Abstract
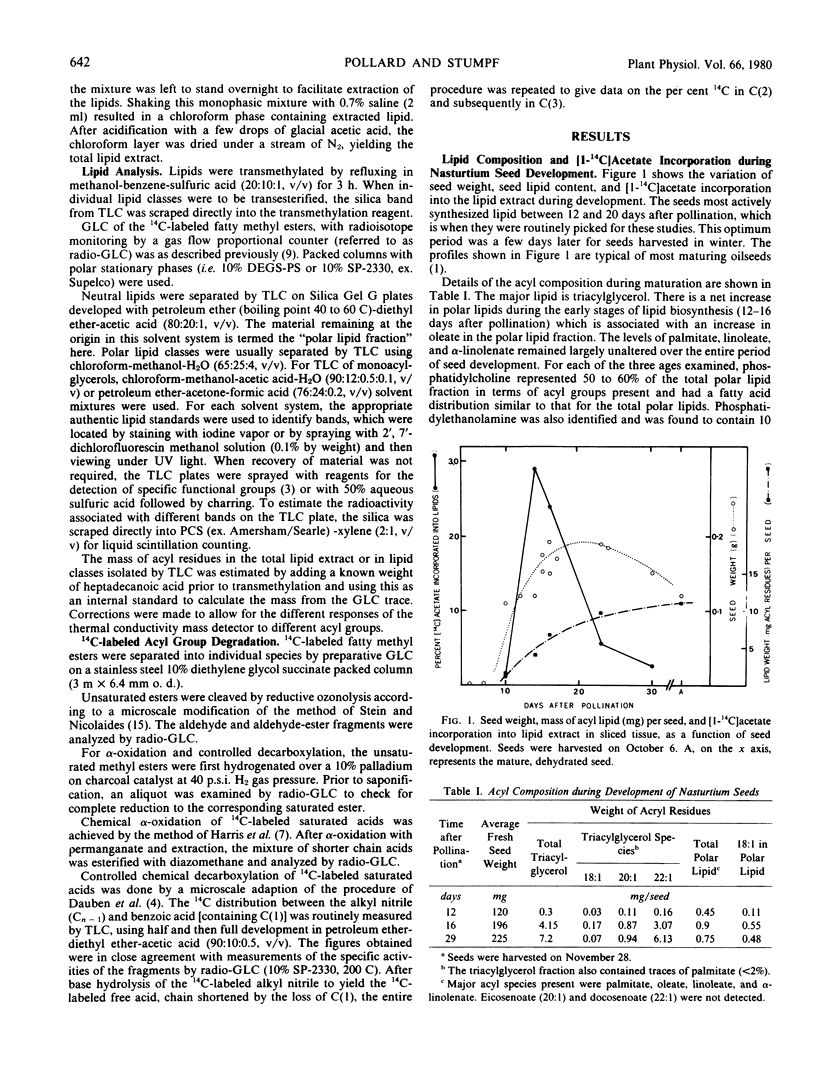
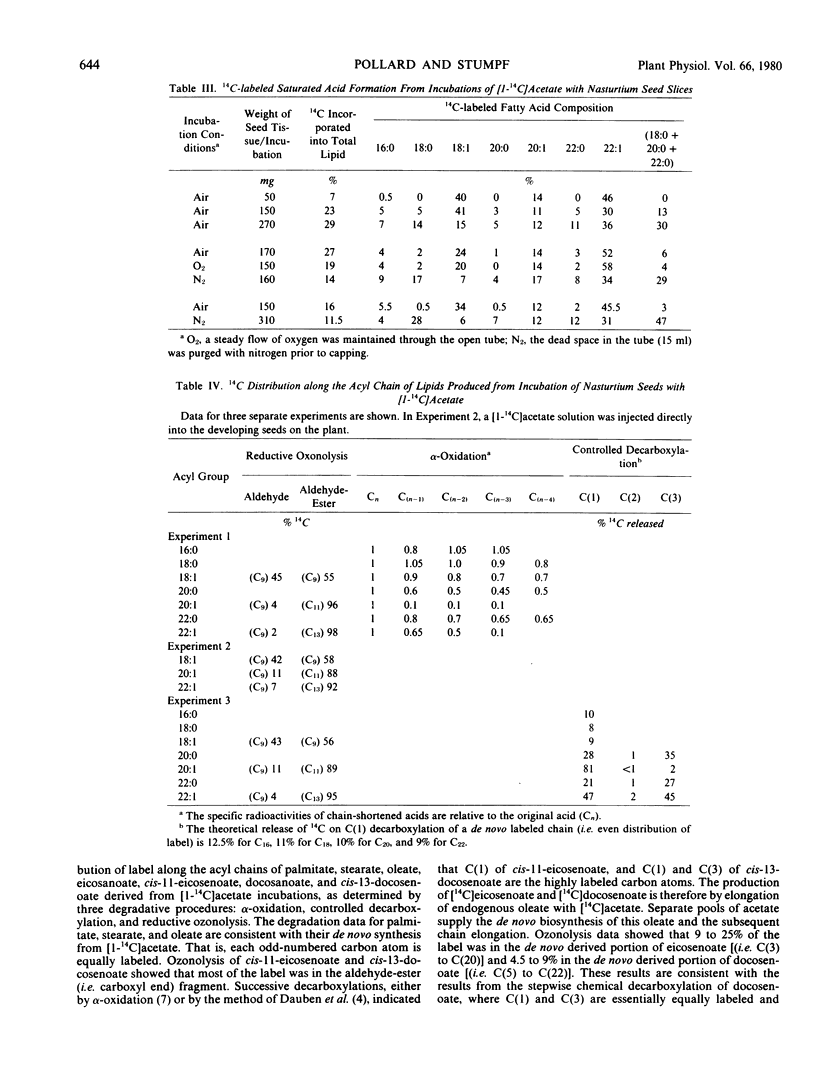
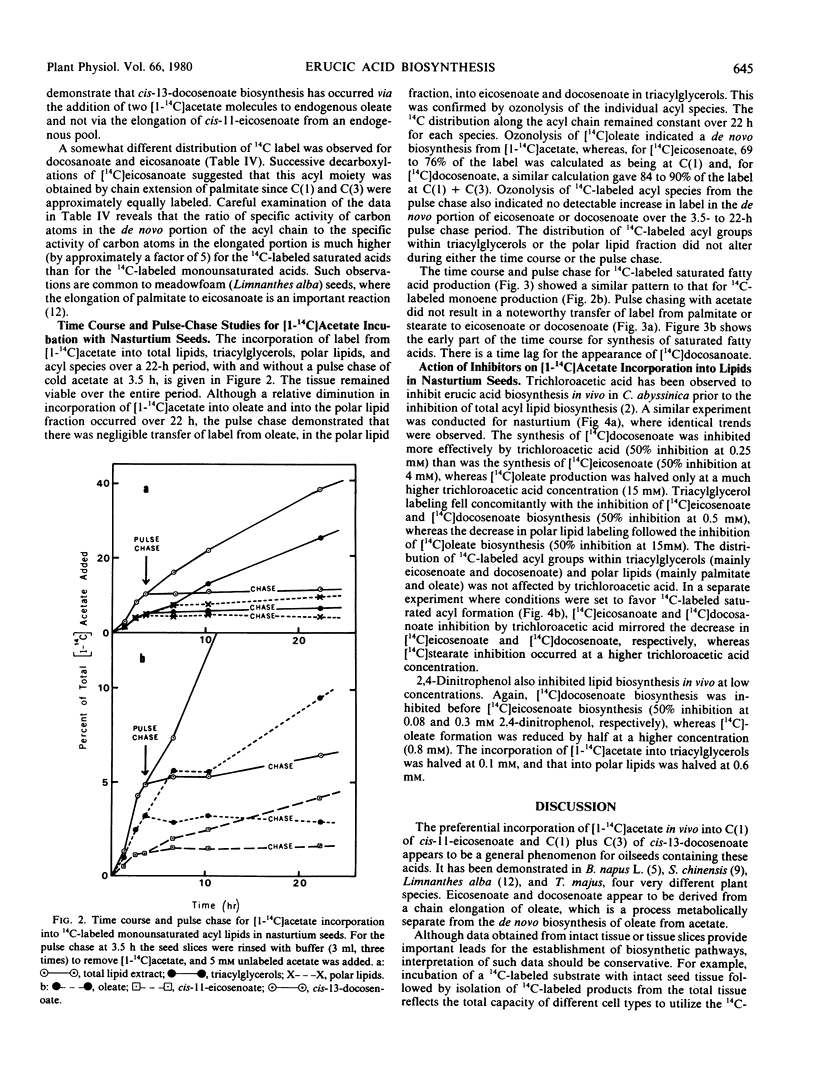
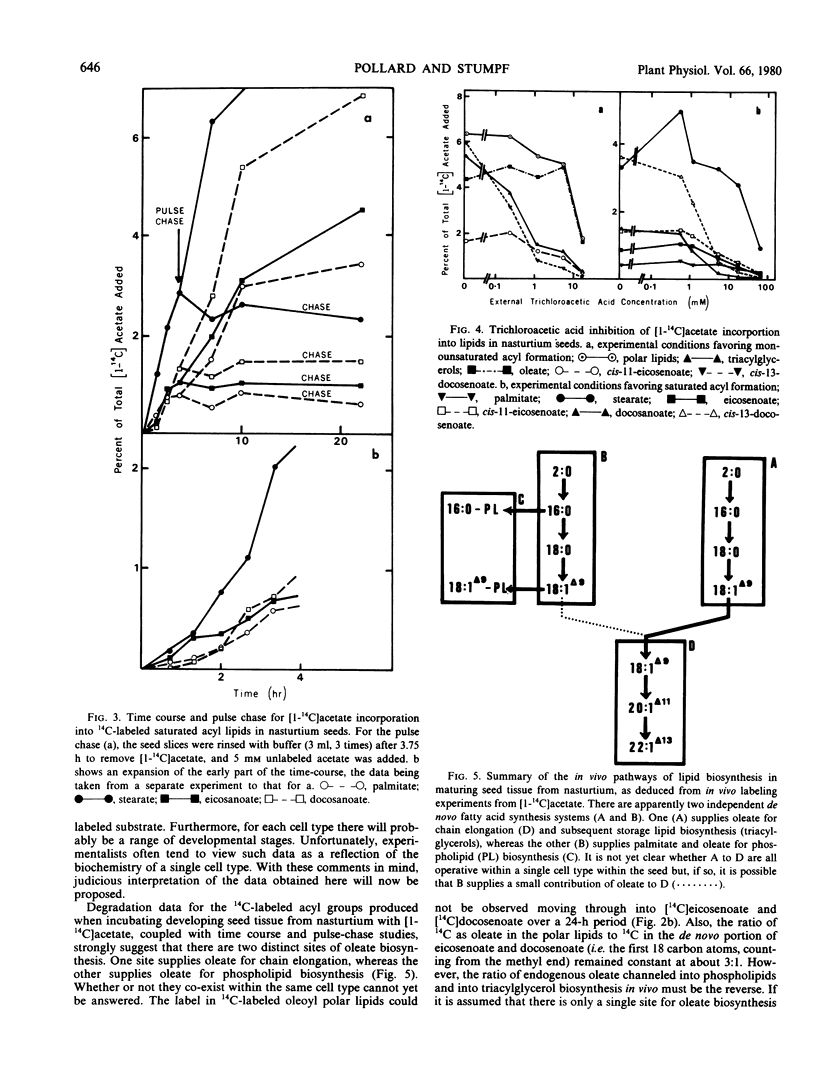
The storage triacylglycerols of nasturtium (Tropaeolum majus) seeds are composed principally of cis-11-eicosenoate and cis-13-docosenoate. To investigate the biosynthesis of these C20 and C22 fatty acids, developing seed tissue was incubated with various 14C-labeled precursors. Incubation with [1-14C]acetate produced primarily cis-11-[1-14C]eicosenoate and cis-13-[1,3-14C]docosenoate in the triacylglycerol fraction, the odd-carbon [U-14C]oleate also formed from [14C] acetate was in the polar lipid fraction. Kinetic data showed that this oleate was not channeled into cis-11-eicosenoate nor cis-13-docosenoate over a 24-hour period. Under suitable conditions, nasturtium seed could also produce [14C]stearate, [14C]eicosenoate, and [14C]docosenoate from [1-14C]acetate. The results are discussed in terms of the number of pathways producing fatty acids. From pool size and other