Abstract
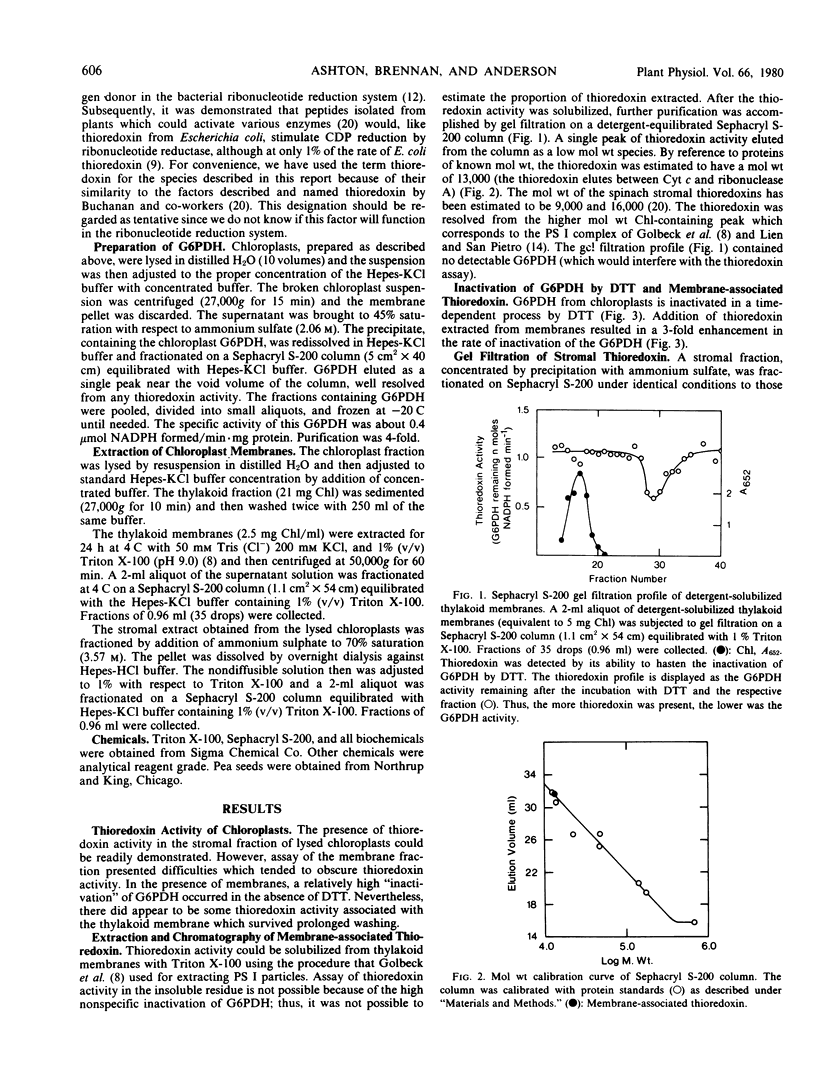
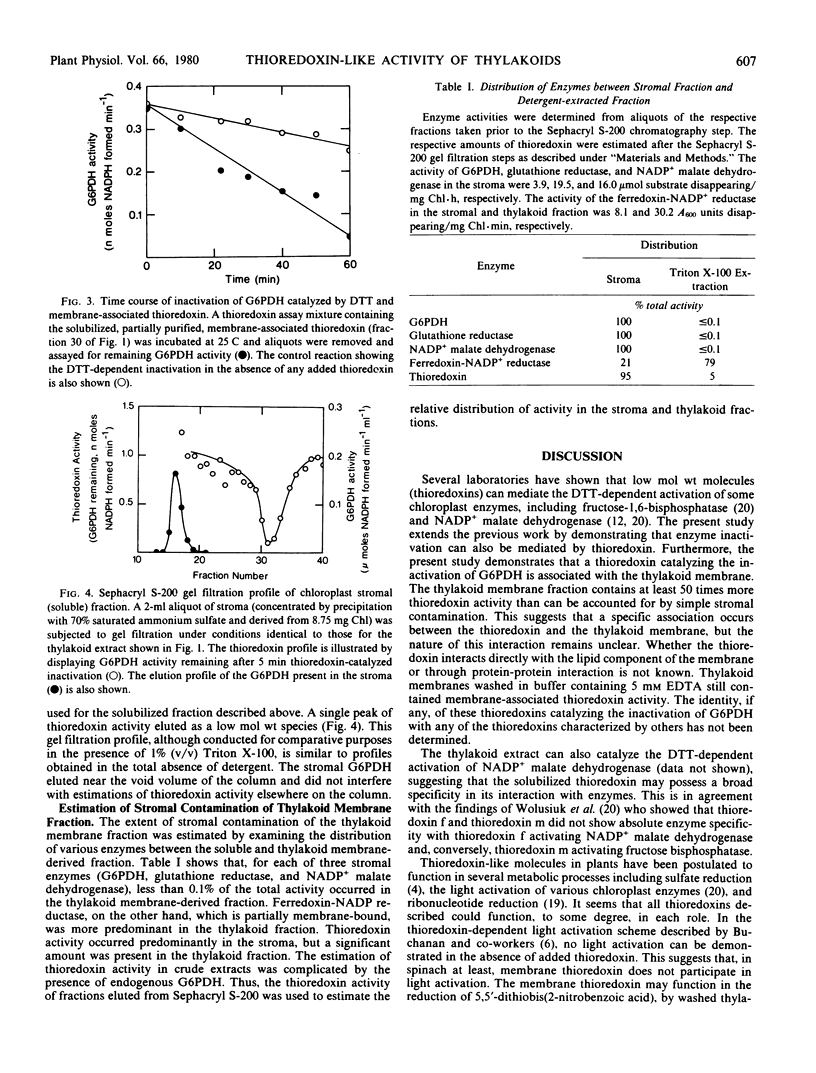
The inactivation of pea leaf chloroplast glucose-6-phosphate dehydrogenase by dithiothreitol can be catalyzed by thioredoxin-like molecules that are present in chloroplasts. This thioredoxin activity occurs predominantly as a soluble species, but washed thylakoid membranes also exhibit some thioredoxin-like activity. The membrane-associated thioredoxin can be extracted by treatment with the detergent Triton X-100. The solubilized thioredoxin appears to have a molecular size similar to that of the soluble thioredoxin which catalyzes the same reaction. The thylakoid-bound activity constitutes only about 5% of the total chloroplast thioredoxin activity. The thioredoxin occurring in the membrane fraction cannot, however, be ascribed to the trapping of stroma since less than 0.1% of three stromal marker enzymes are found in the same thylakoid extract.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Duggan J. X. Light modulation of glucose-6-phosphate dehydrogenase: partial characterization of the light inactivation system and its effects on the properties of the chloroplastic and cytoplasmic forms of the enzyme. Plant Physiol. 1976 Aug;58(2):135–139. doi: 10.1104/pp.58.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Nehrlich S. C., Champigny M. L. Light modulation of enzyme activity: activation of the light effect mediators by reduction and modulation of enzyme activity by thiol-disulfide exchange. Plant Physiol. 1978 Apr;61(4):601–605. doi: 10.1104/pp.61.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi T., Masaki S. Purification and properties of a chloroplast-protein containing photoreducible disulfide bond. J Biochem. 1966 Jul;60(1):90–92. doi: 10.1093/oxfordjournals.jbchem.a128404. [DOI] [PubMed] [Google Scholar]
