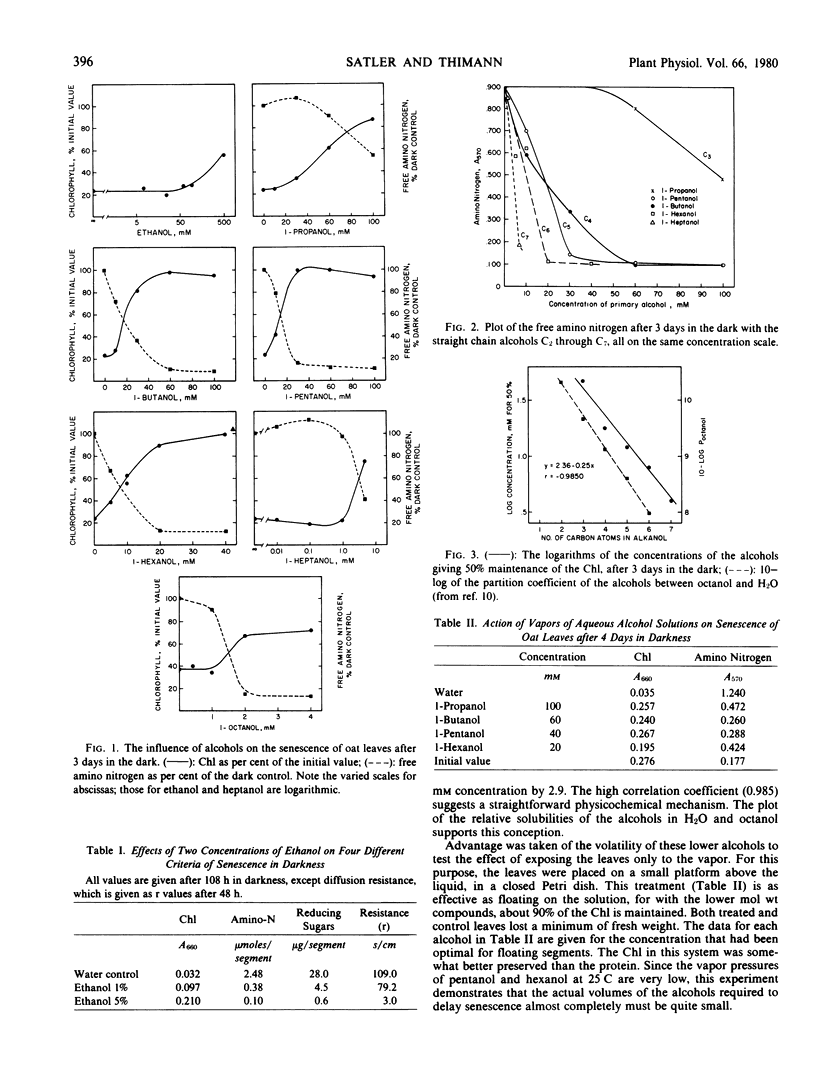
Abstract
Because of the effects of ethanol used as a solvent in other experiments, the action of aliphatic alcohols on leaf senescence in the dark has been studied systematically. These compounds both maintain chlorophyll and prevent proteolysis in the dark, much as do the cytokinins and other senescence-delaying substances. The activity of the straight-chain alcohols increases in a log-linear fashion with increasing chain length up to 1-octanol. Introduction of a branch in the chain or of a second OH group greatly decreases, or in some cases annuls, the antisenescence activity. In all cases, the action on senescence is closely (although not always exactly) paralleled by opening of the stomata. Abscisic acid and exposure to high concentrations of osmoticum, both of which close the stomata, antagonize the action of the alcohols. Some interactions with other agents are noted. The effects are compared with reported effects on seed germination, on hemolysis and animal membranes, and especially on permeability to K+ ions, and a tentative basis for the mechanism of action is advanced.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Glinka Z. Induced changes in permeability of plant cell membranes to water. Plant Physiol. 1972 Apr;49(4):602–606. doi: 10.1104/pp.49.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The effects of long-chain alcohols on membrane lipids and the (Na++K+)-ATPase. Biochim Biophys Acta. 1973 Jul 6;311(3):417–422. doi: 10.1016/0005-2736(73)90322-2. [DOI] [PubMed] [Google Scholar]
- Hansch C., Glave W. R. Structure-activity relationships in membrane-perturbing agents. Hemolytic, narcotic, and antibacterial compounds. Mol Pharmacol. 1971 May;7(3):337–354. [PubMed] [Google Scholar]
- Hegyvary C. Effects of some organic solvents on the reactivity of sodium plus potassium ion-transport ATPase. Biochim Biophys Acta. 1973 Jun 22;311(2):272–291. doi: 10.1016/0005-2736(73)90274-5. [DOI] [PubMed] [Google Scholar]
- Kanemasu E. T., Thurtell G. W., Tanner C. B. Design calibration and field use of a stomatal diffusion porometer. Plant Physiol. 1969 Jun;44(6):881–885. doi: 10.1104/pp.44.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M., Kasai M. The effects of n-alcohols on sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1973 Jul 6;311(3):391–399. doi: 10.1016/0005-2736(73)90319-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenaz G., Parenti-Castelli G., Monsigni N., Silvestrini M. G. Effect of alcohols on the functional organization of the inner mitochondrial membrane. J Bioenerg. 1971 May;2(2):119–127. doi: 10.1007/BF01648927. [DOI] [PubMed] [Google Scholar]
