Abstract
As the number of total joint arthroplasty and internal fixation procedures continues to rise, the threat of infection following surgery has significant clinical implications. These infections may have highly morbid consequences to patients, who often endure additional surgeries and lengthy exposures to systemic antibiotics, neither of which are guaranteed to resolve the infection. Of particular concern is the threat of bacterial biofilm development, since biofilm-mediated infections are difficult to diagnose and effective treatments are lacking. Developing therapeutic strategies have targeted mechanisms of biofilm formation and the means by which these bacteria communicate with each other to take on specialized roles such as persister cells within the biofilm. In addition, prevention of infection through novel coatings for prostheses and the local delivery of high concentrations of antibiotics by absorbable carriers has shown promise in laboratory and animal studies. Biofilm development, especially in an arthoplasty environment, and future diagnostic and treatment options are discussed.
Keywords: antibiotic resistance, arthroplasty, biofilm, coating, infection, planktonic, prosthesis, treatment
Infection in total joint arthroplasties
Rates of total joint arthroplasty placements & infection
Recently, a number of excellent articles have appeared detailing the pathogenesis, diagnosis, prevention and treatment of orthopedic infections and the growing evidence for the role of biofilm formation as a mechanism to explain issues in each of these areas [1–7]. Many of these studies have focused on the clinical perspective. This article discusses these important topics in relation to biofilm formation, not only on the foreign body, but also on adjacent soft tissues and bone in the context of a developmental process as a holistic integration with planktonic and invasive bacteria. We identify possible interventions arising from the consideration of specific tissue and cellular locations that might be considered ‘reservoirs’ of the infecting pathogens (e.g., joint fluid, intracellular areas and biofilms), with the concern that bacteria distributed among these various reservoirs could allow regrowth if all are not killed or removed.
Orthopedic infection continues to receive a high level of attention, due to the fact that nearly 1 million total hip and knee arthroplasties (Figure 1) are performed in the USA each year, and this number is expected to rise in the near term with the aging population [8]. Artificial joints can improve the quality of life for patients, but failure of the prosthetic can also inflict significant morbidity and suffering. The major causes of failure are loosening of the prosthesis from the bone, fracture of the bone or infection at the site of implantation. Although orthopedic infections overall include osteomyelitis and soft-tissue infections (e.g., diabetic foot infections – for a review, see [9]), this article will focus on infection following implantation, a serious complication that can often result in failure requiring revision [10–14].
Figure 1. Diagnosis of infection can be challenging.
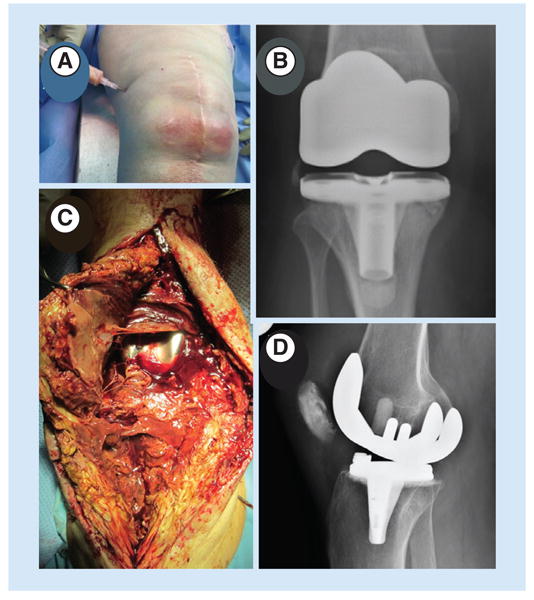
(A) In this patient with a hot knee, diagnosis was straightforward from clinical signs and symptoms and a positive culture from aspirate. (B) Radiograph from a total knee arthroplasty patient for whom diagnosis was more difficult. The aspirate was culture negative and the radiograph revealed no indications of infection. (C) Intraoperative image from (B) showing purulence and slime in the joint space and an intraoperative positive culture. (D) Radiograph from a patient with only subtle signs of a TKA infection, a culture-negative aspirate, a normal erythrocyte sedimentation rate and slightly elevated C-reactive protein, but over 20,000 white blood cells with more than 90% polymorphonuclear leukocytes. Intraoperative cultures showed coagulase-negative Staphylococcus.
In considering periprosthetic infection, there are two major routes and time scales associated with the entry of the pathogenic bacteria. The first is the perioperative period, most commonly via the surgical incision itself, with the source being the patient's endogenous flora or bacteria from the personnel or environment of the operating room (OR). The second is from hematogenous spread, which is generally thought to occur during the postoperative period [15]. The insertion of a foreign body that will reside in the patient for years increases the risk for infection that could ultimately mature into a biofilm. Infections can present in patients acutely and/or persist chronically for years following surgery, and the diagnosis of infection and the type of bacterial growth (i.e., planktonic vs biofilm) remains a difficult task for clinicians [16,17], often requiring highly specialized research equipment and time-consuming methods. Early infections are typically caused by high-virulence bacteria, while those that develop later tend to be caused by low-virulence bacteria [18]. Bacteria noted to cause periprosthetic infection include coagulase-negative staphylococci (30–43%), Staphylococcus aureus (12–23%), streptococci (9–10%), enterococci (3–7%), Gram-negative organisms (3–6%) or anaerobes (2–4%) [19]. However, the picture from culture data is likely misleading, since species such as Propionibacterium acnes, which is generally considered to be nonpathogenic or ‘weakly’ pathogenic, may be inappropriately dismissed as a contamination. This species has now been found to be more common than previously thought when more robust sampling and detection methods are used, such as sonication and nucleic acid detection by PCR [20]. Another issue is the development of specific biofilm phenotypes such as ‘small-colony variants’, which are slow growing and easily missed in routine clinical cultures [21]. Regardless of the species, once bacteria have gained entry to the surgical site, they can persist within the body in three distinct phenotypic modes of growth (Figure 2).
Figure 2. Various configurations of bacteria and biofilms in three different orthopedic patients.
