Abstract
Background
An estimated 4 million Americans have been exposed to the hepatitis C virus (HCV) in the US population. The risk of incident and progressive chronic kidney disease and of mortality in patients with normal kidney function infected with HCV is unclear.
Methods
In a nationally representative cohort of 100,518 HCV+ and 920,531 HCV- US Veterans with normal baseline estimated glomerular filtration rate(eGFR), we examined the association of HCV infection with: (1)all-cause mortality, (2)incidence of decreased kidney function (defined as eGFR <60 ml/min/1.73m2 and 25% decrease in eGFR), (3)ESRD, and (4)rate of kidney function decline. Associations were examined in naïve and adjusted Cox models (for time-to-event analyses) and logistic regression models (for slopes), with sequential adjustments for important confounders. Propensity-matched cohort analysis was used in sensitivity analyses.
Results
The patients’ age was 54.5±13.1(mean±SD) years, 22% were black and 92% male, and the baseline eGFR was 88±16 ml/min/1.73m2. In multivariate adjusted models HCV infection was associated with 2.2 fold higher mortality (fully adjusted hazard ratio(aHR), 95%CI: 2.17(2.13–2.21)), 15% higher incidence of decreased kidney function(aHR, 95%CI: 1.15(1.12–1.17)), 22% higher risk of steeper slopes of eGFR (adjusted odds ratio, 95%CI: 1.22(1.19–1.26)) and 98% higher hazard of ESRD (aHR, 95%CI: 1.98 (1.81–2.16)). Quantitatively similar results were found in propensity-matched cohort analyses.
Conclusions
HCV infection is associated with higher mortality risk, incidence of decreased kidney function and progressive loss of kidney function. Randomized controlled trials are warranted to determine whether treatment of HCV infection can prevent the development and progression of CKD and improve patient outcomes.
Keywords: Chronic Kidney Disease, End Stage Renal Disease, Hepatitis C, Kidney Function and Mortality
Introduction
Chronic hepatitis C virus (HCV) infection, which affects 130–150 million people worldwide, is one of the leading causes of liver cirrhosis and hepatocellular cancer, as well as a leading indication for liver transplantation in developed countries.(1) In addition, several extra-hepatic complications, such as dermatologic, rheumatologic and hematologic disorders, are also associated with chronic HCV.(2) Renal complications, such as albuminuria,(3–5) cryoglobulinemia-induced membranoproliferative glomerulonephritis and other glomerulonephritides,(6) are also well documented in patients with chronic HCV. However, it is not clear whether and to what extent chronic HCV infection affects the development and progression of chronic kidney disease (CKD) at a population level.
Several studies of large databases have recently addressed the association between HCV and kidney disease, with conflicting results,(3, 7–15) and a recent meta-analysis concluded that HCV was not significantly associated with the incidence of reduced estimated glomerular filtration rate (eGFR), but was positively associated with the presence of albuminuria and proteinuria in the general population.(4, 5) However, several of the previous studies had limitations such as modest sample size, low event rates, selection bias and lack of proper end point definition (e.g. the use of a single eGFR <60 ml/min/1.73 m2 to define incident CKD).(7, 8) Furthermore, it remains unclear if HCV infection affects the rate of kidney function deterioration in patients with established CKD, and therefore the incidence of end stage renal disease (ESRD).
We examined the association of HCV infection with the development of decreased kidney function and with progressive loss of kidney function, along with the risk of all-cause mortality in a large, nationally representative contemporary cohort of US veterans. We hypothesized that the presence of HCV infection is associated with higher risk of incidence of low eGFR, with faster renal function deterioration, with higher a risk of developing ESRD, and with higher risk of death.
