Abstract
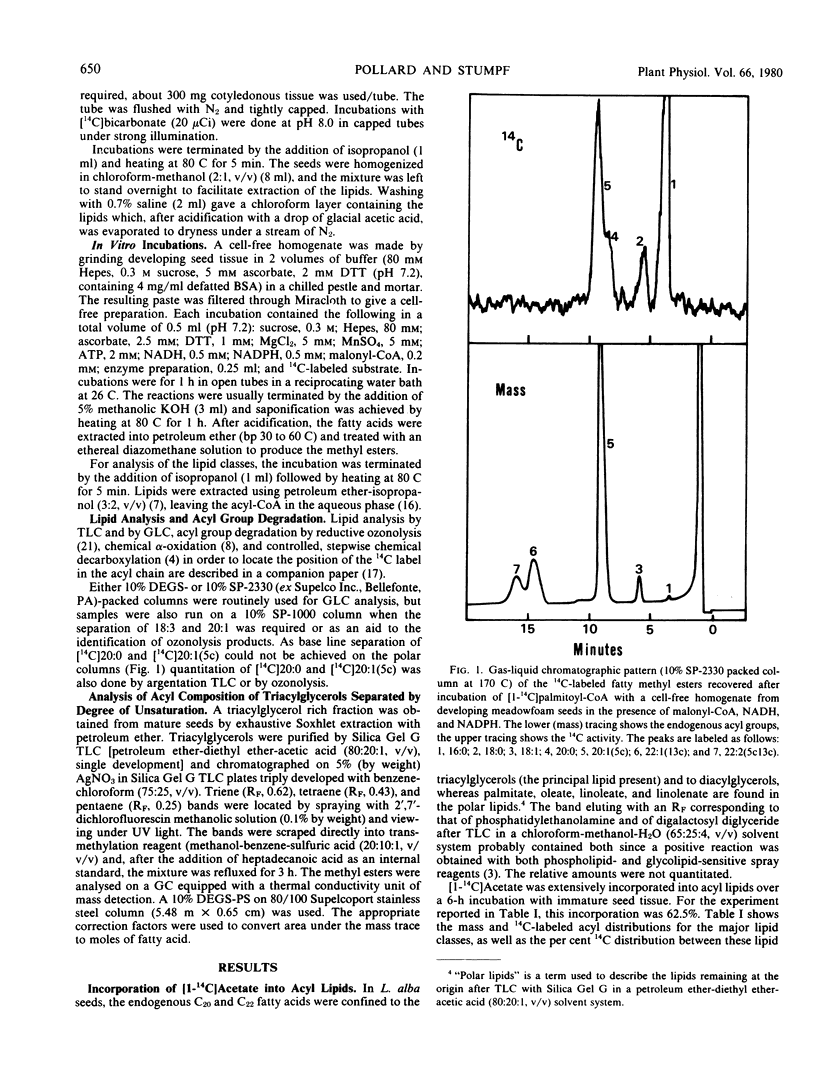
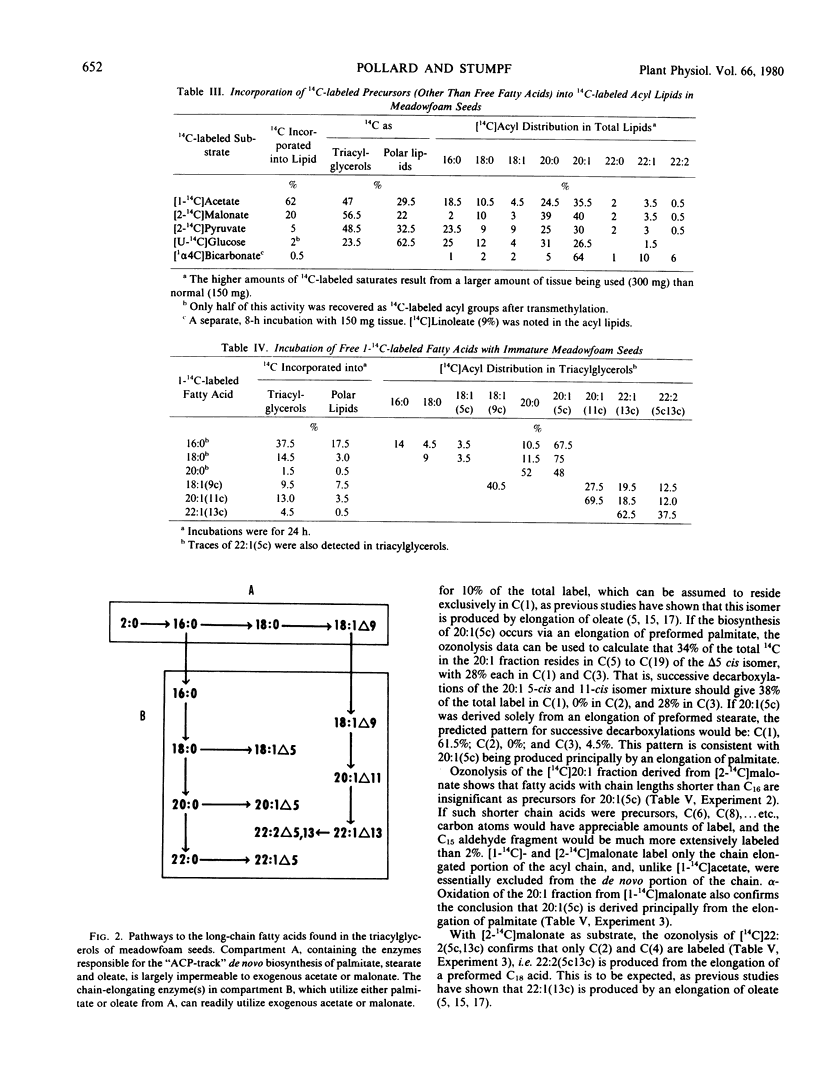
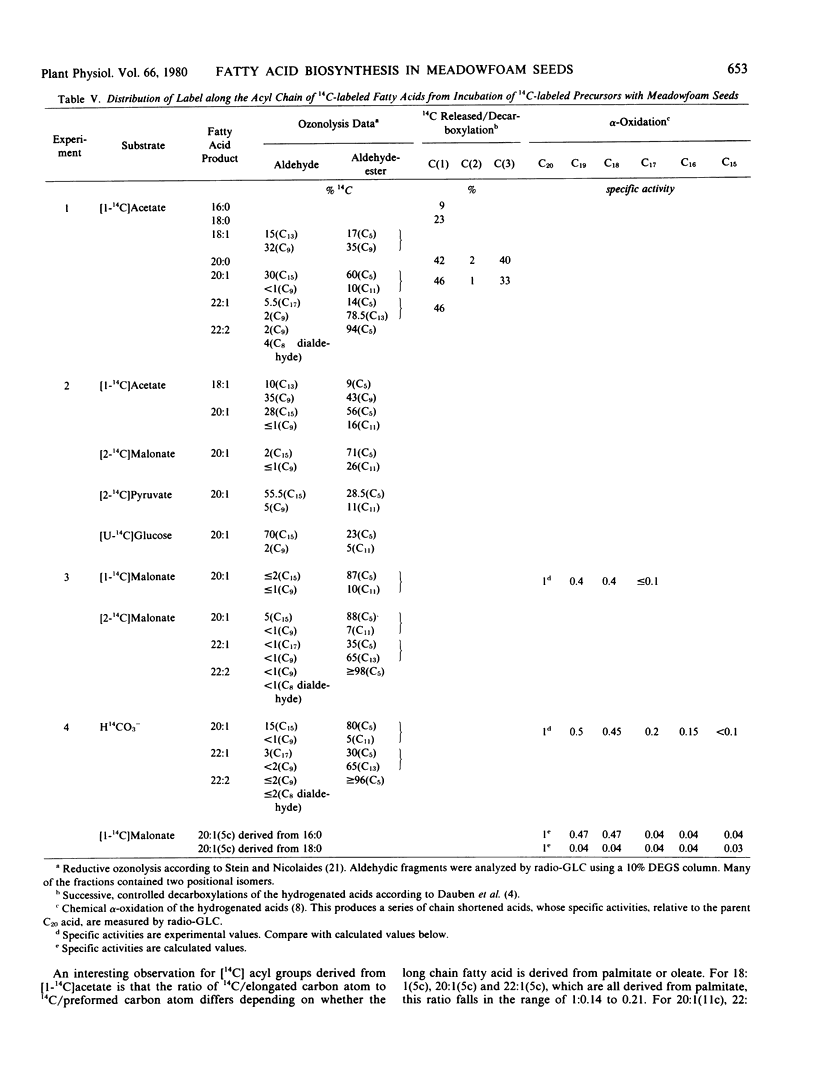
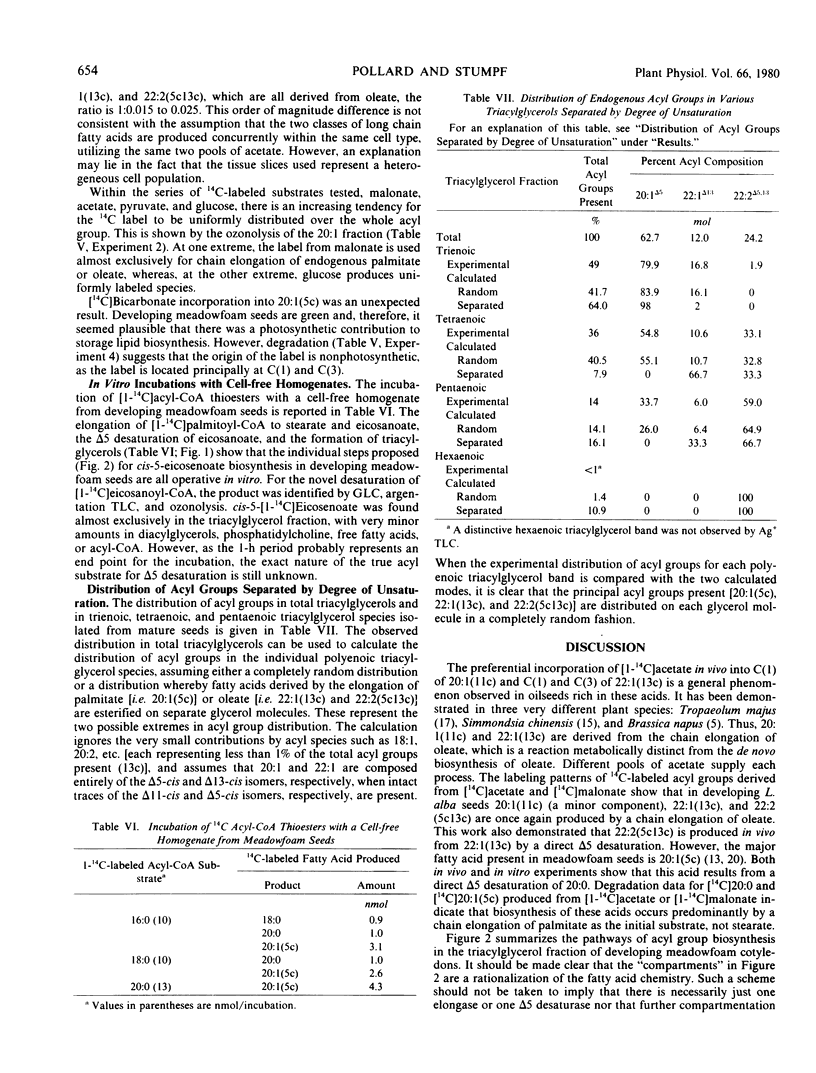
The storage triacylglycerols of meadowfoam (Limnanthes alba) seeds are composed essentially of C20 and C22 fatty acids, which contain an unusual Δ5 double bond. When [1-14C]acetate was incubated with developing seed slices, 14C-labeled fatty acids were synthesized with a distribution similar to the endogenous fatty acid profile. The major labeled product was cis-5-eicosenoate, with smaller amounts of palmitate, stearate, oleate, cis-5-octadecenoate, eicosanoate, cis-11-eicosenoate, docosanoate, cis-5-docosenoate, cis-13-docosenoate, and cis-5,cis-13-docosadienoate. The label from [14C]acetate and [14C]malonate was used preferentially for the elongation of endogenous oleate to produce cis-[14C]11-eicosenoate, cis-13-[14C]docosenoate, and cis-5,cis-13-[14C]docosadienoate and for the elongation of endogenous