Abstract
Heat shock protein 72 (Hsp72) protects cells against a variety of stressors, and multiple studies have suggested that Hsp72 plays a cardioprotective role. As skeletal muscle Hsp72 overexpression can protect against high-fat diet (HFD)-induced insulin resistance, alterations in substrate metabolism may be a mechanism by which Hsp72 is cardioprotective. We investigated the impact of transgenically overexpressing (Hsp72 Tg) or deleting Hsp72 (Hsp72 KO) on various aspects of cardiac metabolism. Mice were fed a normal chow (NC) or HFD for 12 weeks from 8 weeks of age to examine the impact of diet-induced obesity on metabolic parameters in the heart. The HFD resulted in an increase in cardiac fatty acid oxidation and a decrease in cardiac glucose oxidation and insulin-stimulated cardiac glucose clearance; however, there was no difference in Hsp72 Tg or Hsp72 KO mice in these rates compared with their respective wild-type control mice. Although HFD-induced cardiac insulin resistance was not rescued in the Hsp72 Tg mice, it was preserved in the skeletal muscle, suggesting tissue-specific effects of Hsp72 overexpression on substrate metabolism. Comparison of two different strains of mice (BALB/c vs. C57BL/6J) also identified strain-specific differences in regard to HFD-induced cardiac lipid accumulation and insulin resistance. These strain differences suggest that cardiac lipid accumulation can be dissociated from cardiac insulin resistance. Our study finds that genetic manipulation of Hsp72 does not lead to alterations in metabolic processes in cardiac tissue under resting conditions, but identifies mouse strain-specific differences in cardiac lipid accumulation and insulin-stimulated glucose clearance.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-015-0571-6) contains supplementary material, which is available to authorized users.
Keywords: Heart, Hsp72, Lipid, Glucose metabolism, Cardiac insulin resistance
Introduction
Heat shock proteins are ubiquitous, highly conserved proteins that play a fundamental role in the maintenance of cellular homeostasis. Heat shock protein 72 (Hsp72; the inducible form of the 70 kDa (HSP70) family of heat shock proteins) is a well-studied heat shock protein which is induced in response to a variety of stressors. Numerous studies have suggested that the induction or overexpression of Hsp72 may play a cardioprotective role in the setting of ischemia (Hutter et al. 1994, 1996; Mandal et al. 2005; Marber et al. 1995; Radford et al. 1996; Trost et al. 1998). Conversely, deletion of the inducible 70-kDa heat shock protein genes in mice (Hspa1a and Hspa1b) impairs cardiac contractile function and calcium handling and is associated with cardiac hypertrophy (Kim et al. 2006), suggesting that Hsp72 is necessary for maintenance of normal cardiac function. Furthermore, reduced cardiac HSP70/Hsp72 or a mutation in the HSP70-Hom gene is associated with an increased risk of atrial fibrillation in humans (St Rammos et al. 2002; Afzal et al. 2008). Together, the data from both animals and human studies suggest that an elevation in Hsp72 levels may be beneficial across a range of cardiac pathologies, while a decrease in Hsp72 may be detrimental to heart health even under resting conditions.
Many of the cardioprotective effects of Hsp72 induction or overexpression have been suggested to be due to its known chaperoning function allowing for maintenance of normal protein function and return of protein synthesis rapidly following insult to the heart (Trost et al. 1998). An alteration in substrate metabolism could, however, also be a contributing protective factor. Myocardial glucose uptake and metabolism are essential for maintaining myocardial energetics especially under circumstances of stress, such as myocardial ischemia or hypertrophy (Patterson et al. 2009). Indeed, stimulating glucose oxidation protects against acute myocardial infarction and reperfusion injury and is a potential therapeutic target (Ussher et al. 2012). This may be especially important in patients with insulin resistance or type 2 diabetes, as multiple studies have shown defective myocardial glucose uptake and insulin resistance in these patients using fluorine 18-labeled fluorodeoxyglucose approaches (Iozzo et al. 2002; Yokoyama et al. 2000). Once cardiac insulin resistance is established, these hearts display differences in both substrate preference and cardiac function. A loss of metabolic flexibility (where the heart cannot modify fuel oxidation in response to nutrient availability sufficiently) is observed in insulin-resistant animals, where palmitate oxidation increases in parallel with a reduced glucose and lactate oxidation rate (Belke et al. 2000; Chatham and Seymour 2002; Wilson et al. 2007). This disruption to cardiac metabolic flexibility is associated with impaired cardiac function (Calligaris et al. 2013; Park et al. 2005; Wilson et al. 2007) and may contribute to increased severity of ischemic injury in insulin-resistant patients.
Additionally, ectopic cardiac intramyocellular lipid accumulation is believed to directly affect cellular cardiac contractions by spatial hindrance of the contractile machinery, making it more difficult to sustain contractions with appropriate amplitude (Unger and Orci 2001). Furthermore, lipid metabolites are known to induce insulin resistance in various insulin-sensitive peripheral tissues and may contribute to inducing cardiac insulin resistance. Experiments in which lipids are overloaded in cultured cardiomyocytes have demonstrated inhibition of insulin signaling in these cells (Chokshi et al. 2012), while reducing myocardial lipid accumulation in a cohort of patients with heart failure reversed cardiac insulin resistance (Chokshi et al. 2012).
We have previously demonstrated that an elevation in skeletal muscle Hsp72 protein expression provides protection against diet or obesity-induced hyperglycemia, hyperinsulinemia, glucose intolerance, and insulin resistance (Chung et al. 2008). In a subsequent study in Hsp72 Tg mice, we showed that skeletal muscle insulin-stimulated glucose clearance, mitochondrial number, and oxidative metabolism were elevated while intramuscular lipid levels were decreased in skeletal muscle (Henstridge et al. 2014). Thus, Hsp72 overexpression may also promote substrate metabolic flexibility in the heart in a state of insulin resistance or diabetes.
Hence, the aim of this study was to examine the role of Hsp72 in substrate utilization in the heart in response to high-fat feeding. We hypothesized that Hsp72 overexpression will alter substrate utilization in the heart, decrease accumulation of intramyocardial lipids, and protect the myocardium from the deleterious effects of high-fat feeding, while deletion of Hsp72 would lead to mitochondrial dysfunction, lipid accumulation, and cardiac insulin resistance.
Materials and methods
Mouse studies
All experiments were approved by the Alfred Medical Research Education Precinct (AMREP) Animal Ethics Committee, and animals were provided humane care in line with the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23, 1985) and in accordance with the National Health and Medical Research Council of Australia Guidelines on Animal experimentation. Animals were administered their prescribed diet and water ad libitum and housed in a temperature-controlled environment (~22 °C) with a 12-h light–dark cycle. Eight-week-old male mice were fed either regular normal chow (NC) (5 % of total energy from fat) or a high-fat diet (HFD) (43 % of total energy from fat) for 12 weeks. Two groups of Hsp72 Tg mice which overexpressed Hsp72 in the heart, skeletal muscle, and brain were studied. The first group was the Hsp72 Tg mice that had been backcrossed ten times onto the C57BL/6J background. These heterozygous Hsp72 Tg mice (designated Hsp72 Tg) were compared with their wild-type littermates. The second group of mice were on a BALB/c background maintained in a homozygous breeding line and compared with wild-type BALB/c mice (designated Hsp72 Tg BALB/c) (Chung et al. 2008; Marber et al. 1995). The Hsp72 KO mice were purchased from the Mutant Mouse Regional Resource Centers (MMRRC) and crossed with C57BL/6J mice to create heterozygous mice. This line has the two genes responsible for induction of Hsp72 (Hspa1a and Hspa1b) deleted (whole body) (Drew et al. 2014). The colony was then maintained with heterozygous x heterozygous breeding to allow for comparison with wild-type littermates. Heterozygous (−/+) and homozygous (−/−) knockouts were both used in the study. Hsp72 expression levels of the various models are provided in Supplementary Fig. 1.