Methods
Cohort Definition
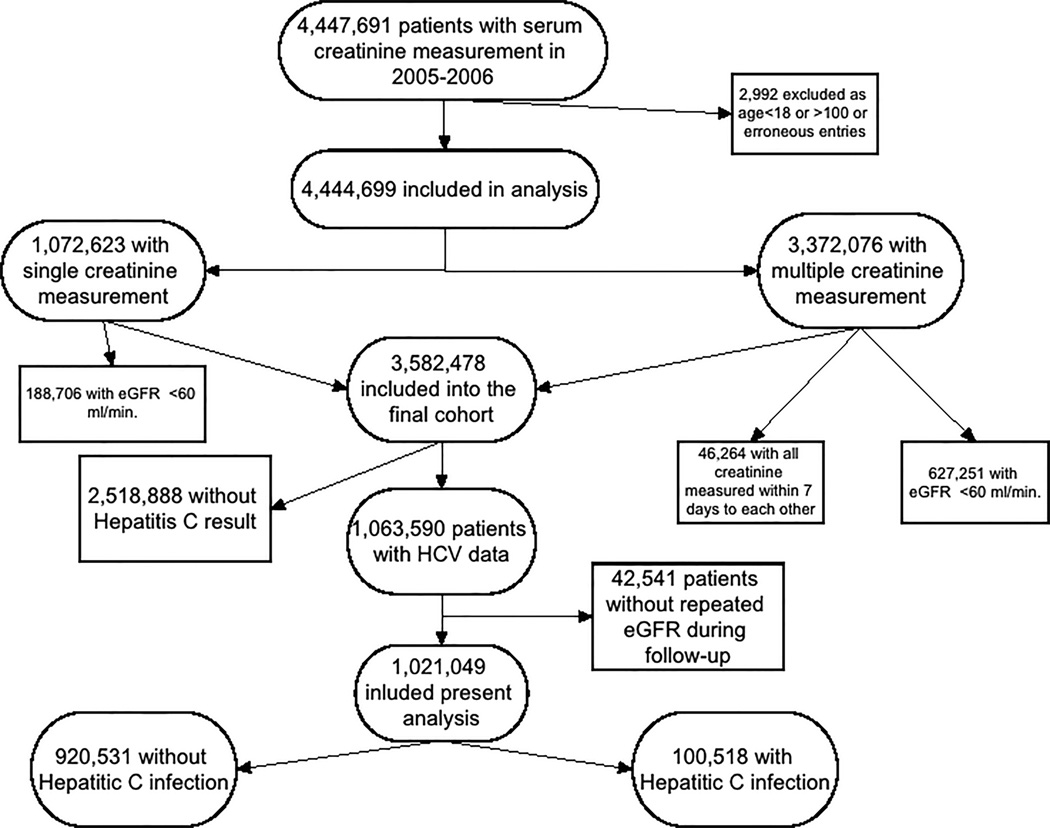
The institutional review committees at the Memphis and Long Beach Veterans Affairs Medical Centers approved the study. Our study utilized data from a cohort study examining risk factors in patients with incident CKD (Racial and Cardiovascular Risk Anomalies in CKD (RCAV) study).(16) The algorithm for cohort definition is shown in Figure 1. Using the national Veterans Affairs (VA) Decision Support System National Data Extracts Laboratory Results files and Corporate Data Warehouse LabChem data files, we extracted serum creatinine levels measured in the course of clinical practice in any VA facility to identify veterans with eGFR of ≥60 ml/min/1.73m2,(17) calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation, which is a reliable and frequently used equation to estimate GFR.(18) We identified 3,582,478 patients with baseline eGFR ≥60 ml/min/1.73m2 among a total of 4,444,699 patients with any available eGFR between October 1, 2004 and September 30, 2006. HCV infection was defined as the presence of a laboratory test result indicating infection, including HCV virus PCR or HCV antibody, any time after October 1, 2004. After exclusion of patients without available HCV test result or repeated serum creatinine measurements during the follow-up period, 1,021,049 patients were included in our final cohort (Figure 1), of which 100,518 were HCV positive on laboratory testing while 920,531 had HCV negative tests (Figure 1). Socio-demographic characteristics, comorbid conditions and laboratory characteristics were obtained, as previously described.(19–22) Information about age, gender and race were obtained through the VA Corporate Data Warehouse (CDW) and from Medicare through the VA-Medicare data merge project.(23)
Figure 1.
Flow chart of the cohort creation
Outcomes
We defined four different outcomes: 1) incidence of eGFR <60 ml/min/1.73m2, 2) slopes of eGFR, 3) incidence of ESRD and 4) all-cause mortality. Incidence of eGFR <60 ml/min/1.73m2 was used in lieu of incidence of CKD, due to unavailability of proteinuria measures on most included participants, and was defined as the presence of two consecutive eGFR values of <60 ml/min/1.73m2 at least 90 days apart with the added stipulation that the decrease in eGFR should be at least 25% from the baseline eGFR. Slopes of eGFR were calculated from all available eGFR values derived from outpatient serum creatinine measurements in each individual during the entire follow-up period, using least squares regression. The median (interquartile range (IQR)) number of serum creatinine measurements used to calculate eGFR slopes was 10 (5–17). Rapid deterioration was defined as slopes of <-5 ml/min/1.73m2/year (i.e. loss of eGFR of >5 ml/min/1.73m2/year).(24) Information on ESRD, defined as initiation of renal replacement therapy or pre-emptive transplant, was obtained from the USRDS database. Data on all-cause mortality was obtained from the VA Vital Status Files (VSF), which contain dates of death or last medical/administrative encounter from all sources in the VA system with sensitivity and specificity of 98.3% and 99.8%, respectively, as compared to the National Death Index as gold standard.(25)
Statistical Analysis
Descriptive analyses were performed and skewed variables were log-transformed. The start of the follow-up period was the date of the first eGFR ≥60 ml/min/1.73m2 during 2005–2006. Patients were followed until death or were censored at the date of last healthcare or administrative visit, or on July 26, 2013.