considerations, the results can be rationalized only in terms of different de novo systems for oleate biosythesis, one supplying oleate for incorporation into phospholipids and the other supplying oleate for chain elongation and subsequent esterification into triacylglycerols. Because of the probable heterogeneous nature of the seed tissue, it is not known if these two systems are operating in different cell types, in the same cell type at different stages of development, or in the same cell type concurrently.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby R. S., Gurr M. I., Nichols B. W. Studies on seed-oil triglycerides. Factors controlling the biosynthesis of fatty acids and acyl lipids in subcellular organelles of maturing Crambe abyssinica seeds. Eur J Biochem. 1974 Oct 1;48(1):209–216. doi: 10.1111/j.1432-1033.1974.tb03758.x. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Blades J., Appleby R. S., Smith C. G., Robinson M. P., Nichols B. W. Studies on seed-oil triglycerides. Triglyceride biosynthesis and storage in whole seeds and oil bodies of Crambe abyssinica. Eur J Biochem. 1974 Apr 1;43(2):281–290. doi: 10.1111/j.1432-1033.1974.tb03411.x. [DOI] [PubMed] [Google Scholar]
- Harris R. V., James A. T. The fatty acid metabolism of Chlorella vulgaris. Biochim Biophys Acta. 1965 Dec 2;106(3):465–473. doi: 10.1016/0005-2760(65)90063-9. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Shine W. E., Stumpf P. K. Fat metabolism in higher plants. Characterization of plant acyl-ACP and acyl-CoA hydrolases. Arch Biochem Biophys. 1978 Aug;189(2):382–391. doi: 10.1016/0003-9861(78)90225-4. [DOI] [PubMed] [Google Scholar]
- Pollard M. R., Stumpf P. K. Biosynthesis of C(20) and C(22) Fatty Acids by Developing Seeds of Limnanthes alba: CHAIN ELONGATION AND Delta5 DESATURATION. Plant Physiol. 1980 Oct;66(4):649–655. doi: 10.1104/pp.66.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling of glycerolipids in the cotyledons of developing oilseeds by [1-14C] acetate and [2-3H] glycerol. Biochem J. 1978 Feb 15;170(2):421–433. doi: 10.1042/bj1700421. [DOI] [PMC free article] [PubMed] [Google Scholar]