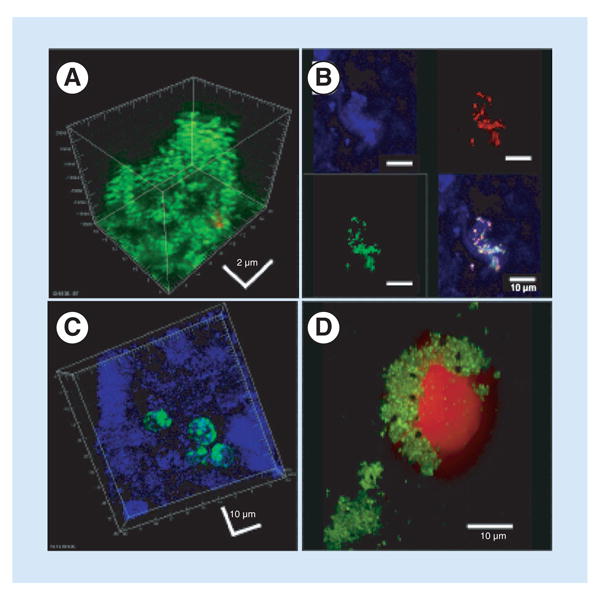
(A) Biofilm of live cocci (green) attached to a screw removed from a fixation device in a nonunion. The biofilm demonstrated classic 3D structure. (B) Patch of biofilm attached to periprosthetic tissue from a failed ankle arthroplasty. The upper left panel shows reflected light demonstrating the surface of the tissue (blue). The upper right panel shows a FISH ‘sau’ probe demonstrating Staphylococcus aureus bacteria (red). The lower left panel shows a FISH ‘Eub’ probe demonstrating all stained bacteria (green). The lower right panel shows an overlay demonstrating the S. aureus biofilm cluster attached to the tissue. (C) Periprosthetic tissue from the same patient as (B), showing bacteria that appear to be intracellular. (D) Intraoperative fluid from a patient with a failed elbow showing clumps of live cocci (green). The large red object is a nucleolus from a host cell that appears to have been ‘attacked’ and damaged by the cocci.
Planktonic
The single-cell form of bacteria is most easily identified and cleared by the host's natural defenses or antibiotic therapy. This type of growth can also serve as a precursor for biofilm development if the bacteria are able to colonize a surface within the patient (either periprosthetic tissue or the prosthesis components themselves). Planktonic bacteria may grow in the joint fluid during an acute episode of infection.
Biofilm
A biofilm is a community of microorganisms in a structural matrix usually adherent to an underlying substratum; these microbes can take on unique phenotypic roles within the 3D biofilm structure in order to evade both antibiotic therapy as well as the natural defenses of the host. Biofilms are commonly associated with a foreign body such as when a prosthetic is implanted into the host, because these abiotic artificial surfaces offer a ready interface to which individual bacteria may attach and eventually form a biofilm. Biofilm bacteria can cause extreme modifications to the local environment, as for example in dental biofilms, which can shift normal physiological conditions to a highly acidic and anaerobic state within just a few tens of microns [22] through their metabolic activity and diffusion-limited mass transfer in the biofilm. There is mounting evidence that over 99% of bacteria reside in biofilms, with staphylococci (specifically S. aureus and Staphylococcus epidermis) being the most common biofilm-forming bacteria [10]. Together, S. epidermis, S. aureus and Pseudomonas aeruginosa make up almost 75% of the biofilms found in medical devices [11,12]. In the context of prosthetic joint infection (PJI), biofilm bacteria can attach to hardware components, cement, bone and fibrous tissue, and detached clumps of biofilm can also be found in the joint fluid [23,24].
Invasive & intracellular
Several species of bacteria are able to enter, survive or even proliferate within host tissue or the host's cells. This phenomenon is most often seen in epithelial and osteoblast cell types, and is used by the bacteria as a means to avoid immune cells and high concentrations of antibiotics present in the extracellular space. Interestingly, S. aureus small-colony variants, a slow-growing phenotype associated with biofilm formation, have been documented inside fibroblasts in PJI cases, thus making a potential link between surface-associated biofilms and invasion [21].
It is possible that each of these three anatomical locations – the overlying fluid, the foreign body or host surface and the subsurface tissue – may represent individual reservoirs of infection, each containing bacteria in different phenotypic states, from which pathogens could repopulate if they are not completely eradicated through means such as washing, antibiotic therapy or surgical debridement. Figure 3 is a schematic showing how pathogenic bacteria might be distributed in a joint space, in this case using an example of a knee.
Figure 3. How bacteria and biofilms might be distributed in a periprosthetic joint infection using a knee as an example.
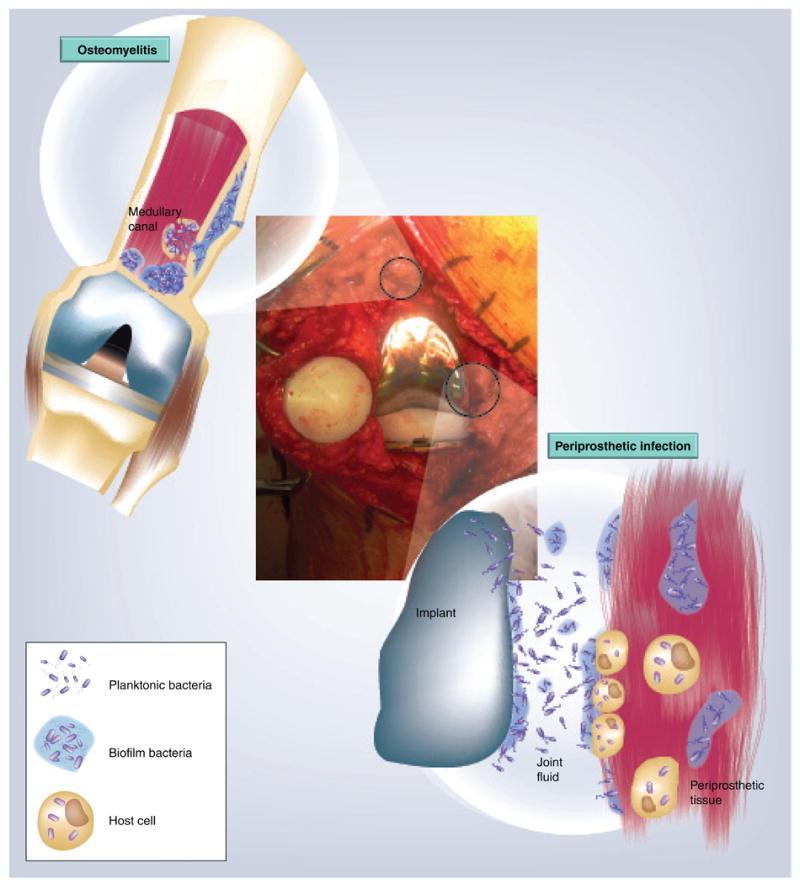
Biofilms can grow on the prostheses components, the surrounding tissue and the fibrous sheath. Each of these can be considered separate, but communicating niches. The joint fluid might contain planktonic cells or clumps of detached biofilm. The periprosthetic tissue can be invaded by the infecting bacteria. There can be migration of bacteria between each of these locations (prosthesis, surface tissue and matrix, subsurface tissue and fluid), which could contribute to survival. If bacteria in one niche are eradicated by debridement or antibiotic therapy, there is potential for the repopulation of pathogens from the other niches.