The association of HCV with mortality was assessed using the Kaplan-Meier method and Cox regressions, by examining crude and adjusted associations of HCV with the studied outcomes and with the composite outcomes of mortality or ESRD and mortality or incident eGFR <60 ml/min/1.73m2. The association between HCV and steeper slopes of eGFR was examined in logistic regression models. Models were adjusted for the following confounders based on a priori considerations: model 1: age, gender, race/ethnicity; model 2: model 1 variables and baseline eGFR; model 3: model 2 variables and comorbidities (diabetes, hypertension, cardiovascular disease, congestive heart failure, cerebro-vascular disease, peripheral vascular disease, lung disease, dementia, rheumatic disease, malignancy, HIV/AIDS and depression); model 4: model 3 variables and systolic blood pressure, diastolic blood pressure and body mass index (BMI); model 5: model 4 variables and various socio-economic parameters (income, marital status, service connection, adherence to medical interventions (defined as the presence of the ICD9-CM code of V15.81 during any inpatient or outpatient encounter), medication adherence (defined as the percentage of days covered (PDC) for any antihypertensive medication throughout follow-up,(16) number of healthcare encounters during follow-up, number of prescribed antihypertensive medications and ACEI/ARB usage throughout follow-up. We did not adjust for presence of liver disease in our main analysis, since liver disease is an effect mediator of HCV rather than a confounder; however, we performed sensitivity analyses with adjustment for liver disease.
The propensity score method was used as sensitivity analysis to account for baseline differences arising from dissimilarities in clinical and demographic characteristics of patients with and without HCV infection.(26) Variables associated with HCV positive status were identified using logistic regression and were used to calculate propensity scores. STATA’s “psmatch2” command suite was used to generate the propensity score-matched cohorts by a 1-to-1 nearest neighbor matching without replacement. As death and development of CKD/ESRD are competing events, the association of HCV with the two outcomes was assessed by means of semi-parametric time-dependent competing-risks regression analyses in the propensity-matched cohort.(27) We performed sensitivity analysis in the sub-cohort of 129,959 patients had been tested for HCV-RNA.
Statistical analyses were performed using STATA MP Version 12 (STATA Corporation, College Station, TX).
Results
Baseline characteristics
The mean±SD age of the cohort at baseline was 54.5±13.1 years, 73% and 22% of patients were white and black, respectively, 21% of the patients were diabetic and the mean baseline eGFR was 87.6±16.3 ml/min/1.73m2. Baseline characteristics of patients categorized by HCV status are shown in Table 1. Patients with HCV were slightly younger, more likely to be black and divorced and to have lower income, liver disease, depression and higher eGFR at baseline. Baseline characteristics were well balanced in the propensity-matched cohort (Table S1).
Table 1.
Baseline characteristics of study population
