Abstract
Aberrant activation of Wnt signaling contributes to ischemia-induced retinal neovascularization in oxygen-induced retinopathy (OIR), although the underlying mechanism is so far unclear. Here, we show that microRNA-184 (miR-184) is significantly down-regulated in the retina of OIR mice, and miR-184 negatively modulates Wnt signaling both in vivo and in vitro. Furthermore, we show that the Wnt receptor, frizzled-7, is a downstream target of miR-184, and delivery of miR-184 mimic inhibits Wnt signaling in the OIR retina. These results suggest that decreased levels of miR-184 are responsible, at least in part, for the aberrant activation of Wnt signaling in ischemia-induced retinal neovascularization.
Keywords: microRNA, neovascularization, oxygen-induced retinopathy, Wnt signaling pathway, gene delivery
1. Introduction
MicroRNAs (miRNAs) are single-stranded, small (19~24 nucleotides) non-coding RNA molecules [1] and serve as negative regulators in gene expression at the posttranscriptional level by partially complementary binding to the 3’ un-translated region (3’UTR) of target mRNAs [2-8]. It is known that miRNAs are widely present in the body and regulate a variety of developmental and physiological processes [3]. Although it is reported that expression changes of some miRNAs are implicated in the development and progression of various diseases including diabetes [9,10], the pathogenic roles of miRNAs in diabetes and its complications remain unclear.
Diabetic retinopathy (DR), one of the major complications of diabetes, is the most common diabetic eye disease and a leading cause of blindness among the working age population in the US [11]. Accumulating evidence suggests that chronic inflammation and oxidative stress in the retina play important pathogenic roles in DR [12-14]. Oxygen-induced retinopathy (OIR) is a well-established animal model of ischemia-induced retinal inflammation and neovascularization (NV) which recapitulates pathologies of proliferative diabetic retinopathy (PDR). Thus, OIR is commonly used as a model of PDR [15].
Wnt signaling is a conserved intracellular signaling pathway consisting of Wnt ligands, their co-receptors and an intracellular signaling cascade and regulates multiple cellular processes, such as cell differentiation, inflammation, carcinogenesis and angiogenesis [16-19]. It has been reported that aberrant-activation of the Wnt signaling in the retina is a major pathogenic mechanism for retinal inflammation and NV in DR and in the OIR model [20-22]. However, the cause of the Wnt signaling activation in OIR is poorly understood.
It was reported that microRNA-184 (miR-184) is abundantly expressed in the cornea and lens [23], and single-base substitution in the miR-184 seed region is associated with corneal diseases and cataract [24-26]. In addition, an earlier study reported that several miRNAs including miR-184 are implicated in ocular NV in the OIR model and the non-ischemia retinopathy (laser-induced choroidal NV) model [27].
Here, we verified that miR-184 was down-regulated in the retina of OIR mice and identified that miR-184 negatively modulates canonical Wnt signaling through the regulation of expression of the Wnt receptor frizzled-7 (Fzd7).
2. Materials and Methods
2.1. Animals
C57BL/6J and Axin2LacZ mice [28] were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained in 12 hr light and 12 hr dark cycles. The Axin2 gene is a known target gene of canonical Wnt signaling, and Axin2LacZ mice were generated in-frame insertion of a nuclear-localized β-galactosidase (NLS-LacZ) to visualize the locations of Wnt signal activation [28,29]. All of the experiments involving mice were approved by the Institutional Animal Care and Use Committees (IACUC) at the University of Oklahoma Health Sciences Center, and performed following the guidelines of the Association for Research in Vision and Ophthalmology (ARVO) statement for the “Use of Animals in Ophthalmic and Vision Research”.
2.2. Oxygen-induced retinopathy (OIR) model
C57BL/6J or Axin2LacZ mice were exposed to 75% O2 from postnatal day 7 (P7) to P12. Age-matched mice with the same genetic background were maintained in constant room air (normoxia control) as the negative control. The retinas were isolated at P16 for RNA or protein analyses.
2.3. Quantitative real-time RT-PCR (qRT-PCR) for microRNA and mRNA expression analyses
RNA extraction and qRT-PCR for miRNA (TaqMan miRNA assay, Lifetech, Carlsbad, CA: miR-184, U6-snRNA) and mRNAs (Vegf-a, Gfap, Hprt1: primer sequences were shown in Table 1) were performed as described previously [30].
Table 1.
