Abstract
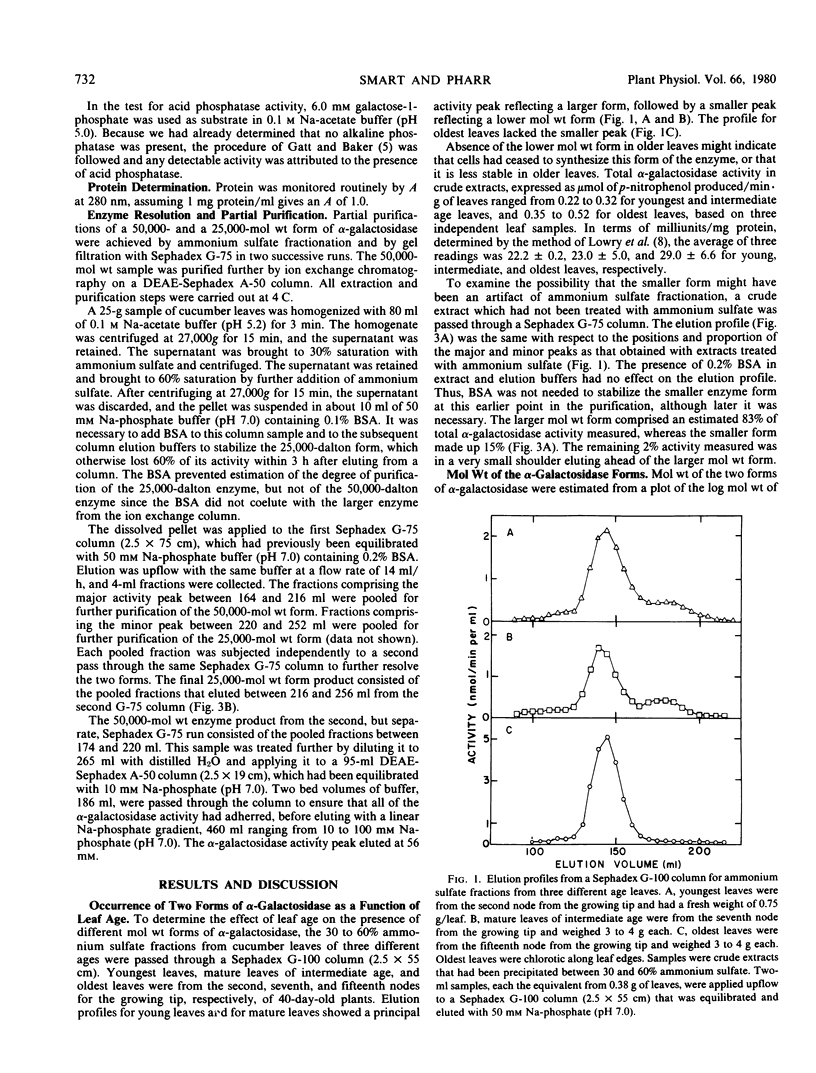
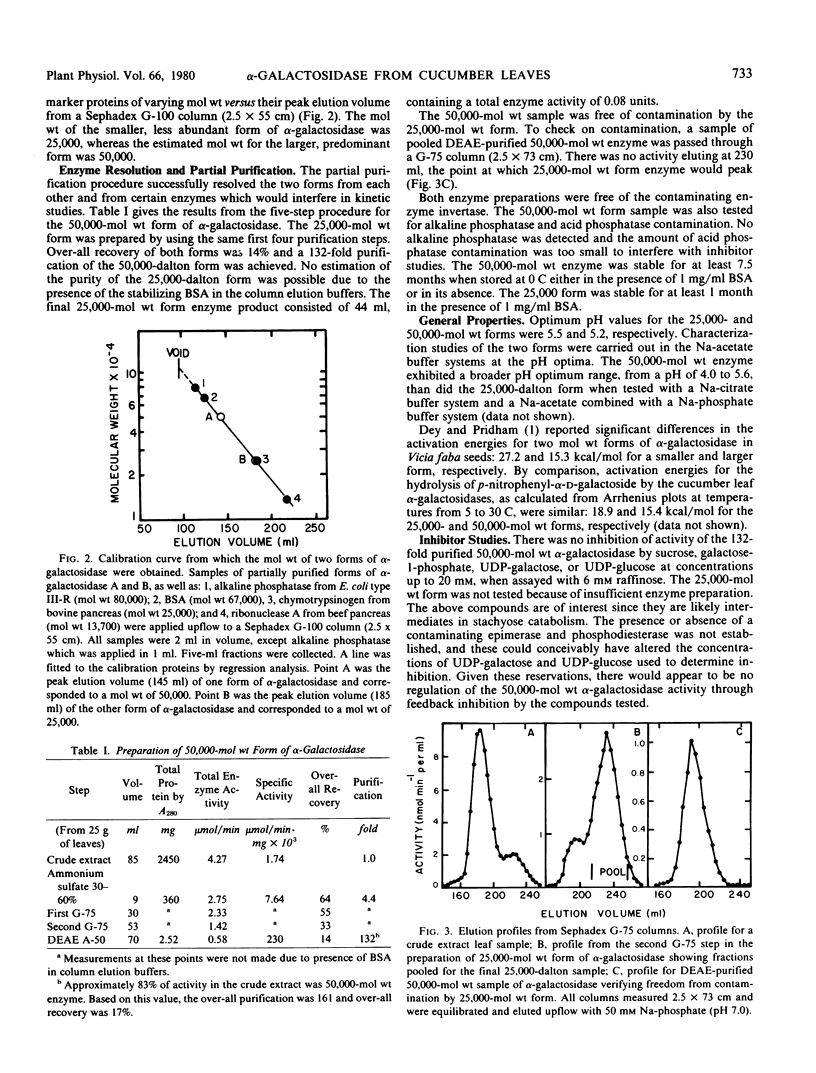
Two forms of α-galactosidase (α-d-galactoside galactohydrolase, E.C. 3.2.1.22) which differed in molecular weight were resolved from Cucumis sativus L. leaves. The enzymes were partially purified using ammonium sulfate fractionation, Sephadex gel filtration, and diethylaminoethyl-Sephadex chromatography. The molecular weights of the two forms, by gel filtration, were 50,000 and 25,000. The 50,000-dalton form comprised approximately 84% of the total α-galactosidase activity in crude extracts from mature leaves and was purified 132-fold. The partially purified 25,000-molecular weight form rapidly lost activity unless stabilized with 0.2% albumin and accounted for 16% of the total α-galactosidase activity in the crude extract. The smaller molecular weight form was not found in older leaves.
The two forms were similar in several ways including their pH optima which were 5.2 and 5.5 for the 50,000- and 25,000-dalton form, respectively, and activation energies, which were 15.4 and 18.9 kilocalories per mole for the larger and smaller forms. Both enzymes were inhibited by galactose as well as by excess concentrations of p-nitrophenyl-α-d-galactoside sub-strate. Km values with this substrate and with raffinose and melibiose were different for each substrate, but similar for both forms of the enzyme. With stachyose, Km values were 10 and 30 millimolar for the 50,000- and 25,000- molecular weight forms, respectively.