| HCV negative (n=920,531) |
HCV positive (n=100,518) |
|
|---|---|---|
| Age (years) | 55±13 | 53±8 |
| Gender (male) | 842,483 (92) | 96,537 (96) |
| Race: | ||
| White | 646,650 (75) | 58,887 (60) |
| African-American | 177,160 (20) | 35,129 (36) |
| Hispanic | 21,452 (2) | 2,158 (2) |
| Other Race | 20,693 (2) | 1,674 (2) |
| Marital status: | ||
| Married | 441,452 (50) | 30,332 (31) |
| Single | 123,439 (14) | 17,714 (18) |
| Divorced | 272,946 (31) | 44,066 (46) |
| Widow | 43,926 (5) | 4,251 (4) |
| Other sociodemographic: | ||
| Income (USD) | 21,111 (11,455–32,858) | 13,952 (9,361–27,615) |
| Non-adherent to medical interventions* | 71,415 (8) | 14,932 (15) |
| Miscallenous: | ||
| Baseline eGFR (ml/min./1.73m2) | 87±16 | 93±16 |
| BMI (kg/m2) | 29±6 | 28±5 |
| Systolic BP (mmHg) | 135±19 | 136±20 |
| Diastolic BP (mmHg) | 78±12 | 81±13 |
| Number of visits | 112 (59–201) | 154 (82–273) |
| Comorbidities: | ||
| Hypertension | 499,312 (54) | 53,230 (53) |
| Diabetes mellitus | 195,781 (21) | 21,152 (21) |
| Cardiovascular Disease** | 90,518 (10) | 7,410 (7) |
| Congestive Heart Failure | 29,635 (3) | 3,256 (3) |
| Cerebrovascular Disease | 43,770 (5) | 4,257 (4) |
| Peripheral Arterial Disease | 40,459 (4) | 4,130 (4) |
| Chronic Lung Disease | 155,446 (17) | 19,399 (19) |
| Dementia | 3,608 (0.4) | 372 (0.4) |
| Rheumatologic Disease | 14,733 (2) | 1,176 (1) |
| Liver Disease | 8,780 (1) | 8,100 (8) |
| All malignancies | 70,789 (8) | 6,622 (7) |
| AIDS/HIV | 9,743 (1) | 2,898 (3) |
| Depression | 115,524 (13) | 19,733 (20) |
| Medications: | ||
| Number of anti-hypertensive medications | 3 (2–4) | 3 (2–4) |
| PDC | 82±28 | 79±28 |
| ACEI or ARB used*** | 474,227 (52) | 52,837 (53) |
Dichotomous/dummy variables are presented as number of patients and percentage; continous variables are presented as mean±SD or median (interquartile range, IQR)
Defined as the presence of the ICD9-CM code V15.81 during any inpatient or outpatient encounter
Cardiovascular Disease was defined as acute myocardial infraction, angina, coronary artery disease, previous coronary artery bypass grafting or percutaneous coronary intervention
ACEI or ARB used any time during follow-up
Abbreviations: ACEI: Angiotensin converting enzyme inhibitor; AIDS: Acquired immundeficiency syndrome; ARB: Angiotensin receptor blocker; BMI: Body mass index; BP: Blood pressure; eGFR: Estimated glomerular filtration rate; HIV: Human Immundeficiency Virus; IQR: Interquartile range; PDC: Percentage of days covered; SD: Standard deviation
Mortality
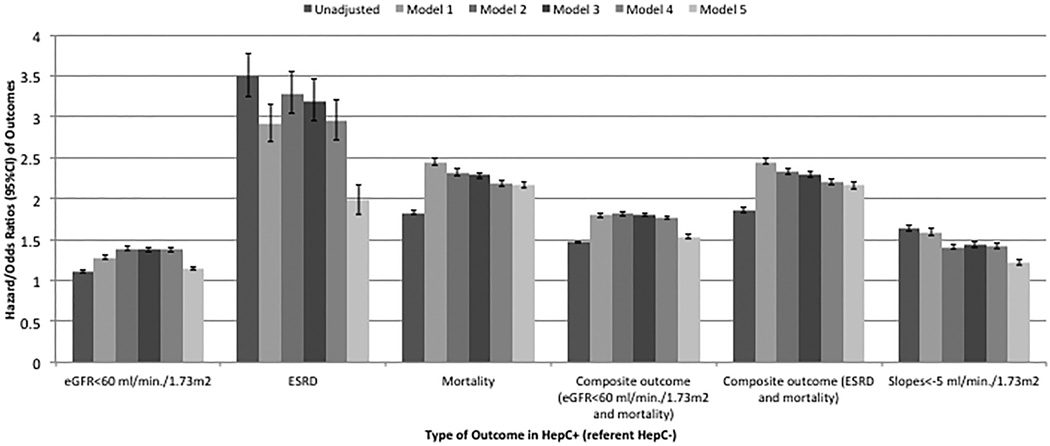
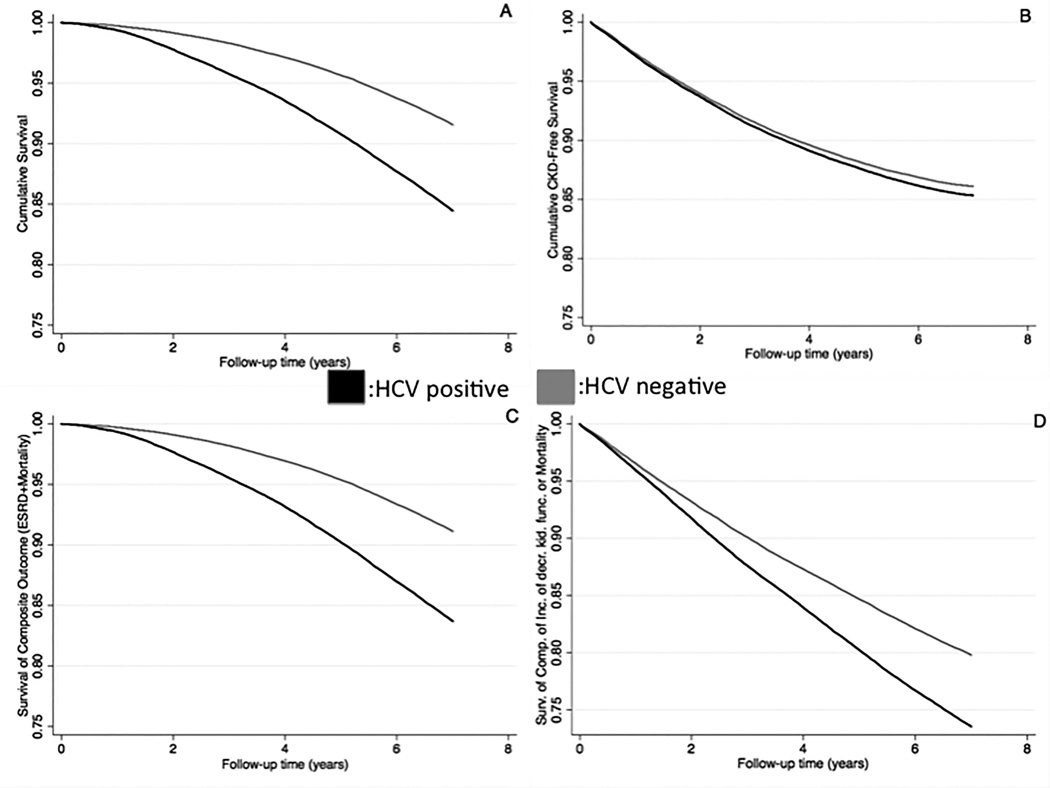
The median follow-up time was 8.0 years (interquartile range: 7.2–8.5 years). There were 18,811 deaths (18.7%, mortality rate 25.8 [25.4–26.2]/1000 patient-years) in the HCV positive group and 98,124 deaths (10.7%, 14.2 [14.1–14.3]/1000 patient-years) in the HCV negative group. Figure 2 shows the association between HCV positive status and mortality in unadjusted and adjusted models. HCV positive status was associated with higher mortality risk in the unadjusted (hazard ratio (HR): 1.83, 95% confidence interval (CI): 1.81–1.86) and fully adjusted model (HR: 2.17, 95%CI: 2.13–2.21) (Table S2). In sensitivity analysis, HCV positive status was associated with higher mortality risk (HR: 1.80, 95%CI: 1.75–1.85) in the propensity score-matched cohort (Table S3). Figure 3 panel A shows the survival probability of HCV positive and negative patients in the propensity score-matched cohort, with HCV positive status showing an association with lower survival probability.
