Abstract
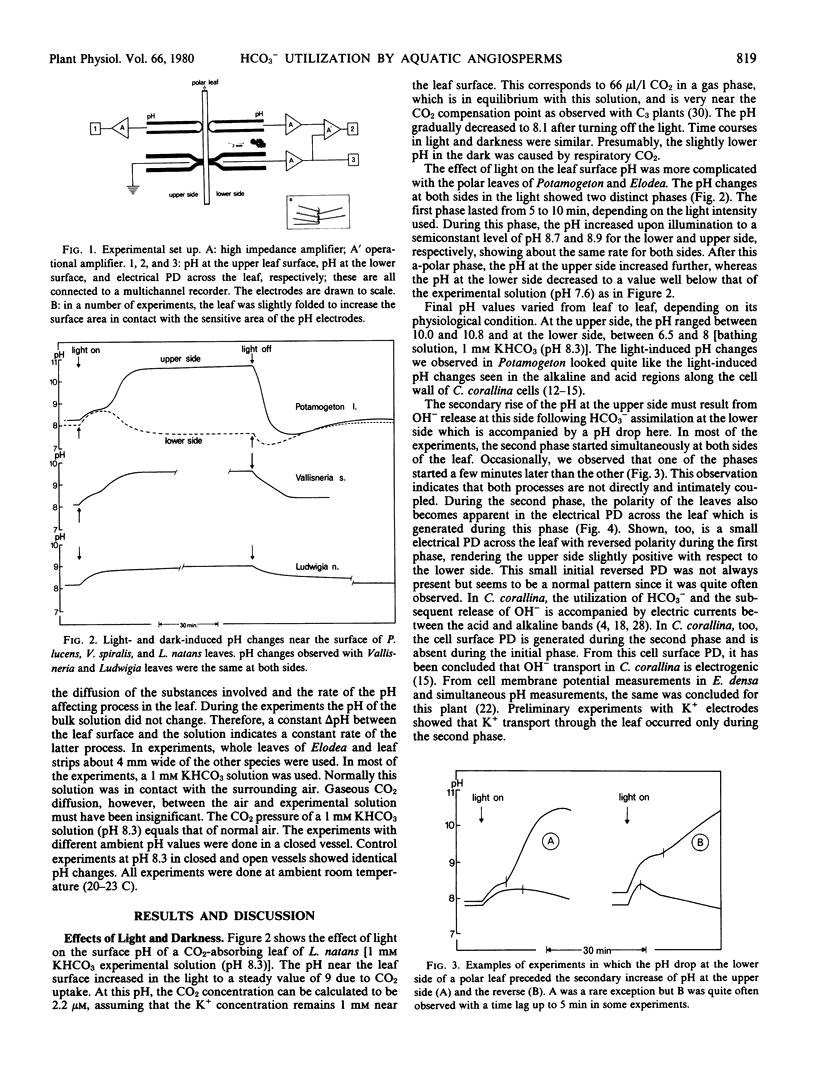
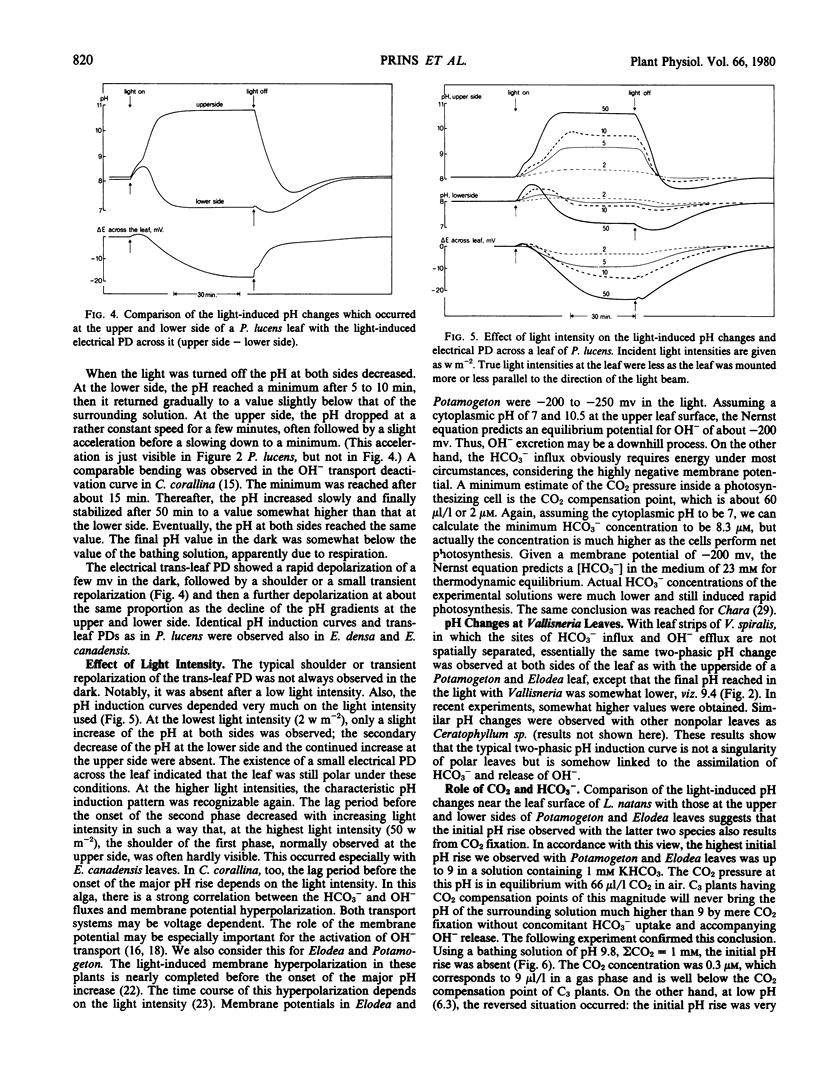
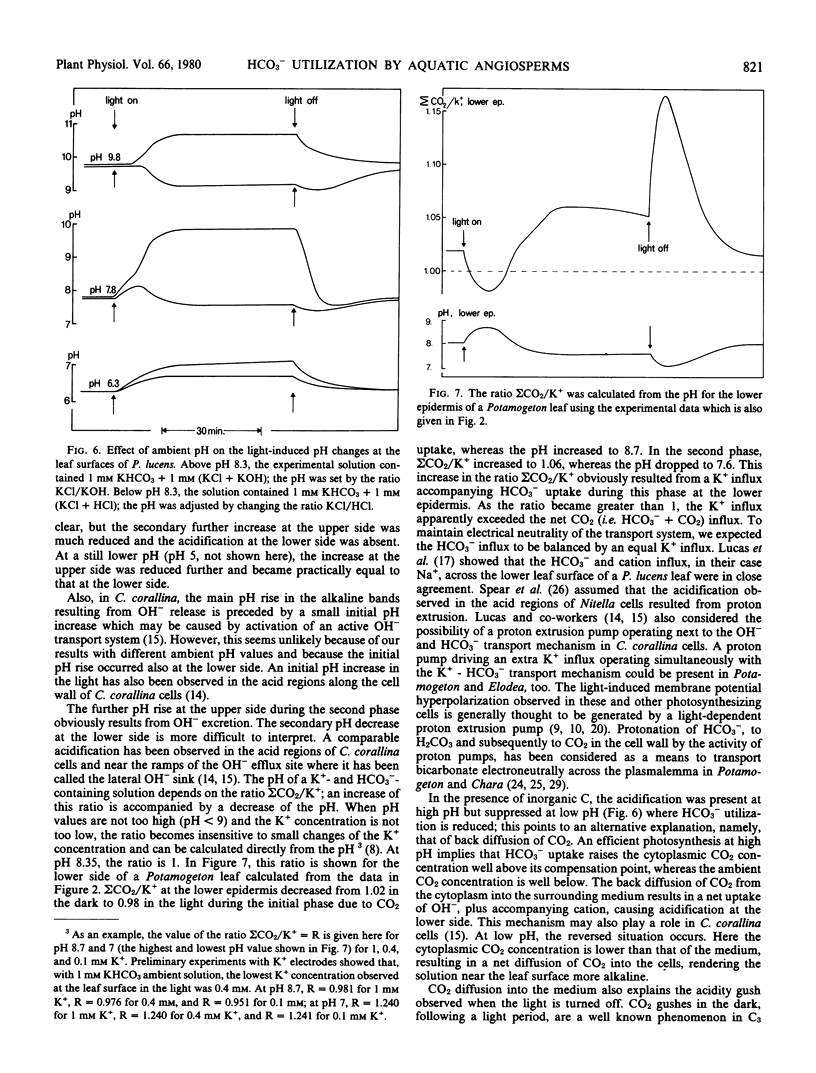
The utilization of HCO3− as carbon source for photosynthesis by aquatic angiosperms results in the production of 1 mole OH− for each mole CO2 assimilated. The OH− ions are subsequently released to the medium. In several Potamogeton and Elodea species, the site of the HCO3− influx and OH− efflux are spatially separated. Described here are light- and dark-induced pH changes at the lower and upper epidermis of the leaves of Potamogeton lucens, Elodea densa, and Elodea canadensis.
In the light, two phases could be discerned. During the first phase, the pH increased at both sides of the leaves. This pH increase apparently resulted from CO2 fixation. During the second, so-called polar phase, the pH at the upper side increased further, but the pH at the lower side dropped below the pH of the ambient solution. The pH drop at the lower epidermis indicates that the K+ influx exceeds the net CO2 (HCO3− + CO2) influx slightly. This may result either from a proton pump driving an extra K+ influx or from CO2 diffusion from the cells into the outer medium previously taken up as HCO3−. In the dark, a CO2 gush was observed at both sides. During the polar phase, the upper side becomes electrically negative with respect to the lower side. Subsequent depolarization in the dark revealed that this potential difference consisted of a fast and a slow component.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Decker J. P. A Rapid, Postillumination Deceleration of Respiration in Green Leaves. Plant Physiol. 1955 Jan;30(1):82–84. doi: 10.1104/pp.30.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J., Ferrier J. M., Dainty J. Plasmalemma transport of OH- in Chara corallina: dynamics of activation and deactivation. J Membr Biol. 1977 Apr 7;32(1-2):49–73. doi: 10.1007/BF01905209. [DOI] [PubMed] [Google Scholar]
- Lucas W. J. HCO(3) Influx across the Plasmalemma of Chara corallina: Physiological and Biophysical Influence of 10 mm K. Plant Physiol. 1978 Apr;61(4):487–493. doi: 10.1104/pp.61.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins H. B., Harper J. R., Higinbotham N. Membrane potentials of vallisneria leaf cells and their relation to photosynthesis. Plant Physiol. 1980 Jan;65(1):1–5. doi: 10.1104/pp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear D. G., Barr J. K., Barr C. E. Localization of hydrogen ion and chloride ion fluxes in Nitella. J Gen Physiol. 1969 Sep;54(3):397–414. doi: 10.1085/jgp.54.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]


