Abstract
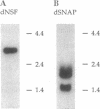
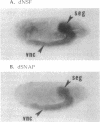
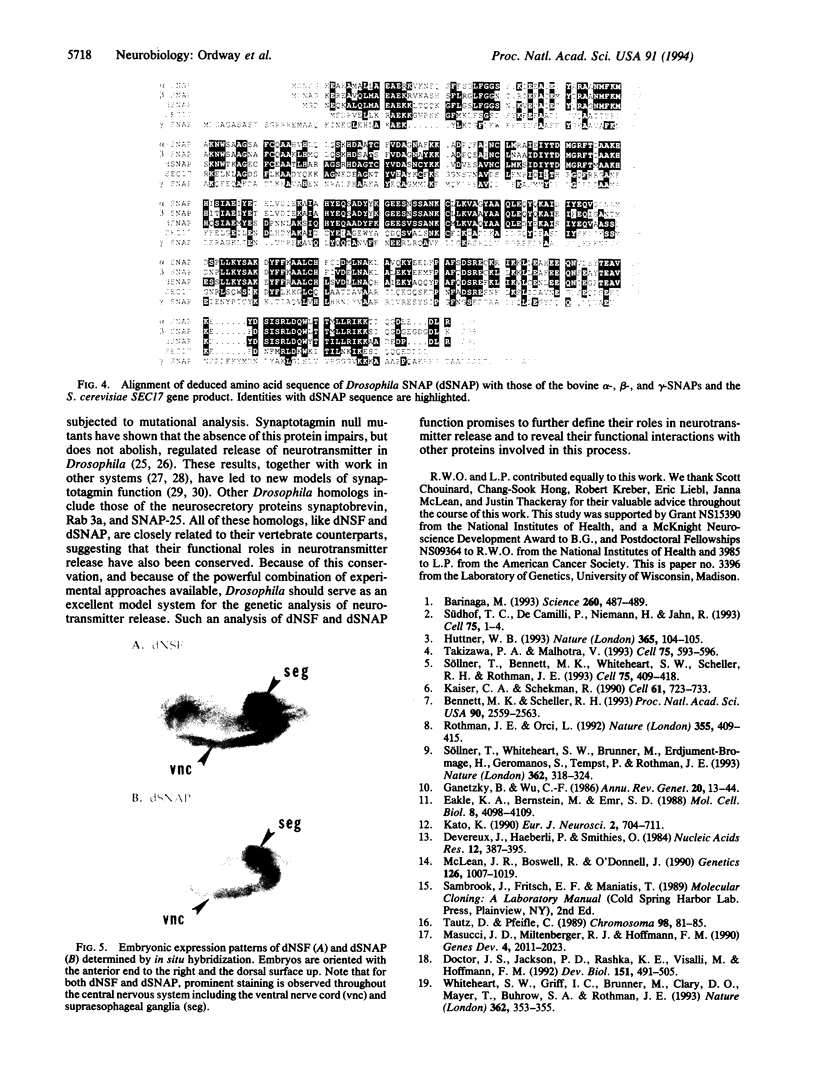
Several lines of investigation have now converged to indicate that the neurotransmitter release apparatus is formed by assembly of cytosolic proteins with proteins of the synaptic vesicle and presynaptic terminal membranes. We are undertaking a genetic approach in Drosophila melanogaster to investigate the functions of two types of cytosolic proteins thought to function in this complex: N-ethylmaleimide-sensitive fusion protein (NSF) and the soluble NSF attachment proteins (SNAPs). We have identified Drosophila homologs of the vertebrate and yeast NSF and SNAP genes. Both Drosophila genes encode polypeptides that closely resemble their vertebrate counterparts and are expressed in the nervous system; neither appears to be in a family of closely related Drosophila genes. These results indicate that the Drosophila NSF and SNAP genes are excellent candidates for mutational analysis of neurotransmitter release.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barinaga M. Secrets of secretion revealed. Science. 1993 Apr 23;260(5107):487–489. doi: 10.1126/science.8475382. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBello W. M., Betz H., Augustine G. J. Synaptotagmin and neurotransmitter release. Cell. 1993 Sep 24;74(6):947–950. doi: 10.1016/0092-8674(93)90716-4. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A., Burgess R. W., Chin A. C., Deitcher D. L., Scheller R. H., Schwarz T. L. Identification and characterization of Drosophila genes for synaptic vesicle proteins. J Neurosci. 1993 Nov;13(11):4924–4935. doi: 10.1523/JNEUROSCI.13-11-04924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A., Parfitt K. D., Schwarz T. L. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993 Jul 2;73(7):1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- Doctor J. S., Jackson P. D., Rashka K. E., Visalli M., Hoffmann F. M. Sequence, biochemical characterization, and developmental expression of a new member of the TGF-beta superfamily in Drosophila melanogaster. Dev Biol. 1992 Jun;151(2):491–505. doi: 10.1016/0012-1606(92)90188-m. [DOI] [PubMed] [Google Scholar]
- Eakle K. A., Bernstein M., Emr S. D. Characterization of a component of the yeast secretion machinery: identification of the SEC18 gene product. Mol Cell Biol. 1988 Oct;8(10):4098–4109. doi: 10.1128/mcb.8.10.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B., Wu C. F. Neurogenetics of membrane excitability in Drosophila. Annu Rev Genet. 1986;20:13–44. doi: 10.1146/annurev.ge.20.120186.000305. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Cell biology. Snappy exocytoxins. Nature. 1993 Sep 9;365(6442):104–105. doi: 10.1038/365104a0. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Archer B. T., 3rd, Robinson K., Mignery G. A., Jahn R., Südhof T. C. rab3A attachment to the synaptic vesicle membrane mediated by a conserved polyisoprenylated carboxy-terminal sequence. Neuron. 