Figure 2.
Association of presence of HCV and outcomes using Cox-proportional models and logistic regression models in the cohort of 1,021,049 patients
Adjusted for:
Model 1: age, gender, race/ethnicity
Model 2: Model 1 and baseline GFR
Model 3: Model 2 and comorbidities (diabetes, hypertension, cardiovascular diseae, congestive heart failure, cerebro-vascular disease, peripheral vascular disease, lung disease, dementia, rheumatic disease, malignancy, HIV/AIDS and depression)
Model 4: Model 3 and systolic BP, diastolic BP and BMI
Model 5: Model 4 and socio-demographic parameters (income, marital status, service connection, complience, drug complience, number of visit, number of medication and ACEI/ARB usage)
Abbreviations: ACEI: Angiotensin converting enzyme inhibitor; AIDS: Acquired immundeficiency syndrome; ARB: Angiotensin receptor blocker; BMI: body mass index; BP: blood pressure; eGFR: Estimated glomerular filtration rate; HIV: Human Immundeficiency Virus
Figure 3.
Kaplan-Meier Survival Curves for various Outcomes (Panel A: All-cause Mortality; Panel B: Incidence of decreased kidney function; Panel C: Composite of ESRD or Mortality; Panel D: Composite of Incidence of decreased kidney function or Mortality) in the Propensity-Matched Cohort (N= 144,178 patients)
Abbreviations: CKD: Chronic kidney disease; ESRD: End stage renal disease
Incidence of eGFR <60 ml/min/1.73m2
The median follow-up time for incident eGFR <60 ml/min/1.73m2 was 7.8 years (interquartile range: 6.8–8.4 years). There were 11,271 events (11.2%, incidence rate 16.7 [16.4–17.0]/1000 patient-years) in the HCV positive group and 95,837 events (10.4%, incidence rate 14.9 [14.8–15.0]/1000 patient-years) in the HCV negative group. Figure 2 shows the association between HCV positive status and new onset eGFR <60 ml/min/1.73m2, with higher risk in the unadjusted (HR: 1.11, 95%CI: 1.09–1.13) and fully adjusted model (HR: 1.15, 95%CI: 1.12–1.17) (Table S2). Figure 3 panel B shows the event-free survival probability of HCV positive and negative patients in the propensity score-matched cohort, with HCV positive status showing an association with slightly higher event rate. HCV positive status was also associated with increased risk of new onset eGFR <60 ml/min/1.73m2 in competing risk regression analysis (Table S3).
Deterioration of kidney function
70,989 veterans had slopes steeper than -5 ml/min/1.73m2/year. Figure 2 shows the association between HCV positive status and the slope of deterioration of the kidney function. HCV positive status was associated with higher risk of rapid deterioration of kidney function in the unadjusted (OR: 1.64, 95%CI: 1.60–1.68) and fully adjusted model (OR: 1.22, 95%CI: 1.19–1.26) (Table S2). Similar results were found in the propensity score-matched cohort (Table S3).
