Abstract
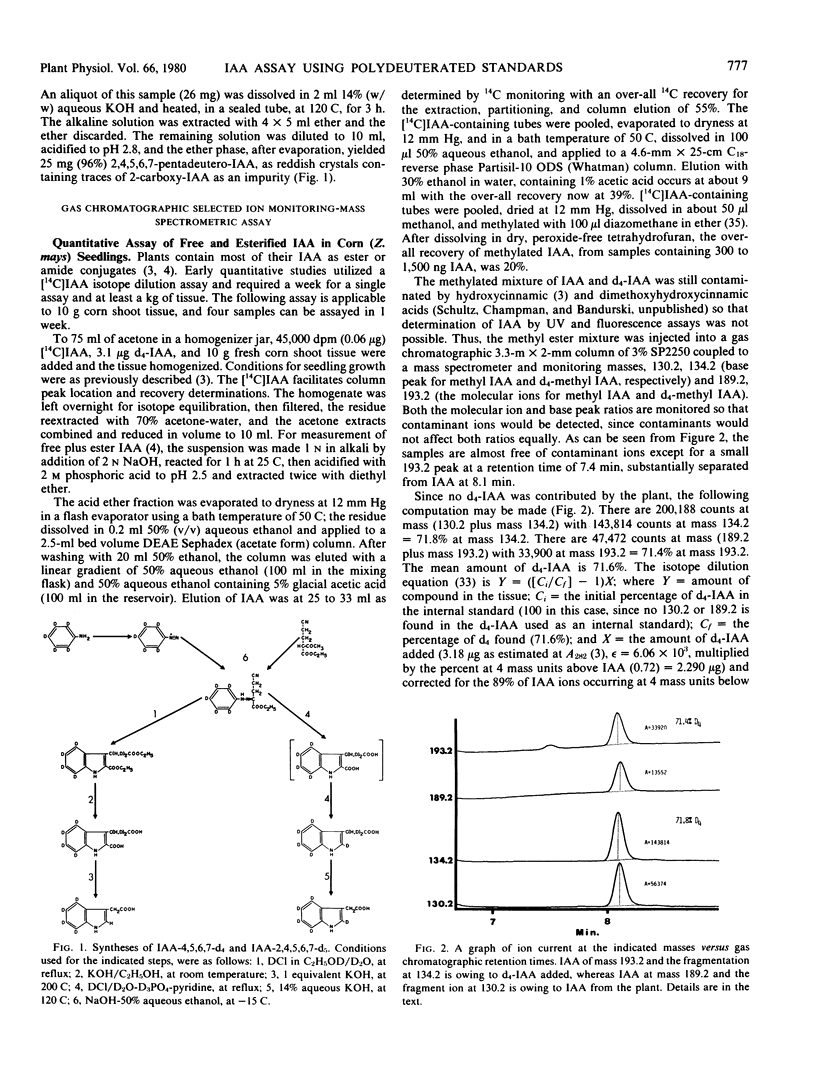
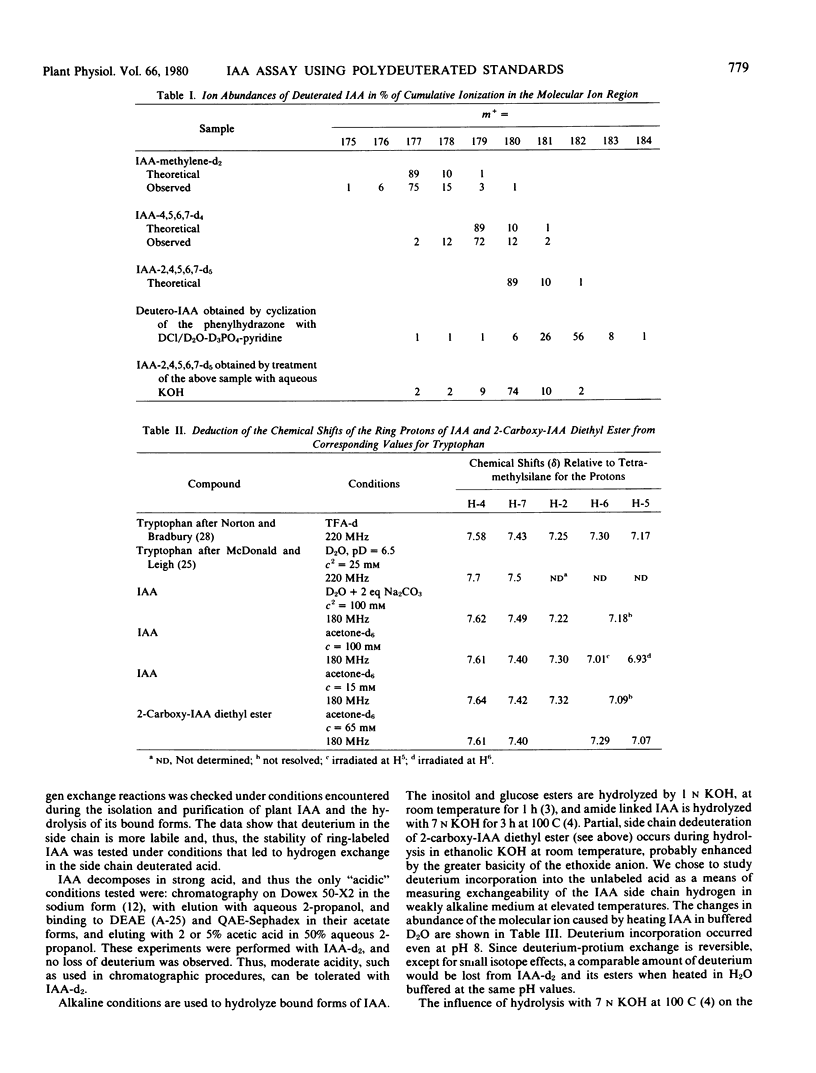
Syntheses are described for tetra and pentadeutero indole-3-acetic acid (IAA) labeled in positions 4, 5, 6, 7 or 2, 4, 5, 6, 7 of the indole moiety. Polydeuterated IAA is proposed as an internal standard for gas chromatographic-mass spectrometric analysis of IAA by selected ion monitoring. Nanogram amounts of IAA may be assayed by monitoring the base peak of IAA at m/z = 130 (134 for d4-IAA) and the molecular ion of the methyl ester of IAA at 189 (193 for d4-IAA). Deuterium in positions 4, 5, 6, and 7 and, to only a slightly lesser extent, that in position 2 of IAA is retained during alkali treatment, thus permitting use of these compounds as internal standards for assay of IAA released by alkaline hydrolysis of ester and amide conjugates. The use of polydeutero internal standards separates the standards from the “isotope cluster” caused by the normal abundance of heavy isotopes and also permits use of reduced mass resolution, thus leading to a 10-fold increase in sensitivity.
Tetradeutero IAA was used as an internal standard for determining free plus ester IAA in alkaline hydrolysates of Zea mays, and showed exact agreement between estimates based on the molecular ion of the methyl ester and those based upon base peak. Application of the method to measuring free IAA in the upper and lower halves of geotropically stimulated Zea shoots showed 61 ± 4% of the free IAA to be on the lower side.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A., Cohen J. D. Photo-regulation of the ratio of ester to free indole-3-acetic acid. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1219–1223. doi: 10.1016/0006-291x(77)91136-6. [DOI] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A. Concentration of Indole-3-acetic Acid and Its Derivatives in Plants. Plant Physiol. 