Abstract
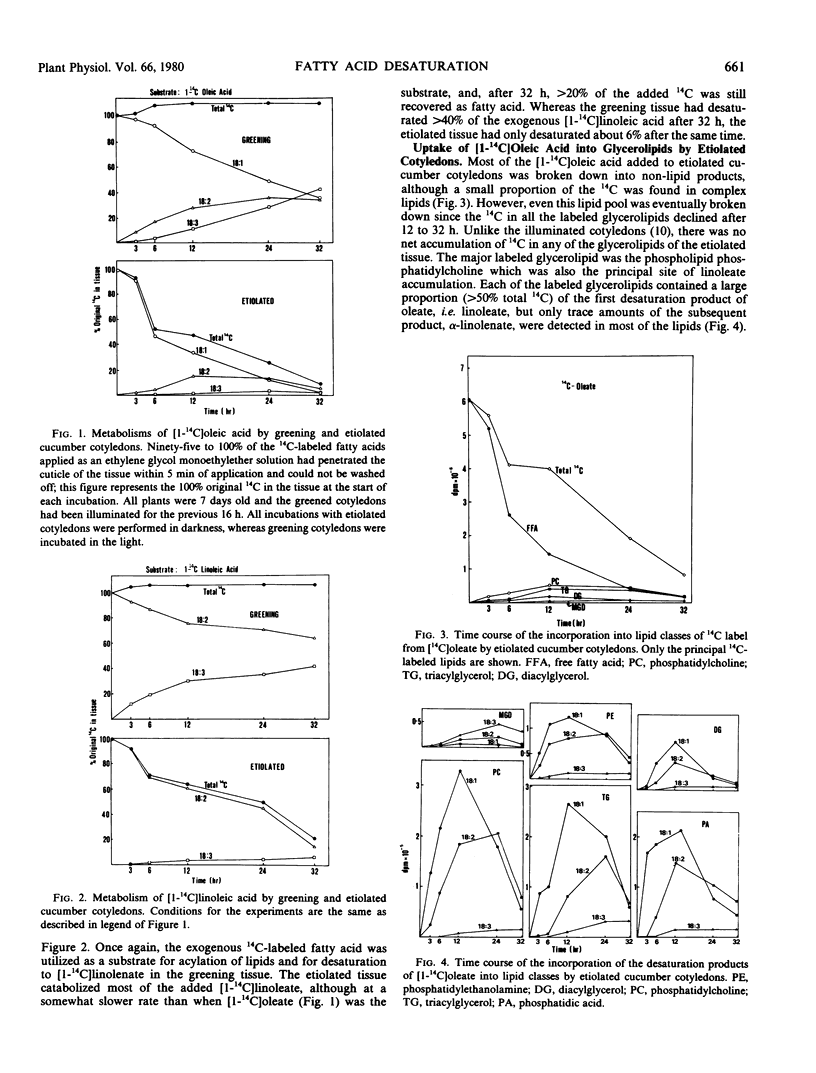
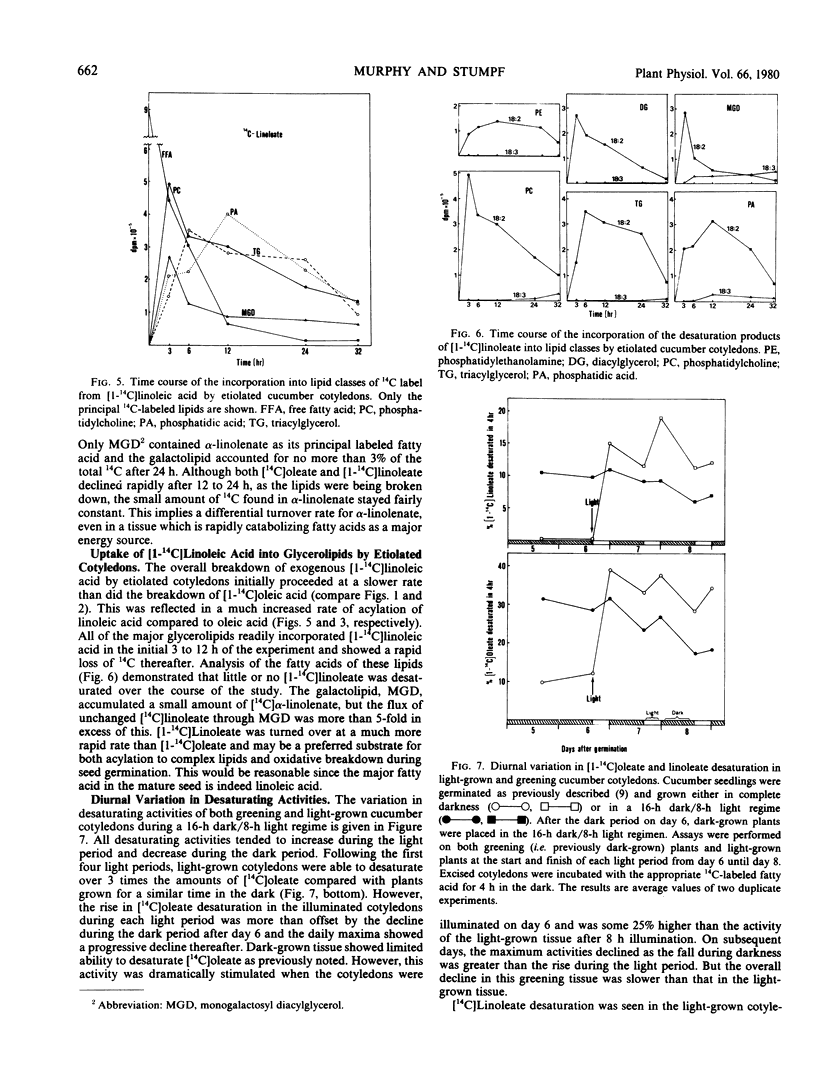
Etiolated Cucumis sativus L. cotyledons preferentially catabolized exogenous [1-14C]oleic acid and [1-14C]linoleic acid with relatively little incorporation into complex lipids or desaturation of the 14C-labeled fatty acids. Following a 16-hour exposure to light, the greening cotyledons efficiently desaturated the exogenous 14C-labeled fatty acids. A small amount of oleate desaturation to linoleate was observed in etiolated tissue, but hardly any linoleate desaturation to α-linolenate was detected. Both oleate and linoleate desaturation showed diurnal variations with maxima at the end of light periods and minima at the end of dark periods. Illumination of etiolated tissue by flashing light, as opposed to continuous light, failed to stimulate either chlorophyll or α-linolenic acid biosynthesis, and both processes could be halted or reversed by 10 micrograms per milliliter cycloheximide. Production of polyunsaturated fatty acids from [1-14C]acetate, [1-14C]oleic acid, and [1-14C]linoleic acid, by greening cucumber cotyledons, was markedly affected by tissue integrity with finely chopped cotyledons having very little capacity for their synthesis and intact seedlings showing the highest rates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W. M., Leaver C. J., Weir E. M., Riezman H. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: I. Developmental Changes in Cotyledonary Protein, RNA, and Enzyme Activities during Germination. Plant Physiol. 