Primer sequences in this study.
| Primer name | Primer Sequence (5′ --- 3′) | Product size |
|---|---|---|
| Vegf-a Fwd | GCCAGCACATAGAGAGAATGAGC | 97 |
| Vegf-a Rev | CAAGGCTCACAGTGATTTTCTGG | |
| Gfap Fwd | ATCGAGATCGCCACCTACAG | 150 |
| Gfap Rev | TACCACGATGTTCCTCTTGA | |
| Hprt1 Fwd | CAGGCCAGACTTTGTTGGAT | 147 |
| Hprt1 Rev | TTGCGCTCATCTTAGGCTTT |
2.4. Western blot analysis
Briefly, equal amounts of total cellular proteins (20 μg) were resolved on 10% SDS-PAGE gel and electro-transferred onto a nitrocellulose membrane. Western blot analysis was conducted as described previously [30]. Rabbit polyclonal antibodies for phosphorylated-LRP6 and non-phosphorylated-β-catenin were purchased from Cell Signaling (Danvers, MA); a rabbit polyclonal antibody for VEGF-A was purchased from Santa Cruz (Dallas, TX); a mouse monoclonal antibody for β-actin was purchased from Sigma Aldrich (Saint Louis, IL) and an anti-Fzd7 mouse monoclonal antibody was purchased from LifeSpan Biosciences (Seattle, WA).
2.5. In vivo and in vitro Wnt signaling activity assays
Wnt signaling activation in the retina of Wnt signaling reporter (Axin2LacZ) mice was evaluated by the Wnt reporter gene, β-galactosidase activity assay using Senescence Cells Histochemical staining kit (Sigma Aldrich). A rat Müller cell line, rMC-1 [31], with stably expressing a firefly luciferase controlled by Wnt/β-catenin system, was generated by lentivirus infection and antibiotic (puromycin, Sigma Aldrich) resistance selection [32]. The equal amounts (12.5 pmol/0.5 mL (25 nM) each) of negative control miRNA mimic or miR-184 mimic (Invitrogen, Carlsbad, CA), and 2.5 pmol/0.5 mL (5 nM) of miR-184 mimic plus 25 pmol/0.5 mL (50 nM) of negative control miRNA inhibitor or miR-184 inhibitor (Invitrogen, Carlsbad, CA) using Lipofectamine RNAiMAX (Invitrogen), and culture media were replaced at 6 hr post-transfection. The cells were treated with 10% Wnt3A-conditioned media (W3A) at 32 hr post-transfection, and a luciferase-based Wnt signaling activity assay (TOP flash assay) was conducted at 48 hr post-transfection following the manufacturer's protocol (Promega Corporation, Madison, WI).
2.6. Verification of downstream target of miR-184
The downstream target of miR-184 was validated with luciferase-based assay as described previously [30]. Briefly, approximately 700 base-pairs of the human Fzd7 3’UTR was cloned into the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega). In addition, a scrambled sequence of miR-184 was cloned into the same vector as a negative control and a reverse complemental sequence of miR-184 was cloned into the same vector as a positive control. Further, we generated a point-mutant and a deletion mutant of the miR-184-binding site in Fzd7 3’UTR to verify the impact of the binding site of miR-184 on Fzd7 expression. For in vitro analysis, the equal amount of assay vectors (0.2 μg each: scrambled, MIR-184, Fzd7-wt, Fzd7-Mut, and Fzd7-Del) were separately transfected into 293A cells (Qbiogen, Montreal, Canada) with 12.5 pmol/0.5 mL (25 nM) of either pre-miRNA negative control or miR-184 mimic (Invitrogen) using Lipofectamine2000 (Invitrogen). At 48 hr post-transfection, dual-luciferase assay (Promega) was conducted following the manufacturer's protocol.
2.7. Nanoparticle formulation and delivery into the eye
A pre-miRNA negative control and a miR-184 mimic (miRIDIAN Mimic, Thermo Fisher Scientific, Chicago, IL) were separately packed in liposome-based nanoparticles as described by Rajala et al [33]. One-μL of the prepared nanoparticle (approximately 5.5 pmol of pre-miRNA) was injected into the vitreous space of OIR mice at P12, and Wnt signaling activity in the retina was evaluated with X-gal staining at P16.
2.8. Statistical analysis
All of quantitative data were expressed as Mean ± SEM and represented as fold of control. At least three individual measurements were conducted and Student's t-test in Microsoft Excel (Microsoft, Redmond, WA) was performed to determine the statistical significance for comparison of two data sets. Significance was denoted as * p<0.05, ** p<0.01 and *** p<0.001, respectively.
