5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- 27.Duncan JE, Goldstein LSB. The genetics of axonal transport and axonal transport disorders. PLoS Genetics. 2006;2:1274–1284. doi: 10.1371/journal.pgen.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dusart I, Sotelo C. Lack of Purkinje cell loss in adult rat cerebellum following protracted axotomy: degenerative changes and regenerative attempts of the severed axons. J Comp Neurol. 1994;347:211–232. doi: 10.1002/cne.903470206. [DOI] [PubMed] [Google Scholar]
- 29.Dusart I, Ghoumari A, Wehrle R, Morel MP, Bouslama-Oueghlani L, Camand E, Sotelo C. Cell death and axon regeneration of Purkinje cells after axotomy: Challenges of classical hypotheses of axon regeneration. Brain Res Reviews. 2005;49:300–316. doi: 10.1016/j.brainresrev.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Rossi F, Gianola S, Corvetti L. The strange case of Purkinje axon regeneration and plasticity. The Cerebellum. 2006;5:174–182. doi: 10.1080/14734220600786444. [DOI] [PubMed] [Google Scholar]
- 31.Sarna JR, Larouche M, Marzban H, Sillitoe RV, Rancourt DE, Hawkes R. Patterned Purkinje cell degeneration in mouse models of Niemann-Pick type C disease. J Comp Neurol. 2003;456:279–291. doi: 10.1002/cne.10522. [DOI] [PubMed] [Google Scholar]
- 32.Higashi Y, Murayama S, Pentchev P, Suzuki K. Cerebellar degeneration in the Niemann-Pick type C mouse. Acta Neuropathol. 1993;85:175–184. doi: 10.1007/BF00227765. [DOI] [PubMed] [Google Scholar]
- 33.Landis S, Mullen RJ. The development and degeneration of Purkinje cells in pcd mutant mice. J Comp Neurol. 1978;177:125–144. doi: 10.1002/cne.901770109. [DOI] [PubMed] [Google Scholar]
- 34.Wang T, Morgan JI. The Purkinje cell degeneration (pcd) mouse: an unexpected molecular link between neuronal degeneration and regeneration. Brain Res. 2007;1140:26–40. doi: 10.1016/j.brainres.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 35.Berezniuk I, Sironi J, Callaway MB, Castro LM, Hirata IZ, Ferro ES, Fricker LD. CCP1/Nna1 functions in protein turnover in mouse brain: Implications for cell death in Purkinje cell degeneration mice. FASEB. 2010;24:1–11. doi: 10.1096/fj.09-147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacheco CD, Lieberman AP. The pathogenesis of Neimann-Pick C disease: a role for autophagy? Expert Rev Mol Med. 2009;10:e26. doi: 10.1017/S146239940800080X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C, Rubinsztein DC, Ballabio A. A block of autophagy in lysosomal storage disorders. Human Molecular Genetics. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- 38.Tai H-C, Shuman EM. Ubiquitin, the proteasome, and protein degradation in neuronal function and dysfunction. Nature Neuroscience Review. 2008;9:826–838. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- 39.Liu JJ, Ding J, Wu C, Bhagavatula P, Cui B, Chu S, Mobley WC, Yang Y. Retrolinkin, a membrane protein, plays an important role in retrograde axonal transport. PNAS. 2007;140:2223–2228. doi: 10.1073/pnas.0602222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu JJ, Ding J, Kowal AS, Nardine T, Allen E, Delcroix JD, Wu C, Mobley W, Fuchs E, Yang Y. BPAG1n4 is essential for retrograde axonal transport in sensory neurons. The Journal of Cell Biology. 2003;163:223–229. doi: 10.1083/jcb.200306075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komatsu M, Wang QJ, Holstein GR, et al. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal regeneration. PNAS. 2007;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ko DC, Milenkovic L, Beier SM, Manuel H, Buchanan J, Scott MP. Cell-autonomous death of cerebellar purkinje neurons with autophagy in Niemann-Pick type C disease. PLoS Genetics. 2005;1:81–95. doi: 10.1371/journal.pgen.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elrick MJ, Pacheco CD, Yu T, et al. Conditional Niemann-Pick C mice demonstrate cell autonomous Purkinje cell neurodegeneration. Human Molec Gen. 2010;19:837–847. doi: 10.1093/hmg/ddp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walkley SU, Wurzelmann S, Siegel DA. Ectopic axon hillock-associated neurite growth is maintained in metabolically-reversed swainsonine-induced neuronal storage disease. Brain Res. 1987;410:89–96. doi: 10.1016/s0006-8993(87)80025-2. [DOI] [PubMed] [Google Scholar]
- 45.Zervas M, Somers KL, Thrall MA, Walkley SU. Critical role for glycosphingolipids in Niemann-Pick disease type C. Current Biology. 2001;11:1283–1287. doi: 10.1016/s0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 46.Davidson CD, Ali NF, Micsenyi MC, Stephney G, Dobrenis K, Ory DS, Vanier MT, Walkley SU. Chronic cyclodextrin administration in Niemann-Pick C disease ameliorates intraneuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE. 2009;4:e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]