Formation of biofilms
Early stage
A biofilm begins when individual, planktonic bacteria adhere to a surface and begin to produce an extracellular polymeric slime (EPS) matrix composed of polysaccharides, DNA and proteins. This matrix can recruit and increase the adhesion of other microbes in the vicinity, which, through growth, can self-organize into a well-defined, highly structured collection of bacteria. The creation and maturation of biofilms are controlled by several factors in the matrix (e.g., exopolysaccharides) and mobility proteins on the bacteria (e.g., pili and flagella) [25]. Individual staphylococcal bacteria adhere to a surface and secrete positively charged homopolymers (polysaccharide intercellular adhesin) to aid in the aggregation of planktonic bacteria. These polysaccharides also create a physical and electrostatic barrier against the positively charged antimicrobial peptides and against phagocytosis by immune cells. Expression of polysaccharide intercellular adhesin has been positively correlated with the virulence of the bacteria [26]. In experiments conducted on Escherichia coli, the most motile strains in a constant flow system were also the best biofilm-forming strains, linking the phenomena of motility and biofilm development [27]. The flagella, which are used for swimming motility, can also act as points of initial adhesion. Once attached, the bacteria can transition from planktonic movement (swimming) to movement along a surface using type IV pilus motility (gliding or twitching). This surface motion can be part of a coordinated activity to form larger, mature biofilm structures. However, motility and motility appendages are not in every case required for the formation of biofilms, as evidenced by the profuse biofilms that can be formed by nonmotile pathogens, such as staphylococci and streptococci.
Maturation
Early in biofilm development, the structure can be less stable and more susceptible to antibiotics and host defenses [28]; however, as the biofilm rapidly matures, the bacteria within becoming increasingly tolerant of antibiotics. An important process in the maturation phase is the production of EPS, which binds the cells together and to the surface. The EPS forms a physical barrier, as well as causing diffusion-limited transport of chemicals into and out of the biofilm. The two main constituents of EPS are polysaccharides and extracellular DNA (eDNA). Recent evidence shows that eDNA can form net-like structures, similar in appearance to the nets used by neutrophils to trap planktonic bacteria [29]. In S. aureus, the release of eDNA is regulated by the cidA gene, which causes lysis of a subpopulation of cells within the biofilm [30]. P. aeruginosa also appears to have a regulated mechanism for the release of eDNA, which is coordinated with the development of biofilm structure [31]. There also appears to be an even higher level of extracellular ordering with respect to the extracellular matrix as shown in a study by Goodman et al., which identified extracellular proteins that linked eDNA polymer strands to form networks in Haemophilus influenzae biofilms [32]. Targeting biofilm EPS components such as eDNA in order to dissipate the biofilm with simultaneous application of antibiotics to kill the released cells is an area of active research.
The close proximity of cells, the high cell density and limited transport through the biofilm creates an environment conducive to cell-to-cell communication through quorum sensing (QS). QS in microbes is a regulatory mechanism that allows bacteria to modify gene transcription based on cell density. In staphylococcal biofilms, this is accomplished through a peptide (autoinducing peptide) that is secreted from the bacterial cells [33,34]. As bacteria aggregate, extracellular autoinducing peptide reaches a threshold concentration and begins binding to a membrane receptor, initiating a signaling cascade that results in the expression of the regulatory RNA, RNAIII. This effector activates the accessory gene regulator agr with the consequent expression of secreted virulence factors [35]. Mutations in agr also cause reduced dispersion, leading to increased biofilm formation [36]. Gram-negative biofilm-forming pathogens such as P. aeruginosa use a different QS system that is mediated by signaling molecules from the homoserine lactone family. Here, an accumulation of QS agents beyond a threshold concentration is also accompanied by an increase in biofilm formation, as well as an increase in secreted virulence factors [37]. The close proximity of cells within the biofilm is also conducive for horizontal gene transfer, which can allow for the transmission of virulence genes within the biofilm, as well as genes that are responsible for antibiotic resistance.
There are other consequences for cells within the biofilm as the biofilm grows and matures. The bacteria on the outside of the biofilm consume nutrients, such as oxygen and glucose, leaving the center of the biofilm relatively devoid of the same, such that centrally situated bacteria can become metabolically inactive. Nutrient deprivation and other stressors such as acidic conditions can cause bacteria within the biofilm to enter a dormant ‘persister’ state [38]. Both persister and small-colony variant populations in the biofilm can survive exposure to high levels of antibiotics due to their lack of metabolic activity.
Dispersal from the biofilm
In addition to its role in the developing biofilm, QS can also play a role in the detachment of bacteria from the mature biofilm in order to spread infection within the host, as previously discussed with respect to agr-mediated dispersal in staphylococcal biofilms [35]. P. aeruginosa has two dispersal mechanisms, one mediated by nitric oxide [39] and the other by cis-2-decanoic acid, a fatty acid messenger [40]. It is thought that coordinated dispersal is a mechanism used by bacteria to colonize new surfaces once nutrients and space have been depleted. Dispersal may therefore play a role in the dissemination of infection and can explain acute infectious episodes that appear as exacerbations of a chronic underlying process.