3. Results
3.1. Activation of canonical Wnt signaling in the retina of OIR mice
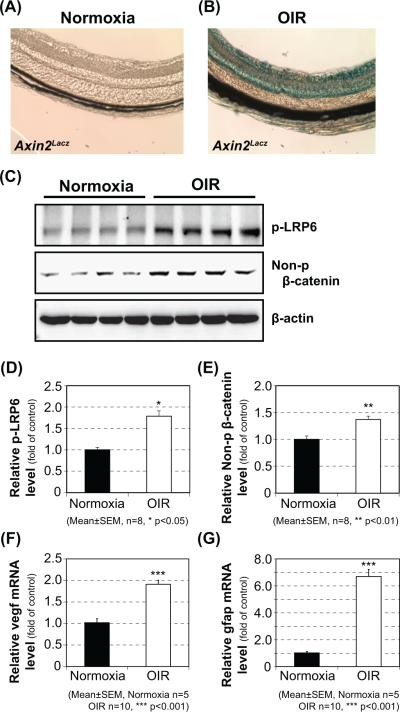
Firstly, we evaluated Wnt signaling activation in the retina of OIR mice. The Axin2LacZ Wnt signal reporter mice [28,29] were placed in the oxygen chamber (75% Oxygen) at P7 for 5 days (OIR model). Wnt signaling activation was evaluated by X-gal staining of retinal sections of normoxia control (Fig. 1A) and OIR (Fig. 1B) Axin2LacZ mice at P16. Substantial blue color staining was observed in the retina of OIR Axin2LacZ mice, but not in the normoxia control, indicating Wnt signaling activation in the OIR retina. In addition, Western blot analyses showed up-regulation of phosphorylated-LRP6 [34] (Fig. 1C, D) and non-phosphorylated-β-catenin [35] in the retinas of OIR C57BL/6J mice (Fig. 1C, E), further verifying activation of Wnt signaling in the OIR retina. Further, mRNA expression of Vegf-a (Fig. 1F), a major angiogenic factor and target gene of Wnt signaling, and Gfap (Fig. 1G), a commonly used stress marker in the retina [36,37], were significantly up-regulated in the retina of OIR mice. These results suggested that canonical Wnt signaling was activated in the retina of OIR mice, consistent with previous reports [20].
Figure 1. Activation of canonical Wnt signaling in the retina of OIR mice.
Wnt signaling activation was evaluated by X-gal staining of retinal sections of normoxia control (A) or OIR (B) Axin2Lacz mice at P16. Western blot analysis of phosphorylated-LRP6 (C, D) and non-phosphorylated-β-catenin (C, E) was performed using the retinas from the OIR C57BL/6J mice and normoxia control. The expression of Vegf-a (F), a major inflammatory factor, and Gfap (G), a commonly used stress marker, at the mRNA level in the retina of OIR mice was analyzed by qRT-PCR.
3.2. Expression level of miR-184 in the retina of OIR mice and Wnt signaling modulation by miR-184
Following the OIR procedure, the retinas were isolated at P16 for RNA extraction. As shown by qRT-PCR, the level of miR-184 was significantly down-regulated in the retina of OIR mice compared to that in the normoxia control (Fig. 2A). Furthermore, an rMC1-TOP cells were generated using rMC1 cells, a rat Müller cell line [31], infected with lentivirus expressing luciferase reporter gene under a promoter containing TCF/β-catenin-binding sites [32] to evaluate Wnt signaling activity in retinal cells. The rMC1-TOP cells were separately transfected with the negative control miRNA, miR-184 mimic or miR-184 inhibitor. In vitro Wnt signaling activation assay (TOP-Flash assay) was conducted at 48 hr post-transfection. Transfection of miR-184 mimic significantly inhibited Wnt signaling activity, whereas the miR-184 inhibitor significantly enhanced Wnt activity (Fig. 2B). It should be noted that the Wnt signaling activity in the cells treated with W3A-conditioned media increased approximately 5.2-fold over that of negative control L-cell conditioned media (data not shown).
Figure 2. Expression of miR-184 in the retina of OIR mice and regulation of Wnt signaling by miR-184.