ESRD
The median follow-up time for incident ESRD was 8.0 years (interquartile range: 7.1–8.5 years). There were 904 incident ESRD patients (0.9%, incidence rate 1.2 [1.1–1.3]/1000 patient-years) in the HCV positive group and 2,479 incident ESRD patients (0.3%, incidence rate 0.36 [0.34–0.37]/1000 patient-years) in the HCV negative group. Figure 2 shows the association between HCV positive status and development of ESRD. HCV positive status was associated with higher risk of development of ESRD in the unadjusted (HR: 3.50, 95%CI: 3.25–3.78) and adjusted model (HR: 1.98, 95%CI: 1.81–2.16) (Table S2). Figure 3 panel C shows the survival curves of the composite outcome (ESRD or mortality) of HCV positive and negative patients in the propensity score-matched cohort, with HCV positive status showing an association with higher risk for the development of ESRD or death. HCV positive status was also associated with increased risk of ESRD in competing risk regression analysis (Table S3).
Sensitivity analyses
Of the 1,021,049 patients, 129,959 had been tested for the presence of HCV-RNA. In the latter group we assessed the association between HCV status and various outcomes separately in patients who were viremic, and in those who were HCV antibody-positive, but non-viremic according to HCV-RNA testing (Table S4). In non-viremic patients, there was no significant association between HCV antibody positive status and renal outcomes. However, HCV antibody and RNA positive (viremic) patients had 10% higher risk for new onset eGFR <60 ml/min/1.73m2 (HR: 1.10, 95%CI: (1.05–1.16)), 62% higher risk for ESRD (HR: 1.62, 95%CI: (1.26–2.07)), and 23% higher risk for rapid deterioration of kidney function (HR: 1.23, 95%CI: (1.14–1.33)) compared to HCV antibody negative patients (Table S4). We also performed sensitivity analyses adjusting for liver disease, in which we found qualitatively similar results for all outcomes (Table S5).
Discussion
In a large cohort of US veterans with baseline eGFR ≥60 ml/min/1.73m2, we examined the association of HCV infection with developing incident eGFR <60 ml/min/1.73m2, on the rate of kidney function decline, and on the development of ESRD and all-cause mortality. Similar to results reported in previous smaller studies,(12, 14) patients with HCV in our cohort were younger, more likely to be African-American and divorced and to have lower income, liver disease, depression and higher eGFR at baseline.
The presence of HCV infection was associated with increased risk of development of eGFR <60 ml/min/1.73m2 and with an almost 2-fold increased risk of ESRD. In addition, the presence of HCV was associated with rapid deterioration of kidney function. The association of HCV with ESRD and with the slopes of eGFR was stronger compared to its association with incident eGFR <60 ml/min/1.73m2, suggesting that the effect of HCV may be more pronounced on the progression of established CKD versus on the development of de novo CKD. These associations were present in HCV-RNA positive (viremic) patients, but not in HCV-RNA negative patients in our sensitivity analyses. Several previous studies found similar associations,(9–12, 28, 29) while other studies reported opposite findings.(7, 8) Potential reasons for the discrepant results in these studies are their smaller sample sizes, selection bias, lack of adjustment for possible confounders and shorter follow-up duration.(7, 8) Another potential problem in some previous studies was the utilization of end points that do not reliably identify CKD, such as the occurrence of a single eGFR <60 ml/min/1.73m2.(8) Prior to ours the largest study examining the association between HCV infection and CKD-related outcomes reported an almost 3-fold higher risk of development of ESRD in HCV-positive patients compared to their HCV-negative counterparts.(12)
There are several potential explanations for the higher risk of adverse renal events in HCV-positive patients. HCV infection is associated with albuminuria and presence of cryoglobulins, amyloid deposition, or HCV-antibody immune complexes, which can be responsible for kidney injury due to systemic immune response to HCV infection.(3, 4, 30) HCV is also believed to have the potential for entry into and replication within renal tissue.(3, 31) In addition to viral cytopathic or immunologic effects, insulin resistance can be a very important host factor in patients with chronic HCV infection.(32) Insulin resistance and hyperinsulinemia cause excess intra-renal production of insulin-like growth factor 1 and transforming growth factor β, thus triggering proliferation of renal cells and up-regulating the expression of angiotensin II type 1 receptors in mesangial cells, which in turn enhances the harmful effects of angiotensin II in the kidney.(33)
In addition to the association with development of ESRD and new onset decreased eGFR, HCV infection was strongly associated with increased risk of all-cause mortality. Several potential mechanisms can explain the association between HCV infection and increased risk of mortality. HCV is a well-known cause of hepatic cirrhosis and hepatocellular carcinoma. In our database, the prevalence of liver disease was 8-fold higher in HCV positive patients than in their HCV negative counterparts.