Clinical complications due to bacterial growth in a biofilm
Antibiotic resistance
Bacterial cells residing in the outermost aspects of the biofilm are most exposed to the host's defenses and antibiotics, but these microbes can themselves mount several protective defenses. The matrix and layers of cells within the biofilm create a physical barrier to slow the diffusional penetration of antibiotics. Metabolic activity in the outer biofilm can create acidic or anoxic areas that might contribute to antibiotic degradation [41]. In addition, secreted polymers that make up the extracellular matrix of the biofilm can bind and deactivate antibiotics, forming an antibiotic ‘sink’ [42]. As previously discussed, the depletion of nutrients in the interior of the biofilm can lead to starvation-induced dormancy and the development of resistant persister cells. Moreover, diffusion-limited transport, binding to matrix components and uptake by bacteria can create a gradient of antibiotic concentration. This gradient exposes a subpopulation of the bacteria within the biofilm to a sublethal dose of antibiotics. Sublethal exposure has been shown to increase the development of antibiotic tolerance by increasing biofilm formation [43]. Because of these various defense strategies when bacteria are grown as a biofilm, the MIC of antibiotics is increased by as much as 1000-fold in P. aeruginosa [44–47] and in biofilm clusters from methicillin-sensitive S. aureus, which exhibited tolerance to antibiotics that is normally associated with methicillin-resistant S. aureus strains [48].
In addition to biofilm-specific mechanisms of tolerance, biofilm bacteria also utilize conventional resistance mechanisms; for example, β-lactamases are released from P. aeruginosa biofilms in order to breakdown extracellular β-lactams (e.g., penicillin and cephalosporin, among others), a common family of antibiotics used to treat orthopedic infection [49,50]. Biofilm bacteria can also upregulate the expression of efflux pumps in order to remove antibiotics that have successfully penetrated into the bacterial intracellular compartment [49].
Avoidance of the host's defenses
Biofilm structure also inhibits the adaptive and innate immune responses of the host [51]. The mere presence of a foreign body within the patient is already relatively inaccessible to the immune response, due to the lack of blood supply to the structure. This restricts the ability of immune cells and antibodies to reach the foreign surface and clear infections [52]. Moreover, the investing layer of scar tissue that typically forms around an embedded implant is also relatively hypovascular, and therefore similarly compromised. When biofilms do form on and about implants, polymorphonuclear leukocytes are unable to effectively phagocytose bacteria due to restricted access to the bacterial cells within, caused by the EPS matrix [53]. Granulocytes isolated from the implant in an animal model had poor bactericidal activity and reduced superoxide production when compared with polymorphonuclear leukocytes from the blood or exudates. This impairment is thought to be caused by the interaction of the granulocyte with the implant or wear particles in the environment [54]. These nonfunctioning immune cells have been referred to as ‘frustrated’ granulocytes [55]. This defective immune response is evidenced by the significant reduction (by at least 10,000-fold) in abscess-forming doses of Staphylococcus when a foreign body is present in animal models and humans [56,57]. Zimmerli et al. and Elek and Conen found similar results in a guinea pig model and showed that reduced opsonization might account for some of this effect [56,57].
Innate immune cells are also able to activate the adaptive immune response through lectin binding in order to produce antibodies [51]. Antibodies, similar to phagocytic immune cells, are more effective early on against biofilm development, but these proteins are not able to penetrate the matrix or layers of bacterial cells once a biofilm has matured [58–60]. In certain situations, such as cystic fibrosis, this ineffective antibody response causes chronic inflammation, which can damage the host's tissues [61]. Similarly, in dental biofilms, chronic inflammation stimulated by the presence of a biofilm causes damage to the surrounding soft tissues, yet is ineffective in resolving the infection [62]. In PJI, there is a correlation with elevated inflammatory parameters, and the local tissue damage caused by inflammation might release nutrients that then become available to the biofilm bacteria, exacerbating the disease.
Role of biofilms in total joint arthroplasties
Diagnosis of infection
While the development of an infection can be accompanied by obvious signs (e.g., fever, swelling and purulent discharge at surgical site, among others), in the absence of a positive culture, differentiating between loosening due to wear reactions (aseptic loosening) and infection is very difficult. Ascertaining whether microbes are growing in a planktonic or biofilm state within the patient represents an even greater challenge. Even if bacteria can be recovered from the patient and identified through in vitro culturing, this technique is not necessarily reflective of the in vivo growth state, since most strains of bacteria can grow in both planktonic and biofilm modes (although recovered biofilm bacteria are frequently difficult to culture – see below). Acute implant infections have been defined as having “signs and symptoms lasting <14–28 days, an unambiguous diagnosis based on histopathology and microbiology, a stable implant, and susceptibility of the microorganism to an effective orally available antimicrobial agent” [63]. Acute infections are classically considered to be caused by rapidly growing planktonic bacteria, which are relatively easy to recover in sampling, since they are typically present in fluids and tissues in high concentrations. Because they are usually rapidly growing in vivo, they are also relatively easy to propagate on nutrient-rich clinical culture media. However, chronic subacute or indolent infections are harder to diagnose. This can be explained by the biofilm phenotype, in which many bacteria are in a metabolically quiescent state induced by nutrient limitation inside the biofilm. Biofilms are often patchy and might be missed in a fluid, swab or tissue biopsy. In multiple studies, simple in vitro culturing of the bacteria only detected biofilms 30% of the time compared with 80–90% using histology and microscopy [64]. Improvement in the methods used to detect the presence and phenotypic state of microbes is of critical importance, as this may help with determining the type and concentration of antibiotic(s) or surgical interventions needed to eliminate or more effectively control the infection.
The recognized difficulty in diagnosing periprosthetic joint infection (Figure 1) led to the formation of a working group of the Musculoskeletal Infection Society, and more recently a specific study group of the Consensus Meeting on Periprosthetic Joint Infection, with the task of defining consensus guidelines for diagnostic purposes [65]. The proposed guidelines consider three criteria, only one of which exclusively relies on direct culture (if a pathogen is cultured from two separate samples). Furthermore, culture of low-virulence pathogens, such as P. acnes, in the absence of other criteria is not considered a definitive diagnostic of infection. It has also been suggested that even pathogens cultured from draining sinuses associated with total knee and hip arthroplasties do not correlate well with the pathogens involved in the PJI and might result in misguided treatment [66]. The other two criteria are: the presence of a sinus communicating directly with the prosthesis; and the presence of four out of six clinical and laboratory findings, including elevated erythrocyte sedimentation rate and C-reactive protein, elevated synovial leukocyte count or neutrophil percentage, presence of purulence in the affected joint, a single culture-positive sample and greater than five neutrophils per high-power field in five high-power fields of histological analysis of the explanted tissue. Since the level of C-reactive protein is often elevated postsurgery, levels prior to surgery should be established, with continued testing following surgery in order to ensure that levels return to baseline. Blood and synovial fluid should be cultured for the presence of both aerobic and anaerobic microbes. If the infection has already caused structural damage to the bone, a radiograph may visualize the subsequent weakness caused by the infection; however, radiographs are highly limited in their ability to confidently diagnose infection. Localized bone resorption caused by infection, appearing in radiographs as a focal lucency, often appears to be indistinguishable from granulomatosis [67] or periprosthetic osteolysis due to wear particles [68].
