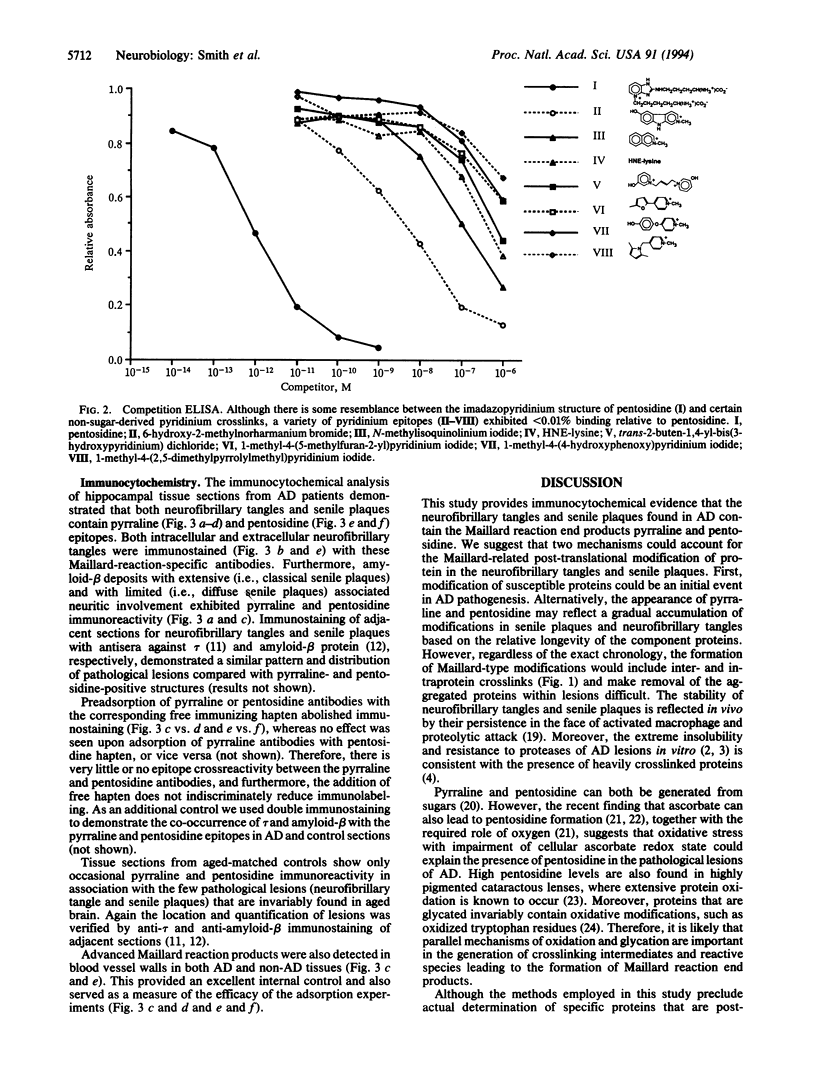
Abstract
During aging long-lived proteins accumulate specific post-translational modifications. One family of modifications, termed Maillard reaction products, are initiated by the condensation between amino groups of proteins and reducing sugars. Protein modification by the Maillard reaction is associated with crosslink formation, decreased protein solubility, and increased protease resistance. Here, we present evidence that the characteristic pathological structures associated with Alzheimer disease contain modifications typical of advanced Maillard reaction end products. Specifically, antibodies against two Maillard end products, pyrraline and pentosidine, immunocytochemically label neurofibrillary tangles and senile plaques in brain tissue from patients with Alzheimer disease. In contrast, little or no staining is observed in apparently healthy neurons of the same brain. The Maillard-reaction-related modifications described herein could account for the biochemical and insolubility properties of the lesions of Alzheimer disease through the formation of protein crosslinks.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee M., Vlassara H., Kooney A., Ulrich P., Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986 Jun 27;232(4758):1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Dyer D. G., Blackledge J. A., Thorpe S. R., Baynes J. W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991 Jun 25;266(18):11654–11660. [PubMed] [Google Scholar]
- Dyrks T., Dyrks E., Hartmann T., Masters C., Beyreuther K. Amyloidogenicity of beta A4 and beta A4-bearing amyloid protein precursor fragments by metal-catalyzed oxidation. J Biol Chem. 1992 Sep 5;267(25):18210–18217. [PubMed] [Google Scholar]
- Fisher G. H., Payan I. L., Chou S. J., Man E. H., Cerwinski S., Martin T., Emory C., Frey W. H., 2nd Racemized D-aspartate in Alzheimer neurofibrillary tangles. Brain Res Bull. 1992 Jan;28(1):127–131. doi: 10.1016/0361-9230(92)90239-t. [DOI] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Crowther R. A., Six J., Lübke U., Vandermeeren M., Cras P., Trojanowski J. Q., Lee V. M. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Sisodia S. S., Price D. L. Neurofibrillary tangles and beta-amyloid deposits in Alzheimer's disease. Curr Opin Neurobiol. 1991 Oct;1(3):441–447. doi: 10.1016/0959-4388(91)90067-h. [DOI] [PubMed] [Google Scholar]
- Grandhee S. K., Monnier V. M. Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J Biol Chem. 1991 Jun 25;266(18):11649–11653. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustke N., Steiner B., Mandelkow E. M., Biernat J., Meyer H. E., Goedert M., Mandelkow E. The Alzheimer-like phosphorylation of tau protein reduces microtubule binding and involves Ser-Pro and Thr-Pro motifs. FEBS Lett. 1992 Jul 28;307(2):199–205. doi: 10.1016/0014-5793(92)80767-b. [DOI] [PubMed] [Google Scholar]
- Hunt J. V., Wolff S. P. Oxidative glycation and free radical production: a causal mechanism of diabetic complications. Free Radic Res Commun. 1991;12-13 Pt 1:115–123. doi: 10.3109/10715769109145775. [DOI] [PubMed] [Google Scholar]
- Jürgens G., Lang J., Esterbauer H. Modification of human low-density lipoprotein by the lipid peroxidation product 4-hydroxynonenal. Biochim Biophys Acta. 1986 Jan 3;875(1):103–114. doi: 10.1016/0005-2760(86)90016-0. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., Beal M. F., Busciglio J., Duffy L. K., Yankner B. A. An in vivo model for the neurodegenerative effects of beta amyloid and protection by substance P. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Monnier V. Immunohistochemical detection of advanced glycosylation end products in diabetic tissues using monoclonal antibody to pyrraline. J Clin Invest. 1992 Apr;89(4):1102–1112. doi: 10.1172/JCI115690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T., Oda O., Inagi R., Iida Y., Araki N., Yamada N., Horiuchi S., Taniguchi N., Maeda K., Kinoshita T. beta 2-Microglobulin modified with advanced glycation end products is a major component of hemodialysis-associated amyloidosis. J Clin Invest. 1993 Sep;92(3):1243–1252. doi: 10.1172/JCI116696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Nagaraj R. H., Sell D. R., Prabhakaram M., Ortwerth B. J., Monnier V. M. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoroge F. G., Sayre L. M., Monnier V. M. Detection of D-glucose-derived pyrrole compounds during Maillard reaction under physiological conditions. Carbohydr Res. 1987 Sep 15;167:211–220. doi: 10.1016/0008-6215(87)80280-x. [DOI] [PubMed] [Google Scholar]