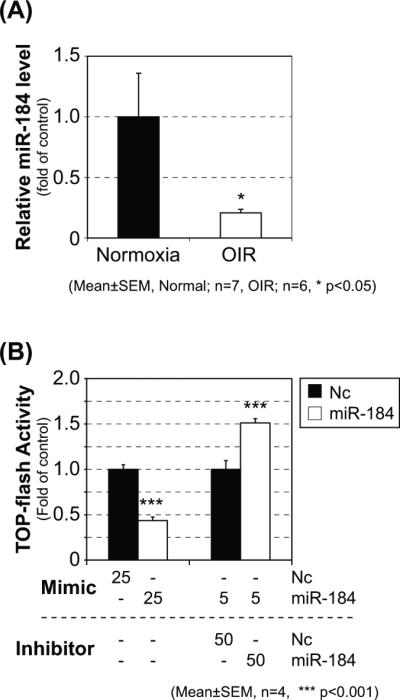
C57BL/6J mice were under room air (normoxia control) or in the oxygen chamber from P7 to P12 (OIR model). Expression levels of miR-184 in the retinas at P16 were evaluated by qRT-PCR (A). MiR-184 mimic and its control (25 nM each) or miR-184 inhibitor and its control (50 nM each plus 5 nM miR-184 mimic) were separately transfected into rMC1-TOP cells stably expressing firefly luciferase under the control of the Wnt/β-catenin system. The cells were treated with W3A-conditioned medium (10%) at 32 hr post-transfection and Wnt signaling activity assay (TOP flash assay) was conducted at 48 hr post-transfection (B).
3.3. Identification of downstream targets of miR-184
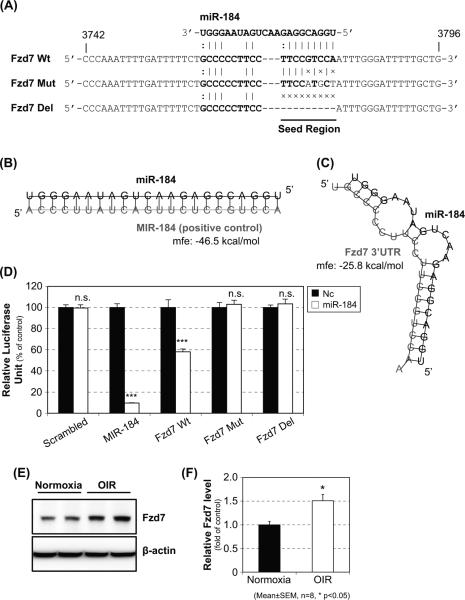
Bioinformatics analysis predicted that the mRNA of frizzled-7 (Fzd7), which is a receptor of canonical Wnt signaling, contains a binding site of miR-184 in the 3’ UTR (Fig. 3A, C). Therefore, the Fzd7 cDNA 3’UTR including the predicted binding site was subcloned into the pmirGLO vector (Fzd7 Wt) (Fig. 3A). The interaction of miR-184 with this binding site in Fzd7 3’UTR was evaluated using luciferase-based assays. The miR-184 mimic transfection significantly down-regulated the expression of firefly luciferase in the cells cotransfected with the positive control (MIR-184) and Wt Fzd7 assay vectors, whereas no effect was observed in the negative control miRNA transfection (Fig. 3D). It should be noted that other predicted downstream target genes of miR-184 related to Wnt signaling did not regulate the expression of firefly luciferase (Suppl. Fig. 1 and 2). To further verify the miR-184-binding site in the 3’UTR of the Fzd7 mRNA, we have mutated and deleted the predicted miR-184-binding site in the assay vectors. Luciferase activity assays showed that miR-184 failed to regulate the reporter gene expression after the binding site was mutated (Fzd7 Mut) or deleted (Fzd7 Del). These results suggest that Fzd7 is a downstream target of miR-184. Furthermore, the protein level of Fzd7 in the retina of OIR mice was evaluated by Western blot analysis, which showed that Fzd7 protein was significantly up-regulated in the retina of OIR mice (Fig. 3E, F).
Figure 3. Verification of downstream target of miR-184.
Approximately 700 bps of the 3’UTR of the Fzd7 cDNA including the predicted miR-184-binding site was subcloned into pmirGLO vector (A; Fzd7 Wt). Further, the predicted binding site of miR-184 (seed region) in the Fzd7 3’UTR was either mutated (A; Fzd7 Mut, 3 nucleotides in seed region were substituted) or deleted (A; Fzd7 Del). Generated pmirGLO vector containing a scrambled sequence of miR-184 was used as a negative control (Scrambled) and (B; MIR-184). The 3’UTR of Fzd7 Wt, Mut, and Del were co-transfected with negative control miRNA mimic (Nc, black bar) or miR-184 mimic (miR-184, white bar). Predicted secondary structure and minimum free energy (mfe) of the miR-184-binding site in the Fzd7 3’UTR were represented (C). At 48 hr post-transfection, luciferase assay was conducted (D). The retinas were collected from normoxia or OIR mice at P16, and Western blot analysis was conducted to evaluate the retinal level of Fzd7 protein (E, F).