- Malik N. S., Thimann K. V. Metabolism of Oat Leaves during Senescence: VI. CHANGES IN ATP LEVELS. Plant Physiol. 1980 May;65(5):855–859. doi: 10.1104/pp.65.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P. S. Mitochondrial permeability for alcohols, aldoses, and amino acids. J Membr Biol. 1973;12(3):287–299. doi: 10.1007/BF01870006. [DOI] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Deferral of leaf senescence with calcium. Plant Physiol. 1973 Sep;52(3):236–239. doi: 10.1104/pp.52.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. M., Halpern L. A. THE EFFECT OF BRANCHING AT C-1 ON THE BIOLOGICAL ACTIVITY OF ALCOHOLS. Proc Natl Acad Sci U S A. 1964 May;51(5):765–768. doi: 10.1073/pnas.51.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley R. M., Thimann K. V. The Metabolism of Oat Leaves during Senescence: I. Respiration, Carbohydrate Metabolism, and the Action of Cytokinins. Plant Physiol. 1974 Sep;54(3):294–303. doi: 10.1104/pp.54.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Satler S. O. Relation between leaf senescence and stomatal closure: Senescence in light. Proc Natl Acad Sci U S A. 1979 May;76(5):2295–2298. doi: 10.1073/pnas.76.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Satler S. Relation between senescence and stomatal opening: Senescence in darkness. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2770–2773. doi: 10.1073/pnas.76.6.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Tetley R. M., Krivak B. M. Metabolism of Oat Leaves during Senescence: V. Senescence in Light. Plant Physiol. 1977 Mar;59(3):448–454. doi: 10.1104/pp.59.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore A., Baltscheffsky H. Inhibitory effects of lower aliphatic alcohols on electron transport phosphorylation systems. 2. Secondary, tertiary, and di-alcohols. Acta Chem Scand. 1965;19(7):1600–1606. doi: 10.3891/acta.chem.scand.19-1600. [DOI] [PubMed] [Google Scholar]
- Thore A., Baltscheffsky H. Inhibitory effects of lower aliphatic alcohols on electron transport phosphorylation systems. I. Straight-chain, primary alcohols. Acta Chem Scand. 1965;19(7):1591–1599. doi: 10.3891/acta.chem.scand.19-1591. [DOI] [PubMed] [Google Scholar]