- Payan I. L., Chou S. J., Fisher G. H., Man E. H., Emory C., Frey W. H., 2nd Altered aspartate in Alzheimer neurofibrillary tangles. Neurochem Res. 1992 Feb;17(2):187–191. doi: 10.1007/BF00966798. [DOI] [PubMed] [Google Scholar]
- Perry G., Cras P., Siedlak S. L., Tabaton M., Kawai M. Beta protein immunoreactivity is found in the majority of neurofibrillary tangles of Alzheimer's disease. Am J Pathol. 1992 Feb;140(2):283–290. [PMC free article] [PubMed] [Google Scholar]
- Perry G., Cras P., Siedlak S. L., Tabaton M., Kawai M. Beta protein immunoreactivity is found in the majority of neurofibrillary tangles of Alzheimer's disease. Am J Pathol. 1992 Feb;140(2):283–290. [PMC free article] [PubMed] [Google Scholar]
- Perry G., Kawai M., Tabaton M., Onorato M., Mulvihill P., Richey P., Morandi A., Connolly J. A., Gambetti P. Neuropil threads of Alzheimer's disease show a marked alteration of the normal cytoskeleton. J Neurosci. 1991 Jun;11(6):1748–1755. doi: 10.1523/JNEUROSCI.11-06-01748.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser K., McCormick R. J., Rucker R. B. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J. 1992 Apr;6(7):2439–2449. doi: 10.1096/fasebj.6.7.1348714. [DOI] [PubMed] [Google Scholar]
- Sayre L. M., Arora P. K., Iyer R. S., Salomon R. G. Pyrrole formation from 4-hydroxynonenal and primary amines. Chem Res Toxicol. 1993 Jan-Feb;6(1):19–22. doi: 10.1021/tx00031a002. [DOI] [PubMed] [Google Scholar]
- Sayre L. M., Singh M. P., Arora P. K., Wang F., McPeak R. J., Hoppel C. L. Inhibition of mitochondrial respiration by analogues of the dopaminergic neurotoxin 1-methyl-4-phenylpyridinium: structural requirements for accumulation-dependent enhanced inhibitory potency on intact mitochondria. Arch Biochem Biophys. 1990 Aug 1;280(2):274–283. doi: 10.1016/0003-9861(90)90330-2. [DOI] [PubMed] [Google Scholar]
- Sayre L. M., Wang F. J., Arora P. K., Riachi N. J., Harik S. I., Hoppel C. L. Dopaminergic neurotoxicity in vivo and inhibition of mitochondrial respiration in vitro by possible endogenous pyridinium-like substances. J Neurochem. 1991 Dec;57(6):2106–2115. doi: 10.1111/j.1471-4159.1991.tb06429.x. [DOI] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Effects of age and diabetes mellitus on the solubility of collagen from human skin, tracheal cartilage and dura mater. Exp Gerontol. 1982;17(3):185–194. doi: 10.1016/0531-5565(82)90024-9. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. End-stage renal disease and diabetes catalyze the formation of a pentose-derived crosslink from aging human collagen. J Clin Invest. 1990 Feb;85(2):380–384. doi: 10.1172/JCI114449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Kalaria R. N., Perry G. Alpha 1-trypsin immunoreactivity in Alzheimer disease. Biochem Biophys Res Commun. 1993 Jun 15;193(2):579–584. doi: 10.1006/bbrc.1993.1663. [DOI] [PubMed] [Google Scholar]
- Snowden J. M. The effects of polymers on the shrinkage temperature of tendon. Biochim Biophys Acta. 1982 Sep 22;707(1):142–146. doi: 10.1016/0167-4838(82)90407-1. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Weisgraber K. H., Huang D. Y., Dong L. M., Salvesen G. S., Pericak-Vance M., Schmechel D., Saunders A. M., Goldgaber D., Roses A. D. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Imoto T., Fujita K., Okazaki K., Motomura M. Selective modification of aspartic acid-101 in lysozyme by carbodiimide reaction. Biochemistry. 1981 Aug 18;20(17):4836–4842. doi: 10.1021/bi00520a005. [DOI] [PubMed] [Google Scholar]