Our results have implications for clinical practice and for future research. Clinicians may wish to counsel patients with HCV infection about their potential for increased risk of kidney disease and mortality in addition to their risk for hepatic complications. Future studies should investigate whether treatment for eradicating HCV, including recently developed drugs,(34–36) alters the risk for the development and/or progression of renal disease.
Our study is notable for its large sample size and event numbers, and for it being representative of veterans in the entire United States. It also has several limitations that need to be acknowledged. A limitation of observational studies such as ours is that they cannot prove causal associations between predictors and outcomes, and therefore we cannot claim that the HCV infection was indeed the primary reason for the worse survival and higher risk of renal end points. The study population consisted mostly of male patients; hence the results may not apply to females. The veteran population is not representative of the entire United States population. We used relatively novel statistical methods such as propensity score matching and competing risk regression analyses in our sensitivity analyses to minimize the effects of bias by indication and competing risk, but even these methods do not address unmeasured confounders, such as smoking history. In addition, we did not have information about the causes of death and therefore we cannot analyze associations with cardiovascular, infectious, and malignancy-related mortality. We did not have rigorous and homogenous diagnostic approaches for assessing HCV exposure, which may lead to non-differential misclassification. Such rigorous case ascertainment is, however, not feasible in large administrative cohorts such as ours. Additionally, we do not have data about albuminuria in our database; consequently we can only comment on associations between HCV and eGFR-related outcomes, but not on associations with properly defined incident CKD. Nevertheless, by using ESRD as one of our end points we can infer that HCV is associated with the development and/or progression of CKD. Lastly, we used diagnostic codes to define comorbid conditions that could act as confounders in the association of HCV infection with adverse outcomes, which may underestimate their prevalence.
Conclusions
In our large and contemporary cohort of more than 1 million US veterans, HCV infection is associated with higher mortality risk and with the development and progression of kidney disease. Randomized controlled trials are indicated to determine if treatment of HCV infection can prevent the development and progression of CKD and lower the risk of renal disease and mortality.
Supplementary Material
Acknowledgments
We thank Ms. Yin Su for her help creating materials for data presenation.
Funding Sources
This study is supported by grant 1R01DK096920 from the NIH to CPK and KKZ, and by resources from the US Department of Veterans Affairs. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
List of abbreviations
- ACEI
Angiotensin converting enzyme inhibitor
- AIDS
Acquired immundeficiency syndrome
- ARB
Angiotensin receptor blocker
- BMI
Body mass index
- BP
Blood pressure
- HCV
Chronic hepatitis C virus
- CDW
Corporate Data Warehouse
- CKD
chronic kidney disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- eGFR
Estimated glomerular filtration rate
- ESRD
end stage renal disease
- HIV
Human Immundeficiency Virus
- RCAV
Racial and Cardiovascular Risk Anomalies in CKD study
- RNA
Ribonucleic acid
- IQR
Interquartile range
- USRDS
United States Renal Data System
- PCR
Polymerase chain reaction
- PDC
Percentage of days covered
- SD
Standard deviation
- VA
Veterans Affairs
- VSF
Vital Status Files
Footnotes
Conflict of interest
None.
Disclosures
CPK and BMW are employees of the Department of Veterans affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs. The results of this paper have not been published previously in whole or part, except for abstract submission to the 46th Annual American Society of Nephrology Conference, November 11–14, 2014, Philadelphia, PA.
REFERENCES
- 1.WHO. Hepatitis C. Fact sheet N°164. World Health Organization. 2014
- 2.Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsui JI, Vittinghoff E, Shlipak MG, O'Hare AM. Relationship between hepatitis C and chronic kidney disease: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2006;17:1168–1174. doi: 10.1681/ASN.2005091006. [DOI] [PubMed] [Google Scholar]