3.4. Regulation of Wnt signaling by miR-184 in the retina of OIR mice
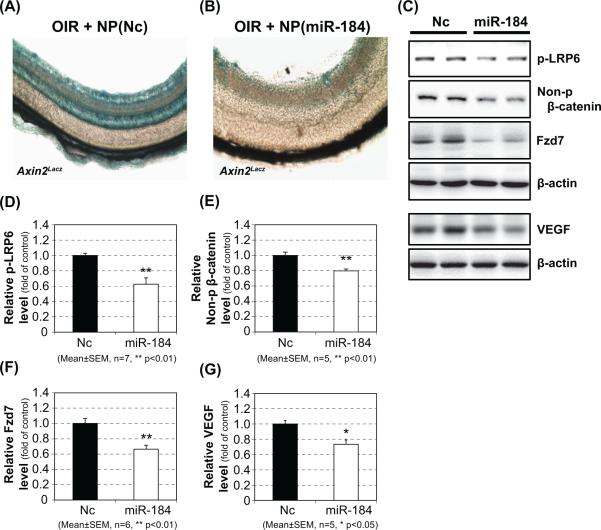
To elucidate the functional role of miR-184 in vivo, the negative control miRNA mimic and miR-184 mimic were separately packed in nanoparticles and injected into the vitreous space of OIR mice at P12. Its impact on Wnt signaling was examined at P16. A retinal section of OIR Axin2Lacz mice injected with the negative control nanoparticle showed intense X-gal staining (Fig. 4A) indicating activation of Wnt signaling. This was consistent with the result in the retinal section of OIR mice without miRNA injection as shown in Figure 1B. On the other hand, the retinal section of OIR mice injected with miR-184 nanoparticles showed substantial reduction of X-gal staining (Fig. 4B) compared to that injected with the negative control miRNA (Fig. 4A), indicating that Wnt signaling was attenuated by miR-184 delivery. In addition, Western blot analyses showed significant reductions of phosphorylated-LRP6 (Fig. 4C, D) and non-phosphorylated-β-catenin (Fig. 4C, E) in the retina of OIR mice injected with miR-184, suggesting attenuation of Wnt signaling. Furthermore, expression levels of Fzd7, which is identified as a downstream target of miR-184 in this study, and Vegf-a, a target gene of Wnt signaling, were reduced in the retina of OIR mice with miR-184 injection (Fig. 4C, F).
Figure 4. Delivery of miR-184 into the retina of OIR mice and its impact on Wn signaling.
Wnt signaling activation was evaluated by X-gal staining of retinal sections on negative control nanoparticle-injected (A, OIR+NP(Nc)) or miR-184 nanoparticle-injected (B, OIR+NP(miR-184)) OIR-Axin2 mice at P16. Western blot analyses of phosphorylated-LRP6 (C, D) and using negative control or miR-184 nanoparticle-injected OIR retina at P16. Further, expression of Fzd7 (C, F), a downstream target of miR-184, and Vegf-a (C, G), a target gene of Wnt signaling, in the retina of the miRNAs-injected OIR mice was evaluated by Western blot analysis.
4. Discussion
Our earlier studies suggested that Wnt signaling is activated in the diabetic retina, which plays a major pathogenic role in retinal inflammation and NV in DR [20,38,39]. However, the role of miRNAs in the Wnt signaling regulation in DR is not well- understood. In the present study, we identified the functional role of miR-184 in ischemia-induced retinal inflammation and NV (oxygen-induced retinopathy, OIR), a mouse model of PDR. We firstly verified that Wnt signaling was activated (Fig. 1), while the expression of miR-184 was significantly down-regulated in the retina of OIR mice (Fig. 2A). These results were consistent with earlier studies [20,27,38,39].
Then, we generated a rat Müller cell line stably expressing a Wnt signaling reporter gene, a firefly luciferase controlled by the Wnt/β-catenin system (rMC1-TOP) to examine whether Wnt signaling in Müller cells is regulated by miR-184, since Wnt signaling activation was predominantly observed in the cells in the inner retina (Fig. 1A). Wnt signaling in the rMC1-TOP cells was significantly inhibited by transfection of the miR-184 mimic, while enhanced by the miR-184 inhibitor (Fig. 2B). These results strongly suggest that there is a link between the reduction of miR-184 expression and activation of Wnt signaling in the retina of OIR mice.