Metabolic testing
Body composition analysis
Fat mass and lean body mass were determined using the EchoMRI 4-in-1 (Echo Medical Systems, Houston, USA).
Intravenous insulin with glucose tracer experiments
Mice were anesthetized and had their jugular vein cannulated. A bolus injection of insulin (0.6 U/kg of lean body mass) with [3H]deoxyglucose ([3H]2-DG); 10 μCi; PerkinElmer, Waltham, MA) was administered via the cannula. Blood (10 μL) was taken at 0, 2, 5, 10, 15, 25, and 35 min from the tail for the determination of blood glucose and [3H]2-DG. Following the final time point, tissues were collected, snap frozen, and stored at −80 °C for later glucose clearance analysis.
Ex vivo assays
Palmitate oxidation
Tissues were removed, weighed, and immediately placed in ice-cold homogenizing buffer (250 mM sucrose, 10 mM Tris-HCl, 1 mM EDTA, pH 7.4) at 1:19 w/v. Tissues were minced with scissors before being homogenized with a mortar and pestle. Fifty microliters of homogenate was added to 450 μL oxidation buffer (111 mM sucrose, 11.1 mM Tris-HCl, 5.56 mM KH2PO4, 1.11 mM MgCl2, 88.9 mM KCl, 0.222 mM EDTA, 1.11 mM DTT, 2.22 mM ATP, 0.33 % fatty acid-free BSA, 2.22 mM carnitine, 0.056 mM CoA, 0.111 mM malate, 222 μM palmitate + 0.5 μCi/mL [1-14C]-palmitic acid pH 7.4) in a tightly capped 7-mL scintillation vial containing an Eppendorf with 100 μL 1 M NaOH. The vial was incubated in a shaking water bath at 30 °C for 90 min. The buffer was acidified by the addition of 100 μL 1 M perchloric acid, and the vials were recapped and left for 2 h on an orbital shaker to trap CO2. Then, 100 μL NaOH was added to scintillation fluid to measure complete oxidation while the acid-soluble metabolites were measured by centrifuging the buffer for 5 min at 13,000 rpm and taking 100 μL to add to the scintillation fluid.
Glucose oxidation
Hearts were excised and a 5 % homogenate prepared in ice-cold homogenization buffer (250 mM sucrose, 10 mM Tris-HCl, 1 mM EDTA, pH 7.4). Glucose oxidation was initiated by the addition of oxidation buffer (0.5 μCi/mL [14C]-glucose, 111 mM sucrose, 11.1 mM Tris-HCl, 5.56 mM KH2PO4, 1.11 mM MgCl2, 88.9 mM KCl, 1.11 mM DTT, 2.22 mM ATP, 2 % fatty acid-free BSA, pH 7.4) to homogenates, followed by incubation at 37 °C for 2 h. One molar of HClO4 acid was added to allow [14CO2] produced from oxidation to be released and captured in 1 M NaOH. NaOH was removed and [14C] measured using a scintillation β-counter.
Isolated mitochondrial respiration
Oxygen consumption rates were measured in isolated mitochondria from the hearts of mice fed a NC or HFD based on the methods of Liesa et al. (2011). Briefly, hearts were incubated and minced in ice-cold fiber relaxation buffer (KCl 100 mM, EGTA 5 mM, HEPES 5 mM, pH 7.4) and then homogenized in 2 mL HES buffer (HEPES 5 mM, EDTA 1 mM, sucrose 0.25 mM, pH 7.4) with a glass dounce homogenizer. The homogenate was centrifuged at 500g for 10 min at 4 °C before the supernatant was recentrifuged at 500g. The supernatant was centrifuged at 9000g for 15 min at 4 °C, and the mitochondrial pellet resuspended in 150 μL HES buffer with 0.2 % BSA fatty acid free. Protein was quantified with BCA (Pierce Reagent kit), and the value of the protein measured in HES-BSA 0.2 % buffer alone was subtracted. Basal, ADP-stimulated state III (3 mM), and carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP)-stimulated uncoupled respiration (1 μM) were measured in isolated mitochondria preps in an XFe-96 Seahorse Bioanalyser (2.5 μg/well centrifuged at 2000g for 15 min to adhere the mitochondria to the bottom of the plate) in the ice-cold mitochondrial assay buffer (MAS: sucrose 70 mM, mannitol 220 mM, KH2PO4 5 mM, MgCl2 5 mM, HEPES 2 mM, EGTA 1 mM, BSA fatty acid free 0.2 %, pH 7.4 adjusted with KOH 1 mol/L). The MAS buffer was supplemented with the presence of complex 1 substrates (10 mM pyruvate, 10 mM malate).
Biochemical analysis
Lipidomics
Lipid content was determined in the cardiac tissue using the methods previously described (Henstridge et al. 2012; Matthews et al. 2010; Weir et al. 2013). Briefly, samples (20–30 mg wet weight) were homogenized in 100 μL PBS buffer, pH 7.47. Lipids were extracted from 25 μg of protein using 20 volumes of chloroform/methanol (2:1) in a single phase extraction process, recovering all lipids in a single phase suitable for liquid chromatography–mass spectrometry analysis. Lipid analyses were performed by liquid chromatography, electrospray ionization–tandem mass spectrometry using an HP 1200 liquid chromatography system combined with a AB Sciex API 4000 Q/TRAP mass spectrometer with a turbo ion spray source (350 °C) and Analyst 1.5 data system. The lipid classes investigated included triacylglycerol (TG), diacylglycerol (DG), and ceramide. Individual lipid species of each class of lipid were summed to give the total pool for each lipid class.
Determination of plasma and tissue radioactivity
Blood samples (10 μL) were deproteinized with barium hydroxide (0.3 N) and zinc sulfate (0.3 N), and [3H]2-DG radioactivity was determined by liquid scintillation counting. Accumulation of [3H]2-DG was determined in an aqueous extract of tissue after homogenization. Free and phosphorylated [3H]2-DG were separated by ion exchange chromatography on Dowex l-X8 columns (acetate form). The area under the tracer disappearance curve of [3H]2-DG together with the radioactivity for the phosphorylated [3H]2-DG from individual tissues (gastrocnemius, quadriceps, white adipose tissue (WAT), and heart) was used to calculate the tissue-specific glucose clearance (Kg′).
Western blotting
Heart samples were lysed and protein concentration was determined and resolved by SDS-PAGE as previously described (Henstridge et al. 2012). Immunoblotting was performed using the following primary antibodies: Hsp72, Hsp90 (Enzo Life Sciences (formerly Stressgen), PA, USA), and GAPDH (Cell Signaling, Danvers, USA).