Molecular methods of diagnosis
Currently, diagnosis is based on standard microbiological cultures of recovered clinical samples, clinical signs and a consideration of various laboratory results, as described above [65]. However, due to the inadequacy of cultures for detecting biofilm bacteria, more recent studies have utilized molecular methods in order to assay for bacterial DNA and RNA directly. Many of these methods involve PCR amplification and sequencing of the nucleic acid mix isolated from clinical samples using either microbe-specific or broad-range primers, often derived from bacterial 16S rDNA sequences. This can allow for the simultaneous identification of multiple microbes that may be present in any given sample or patient. The use of PCR amplification and molecular analysis (in conjunction with cultures) has shown several advantages over cultures alone in the detection and identification of microbes:
PCR in conjunction with culturing from biopsy or joint fluid has detected resident bacteria in 9% of culture-negative cases [69]. However, other studies report positive detection rates of between 70 and 80% [70];
PCR has been used to improve the diagnosis of other infections, including port-related bloodstream infections, likely due to its ability to detect slow-growing microbes [71];
A broader range of microbes have been identified as the putative cause of orthopedic infections due to greater sensitivity and the ability to identify bacteria without having to first ‘target’ specific suspected pathogens [72].
While PCR-based methods show promise for the improved diagnosis of infections in the clinic, several issues still need to be addressed. Due to the sensitivity of PCR, contamination remains a concern that requires specific controls/multiple samples. There is still some debate as to whether the DNA from ‘unusual’ organisms detected by PCR is actually from contaminating bacteria, or even from the fluids and instruments used during surgery.
Investigation of the implant & surrounding tissue upon removal
On contemplating a potentially infected implant and its removal, a preoperative diagnosis and the identification of the organism and its method of growth would be ideal, but in many cases, the presence and identification of pathogens can only be resolved after the removal of the implant. During these procedures, surgeons should also collect periprosthetic tissue samples for pathological examination. Upon removal of the prosthetic, its surface can be tested using sensitive and accurate histopathic and microscopic examination in order to determine the presence and structure of adhered bacteria [73], although it is possible that the physical trauma of implant removal may mechanically dissociate the biofilm from the implant.
A technique that has proven useful in increasing the sensitivity of culturing for the detection of pathogens associated with failed orthopedic prostheses is sonication [74,75]. Briefly, the recovered implants are sonicated in a bath sonicator in a container containing buffered saline, and the sonicate is then cultured. In a 331-patient study looking at revised hips and knees, prior sonication increased the sensitivity of culturing for the detection of pathogens from 60.8% from homogenized periprosthetic tissue to 78.5% [74]. It is presumed that the reason for the increased sensitivity was that the bacteria were present as an adherent biofilm, which was more effectively dislodged, but not killed, by sonication. Interestingly, it was noted in the same study that presonication was particularly useful in increasing culture sensitivity from patients who had received antimicrobial therapy within 14 days before surgery. The authors hypothesized that this was due to the fact that “planktonic bacteria present in tissue are more susceptible to anti-infective agents than are biofilm bacteria.” However, our direct examination shows adherent biofilms on periprosthetic tissue as well as orthopedic hardware [23,24]; similar observations have been made for biofilms on periprosthetic tissue associated with other types of indwelling medical devices [76]. An alternative explanation is that the large volume of buffer required to immerse the implant for sonication might more effectively dilute residual antibiotics.
Dislodged biofilm bacteria in explant sonicate have also been detected using PCR and microscopic techniques. Tunney et al. used this technique as early as 1999 [77]. They reported that aggregates of bacteria (containing up to 300 cells) were only observed after sonication of the material. Furthermore, they reported that when using PCR targeting bacterial genes, they were able to identify infecting microbes at a higher rate (72%) than either immunofluorescence microscopy (63%) or cultures (22%) of the sonicate. Microscopic staining of the sonicate, although slightly less sensitive for detecting pathogens when compared with PCR, could discern whether bacteria were present in aggregates as biofilms. Culturing and PCR from the sonicate have also been shown to increase sensitivity for detecting pathogens when compared with simply vortexing of the removed prostheses in fluid. More recently, sonication of retrieved implants was combined with multiplex PCR, which enabled the detection of multiple bacterial and fungal pathogens [20]. In another approach for the detection of pathogens following sonication, microcalorimetry was able to detect infection within 12 h [78].
Another approach is direct microscopic examination of the explanted prosthesis, cement or periprosthetic tissue in order to detect pathogens. This method has the advantage that it is arguably the only method that can directly confirm the presence of biofilms in situ going on the assumption that aggregates of bacteria firmly adhered to the surface represent a ‘growth in place’ process, that is, they were not merely casual contaminants which had attached during surgery and specimen collection but had actually grown on the surfaces over time. Microscopy can also distinguish surface-adhered populations from intracellular pathogens. Confocal microscopy is useful for the examination of periprosthetic tissue since it can penetrate approximately 100–200 μm into the tissue and thus identify invasive as well as superficially adhered pathogens.