Next, we investigated a new downstream target of miR-184 implicated in canonical Wnt signaling. Through the bioinformatics analysis, mutagenesis of the predicted miR-184-binding site and luciferase-based assays, for the first time we identified that Fzd7 is a direct target gene of miR-184 among a number of candidate target genes in Wnt signaling (Fig. 3 and Suppl. Fig. 1 and 2). In addition, Western blot analysis showed that Fzd7 protein was increased in the OIR mouse retina (Fig. 3E). This provides a further support that Fzd7 is the target gene of miR-184. For comparison, we also examined a luciferase assay vector containing another putative binding site of miR-184 in the 3’UTR of Fzd4 (accession number NM_008055.4: nucleotide number, 3592-3612), and it did not reduce the luciferase expression by miR-184 mimic transfection (Suppl. Fig. 1 and 2). This result is consistent in-line with a previous observation that Fzd4 protein was not reduced in the OIR mouse retina by pre-miR-184 (miR-184 mimic) injection [27].
In addition to miR-184, other miRNAs (miR-1, 23b, 27a) were recently identified as miRNAs regulating Fzd7 in stem cells and cancer cells [40-42], suggesting that these miRNAs may modulate Wnt signaling in the same fashion as miR-184. It was also reported that Fzd7 is involved in both canonical and non-canonical Wnt signaling in cancer cells [43], and Wnt3A acts as a ligand of Fzd7 in canonical Wnt signaling [44]. Moreover, it was suggested that Fzd7 is a target gene of canonical Wnt signaling, since there is a TCF/β-catenin-binding site in the promoter region of the Fzd7 gene [45]. This suggests that Fzd7 may create a positive feedback loop in canonical Wnt signaling, and down-regulation of Fzd7 expression by miR-184 may be ascribed to the modulation of the canonical Wnt signaling.
Interestingly, Dismuke et al. recently showed that miR-184 abundantly exists in exosomes of the intraocular fluid [46]. As described earlier, it was reported that the major sources of miR-184 in the eye are the cornea and lens [23,47]. It is well-established that exosomes containing unique miRNA species are implicated in cell-to-cell communication [48,49]. This exosome-mediated delivery of miR-184 from the cornea and lens might be an additional mechanism responsible for the elevated level of miR-184 in the retina, in addition to up-regulation in retinal cells.
To further establish the causative role of miR-184 in Wnt signaling activation in the retina, we showed that delivery of the miR-184 mimic formulated in nanoparticles substantially inhibited Wnt signaling in the retina of OIR mice (Fig. 4B), compared to the negative control nanoparticle (Fig. 4A). Although the present study did not conduct the functional assays on inflammatory responses, vascular leakage and neovascularization in the retina, our previous studies have demonstrated that inhibition of Wnt signaling in the OIR model ameliorates these pathogenic features [20,38,39].
Another recent study also showed that the level of miR-184 was down-regulated in a mouse model of spinal cord of diabetic neuropathic pain [50]. It was reported that Wnt signaling regulates neuroinflammation to promote chronic pain, and the miR-184 reduction-mediated activation of Wnt signaling causes the development of neuropathic pain [51]. The present study may reveal the therapeutic potential using miR-184 nanoparticles as a new class of drugs for diseases associated with dysregulation of Wnt signaling.
In summary, the present study for the first time showed that delivery (or over-expression) of miR-184 in the retina with ischemia-induced retinal NV inhibits Wnt signaling as well as vegf-a (a major proinflammatory and angiogenic factor) expression. This may offer a new therapeutic strategy to prevent the inflammatory responses, vascular leakage and NV through canonical Wnt signaling in DR.
Supplementary Material
Highlights.
MiR-184 is down-regulated in the retina of OIR mice.
Canonical Wnt signaling is regulated by miR-184.
Fzd7 is a downstream target of miR-184.
Delivery of precursor of miR-184 inhibits Wnt signaling in the retina of OIR mice.
Acknowledgements
This study was supported by National Institutes of Health grants, EY012231, EY018659, EY019309, EY016507, and GM104934, grants from Juvenile Diabetes Research Foundation (JDRF), 2-SRA-2014-147-Q-R, American Heart Association (AHA), 14PRE20460229, IRRF and Oklahoma Center for the Advancement of Science & Technology (OCAST), HR12-103 and HR13-076. We thank for Dr. William Berry at OUHSC to kindly provided lentiviruses expressing TOP-firefly luciferase and renilla luciferase.