Statistics
Logarithmic transformation was applied to end points not meeting normality assumptions. All data are presented as mean ± standard error of the mean (SEM) and statistical significance was set at p < 0.05. Data were analyzed by two-way analysis of variance and Tukey post hoc tests. Asterisk indicates a diet effect and dagger indicates a genotype effect. In the figures, a straight line represents a main effect, while lines with ticks between two specific groups represent a significant interaction.
Results
Hsp72 Tg mice backcrossed onto a C57BL/6J background gain less fat mass on a HFD but have normal heart size
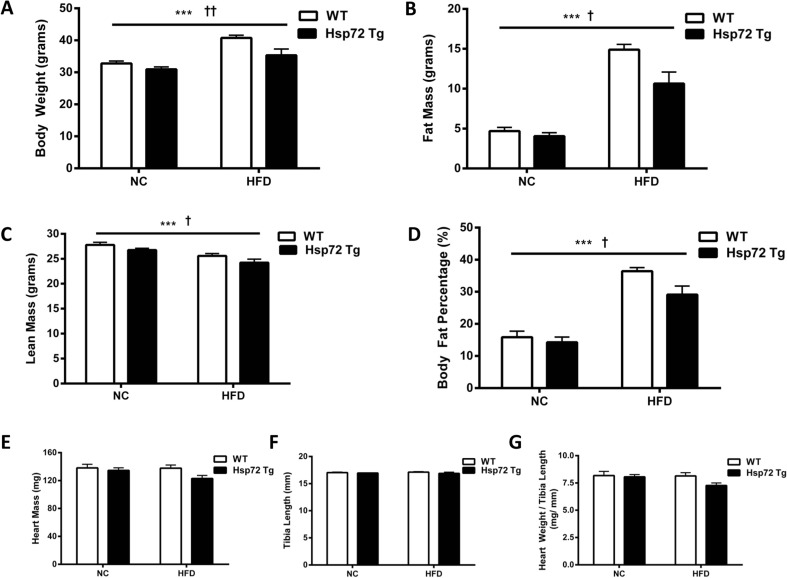
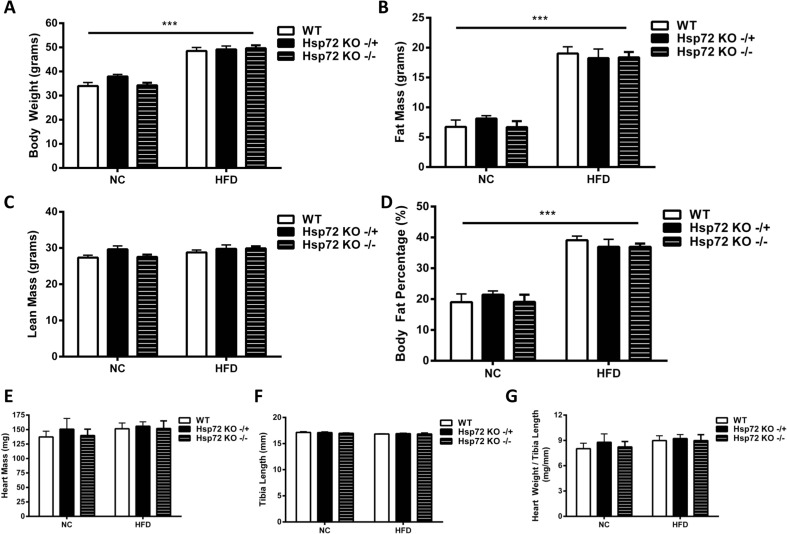
We performed a comprehensive cardiac metabolic analysis of both gain of function (Hsp72 Tg) and loss of function (Hsp72 KO) colonies of mice. Under HFD conditions, heterozygous Hsp72 Tg mice on a C57BL/6J background were leaner compared with littermate control mice (Fig. 1a–d), while no difference in heart mass was observed even when mass was expressed relative to body size (Fig. 1e–g).
Fig. 1.
Hsp72 Tg mice are protected from HFD-induced weight gain and have normal heart size. Mice were fed a normal chow (NC) or high-fat diet (HFD) for 12 weeks from 8 weeks of age. a Body weight, b fat mass, c lean mass, and d body fat percentage, n = 8–9. (Body composition characteristics of this cohort of mice were included as part of a larger population of mice in Henstridge et al. (2014). e Heart mass, n = 8–15; f tibia length, n = 7–10; and g heart weight to tibia length ratio, n = 7–10 per group. ***p < 0.001 for dietary effect; † p < 0.05, †† p < 0.01 for genotype effect. All data are presented as mean ± SEM
Hsp72 Tg mice have lower blood glucose levels following insulin administration which is due to skeletal muscle and not cardiac tissue glucose clearance
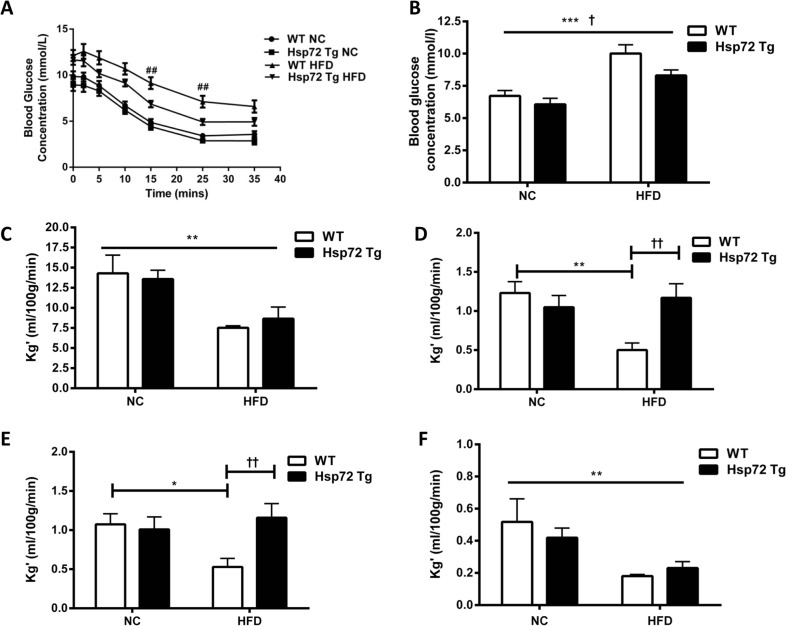
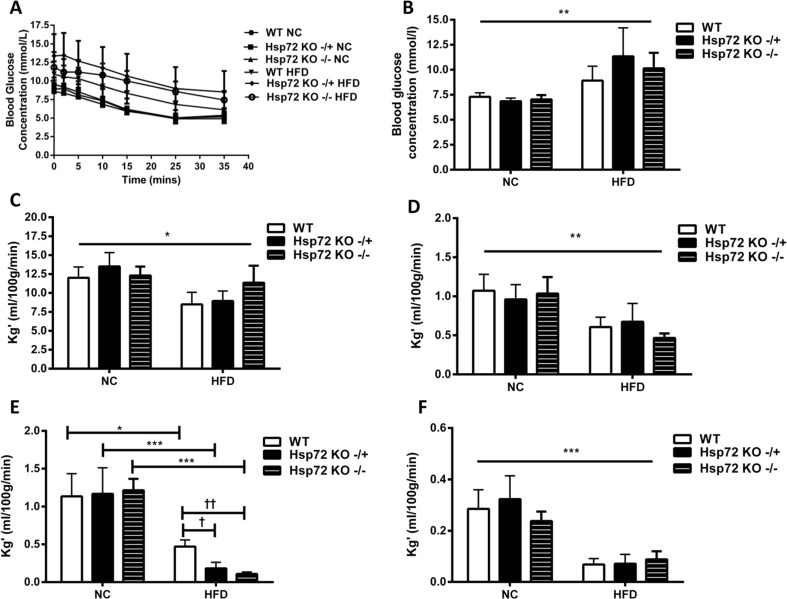
We have previously demonstrated a role for Hsp72 overexpression in modulating skeletal muscle insulin action (Chung et al. 2008; Henstridge et al. 2014). To characterize whether this also held true in cardiac muscle, we evaluated cardiac glucose clearance after an insulin bolus using labeled 2-DG tracer. While high-fat feeding resulted in higher plasma glucose concentrations following insulin stimulation (Fig. 2a, b), the high-fat-fed Hsp72 Tg mice were protected with significantly lower glucose levels (Fig. 2a, b). Next, we analyzed the tissue-specific glucose clearance of various insulin-sensitive peripheral tissues. There was a significant reduction in cardiac glucose clearance with high-fat feeding (Fig. 2c), but no impact of Hsp72 overexpression. In contrast, there was a marked increase in glucose clearance rates in two different skeletal muscles (quadriceps and gastrocnemius) of Hsp72 Tg mice on the HFD (Fig. 2d, e), while glucose clearance into WAT decreased with HFD and was unaltered by Hsp72 overexpression (Fig. 2f).