1978 Oct;62(4):542–549. doi: 10.1104/pp.62.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol P. K. Rapid Metabolic Changes in the Wounding Response of Leaf Discs following Excision. Plant Physiol. 1976 Jan;57(1):80–84. doi: 10.1104/pp.57.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. J., Stumpf P. K. In Vivo Pathway of Oleate and Linoleate Desaturation in Developing Cotyledons of Cucumis sativus L. Seedlings. Plant Physiol. 1980 Oct;66(4):666–671. doi: 10.1104/pp.66.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. J., Stumpf P. K. Light-dependent Induction of Polyunsaturated Fatty Acid Biosynthesis in Greening Cucumber Cotyledons. Plant Physiol. 1979 Feb;63(2):328–335. doi: 10.1104/pp.63.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Subcellular distribution of gluconeogenetic enzymes in germinating castor bean endosperm. Plant Physiol. 1979 Jul;64(1):31–37. doi: 10.1104/pp.64.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler P. A. Aging of the photosynthetic apparatus. IV. Similarity between the effects of aging and unsaturated fatty acids on isolated spinach chloroplasts as expressed by volume changes. Biochim Biophys Acta. 1972 Aug 17;275(2):182–191. doi: 10.1016/0005-2728(72)90039-4. [DOI] [PubMed] [Google Scholar]
- Siegenthaler P. A. Inhibition of photosystem II electron transport in chloroplasts by fatty acids and restoration of its activity by Mn2+. FEBS Lett. 1974 Mar 1;39(3):337–340. doi: 10.1016/0014-5793(74)80144-4. [DOI] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]