Full text
PDF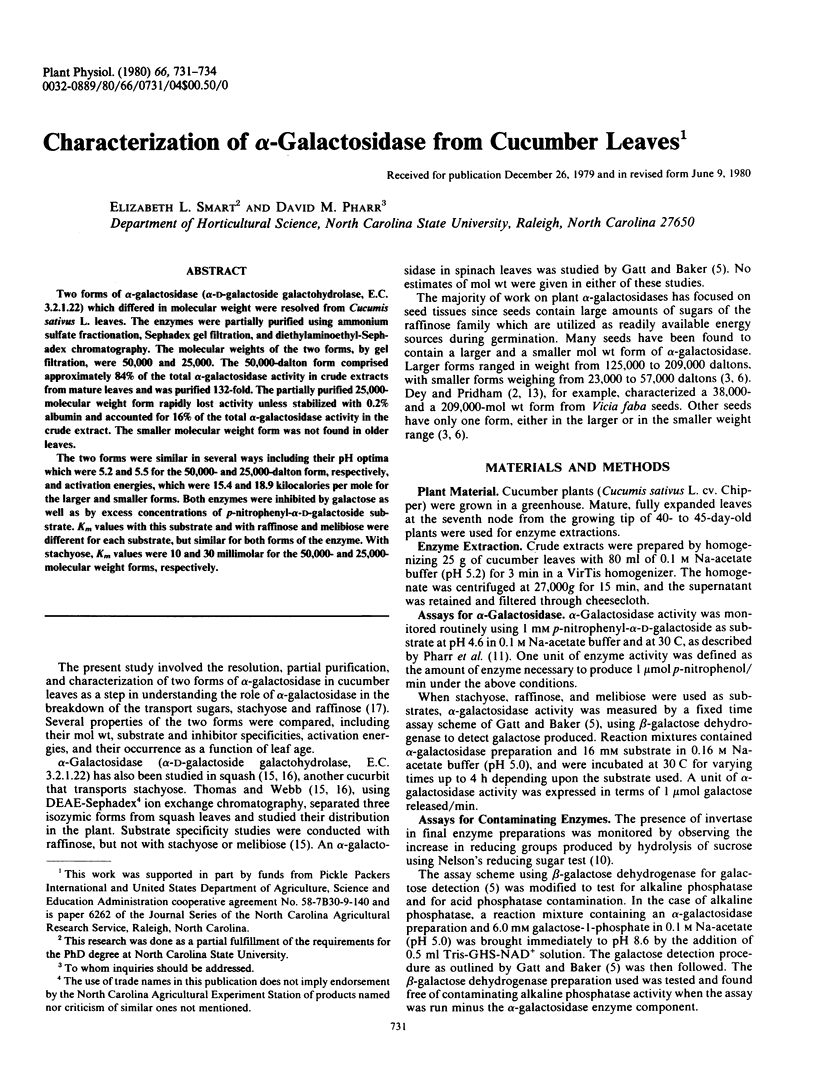

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dey P. M., Pridham J. B. Biochemistry of -galactosidases. Adv Enzymol Relat Areas Mol Biol. 1972;36:91–130. doi: 10.1002/9780470122815.ch3. [DOI] [PubMed] [Google Scholar]
- Dey P. M., Pridham J. B. Purification and properties of alpha-galactosidases from Vicia faba seeds. Biochem J. 1969 Jun;113(1):49–55. doi: 10.1042/bj1130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH D. The raffinose family of oligosaccharides. Adv Carbohydr Chem. 1954;9:149–184. doi: 10.1016/s0096-5332(08)60375-6. [DOI] [PubMed] [Google Scholar]
- Gatt S., Baker E. A. Purification and separation of alpha- and beta-galactosidases from spinach leaves. Biochim Biophys Acta. 1970 Apr 22;206(1):125–135. doi: 10.1016/0005-2744(70)90089-6. [DOI] [PubMed] [Google Scholar]
- Hankins C. N., Kindinger J. I., Shannon L. M. Legume alpha-Galactosidases Which Have Hemagglutinin Properties. Plant Physiol. 1980 Apr;65(4):618–622. doi: 10.1104/pp.65.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Suzuki H., Li S. C., Li Y. T. Alpha-galactosidase from Mortierella vinacea. Crystallization and properties. J Biol Chem. 1970 Feb 25;245(4):781–786. [PubMed] [Google Scholar]
- Thomas B., Webb J. A. Distribution of alpha-Galactosidase in Cucurbita pepo. Plant Physiol. 1978 Nov;62(5):713–717. doi: 10.1104/pp.62.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey J. A. LAW AND LEGISLATION. Am J Public Health (N Y) 1926 Feb;16(2):203–206. doi: 10.2105/ajph.16.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]