Fig. 2.
On a HFD, Hsp72 Tg mice maintain whole body insulin responsiveness and skeletal muscle glucose clearance but not cardiac glucose clearance. Mice were fed a normal chow (NC) or high-fat diet (HFD) for 12 weeks from 8 weeks of age. a Blood glucose response following an intravenous injection of insulin and b average blood glucose concentration over the 35-min period post insulin/tracer injection. c Cardiac glucose clearance rate, d skeletal muscle glucose clearance (quadriceps), e skeletal muscle glucose clearance (gastrocnemius), and f white adipose tissue (epididymal fat pad) glucose clearance, n = 6–8 per group. *p < 0.05, **p < 0.01, ***p < 0.001 for dietary effect; † p < 0.05, †† p < 0.01 for genotype effect; ## p < 0.01 for a direct comparison between WT HFD and Hsp72 Tg HFD groups by a t test. All data are presented as mean ± SEM
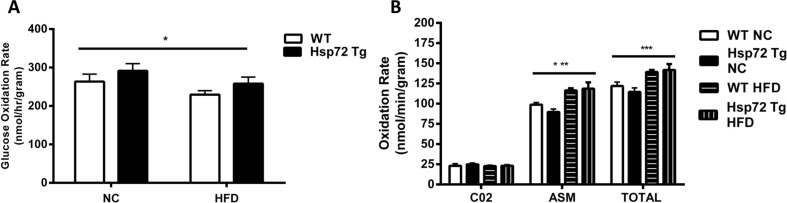
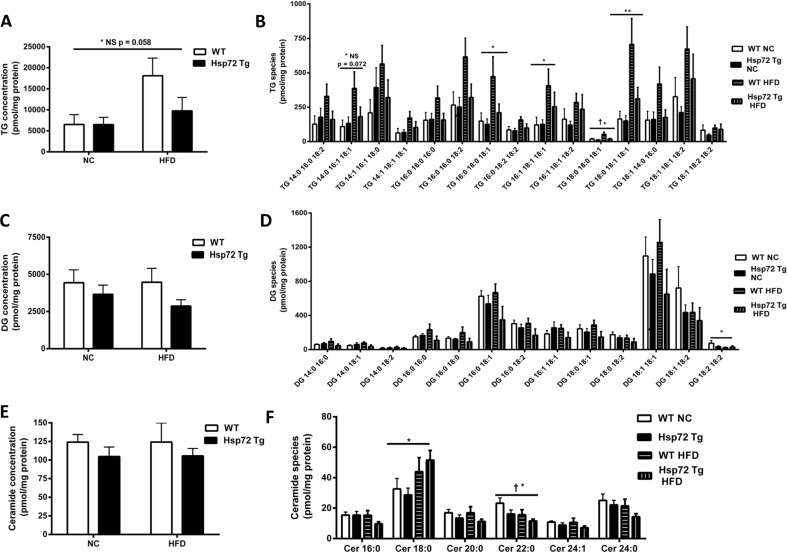
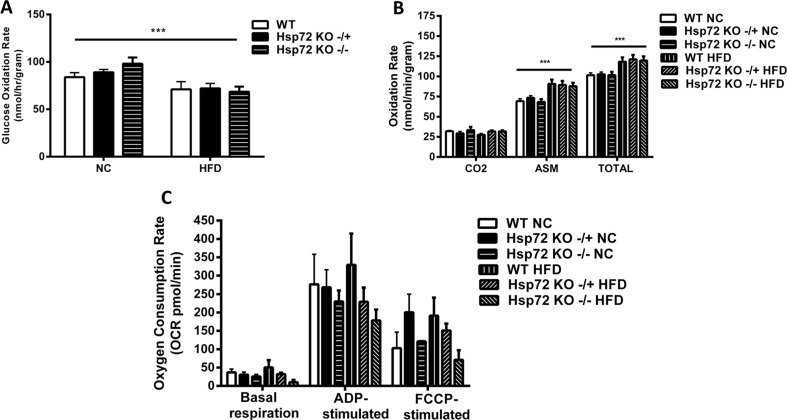
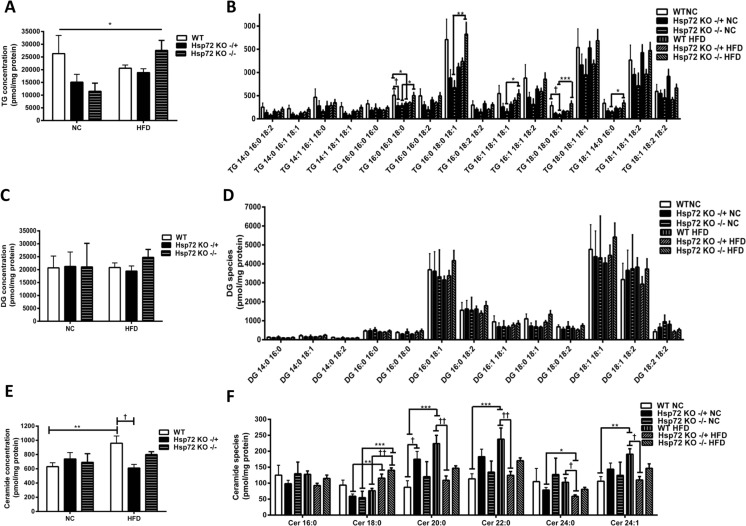
In complementary experiments, we further analyzed the cardiac phenotype in these mice by measuring glucose and palmitate oxidation rates. We observed a significant decrease in glucose oxidation rates (Fig. 3a) and a significant increase in fatty acid oxidation rate (Fig. 3b) with the HFD. However, in line with the insulin-stimulated glucose clearance data, there was no effect of Hsp72 Tg overexpression on these measures. As we have previously observed that Hsp72 Tg mice have a decrease in intramuscular lipid levels after HFD (Henstridge et al. 2014), we hypothesized a similar finding for the heart. Therefore, we performed a comprehensive cardiac lipidomic analysis on the tissues using mass spectroscopy. There was a trend for an increase in cardiac total TG levels with high-fat feeding (p = 0.058) (Fig. 4a) and a decrease with Hsp72 Tg overexpression that did not reach statistical significance. While total TG content did not reach statistical significance, numerous TG molecular species such as TG 16:0_16:0_18:1, TG 16:1_18:1_18:1, TG 18:0_18:0_18:1, and TG 18:0_18:1_18:1 were significantly elevated with the HFD (Fig. 4b). DG is thought to be a more deleterious lipid species than TG; however, there was no HFD or Hsp72 overexpression-induced effect on DG accumulation in the mice (Fig. 4c, d). Ceramides are thought to be a pathogenic lipid species because they promote inflammatory signaling (Holland and Summers 2008). Analysis of total ceramide levels was not altered in these tissues with diet or genotype (Fig. 4e) although Cer 18:0 was elevated with HFD and Cer 22:0 decreased with HFD and Hsp72 overexpression (Fig. 4f).