1991 Jul;7(1):101–109. doi: 10.1016/0896-6273(91)90078-e. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990 May 18;61(4):723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Kato Kikuya. A Collection of cDNA Clones with Specific Expression Patterns in Mouse Brain. Eur J Neurosci. 1990;2(8):704–711. doi: 10.1111/j.1460-9568.1990.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Littleton J. T., Stern M., Schulze K., Perin M., Bellen H. J. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993 Sep 24;74(6):1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- Masucci J. D., Miltenberger R. J., Hoffmann F. M. Pattern-specific expression of the Drosophila decapentaplegic gene in imaginal disks is regulated by 3' cis-regulatory elements. Genes Dev. 1990 Nov;4(11):2011–2023. doi: 10.1101/gad.4.11.2011. [DOI] [PubMed] [Google Scholar]
- McLean J. R., Boswell R., O'Donnell J. Cloning and molecular characterization of a metabolic gene with development functions in Drosophila. I. Analysis of the head function of Punch. Genetics. 1990 Dec;126(4):1007–1019. doi: 10.1093/genetics/126.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M. L., Grundahl K., Meyer B. J., Rand J. B. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993 Jul 2;73(7):1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Johnston P. A., Ozcelik T., Jahn R., Francke U., Südhof T. C. Structural and functional conservation of synaptotagmin (p65) in Drosophila and humans. J Biol Chem. 1991 Jan 5;266(1):615–622. [PubMed] [Google Scholar]
- Popov S. V., Poo M. M. Synaptotagmin: a calcium-sensitive inhibitor of exocytosis? Cell. 1993 Jul 2;73(7):1247–1249. doi: 10.1016/0092-8674(93)90352-q. [DOI] [PubMed] [Google Scholar]
- Risinger C., Blomqvist A. G., Lundell I., Lambertsson A., Nässel D., Pieribone V. A., Brodin L., Larhammar D. Evolutionary conservation of synaptosome-associated protein 25 kDa (SNAP-25) shown by Drosophila and Torpedo cDNA clones. J Biol Chem. 1993 Nov 15;268(32):24408–24414. [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993 Nov 5;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Baumert M., Perin M. S., Jahn R. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. Neuron. 1989 May;2(5):1475–1481. doi: 10.1016/0896-6273(89)90193-1. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., De Camilli P., Niemann H., Jahn R. Membrane fusion machinery: insights from synaptic proteins. Cell. 1993 Oct 8;75(1):1–4. [PubMed] [Google Scholar]
- Takizawa P. A., Malhotra V. Coatomers and SNAREs in promoting membrane traffic. Cell. 1993 Nov 19;75(4):593–596. doi: 10.1016/0092-8674(93)90477-8. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Whiteheart S. W., Griff I. C., Brunner M., Clary D. O., Mayer T., Buhrow S. A., Rothman J. E. SNAP family of NSF attachment proteins includes a brain-specific isoform. Nature. 1993 Mar 25;362(6418):353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]