palmitate to produce the remaining C20 and C22 acyl species. The Δ5 desaturation of the preformed acyl chain and chain elongation of oleate and palmitate were demonstrated in vivo by incubation of the appropriate 1-14C-labeled free fatty acids. Using [1-14C]acyl-CoA thioesters as substrates, these enzyme activities were also demonstrated in vitro with a cell-free homogenate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby R. S., Gurr M. I., Nichols B. W. Studies on seed-oil triglycerides. Factors controlling the biosynthesis of fatty acids and acyl lipids in subcellular organelles of maturing Crambe abyssinica seeds. Eur J Biochem. 1974 Oct 1;48(1):209–216. doi: 10.1111/j.1432-1033.1974.tb03758.x. [DOI] [PubMed] [Google Scholar]
- Hara A., Radin N. S. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978 Oct 1;90(1):420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- Harris R. V., James A. T. The fatty acid metabolism of Chlorella vulgaris. Biochim Biophys Acta. 1965 Dec 2;106(3):465–473. doi: 10.1016/0005-2760(65)90063-9. [DOI] [PubMed] [Google Scholar]
- Jaworski J. G., Stumpf P. K. Fat metabolism in higher plants. Properties of a soluble stearyl-acyl carrier protein desaturase from maturing Carthamus tinctorius. Arch Biochem Biophys. 1974 May;162(1):158–165. doi: 10.1016/0003-9861(74)90114-3. [DOI] [PubMed] [Google Scholar]
- MCMAHON V., STUMPF P. K. SYNTHESIS OF LINOLEIC ACID BY PARTICULATE SYSTEM FROM SAFFLOWER SEEDS. Biochim Biophys Acta. 1964 Jun 15;84:359–361. doi: 10.1016/0926-6542(64)90065-4. [DOI] [PubMed] [Google Scholar]
- Nichols B. W., Safford R. Conversion of lipids to fatty alcohols and lysolipids by NaBH4. Chem Phys Lipids. 1973 Oct;11(3):222–227. doi: 10.1016/0009-3084(73)90024-8. [DOI] [PubMed] [Google Scholar]
- Pollard M. R., Stumpf P. K. Long Chain (C(20) and C(22)) Fatty Acid Biosynthesis in Developing Seeds of Tropaeolum majus: AN IN VIVO STUDY. Plant Physiol. 1980 Oct;66(4):641–648. doi: 10.1104/pp.66.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint J. F., Fulco A. J. The biosynthesis of unsaturated fatty acids by bacilli. V. In vivo substrate specificities of fatty acid desaturases. J Biol Chem. 1973 Oct 10;248(19):6885–6895. [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling of glycerolipids in the cotyledons of developing oilseeds by [1-14C] acetate and [2-3H] glycerol. Biochem J. 1978 Feb 15;170(2):421–433. doi: 10.1042/bj1700421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S., Appelqvist L. A. The biosynthesis of linoleate from oleoyl-CoA via oleoyl-phosphatidylcholine in microsomes of developing safflower seeds. Eur J Biochem. 1978 Oct;90(2):223–229. doi: 10.1111/j.1432-1033.1978.tb12594.x. [DOI] [PubMed] [Google Scholar]
- Vijay I. K., Stumpf P. K. Fat metabolism in higher plants. XLVI. Nature of the substrate and the product of oleyl coenzyme A desaturase from Carthamus tinctorius. J Biol Chem. 1971 May 10;246(9):2910–2917. [PubMed] [Google Scholar]