Fig. 3.
High-fat feeding causes a decrease in glucose oxidation and an increase in palmitate oxidation rate which is not altered by overexpression of Hsp72. Mice were fed a normal chow (NC) or high-fat diet (HFD) for 12 weeks from 8 weeks of age. a Glucose oxidation rate, n = 6–9 per group. b Palmitate oxidation rate, n = 6–10 per group. The palmitate oxidation rates were calculated in the CO2 component and the acid-soluble metabolite (ASM) fraction, and the CO2 and ASM fractions summed to calculate total oxidation rate. Asterisk indicates trend for a main effect of diet. All data are presented as mean ± SEM. *p < 0.05, ***p < 0.001 for dietary effect. All data are presented as mean ± SEM
Fig. 4.
Lipidomic analysis of cardiac triacylglycerol (TG), diacylglycerol (DG), and ceramide in Hsp72 Tg mice fed a NC or HFD. Mice were fed a NC or HFD for 12 weeks from 8 weeks of age. a Total TG levels and b individual cardiac TG molecular lipid species. c Total DG levels and d individual cardiac DG molecular lipid species. e Total ceramide levels and f individual cardiac ceramide molecular lipid species. n = 5–9 per group. *p < 0.05, **p < 0.01 for dietary effect; † p < 0.05 for genotype effect. All data are presented as mean ± SEM
Deletion of Hsp72 does not alter body composition characteristics, heart mass, or insulin-stimulated glucose clearance after 12 weeks of HFD feeding
Next we performed the inverse experiments by analyzing the hearts from mice with Hsp72 deletion (Hsp72 KO). Body composition analysis revealed an increase in body weight, fat mass, and body fat percentage (Fig. 5a–d) with high-fat feeding, but in line with previous analysis of these mice at 20 weeks of age (Drew et al. 2014), no difference in weight was detected with the deletion of Hsp72. Consistent with what was observed with the Hsp72 Tg mice, no difference in heart mass was detected (Fig. 5e–g). Moreover, when insulin-stimulated glucose clearance was analyzed, the HFD induced hyperglycemia, but no difference was noted with the deletion of Hsp72 (Fig. 6a, b). Tissue-specific glucose clearance analysis revealed a decrease in cardiac clearance with the HFD but no difference in the Hsp72 KO groups compared to wild type (Fig. 6c). Similar findings were also found for the skeletal muscle and WAT (Fig. 6d–f), although the Hsp72 KO −/+ and −/− groups were more insulin resistant specifically in the gastrocnemius muscle on the HFD (Fig. 6e).
Fig. 5.
At 20 weeks of age, Hsp72 KO do not have a whole body weight phenotype and have normal heart size. Mice were fed a normal chow (NC) or high-fat diet (HFD) for 12 weeks from 8 weeks of age. a Body weight, b fat mass, c lean mass, and d body fat percentage, n = 6–9. e Heart mass, f tibia length, and g heart weight to tibia length ratio, n = 3–6 per group. ***p < 0.001 for dietary effect. All data are presented as mean ± SEM
Fig. 6.
On a HFD, Hsp72 KO mice have similar whole body insulin responsiveness and skeletal muscle and cardiac glucose clearance as wild-type mice. Mice were fed a normal chow (NC) or high-fat diet (HFD) for 12 weeks from 8 weeks of age. a Blood glucose response following an intravenous injection of insulin and b average blood glucose concentration over the 35-min period, n = 4–9. c Cardiac glucose clearance rate, d skeletal muscle glucose clearance (quadriceps), e skeletal muscle glucose clearance (gastrocnemius), and f white adipose tissue (epididymal fat pad) glucose clearance, n = 4–7 per group. *p < 0.05, **p < 0.05, ***p < 0.001 for dietary effect. † p < 0.05, †† p < 0.05 for genotype effect. All data are presented as mean ± SEM
Deletion of Hsp72 does not impact cardiac glucose or palmitate oxidation rates or the ability of the mitochondria to respire
In parallel to the results obtained in the Hsp72 Tg cohort, high-fat feeding resulted in a decrease in cardiac glucose oxidation rate (Fig. 7a) and an increase in palmitate oxidation rate (Fig. 7b). No effect of Hsp72 deletion was detected for either parameter (Fig. 7a, b). As it has been noted that Hsp72 KO mice have a disrupted skeletal muscle mitochondrial morphology (Drew et al. 2014), we isolated fresh mitochondria from the hearts of these mice and analyzed their ability to consume oxygen in the basal state and in response to the addition of ADP and the oxidative phosphorylation uncoupling agent FCCP. There was no difference between the groups in relation to high-fat feeding or the deletion of Hsp72 in the basal state. While ADP and FCCP stimulated respiration, there was no diet or genotype effect on these rates (Fig. 7c). Additionally, we analyzed the lipids in the cardiac tissue of the Hsp72 KO mice. While there were some small changes in various TG species TG 16:0_16:0_18:0, TG 16:0_18:0_18:1, TG 16:1_18:1_18:1, TG 18:0_18:0_18:1, and TG 18:1_14:0_16:0 predominantly between the Hsp72 KO −/− NC vs. HFD groups (Fig. 8b), the total pool of TG was only modestly altered by HFD (Fig. 8a). Similar to the Hsp72 TG mice, no difference was observed between the groups for DG accumulation (Fig. 8c, d). Finally, while the HFD increased cardiac ceramide levels in the HFD wild-type group (Fig. 8e), deletion of Hsp72 blunted this effect (Fig. 8e) and was most obviously seen in the Cer 20:0, Cer 22:0, and Cer 24:1 molecular species (Fig. 8f).
Fig. 7.