Abbreviations
- OIR
oxygen-induced retinopathy
- NV
neovascularization
- miRNA
microRNA
- DR
Diabetic retinopathy
- 3’UTR
3’ un-translated region
- Fzd7
frizzled-7
- Vegf-a
vascular endothelial growth factor-a
- Gfap
glial fibrillary acidic protein
- Hprt1
hypoxanthine phosphoribosyltransferase 1
- TCF/LEF
T-cell factor/lymphoid enhancing factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Landgraf P, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu S. microRNA expression in the eyes and their significance in relation to functions. Prog. Retin. Eye Res. 2009;28:87–116. doi: 10.1016/j.preteyeres.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 2010;11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 4.Kolfschoten IG, Roggli E, Nesca V, Regazzi R. Role and therapeutic potential of microRNAs in diabetes. Diabetes Obes. Metab. 2009;11:118–129. doi: 10.1111/j.1463-1326.2009.01118.x. [DOI] [PubMed] [Google Scholar]
- 5.Meister G. miRNAs get an early start on translational silencing. Cell. 2007;131:25–28. doi: 10.1016/j.cell.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 7.van den Berg A, Mols J, Han J. RISC-target interaction: cleavage and translational suppression. Biochim. Biophys. Acta. 2008;1779:668–677. doi: 10.1016/j.bbagrm.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang G. siRNA and miRNA: an insight into RISCs. Trends. Biochem. Sci. 2005;30:106–114. doi: 10.1016/j.tibs.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Clin. Chem. 2009;55:623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- 10.Pandey AK, Agarwal P, Kaur K, Datta M. MicroRNAs in diabetes: tiny players in big disease. Cell. Physiol. Biochem. 2009;23:221–232. doi: 10.1159/000218169. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention General information and national estimates on diabetes in the United States. National Diabetes Fact Sheet. 2007 [Google Scholar]
- 12.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Antonetti DA, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 15.Stahl A, et al. The mouse retina as an angiogenesis model. Invest. Ophthalmol. Vis. Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 17.George SJ. Wnt pathway: a new role in regulation of inflammation. Arterioscler. Thromb. Vasc. Biol. 2008;28:400–402. doi: 10.1161/ATVBAHA.107.160952. [DOI] [PubMed] [Google Scholar]
- 18.Masckauchan TN, Kitajewski J. Wnt/Frizzled signaling in the vasculature: new angiogenic factors in sight. Physiology (Bethesda) 2006;21:181–188. doi: 10.1152/physiol.00058.2005. [DOI] [PubMed] [Google Scholar]
- 19.Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11:63–69. doi: 10.1007/s10456-008-9095-3. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, et al. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am. J. Pathol. 2009;175:2676–2685. doi: 10.2353/ajpath.2009.080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, Ma JX. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2010;51:4371–4379. doi: 10.1167/iovs.09-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou T, Zhou KK, Lee K, Gao G, Lyons TJ, Kowluru R, Ma JX. The role of lipid peroxidation products and oxidative stress in activation of the canonical wingless-type MMTV integration site (WNT) pathway in a rat model of diabetic retinopathy. Diabetologia. 2011;54:459–468. doi: 10.1007/s00125-010-1943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan DG, Oliveira-Fernandes M, Lavker RM. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol. Vis. 2006;12:1175–1184. [PubMed] [Google Scholar]
- 24.Hughes AE, Bradley DT, Campbell M, Lechner J, Dash DP, Simpson DA, Willoughby CE. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am. J. Hum. Genet. 2011;89:628–633. doi: 10.1016/j.ajhg.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iliff BW, Riazuddin SA, Gottsch JD. A single-base substitution in the seed region of miR-184 causes EDICT syndrome. Invest Ophthalmol Vis Sci. 2012;53:348–353. doi: 10.1167/iovs.11-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bykhovskaya Y, Caiado Canedo AL, Wright KW, Rabinowitz YS. C.57 C > T Mutation in MIR 184 is Responsible for Congenital Cataracts and Corneal Abnormalities in a Five-generation Family from Galicia, Spain. Ophthalmic Genet. 2013:1–4. doi: 10.3109/13816810.2013.848908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen J, Yang X, Xie B, Chen Y, Swaim M, Hackett SF, Campochiaro PA. MicroRNAs regulate ocular neovascularization. Mol. Ther. 2008;16:1208–1216. doi: 10.1038/mt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Alam D, et al. Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2(LacZ) during murine lung development and repair. PLoS One. 2011;6:e23139. doi: 10.1371/journal.pone.0023139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray AR, Chen Q, Takahashi Y, Zhou KK, Park K, Ma JX. MicroRNA-200b downregulates oxidation resistance 1 (Oxr1) expression in the retina of type 1 diabetes model. Invest. Ophthalmol. Vis. Sci. 2013;54:1689–1697. doi: 10.1167/iovs.12-10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Muller cell line. Invest. Ophthalmol. Vis. Sci. 1998;39:212–216. [PubMed] [Google Scholar]