This utility of combining microscopic methods with PCR was shown in a 2006 study by Hall-Stoodley et al. in which pathogens in biofilms were demonstrated in middle-ear infections [64]. This study successfully used a combination of live dead staining and FISH to determine the presence and viability of bacteria, and also to identify the species of bacteria (using 16S rRNA species specific FISH probes). A similar approach was successfully applied for the identification of pathogens in biofilms in periprosthetic orthopedic infections [23,24] and on implanted suture material in the abdominal wall [79]. A limitation to this technique, however, is that FISH requires significant washing steps that could dislodge possible biofilms. One way of overcoming this issue is that the samples can be cryoembedded in an embedding compound such as paraffin, thin sectioned and mounted on a slide before staining. However, in our experience, looking down directly at the surfaces in plain view provides a better representation of the distribution of bacteria and biofilms than would be found from thin sections. Thus, there is a compromise between embedding to preserve structure and being able to directly scan the exposed surface and the near-surface layers. While implant removal and inspection using nonroutine clinical methods improves the diagnosis of the type of bacterial growth, it also increases the likelihood of in vitro contamination due to the extra handling of the sample in a laboratory setting [80]. Moreover, although direct examination of the device components and intraoperative samples using a variety of research techniques improves our academic understanding of the infection from a failure analysis perspective, it is of limited use clinically, since the decision to explant has already been made without the benefit of this information.
Prevention & treatment of periprosthetic infections
Infection prevention
The first line of defense is containment in the OR environment. Measures to protect the sterile field include high efficiency particulate airfiltered air handling, limiting personnel and traffic, the use of spacesuit scrubs, disinfection of the skin and shielding of as much of the patient's skin as possible with surgical drapes. However, despite these methods, it is impossible to completely sterilize the ‘sterile field’, and it is likely unavoidable that some bacteria will gain access to the incision site. What percentage of such bacteria arrive via the external environment of the OR versus those originating from the patient's own body (e.g., the skin or blood) is not known, although nasal carriage of S. aureus has been shown to increase the risk of infection following orthopedic surgery [81]. In addition to containing pathogens by environmental controls, prophylactic antibiotics remain an effective method for reducing the likelihood of infection following surgery [82], although there is a narrow window of effectiveness for these antibiotics, with Widmer reporting that if antibiotics are given less than 30 min before incision, they are not beneficial [83].
As a possible means to slow the threat of antibiotic-resistant biofilms on prosthetic joints in patients, researchers are testing novel materials with which to construct prosthetics that will impede bacterial colonization on the artificial surface. For example, cobalt–chromium inhibited bacterial growth to a greater extent than titanium [84], while rough titanium has been reported to increase bacterial colonization when compared with smooth titanium in a rabbit model of orthopedic infection [85]. Part of this effect might be that the roughness increases the surface area and also provides protective depressions for the bacteria, but also rough surfaces increase the attachment of host matrix proteins such as fibronectin, which may in turn explain the increased attachment of bacteria such as staphylococci, which express matrix receptors [86]. It should be noted that while different materials have shown promise in vitro, to date, they have not shown efficacy in vivo [87].
Treating prosthetic infection
Once a patient develops a prosthetic infection that cannot be cleared by antibiotic therapy, often the only viable recourse is to remove the affected implant. For example, antibiotic administration without device removal was only found to be successful 22–37% of the time when treating catheter-associated bacteremia [88–92]. In cases of PJI, device removal is accomplished in one of two ways.
One stage
The prosthesis is removed and a new device is implanted in one surgery. This technique requires the surrounding tissue to be clear of infection and secondary damage, the microorganism to be susceptible to antibiotics and the patient not exhibiting any severe comorbidities [93–96].
Two stage
A two-stage procedure is used to try to ensure that all pathogens have been cleared before reimplantation because of the high risk of reinfection associated with placing hardware into a space that might contain retained pathogens. The period between stages allows for antibiotic therapy and surveillance before proceeding to the second stage. The implant is removed and intravenous antibiotics are typically administered for 2–8 weeks. A second prosthetic is then implanted after completion of antibiotic treatment [16,17,97–101].
Surgical spacer
During the two-stage surgical procedure (described above), an antibiotic-impregnated cement spacer is usually used to manage the dead space that results after explantation of the prosthesis while also providing localized delivery of antibiotics. The standard of care to date has involved the use of spacers made of poly(methyl methacrylate) (PMMA). Spacers are used to maintain the joint space during a two-stage revision and can also be formed to provide an articulate surface. PMMA is usually made up in the operating room by mixing powder and liquid components together in order to initiate the polymerization process. At this point, antibiotics can be added so that the cement spacer has an eluting antimicrobial function as well as a mechanical function. In addition to its use as a spacer, antibiotic PMMA beads have also been used to pack into wounds as an antimicrobial delivery system.
Future directions in the prevention & treatment of biofilms in orthopedics
Due to the recognized difficulty in eradicating biofilms once they have formed, the prevention of infection following prosthetic or device implantation continues to be the focus of intense research in orthopedics [102] and other specialties. However, biofilms can also grow on periprosthetic tissue, so it is not only device protection that is important. Ideally, a surface or material would be able to project its antimicrobial effect to adjacent tissues and fluids, as well as to components that are not amenable to coating due to the type of material, size, geometry or manufacturing process. Incorporating conventional antibiotics or antimicrobial agents such as silver to kill cells on contract is one approach, while another is to disrupt the latter stages of biofilm formation by initiating dispersal, interrupting cell signaling or digesting the EPS matrix.
Prosthetic antimicrobial coatings
Biosurfactants
Various groups have begun testing compounds that feature both hydrophilic and hydrophobic moieties (glycolipids, lipopeptides, polysaccharide–protein complexes, phospholipids, fatty acids and neutral lipids) in an attempt to reduce the ability of microbes to bind to the surface of prosthetics (for a review, see [103]). The results thus far have been mixed, with studies suggesting that an initial cohort of microbes is able to adhere to these coated surfaces and subsequently modify the surface by creating a conditioning film that increases the binding of more microbes [104]. In addition, similar to antibiotics that only function against specific bacteria, many coatings only function against certain types of bacteria, prompting the notion of using multiple agents [105].
A recent report found that positively charged biomaterial surfaces were successful in slowing the adhering of Gram-negative but not Gram-positive bacteria [106]. In addition to coating prosthetic surfaces with charged molecules, the use of macromolecules (i.e., heparin and heparin and polypeptides) to form a hydrated layer on the artificial surface has also shown promise in reducing the colonization and formation of biofilms by interfering with the binding of the bacteria to the surface of multiple substrates [107–110].