High-fat feeding causes a decrease in glucose oxidation and an increase in palmitate oxidation rate which is not altered by deletion of Hsp72. Mice were fed a normal chow (NC) or high-fat diet (HFD) for 12 weeks from 8 weeks of age. a Glucose oxidation rate, n = 7–9 per group. b Palmitate oxidation rate, n = 7–8 per group. The palmitate oxidation rates were calculated in the CO2 component and the acid-soluble metabolite (ASM) fraction, and the CO2 and ASM fractions summed to calculate total oxidation rate. c Oxygen consumption rates in isolated cardiac mitochondrial fractions, n = 4–7. ***p < 0.001 for dietary effect. All data are presented as mean ± SEM
Fig. 8.
Cardiac lipidomic analysis of triacylglycerol (TG), diacylglycerol (DG), and ceramide lipids in Hsp72 KO NC or HFD mice. Mice were fed a NC or high-fat diet for 12 weeks from 8 weeks of age. a Total TG levels and b individual cardiac TG molecular lipid species. c Total DG levels and d individual cardiac DG molecular lipid species. e Total ceramide levels and f individual cardiac ceramide molecular lipid species. n = 4–6 per group. *p < 0.05, **p < 0.01, ***p < 0.001 for dietary effect; † p < 0.05, †† p < 0.01 for genotype effect. All data are presented as mean ± SEM
Overexpression of Hsp72 does not alter heart size, insulin-stimulated glucose clearance, or fatty acid oxidation rate in Hsp72 BALB/c mice
As our previous work on Hsp72 overexpression in skeletal muscle was predominantly carried out in mice on a BALB/c background (Chung et al. 2008; Henstridge et al. 2014) and considering different mouse strains can have different total levels and rates of lipid accumulation (Kahle et al. 2013; Montgomery et al. 2013), we also conducted analyses on the hearts from these mice. Similar to the Hsp72 Tg mice on the C57BL/6J background, no difference in heart mass was observed (Supp Fig. 2A) even when mass was expressed relative to body size (Supp Fig. 2B, C). To test the insulin-stimulated response, we coinjected insulin with a 2-DG tracer. Surprisingly, we saw no HFD-induced decrease in cardiac glucose clearance rates or any effect of the transgenic overexpression (Supp Fig. 2D). As we had also previously observed an increase in palmitate fatty acid oxidation rates in the skeletal muscle of these transgenic mice (Henstridge et al. 2014), we assessed this measure in the cardiac tissue. While there was a trend for an increase in total oxidation rate with high-fat feeding (due to an increase in the acid-soluble metabolite fraction) genetic overexpression of Hsp72 had no impact (Supp Fig. 2E).
High-fat feeding and overexpression of Hsp72 alters the cardiac lipidomic profile in Hsp72 BALB/c mice
Lipidomic analysis demonstrated a significant increase in total TG accumulation with high-fat feeding (Supp Fig. 3A) which was predominantly due to increases in the TG 16:0_18:0_18:1, TG 16:1_18:1_18:1, TG 18:0_18:1_18:1, and TG 18:1_18:1_18:2 molecular species (Supp Fig. 3B). There was a trend for genetic overexpression of Hsp72 to decrease cardiac TG (Supp Fig. 3A) primarily due to decreases in some of the shorter TG species TG 14:0_16:0_18:2, TG 14:0_16:1_18:1, TG 14:1_16:1_18:0, TG 14:1_18:1_18:1 as well as TG 16:1_18:1_18:1 and TG 18:1_14:0_16:0 (all p values between 0.05 and 0.07) (Supp Fig. 3B). DG concentration was elevated with high-fat feeding but not altered with Hsp72 expression (Supp Fig. 3C) with significant increases in DG 14:0_18:2, DG 18:0_18:1, DG 18:0_18:1, DG 18:0_20:4, DG 16:0_22:5, and DG 16:0_22:6 (Supp Fig. 3D). Surprisingly, total ceramide was elevated with high-fat feeding in the Hsp72 Tg group only (Supp Fig. 3E) due to greater Cer 20:0, Cer 22:0, and Cer 24:0 than in the wild-type HFD group (Supp Fig. 3F).
Discussion
Despite the documented alterations in metabolic processes in the skeletal muscle of Hsp72 gain of function (Hsp72 Tg) or Hsp72 loss of function models (Hsp72 KO) (Chung et al. 2008; Drew et al. 2014; Henstridge et al. 2014), our study finds little evidence that these changes also occur in cardiac tissue. This suggests that Hsp72 may play an organ-specific role in modulating metabolic processes. Further, while our primary aim was to investigate the effects of genetic manipulation of Hsp72 in cardiac metabolism, our study has identified mouse strain-specific differences in relation to cardiac lipid accumulation and insulin-stimulated glucose clearance. These are important considerations for researchers designing future experiments in the cardiac metabolism field.
Given the similarities between skeletal muscle and cardiac muscle (e.g., both cardiac and skeletal muscles are striated muscle, containing contractile proteins for force generation arranged in sarcomeres), we hypothesized that many of the previous findings we had identified in the skeletal muscle upon Hsp72 genetic manipulation would hold true in cardiac tissue. However, this was not the case, with cardiac insulin-stimulated glucose clearance, glucose oxidation, and palmitate oxidation all being similar in Hsp72 Tg and Hsp72 KO mice compared to their relevant control groups. Due to the maintenance of insulin-stimulated glucose clearance in the Hsp72 Tg HFD mice, into two different skeletal muscles (quadriceps and gastrocnemius) (in Fig. 1d, e), and the lack of an effect in the heart (Fig. 1c), there are likely tissue-specific effects of Hsp72 genetic manipulation. This could be due to the differences in substrate utilization between skeletal muscle and cardiac muscle. Under resting conditions, the heart is far more reliant on the use of fatty acids (up to 80 %) than skeletal muscle is. Given Hsp72 overexpression failed to improve HFD-induced cardiac insulin resistance, it is unlikely that targeting Hsp72 with mimetics (which would not increase Hsp72 expression to as high a level) would be a viable treatment option for cardiac insulin resistance.
A somewhat unexpected result was observed in relation to ceramide levels in the various models. Total ceramide concentration was elevated in the cardiac tissue from the Hsp72 Tg BALB/c mice (Supp Fig. 3E), while deletion of Hsp72 protected against the HFD-induced rise in ceramide concentration that was observed in wild-type control mice (Fig. 8e). Interestingly, strengthening the ceramide/Hsp72 connection, elevating Hsp72 levels has previously been demonstrated to suppress ceramide-mediated apoptosis (Ahn et al. 1999; Buzzard et al. 1998). Closer inspection of the ceramide molecular species data revealed that two of the ceramide species that were most increased within the Hsp72 Tg BALB/c HFD group (Cer 20:0 and Cer 22:0) were also the species that were most decreased in the Hsp72 KO analysis. Hsp72 may therefore be involved in the differential modulation of ceramide synthesis of differing chain length. This is likely achieved through the differential modulation of ceramide synthase 1 (CerS 1), reported to be specific for the synthesis of Cer 18:0; CerS 4 which also shows specificity for C18:0 and C20:0 acyl chains and CerS 2 which shows specificity for the longer chain acyl species C20:0, C22:0, C24:0, and C24:1 (Riebeling et al. 2003). Interestingly these enzymes show tissue-specific expression with CerS 1 expressed primarily in muscle; CerS4 in the kidney, heart, spleen, and lung; and CerS2 primarily in the liver. While ceramide, as a class, has been associated with insulin resistance and type 2 diabetes, Cer 18:0 specifically has been shown to have a strong association with type 2 diabetes and prediabetes in plasma (Meikle et al. 2013), while its expression in muscle has been shown to be inversely associated with alterations in glucose tolerance in a mouse model of insulin resistance (Frangioudakis et al. 2010). Herein, we show in our three mouse models that Cer 18:0 is increased in cardiac tissue upon high-fat feeding (Figs. 4f and 8f, Supp Fig. 3F); however, overexpression of Hsp72 has no effect on its accumulation. Whether the changes observed in Cer 18:0 with high-fat feeding is derived from cardiac tissue or represents transfer of Cer 18:0 from the muscle is unknown and requires further investigation.
