Abstract
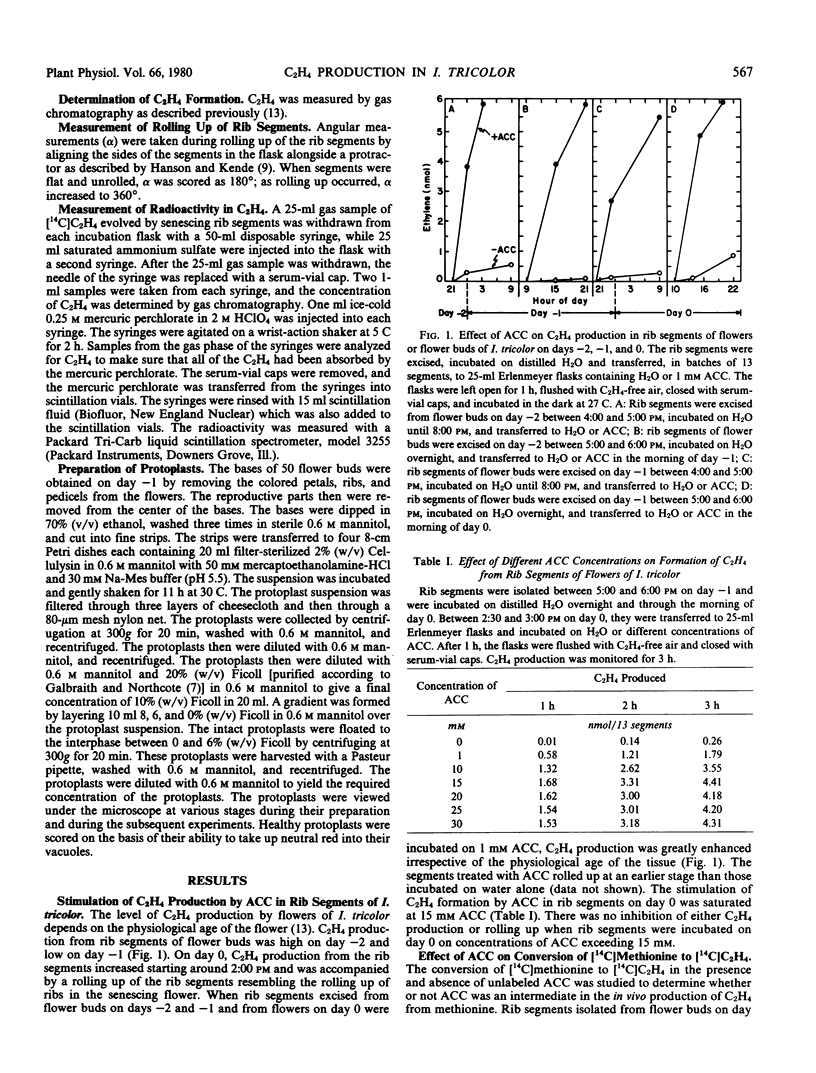
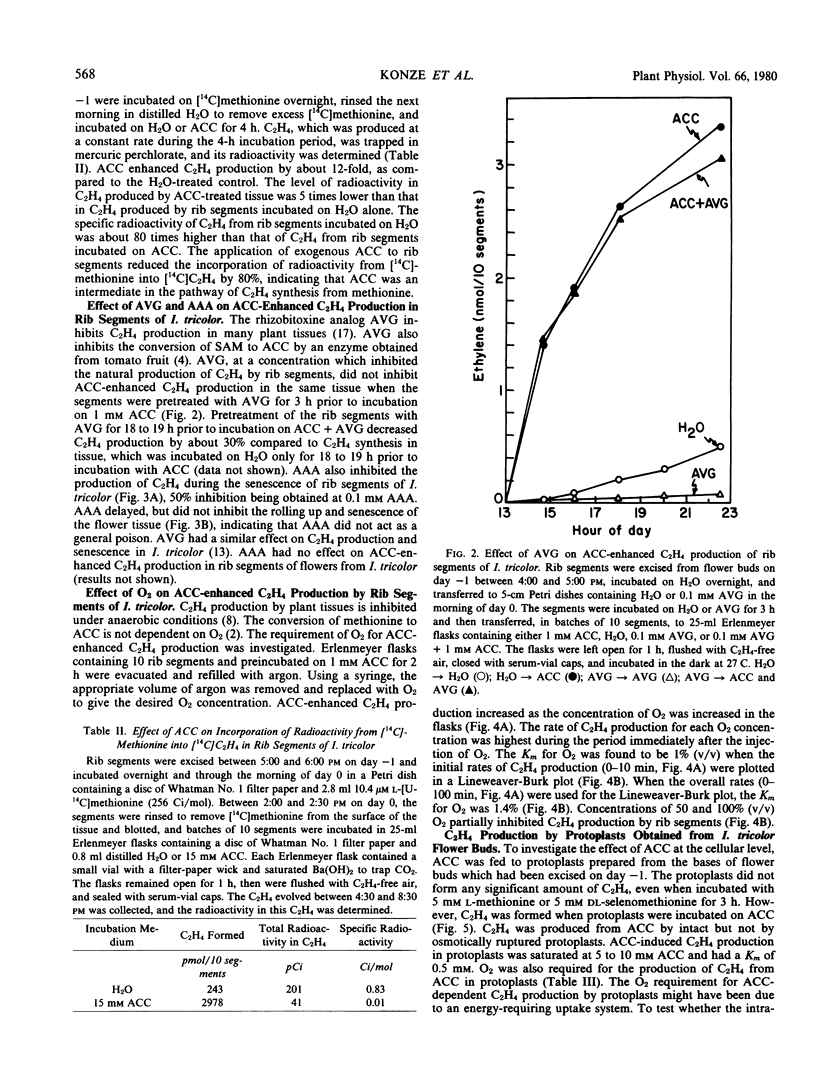
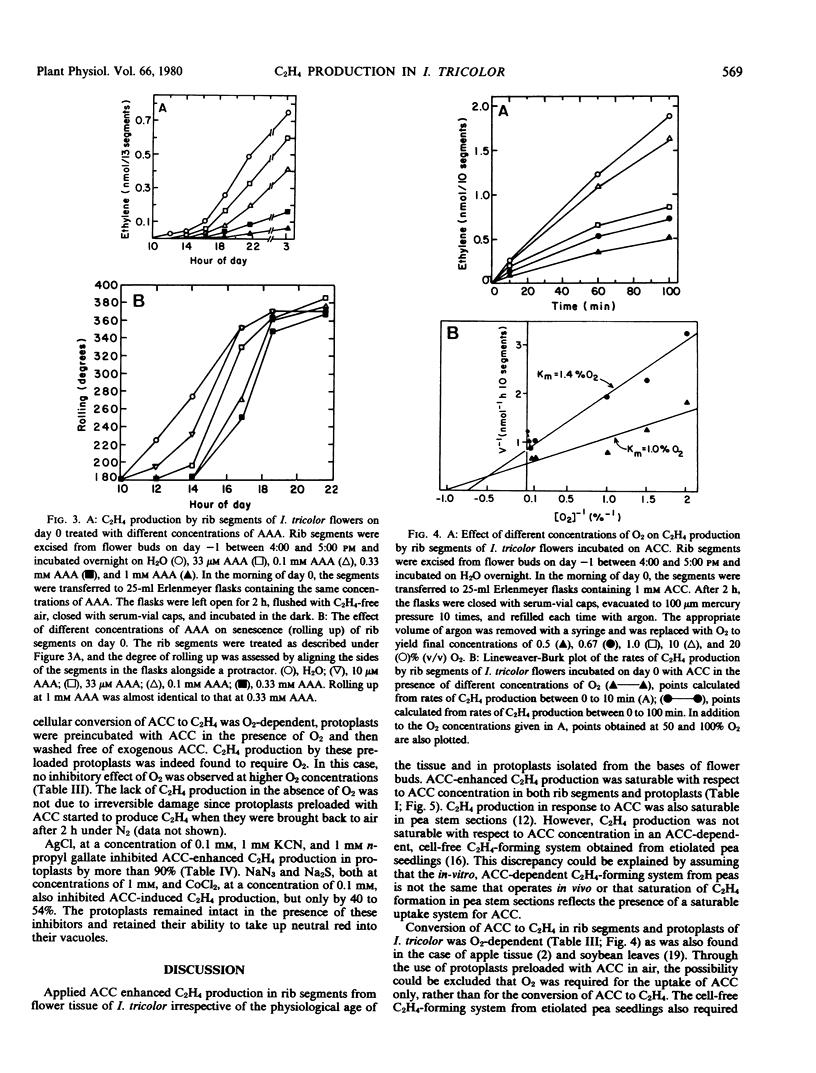
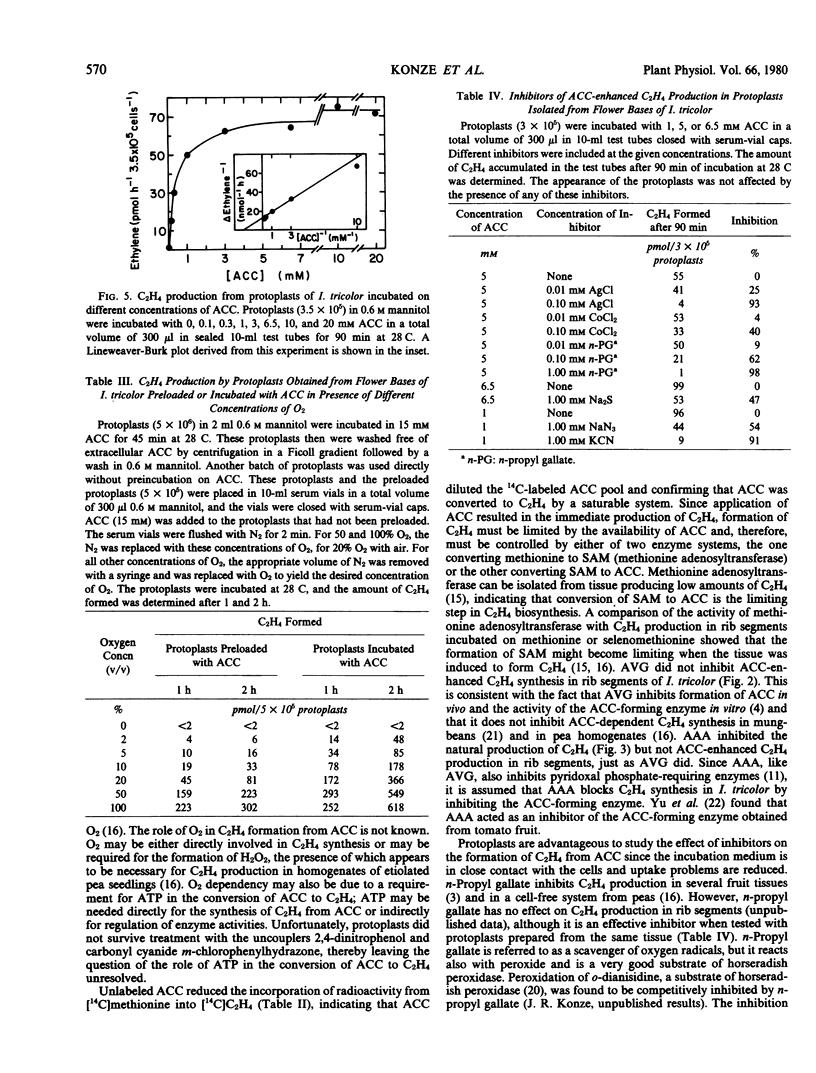
Application of 1-aminocyclopropane-1-carboxylic acid (ACC) to rib segments excised from flowers of Ipomoea tricolor Cav. resulted in the formation of C2H4 in greater quantities than produced under natural conditions. The ability of ACC to enhance C2H4 production was independent of the physiological age of the tissue and its capacity to synthesize C2H4 without applied ACC. When ACC was fed to rib segments that had been treated with [14C]methionine, incorporation of radioactivity into C2H4 was reduced by 80%. Aminoethoxyvinylglycine and aminooxyacetic acid inhibited C2H4 production in rib segments of I. tricolor but had no effect on ACC-enhanced C2H4 production. Protoplasts obtained from flower tissue of I. tricolor did not form C2H4, even when incubated with methionine or selenomethionine. They