Antimicrobial metal & metal oxide nanoparticles
Silver has a long history of antimicrobial activity, including medical device coating [111]. It has been reported to kill many different strains of bacteria, but the exact fatal mechanism remains unknown. Silver is known to bind to DNA, RNA and phosphoproteins [111], and it can also interfere with metabolism through the binding of thiol groups [112,113] and by causing the production of reactive oxygen species [114]. Unlike some other coatings that have been tested, silver nanoparticles can kill both Gram-negative and Gram-positive organisms [115]. These nanoparticles proved successful in a study of biofilm prevention on catheters exposed to E. coli, Enterococcus spp. and S. aureus [116]. Nanoparticle silver ion coatings also inhibited biofilm formation on titanium implants in a rabbit model [117]. While silver is currently the most intensively studied metal in this context, other metals are also being considered for their potential antibiofilm efficacy. Among these are zinc, titanium, iron and copper. Zinc oxide is thought to disrupt the cell wall of both Gram-positive and Gram-negative bacteria, eventually leading to elevated membrane permeability and cell damage [118–120]. However, most work to date has been carried out with zinc oxide against planktonic bacteria; against dental pathogens, zinc oxide showed less of an ability to inhibit biofilms than silver [121,122]. Copper has shown promise as an antibiofilm agent for hospital and public surfaces; however, potential toxicity issues as a nanoparticle may limit its use for indwelling devices [123].
Antibiotic-coated metals
Direct tethering of antibiotics to metal orthopedic implants has promise to kill bacteria when they first come into contact with a prosthesis, and hence halt the progression of biofilm formation. Vancomycin and gentamycin were tethered to 316L-grade stainless steel by self-assembled monolayers and they retained their potency against S. aureus over periods of up to 48 h [124]. In addition, titanium implants with vancomycin covalently bound to the surface were found to reduce bacterial colonization and improve bone healing in an ovine model of implant infection [125]. While the reservoir of deliverable antibiotics is limited, using a tethering approach for the protection of the implant surface against colonizing bacteria immediately following implantation might be enough to significantly reduce the risk of infection, although such an effect is likely to be limited to the immediate vicinity of the implant surface.
While coatings offer the advantage of protecting a particular component, there are still issues regarding the longevity of the coating or possible reactions with the underlying material. Laboratory studies assessing surface protection of a coating tend to be of short duration and the influence of these coatings with respect to the long-term effects on wear or particle cytotoxicity remains to be evaluated.
Localized antibiotic delivery
Cements
Both absorbable and nonabsorbable cements can be utilized for drug delivery even though, in many cases, they are used for a different primary function, such as to provide mechanical stability, dead space preservation, stimulation of bone repair or osteoconductivity. Nonbiodegradable acrylic-based polymer cements (e.g., PMMA) and biodegradable mineral-based bone cements (e.g., calcium sulfate and/or phosphate) can be loaded with single or multiple antibiotics. These materials can deliver very high local concentrations of antimicrobial agents to the surgical site, which otherwise could not be achieved systemically due to toxicity. PMMA has the advantage of being mechanically very strong, while absorbable cements can offer better release characteristics, such that therapeutic levels are maintained over longer periods. The addition of antibiotics to commercially available cements and mixing procedures will affect the microstructure of the cement, which in turn influences the mechanical properties and elution characteristics. Both antibiotic-loaded polymer and mineral-based cements are routinely formed into beads specifically for drug delivery and dead space management. Release is generally controlled by diffusion and is difficult to control, and with a nonabsorbable material, there is often a rapid ‘burst’ release of antibiotic over the first few hours to days. One concern is that if all bacteria in the vicinity are not killed during the initial burst, those remaining will be exposed to subinhibitory concentrations, with a possible consequence of increasing the likelihood of emerging antibiotic resistance. However, despite the fact that the high local concentration of antibiotic can be relatively short lived, eluting cements may reduce the risk of the establishment of a chronic infection in the initial postsurgical period by killing planktonic cells before they can establish a biofilm. Clinical evidence pointing to the optimal parameters required for infection control are difficult to interpret due to the variability in brand of cement, concentration and type of antibiotics, mixing techniques, anatomical location and shape and size parameters of the formed cement; however, the general consensus is that antibiotic-loaded acrylic cement is useful in the treatment and management of chronic orthopedic infections [126,127]. More recently, bioabsorbable materials such as calcium sulfate and calcium phosphate have regained the interest of researchers and clinicians. Calcium sulfate has been used as a bone graft substitute for over 100 years [128], but has more recently been utilized for additional functions in a pelletized form as a local antibiotic delivery mechanism [129], although adverse reactions to the pelletized form have been reported [128]. Synthetic calcium sulfate beads have been recently introduced in the hope of reducing the risk of adverse reaction and have been shown to clear osteomyelitis in a rabbit model [130]. In addition, the elution characteristics can be controlled by bead size and composition [131], and they do not provide a permanent artificial surface for bacterial colonization. While absorbable and nonabsorbable carriers have been found to be reasonably effective in the treatment of osteomyelitis [132], if no long-term mechanical function is required, absorbable carriers have the advantage of not providing a permanent surface for possible biofilm infection and would not require surgical removal in another procedure.
Nonantibiotic biofilm targets
In addition to the targeted killing of individual bacteria, as is performed with conventional antibiotics, our growing understanding of the chemical composition, mechanical properties and biological processes of biofilms has led to the development of strategies to target biofilm-specific components and processes.
Vaccines
In order to circumvent issues with antibiotic resistance, several groups have studied the use of biofilm-specific vaccines in order to help the immune system recognize the infecting microbes in patients. Unfortunately, there are several hurdles to overcome. First, the genetic expression in bacteria shifts as the bacteria aggregate from a planktonic form to a structured biofilm [36]; therefore, vaccines derived in the laboratory against epitopes expressed on the surface of planktonic bacteria may not be successful against biofilm bacteria. Protein-based vaccines targeting adhesive matrix molecules or cell wall proteins have also shown only limited success [133,134]. Another problem is masking, in which biofilm-specific cell surface epitopes might be ‘hidden’ from vaccine surveillance by the EPS. More recently, testing of a multicomponent vaccine has been conducted with better results. This vaccine combined four cell wall and membrane-associated proteins that have been shown to be immunogenic during the maturation of the biofilm and found that a combination of antibiotics and vaccination significantly increased the percentage of mice that could clear an methicillin-resistant S. aureus infection (87.5% clearance with vaccination and antibiotics, 55.6% with only antibiotics, 22.2% with only vaccination and 33.3% clearance in controls) [135]. This study suggests that while vaccines may not be sufficient to protect the host, they could function to improve clinical outcomes when coupled with antibiotic therapy.