- 4.Liangpunsakul S, Chalasani N. Relationship between hepatitis C and microalbuminuria: results from the NHANES III. Kidney Int. 2005;67:285–290. doi: 10.1111/j.1523-1755.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 5.Fabrizi F, Martin P, Dixit V, Messa P. Hepatitis C virus infection and kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2012;7:549–557. doi: 10.2215/CJN.06920711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabrizi F, Plaisier E, Saadoun D, Martin P, Messa P, Cacoub P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis. 2013;61:623–637. doi: 10.1053/j.ajkd.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 7.Moe SM, Pampalone AJ, Ofner S, Rosenman M, Teal E, Hui SL. Association of hepatitis C virus infection with prevalence and development of kidney disease. Am J Kidney Dis. 2008;51:885–892. doi: 10.1053/j.ajkd.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asrani SK, Buchanan P, Pinsky B, Rey LR, Schnitzler M, Kanwal F. Lack of association between hepatitis C infection and chronic kidney disease. Clin Gastroenterol Hepatol. 2010;8:79–84. doi: 10.1016/j.cgh.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satapathy SK, Lingisetty CS, Williams S. Higher prevalence of chronic kidney disease and shorter renal survival in patients with chronic hepatitis C virus infection. Hepatol Int. 2011 doi: 10.1007/s12072-011-9284-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen YC, Chiou WY, Hung SK, Su YC, Hwang SJ. Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: a 6-year nationwide cohort study across Taiwan. BMC Nephrol. 2013;14:187. doi: 10.1186/1471-2369-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su FH, Su CT, Chang SN, Chen PC, Sung FC, Lin CC, Yeh CC. Association of hepatitis C virus infection with risk of ESRD: a population-based study. Am J Kidney Dis. 2012;60:553–560. doi: 10.1053/j.ajkd.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Tsui JI, Vittinghoff E, Shlipak MG, Bertenthal D, Inadomi J, Rodriguez RA, O'Hare AM. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271–1276. doi: 10.1001/archinte.167.12.1271. [DOI] [PubMed] [Google Scholar]
- 13.Lee JJ, Lin MY, Yang YH, Lu SN, Chen HC, Hwang SJ. Association of hepatitis C and B virus infection with CKD in an endemic area in Taiwan: a cross-sectional study. Am J Kidney Dis. 2010;56:23–31. doi: 10.1053/j.ajkd.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Lee JJ, Lin MY, Chang JS, Hung CC, Chang JM, Chen HC, Yu ML, et al. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One. 2014;9:e100790. doi: 10.1371/journal.pone.0100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crook ED, Penumalee S, Gavini B, Filippova K. Hepatitis C is a predictor of poorer renal survival in diabetic patients. Diabetes Care. 2005;28:2187–2191. doi: 10.2337/diacare.28.9.2187. [DOI] [PubMed] [Google Scholar]
- 16.Gosmanova EO, Lu JN, Streja E, Cushman WC, Kalantar-Zadeh K, Kovesdy CP. Association of medical treatment non-adherene with all-cause mortality in newly treated hypertensive us veterans. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.03805. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens PE, Levin A Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molnar MZ, Kalantar-Zadeh K, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Quarles DL, et al. ACE Inhibitor and Angiotensin Receptor Blocker Use and Mortality in Patients with Chronic Kidney Disease. J Am Coll Cardiol. 2014;63:650–658. doi: 10.1016/j.jacc.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar-Zadeh K. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125:677–684. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar-Zadeh K. Outcomes associated with microalbuminuria: effect modification by chronic kidney disease. J Am Coll Cardiol. 2013;61:1626–1633. doi: 10.1016/j.jacc.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VIReC Research User Guide; VHA Medical SAS Inpatient Datasets FY2006-2007. Hines, IL: U.S. Department of Veterans Affairs. VA Information Resource Center; 2007. [Google Scholar]
- 23.US Department of Veterans Affairs VA Information Resource Center Data Quality Update: Race. 2009 [Google Scholar]
- 24.KDIGO. 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements. 2013:136–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 25.Arnold NSM, Maynard C, Hynes DM. In: VIReC Technical Report 2: VA-NDI Mortality Data Merge Project. Center VIR, editor. Hines, IL: 2006. [Google Scholar]
- 26.D'Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 28.Noureddine LA, Usman SA, Yu Z, Moorthi RN, Moe SM. Hepatitis C increases the risk of progression of chronic kidney disease in patients with glomerulonephritis. Am J Nephrol. 2010;32:311–316. doi: 10.1159/000319456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014;85:1200–1207. doi: 10.1038/ki.2013.455. [DOI] [PubMed] [Google Scholar]
- 30.Barsoum RS. Hepatitis C virus: from entry to renal injury--facts and potentials. Nephrol Dial Transplant. 2007;22:1840–1848. doi: 10.1093/ndt/gfm205. [DOI] [PubMed] [Google Scholar]
- 31.Valli MB, Serafino A, Crema A, Bertolini L, Manzin A, Lanzilli G, Bosman C, et al. Transmission in vitro of hepatitis C virus from persistently infected human B-cells to hepatoma cells by cell-to-cell contact. J Med Virol. 2006;78:192–201. doi: 10.1002/jmv.20527. [DOI] [PubMed] [Google Scholar]
- 32.Ratziu V, Heurtier A, Bonyhay L, Poynard T, Giral P. Review article: an unexpected virus-host interaction--the hepatitis C virus-diabetes link. Aliment Pharmacol Ther. 2005;22(Suppl 2):56–60. doi: 10.1111/j.1365-2036.2005.02598.x. [DOI] [PubMed] [Google Scholar]
- 33.Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009;4:207–220. doi: 10.2215/CJN.03710708. [DOI] [PubMed] [Google Scholar]
- 34.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 35.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 36.Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, Everson GT, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370:222–232. doi: 10.1056/NEJMoa1306227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.