produced C2H4 upon incubation with ACC, however. ACC-dependent C2H4 production in protoplasts was inhibited by n-propyl gallate, AgCl, CoCl2, KCN, Na2S, and NaN3. ACC-dependent C2H4 synthesis in rib segments and protoplasts was dependent on O2, the Km for O2 being 1.0 to 1.4% (v/v). These results confirm the following pathway for C2H4 biosynthesis in I. tricolor. methionine [selenomethionine] → S-adenosylmethionine [selenoadenosylmethionine] → ACC → C2H4.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. O., Yang S. F. Methionine metabolism in apple tissue: implication of s-adenosylmethionine as an intermediate in the conversion of methionine to ethylene. Plant Physiol. 1977 Dec;60(6):892–896. doi: 10.1104/pp.60.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. E., Lieberman M., Anderson J. D. Inhibition of ethylene production in fruit slices by a rhizobitoxine analog and free radical scavengers. Plant Physiol. 1978 Jun;61(6):886–888. doi: 10.1104/pp.61.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P. Ethylene in plant growth. Proc Natl Acad Sci U S A. 1973 Feb;70(2):591–597. doi: 10.1073/pnas.70.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith D. W., Northcote D. H. The isolation of plasma membrane from protoplasts of soybean suspension cultures. J Cell Sci. 1977 Apr;24:295–310. doi: 10.1242/jcs.24.1.295. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Ethylene-enhanced Ion and Sucrose Efflux in Morning Glory Flower Tissue. Plant Physiol. 1975 Apr;55(4):663–669. doi: 10.1104/pp.55.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Methionine metabolism and ethylene biosynthesis in senescent flower tissue of morning-glory. Plant Physiol. 1976 Apr;57(4):528–537. doi: 10.1104/pp.57.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John R. A., Charteris A. The reaction of amino-oxyacetate with pyridoxal phosphate-dependent enzymes. Biochem J. 1978 Jun 1;171(3):771–779. doi: 10.1042/bj1710771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Hanson A. D. Relationship between Ethylene Evolution and Senescence in Morning-Glory Flower Tissue. Plant Physiol. 1976 Apr;57(4):523–527. doi: 10.1104/pp.57.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konze J. R., Kende H. Interactions of Methionine and Selenomethionine with Methionine Adenosyltransferase and Ethylene-generating Systems. Plant Physiol. 1979 Mar;63(3):507–510. doi: 10.1104/pp.63.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konze J. R., Schilling N., Kende H. Enhancement of ethylene formation by selenoamino acids. Plant Physiol. 1978 Sep;62(3):397–401. doi: 10.1104/pp.62.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. Regulation of Auxin-induced Ethylene Production in Mung Bean Hypocotyls: Role of 1-Aminocyclopropane-1-Carboxylic Acid. Plant Physiol. 1979 Mar;63(3):589–590. doi: 10.1104/pp.63.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]