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. The isolation of spinach chloroplasts in pyrophosphate media. Plant Physiol. 1968 Sep;43(9):1415–1418. doi: 10.1104/pp.43.9.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbeck J. H., Lien S., San Pietro A. Isolation and characterization of a subchloroplast particle enriched in iron-sulfur protein and P700. Arch Biochem Biophys. 1977 Jan 15;178(1):140–150. doi: 10.1016/0003-9861(77)90178-3. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Buchanan B. B., Wolosiuk R. A. Photosynthetic regulatory protein from rabbit liver is identical with thioredoxin. FEBS Lett. 1977 Oct 15;82(2):351–354. doi: 10.1016/0014-5793(77)80619-4. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Reduction of disulfides by thioredoxin. Exceptional reactivity of insulin and suggested functions of thioredoxin in mechanism of hormone action. J Biol Chem. 1979 Sep 25;254(18):9113–9119. [PubMed] [Google Scholar]
- Kagawa T., Hatch M. D. Regulation of C4 photosynthesis: characterization of a protein factor mediating the activation and inactivation of NADP-malate dehydrogenase. Arch Biochem Biophys. 1977 Nov;184(1):290–297. doi: 10.1016/0003-9861(77)90353-8. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- Lien S., San Pietro A. Interaction of plastocyanin and P700 in PSI reaction center particles from C. reinhardi and spinach. Arch Biochem Biophys. 1979 Apr 15;194(1):128–137. doi: 10.1016/0003-9861(79)90602-7. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R., Beck E. On the Mechanism of Activation by Light of the NADP-dependent Malate Dehydrogenase in Spinach Chloroplasts. Plant Physiol. 1979 Nov;64(5):744–748. doi: 10.1104/pp.64.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Wanger W., Follmann H. A thioredoxin from green algae. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1044–1051. doi: 10.1016/s0006-291x(77)80083-1. [DOI] [PubMed] [Google Scholar]
- Wolosiuk R. A., Crawford N. A., Yee B. C., Buchanan B. B. Isolation of three thioredoxins from spinach leaves. J Biol Chem. 1979 Mar 10;254(5):1627–1632. [PubMed] [Google Scholar]