- 32.McBride JD, et al. Elevated circulation levels of an antiangiogenic SERPIN in patients with diabetic microvascular complications impair wound healing through suppression of Wnt signaling. J. Invest. Dermatol. 2014;134:1725–1734. doi: 10.1038/jid.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajala A, Wang Y, Zhu Y, Ranjo-Bishop M, Ma JX, Mao C, Rajala RV. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 2014;14:5257–5263. doi: 10.1021/nl502275s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol. Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 35.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J. Biol. Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 36.Lieth E, Gardner TW, Barber AJ, Antonetti DA. Retinal neurodegeneration: early pathology in diabetes. Clin. Experiment. Ophthalmol. 2000;28:3–8. doi: 10.1046/j.1442-9071.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 37.Wu KH, Madigan MC, Billson FA, Penfold PL. Differential expression of GFAP in early v late AMD: a quantitative analysis. Br. J. Ophthalmol. 2003;87:1159–1166. doi: 10.1136/bjo.87.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee K, Hu Y, Ding L, Chen Y, Takahashi Y, Mott R, Ma JX. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes. 2012;61:2948–2957. doi: 10.2337/db11-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Zhang B, McBride JD, Zhou K, Lee K, Zhou Y, Liu Z, Ma JX. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes. 2013;62:4228–4238. doi: 10.2337/db12-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu TY, et al. Overexpression of microRNA-1 promotes cardiomyocyte commitment from human cardiovascular progenitors via suppressing WNT and FGF signaling pathways. J. Mol. Cell. Cardiol. 2013;63:146–154. doi: 10.1016/j.yjmcc.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, et al. Genome-wide functional screening of miR-23b as a pleiotropic modulator suppressing cancer metastasis. Nat. Commun. 2011;2:554. doi: 10.1038/ncomms1555. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, Ma T, Huang C, Zhang L, Lv X, Xu T, Hu T, Li J. MiR-27a modulates the MDR1/P-glycoprotein expression by inhibiting FZD7/beta-catenin pathway in hepatocellular carcinoma cells. Cell. Signal. 2013;25:2693–2701. doi: 10.1016/j.cellsig.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Ueno K, Hirata H, Hinoda Y, Dahiya R. Frizzled homolog proteins, microRNAs and Wnt signaling in cancer. Int. J. Cancer. 2013;132:1731–1740. doi: 10.1002/ijc.27746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, Wands JR. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J. Hepatol. 2008;48:780–791. doi: 10.1016/j.jhep.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katoh M. Comparative integromics on FZD7 orthologs: conserved binding sites for PU.1, SP1, CCAAT-box and TCF/LEF/SOX transcription factors within 5'-promoter region of mammalian FZD7 orthologs. Int. J. Mol. Med. 2007;19:529–533. [PubMed] [Google Scholar]
- 46.Dismuke WM, Challa P, Navarro I, Stamer WD, Liu Y. Human aqueous humor exosomes. Exp. Eye Res. 2015;132C:73–77. doi: 10.1016/j.exer.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karali M, Peluso I, Gennarino VA, Bilio M, Verde R, Lago G, Dolle P, Banfi S. miRNeye: a microRNA expression atlas of the mouse eye. BMC Genomics. 2010;11:715. doi: 10.1186/1471-2164-11-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc. Res. 2013;100:7–18. doi: 10.1093/cvr/cvt161. [DOI] [PubMed] [Google Scholar]
- 49.Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am. J. Physiol. Cell Physiol. 2014;306:C621–633. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 50.Gong Q, et al. Altered microRNAs expression profiling in mice with diabetic neuropathic pain. Biochem. Biophys. Res. Commun. 2015;456:615–620. doi: 10.1016/j.bbrc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.