Another finding from the study was the strain-specific differences that were observed. While the HFD-fed mice on a BALB/c background displayed cardiac lipid accumulation as evidenced by an increase in TG and DG lipids, this did not correspond with cardiac insulin resistance (Supp Fig. 2D). That is, in these mice, cardiac lipid accumulation was dissociated from cardiac insulin resistance. Conversely, the mice on a C57BL/6J background displayed modest increases in TG and no increase in DG lipid accumulation but did have HFD-induced cardiac insulin resistance (Figs. 2c and 6c). A recent report documented the skeletal muscle and liver lipid accumulation in five different strains of mice fed a HFD (Montgomery et al. 2013). In accordance with the present study, this previous study (Montgomery et al. 2013) also identified strain-specific differences in organ lipid accumulation between BALB/c and C57BL/6 mice. While C57BL/6 mice have increased skeletal muscle TG and liver TG and DG, the BALB/c mice had increased skeletal muscle TG but no such increase in TG and DG in the liver that was observed with C57BL/6 mice (Montgomery et al. 2013). While this previous study did not analyze the lipids in the heart, it is tempting to speculate, given our data, that the lipids in the liver were not elevated as they were preferentially deposited in the heart. Interestingly, different cardiac phenotypes can be detected not only between different strains of mice but even between different substrains of the same strain of mouse. A recent paper was able to clearly identify substrain-specific differences in response to cardiac pressure overload within C57BL/6 mice (Garcia-Menendez et al. 2013). Together, these data indicates that cardiac lipid accumulation is not necessary to cause cardiac insulin resistance, and when cardiac lipid accumulation is present, this does not automatically result in cardiac insulin resistance. Given these differences, it may be advantageous for researchers to utilize the BALB/c strain to investigate aspects of cardiac lipid accumulation, while the C57BL/6J model may be best utilized for studies into cardiac insulin resistance.
A limitation to this study was that besides the use of the HFD, the experimentation took place under stress-free conditions. Thus, all of the measures were made in the basal state when the cardiac tissue is not compromised. It is possible that under more stressful conditions (e.g., ischemia reperfusion, post myocardial infarction, heart failure, atrial fibrillation, and intense acute exercise), we may have observed a different response. Future studies are warranted to investigate such an effect. Also all mice examined in this study were male, and as gender-specific regulation of myocardial Hsp72 has been described (Paroo et al. 2002), we cannot rule out a different observation if females were studied.
In summary, this study demonstrates that genetic modification of cardiac Hsp72 does not have an effect on cardiac metabolism under resting NC or HFD conditions. Further, specific differences between mouse strains in relation to lipid accumulation and insulin resistance were identified which should be considered in future experimental design.
Electronic supplementary material
Protein expression carried out by Western blotting for Hsp72 and Hsp90 protein levels in the cardiac tissues of the genetic models used in the study. GAPDH was used as a loading control. Samples from the three mouse models were ran on separate gels under different exposure conditions. (PPTX 324 kb)
Hsp72 Tg BALB/c mice have normal heart size, cardiac insulin-stimulated glucose clearance and palmitate fatty acid oxidation rates. Mice were fed a normal chow (NC) or high fat diet (HFD) for 12 weeks from 8 weeks of age. (A) Heart mass, (B) tibia length, (C) heart weight / tibia length ratio, n =5-8 per group. D) Mice were stimulated with 0.6U/kg of lean body mass insulin and glucose clearance into the heart measured, n = 5-9 per group. (E) Palmitate oxidation rates were measured as an indicator of fatty acid oxidation rates. The oxidation was calculated in the CO2 component and the acid soluble metabolite (ASM) fraction and the CO2 and ASM fractions summed to calculate total oxidation rate, n = 4-10 per group. * indicates trend for a main effect of diet. All data are presented as mean ± SEM. (PPTX 329 kb)
Hsp72 Tg BALB/c mice have an altered cardiac lipidomic profile. Lipidomic analysis of cardiac triacylglycerol (TG), diacylglycerol (DG) and ceramide in NC or HFD mice. Mice were fed a normal chow (NC) or high fat diet (HFD) for 12 weeks from 8 weeks of age. (A) Total TG levels and (B) individual cardiac TG molecular lipid species. (C) Total DG levels and D) individual cardiac DG molecular lipid species (E) Total ceramide levels and (F) individual cardiac ceramide molecular lipid species. n = 6-8 per group. * p = <0.05, ** p = <0.01, *** p = <0.001 for dietary effect, † p = <0.05, †† p = <0.01, for genotype effect. All data are presented as mean ± SEM. (PPTX 539 kb)
Acknowledgments
We wish to acknowledge Deb Ramsey and her team at the AMREP AS for their assistance with the animal studies, Jacqui Weir for technical assistance, and Hélène Kammoun for critically reviewing the manuscript. This study was supported by grants from the National Health and Medical Research Council of Australia (NHMRC Project Grant 1004441) and the Victorian Government Operational Infrastructure Support Program. MAF and BAK (1059454) are Senior Principal Research Fellows of the NHMRC. PJM is a Senior Research Fellow of the NHMRC. DCH is supported by a National Heart Foundation (NHF) Biomedical Postdoctoral Fellowship and an Australian Diabetes Society (ADS) Skip Martin Fellowship. The original Hsp72 Tg mouse line was provided by Prof Ruben Mestril.
Conflict of interest
MAF is Chief Scientific Officer of N-Gene Research Laboratories Ltd.