1977 Aug;60(2):211–213. doi: 10.1104/pp.60.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A. Concentrations of Indole-3-acetic Acid and Its Esters in Avena and Zea. Plant Physiol. 1974 Sep;54(3):257–262. doi: 10.1104/pp.54.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertilsson L., Palmér L. Indole-3-acetic acid in human cerebrospinal fluid: identification and quantification by mass fragmentography. Science. 1972 Jul 7;177(4043):74–76. doi: 10.1126/science.177.4043.74. [DOI] [PubMed] [Google Scholar]
- Bradbury J. H., Norton R. S. Carbon-13 NMR spectra of tryptophan, tryptophan peptides and of native and denatured proteins. Biochim Biophys Acta. 1973 Nov 11;328(1):10–19. doi: 10.1016/0005-2795(73)90324-3. [DOI] [PubMed] [Google Scholar]
- Caruso J. L., Smith R. G., Smith L. M., Cheng T. Y., Daves G. D. Determination of Indole-3-acetic Acid in Douglas Fir Using a Deuterated Analog and Selected Ion Monitoring: Comparison of Microquantities in Seedling and Adult Tree. Plant Physiol. 1978 Dec;62(6):841–845. doi: 10.1104/pp.62.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M. GROUP MEDICINE. Am J Public Health (N Y) 1919 May;9(5):358–362. doi: 10.2105/ajph.9.5.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann A. The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977 Feb 11;132(2):267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- Hoskins J. A., Pollitt R. J. Quantitative aspects of urinary indolo-3-acetic acid and 5-hydroxyindole-3-acetic acid excretion. J Chromatogr. 1975 Jun 18;109(2):436–438. doi: 10.1016/s0021-9673(01)91824-2. [DOI] [PubMed] [Google Scholar]
- Norton R. S., Bradbury J. H. Kinetics of hydrogen-deuterium exchange of tryptophan and tryptophan peptides in deutero-trifluoroacetic acid using proton magenetic resonance spectroscopy. Mol Cell Biochem. 1976 Aug 30;12(2):103–111. doi: 10.1007/BF01731556. [DOI] [PubMed] [Google Scholar]