References
- Afzal AR, et al. Association of Met439Thr substitution in heat shock protein 70 gene with postoperative atrial fibrillation and serum HSP70 protein levels. Cardiology. 2008;110:45–52. doi: 10.1159/000109406. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Ko YG, Park WY, Kang YS, Chung HY, Seo JS. Suppression of ceramide-mediated apoptosis by HSP70. Mol Cell. 1999;9:200–206. [PubMed] [Google Scholar]
- Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998;273:17147–17153. doi: 10.1074/jbc.273.27.17147. [DOI] [PubMed] [Google Scholar]
- Calligaris SD, et al. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS One. 2013;8:e60931. doi: 10.1371/journal.pone.0060931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham JC, Seymour AM. Cardiac carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc Res. 2002;55:104–112. doi: 10.1016/S0008-6363(02)00399-1. [DOI] [PubMed] [Google Scholar]
- Chokshi A, et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew BG, et al. HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes. 2014;63:1488–1505. doi: 10.2337/db13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioudakis G, Garrard J, Raddatz K, Nadler JL, Mitchell TW, Schmitz-Peiffer C. Saturated- and n-6 polyunsaturated-fat diets each induce ceramide accumulation in mouse skeletal muscle: reversal and improvement of glucose tolerance by lipid metabolism inhibitors. Endocrinology. 2010;151:4187–4196. doi: 10.1210/en.2010-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Menendez L, Karamanlidis G, Kolwicz S, Tian R. Substrain specific response to cardiac pressure overload in C57BL/6 mice. Am J Physiol Heart Circ Physiol. 2013;305:H397–H402. doi: 10.1152/ajpheart.00088.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge DC, et al. Skeletal muscle-specific overproduction of constitutively activated c-Jun N-terminal kinase (JNK) induces insulin resistance in mice. Diabetologia. 2012;55:2769–2778. doi: 10.1007/s00125-012-2652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge DC, et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63:1881–1894. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter MM, Sievers RE, Barbosa V, Wolfe CL. Heat-shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein induced and the degree of myocardial protection. Circulation. 1994;89:355–360. doi: 10.1161/01.CIR.89.1.355. [DOI] [PubMed] [Google Scholar]
- Hutter JJ, Mestril R, Tam EK, Sievers RE, Dillmann WH, Wolfe CL. Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation. 1996;94:1408–1411. doi: 10.1161/01.CIR.94.6.1408. [DOI] [PubMed] [Google Scholar]
- Iozzo P, Chareonthaitawee P, Dutka D, Betteridge DJ, Ferrannini E, Camici PG. Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes. 2002;51:3020–3024. doi: 10.2337/diabetes.51.10.3020. [DOI] [PubMed] [Google Scholar]
- Kahle M, et al. Phenotypic comparison of common mouse strains developing high-fat diet-induced hepatosteatosis. Mol Metab. 2013;2:435–446. doi: 10.1016/j.molmet.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Suarez J, Hu Y, McDonough PM, Boer C, Dix DJ, Dillmann WH. Deletion of the inducible 70-kDa heat shock protein genes in mice impairs cardiac contractile function and calcium handling associated with hypertrophy. Circulation. 2006;113:2589–2597. doi: 10.1161/CIRCULATIONAHA.105.598409. [DOI] [PubMed] [Google Scholar]
- Liesa M, et al. Mitochondrial transporter ATP binding cassette mitochondrial erythroid is a novel gene required for cardiac recovery after ischemia/reperfusion. Circulation. 2011;124:806–813. doi: 10.1161/CIRCULATIONAHA.110.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal K, Torsney E, Poloniecki J, Camm AJ, Xu Q, Jahangiri M. Association of high intracellular, but not serum, heat shock protein 70 with postoperative atrial fibrillation. Ann Thorac Surg. 2005;79:865–871. doi: 10.1016/j.athoracsur.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews VB, et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53:2431–2441. doi: 10.1007/s00125-010-1865-y. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS One. 2013;8:e74341. doi: 10.1371/journal.pone.0074341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ, Turner N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia. 2013;56:1129–1139. doi: 10.1007/s00125-013-2846-8. [DOI] [PubMed] [Google Scholar]
- Park SY, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54:3530–3540. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- Paroo Z, Haist JV, Karmazyn M, Noble EG. Exercise improves postischemic cardiac function in males but not females: consequences of a novel sex-specific heat shock protein 70 response. Circ Res. 2002;90:911–917. doi: 10.1161/01.RES.0000016963.43856.B1. [DOI] [PubMed] [Google Scholar]
- Patterson B, Fields AV, Shannon RP. New insights into myocardial glucose metabolism: surviving under stress. Curr Opin Clin Nutr Metab Care. 2009;12:424–430. doi: 10.1097/MCO.0b013e32832c4167. [DOI] [PubMed] [Google Scholar]
- Radford NB, et al. Cardioprotective effects of 70-kDa heat shock protein in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:2339–2342. doi: 10.1073/pnas.93.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebeling C, Allegood JC, Wang E, Merrill AH, Jr, Futerman AH. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J Biol Chem. 2003;278:43452–43459. doi: 10.1074/jbc.M307104200. [DOI] [PubMed] [Google Scholar]
- St Rammos K, Koullias GJ, Hassan MO, Argyrakis NP, Voucharas CG, Scarupa SJ, Cowte TG. Low preoperative HSP70 atrial myocardial levels correlate significantly with high incidence of postoperative atrial fibrillation after cardiac surgery. Cardiovasc Surg. 2002;10:228–232. doi: 10.1016/S0967-2109(01)00138-7. [DOI] [PubMed] [Google Scholar]
- Trost SU, Omens JH, Karlon WJ, Meyer M, Mestril R, Covell JW, Dillmann WH. Protection against myocardial dysfunction after a brief ischemic period in transgenic mice expressing inducible heat shock protein 70. J Clin Invest. 1998;101:855–862. doi: 10.1172/JCI265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J. 2001;15:312–321. doi: 10.1096/fj.00-0590. [DOI] [PubMed] [Google Scholar]
- Ussher JR, et al. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res. 2012;94:359–369. doi: 10.1093/cvr/cvs129. [DOI] [PubMed] [Google Scholar]
- Weir JM, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res. 2013;54:2898–2908. doi: 10.1194/jlr.P035808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J. 2007;406:457–467. doi: 10.1042/BJ20070392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama I, et al. Role of insulin resistance in heart and skeletal muscle F-18 fluorodeoxyglucose uptake in patients with non-insulin-dependent diabetes mellitus. J Nucl Cardiol. 2000;7:242–248. doi: 10.1016/S1071-3581(00)70013-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protein expression carried out by Western blotting for Hsp72 and Hsp90 protein levels in the cardiac tissues of the genetic models used in the study. GAPDH was used as a loading control. Samples from the three mouse models were ran on separate gels under different exposure conditions. (PPTX 324 kb)
Hsp72 Tg BALB/c mice have normal heart size, cardiac insulin-stimulated glucose clearance and palmitate fatty acid oxidation rates. Mice were fed a normal chow (NC) or high fat diet (HFD) for 12 weeks from 8 weeks of age. (A) Heart mass, (B) tibia length, (C) heart weight / tibia length ratio, n =5-8 per group. D) Mice were stimulated with 0.6U/kg of lean body mass insulin and glucose clearance into the heart measured, n = 5-9 per group. (E) Palmitate oxidation rates were measured as an indicator of fatty acid oxidation rates. The oxidation was calculated in the CO2 component and the acid soluble metabolite (ASM) fraction and the CO2 and ASM fractions summed to calculate total oxidation rate, n = 4-10 per group. * indicates trend for a main effect of diet. All data are presented as mean ± SEM. (PPTX 329 kb)
Hsp72 Tg BALB/c mice have an altered cardiac lipidomic profile. Lipidomic analysis of cardiac triacylglycerol (TG), diacylglycerol (DG) and ceramide in NC or HFD mice. Mice were fed a normal chow (NC) or high fat diet (HFD) for 12 weeks from 8 weeks of age. (A) Total TG levels and (B) individual cardiac TG molecular lipid species. (C) Total DG levels and D) individual cardiac DG molecular lipid species (E) Total ceramide levels and (F) individual cardiac ceramide molecular lipid species. n = 6-8 per group. * p = <0.05, ** p = <0.01, *** p = <0.001 for dietary effect, † p = <0.05, †† p = <0.01, for genotype effect. All data are presented as mean ± SEM. (PPTX 539 kb)