Another approach is to direct the immune system to attack not the bacterial cells themselves, but components of the biofilm EPS that play a structural role. Goodman et al. used antisera against a DNA binding protein, DNABII, which connects strands of eDNA in nontypeable H. influenzae biofilms in order to significantly reduce biofilm formation by this organism [32]. The EPS of S. aureus biofilms is known to contain eDNA, which plays a structural role [136,137], and it is possible that a similar vaccination against EPS-connecting proteins (if they exist in S. aureus) might prevent biofilm formation by interfering with biofilm assembly.
Biofilm-disaggregating agents
Using agents that disrupt the EPS, which holds biofilm bacteria together and attaches them to a surface, is another antibiofilm strategy. An enzyme that has shown promise in this regard is the DNA-digesting enzyme, DNase I. This enzyme has been shown to break down eDNA in vitro [138]. Since eDNA is a major component of the EPS [139], this significantly disrupts the biofilm and enhances the efficacy of antimicrobials [140,141]. There are a number of advantages in targeting eDNA; it is common in biofilms formed by many types of pathogens, it is linear and it has only one basic linkage (phosphodiester bonds), which can cleaved be nucleases.
The other major component of EPS is polysaccharides. However, unlike DNA, there are many types of biofilm polysaccharides produced by different pathogens. In addition, they are often branching, they can be homo- or co-polymers and there are different types of bonds holding the sugar monomers together. Thus, enzymes to digest polysaccharides need to be targeted to a particular species or genus. Staphylococci, which are the most common pathogens of orthopedic concern, produce poly-N-acetylglucosamine. Poly-N-acetylglucosamine can be digested by dispersin B, a glycoside hydrolase, which has been shown to function both independently and in conjunction with antibiotics to eliminate or inhibit biofilm development on polyurethanes and catheters [142,143].
While digestion of the EPS shows promise, current thinking suggests that it should be used in combination with conventional antibiotics, since breaking up biofilms will actually release live bacterial cells into the surrounding environment. Given the complexity of the chemical and physical structure of the EPS, combined with the fact that the host matrix can also be associated with the biofilm, it is likely that multiple components will need to be targeted in order to achieve complete digestion.
QS inhibitors
Because QS is important in the regulation of secreted virulence factors and in biofilm formation, many groups have tried to identify small-molecule inhibitors to disrupt this form of communication within the biofilm [37]. Many Gram-negative biofilm-forming bacteria use the acetylated homoserine lactone-based las quorum sensing signaling system, in which the signal molecule, an acetylated homoserine lactone, is secreted into the surrounding space, where it can be taken up by adjacent bacteria to bind with the LasR transcriptional regulator. This in turn can increase the expression of multiple virulence factors, and also increase biofilm formation. Inhibitors that target the las quorum sensing system system in P. aeruginosa accelerated bacterial clearance and reduced pathology in a mouse model of lung infection [144,145]. Importantly, these inhibitors also increased the survival time of mice against lethal P. aeruginosa infections [145]. In addition to receptor antagonists to disrupt the ability of bacteria to communicate through QS, enzymes that can breakdown QS signaling peptides have also been shown to be effective in a Caenorhabditis elegans model of infection [146]. Although proof-of-concept trials have been successfully carried out in the laboratory, these agents have yet to be tested in the clinical setting.
Biofilm-dispersal agents
Unlike disaggregation, which breaks apart a biofilm by digestion, dispersal signals are regulated by density-dependent QS, causing natural dispersal of bacteria from biofilms [40]. It is thought that natural dispersal can be induced by the exogenous administration of these signaling molecules and, as is the case with disaggregation, they would need to be used in combination with antibiotics in order to kill any released bacteria. In terms of dispersal, the two signals that show the greatest promise are nanomolar nitric oxide [39] and cis-2-decanoic acid, a fatty acid messenger [40]. Current research suggests that nitric oxide may be more effective at dispersing motile strains of bacteria and thus may be useful against a number of Gram-negative orthopedic pathogens, but it is not clear whether it would have activity against staphylococci or streptococci. There are also issues with the delivery of nitric oxide to deep tissues due to the fact that it is highly labile, and potential cytotoxicity is also a concern. Cis-2-decanoic acid has been shown to reduce biofilm formation by a number of pathogens, including S. aureus [147], and in combination with antibiotics, it has been shown to completely prevent biofilm formation. It is likely that delivery would have to be through local release from a carrier.
Attacking the persister/dormant state
Another potential biofilm target is the dormant or persister population within biofilms, which are thought to be responsible for the high levels of antibiotic tolerance. One possibility is adding factors to actually stimulate biofilm bacterial metabolism, such that metabolically active bacteria are rendered vulnerable to otherwise-ineffective antibiotics. It has been shown that a methicillin-sensitive strain of S. aureus was highly tolerant of oxacillin as a biofilm, and when the biofilm was dispersed in spent media or buffer, the bacteria were still tolerant [48]. However, in the presence of nutrients (fresh media) and antibiotics, the cells became sensitive again. In order to be used therapeutically, growth factors would likely need to be added in combination with a dispersal agent and an antibiotic, but the concept of activating dormant biofilm bacteria obviously has potential to worsen the infection.
A novel alternative approach is to attack the persister cells while in the dormant state. It has recently been shown that the cytoplasmic protease ClpP in S. aureus can be activated by the antibiotic acyldepsipeptide (ADEP4) in order to kill persister cells by causing them to digest themselves from within. In combination with rifampicin, ADEP4 was active against in vitro biofilms and was able to eradicate infection from a mouse thigh, even in the presence of a foreign body [148]. While these results are very promising, it was noted that not all cells have the ClpP protein, and the spread of ADEP4-resistant strains in the population was rapid. It remains to be seen whether this approach has potential for the treatment of biofilm infections associated with orthopedic and other implants.
Conclusion & future perspective
Antimicrobial control of the OR environment due to the pioneering work of people like Joseph Lister and John Charney has dramatically reduced the rates of surgical site infection from the late-19th century to the current day. However, further significant reductions have remained elusive. The difficulty in the prevention and treatment of biofilms is a major obstacle in further reducing infection rates and the devastating impacts of infection. The ability of bacteria to overcome antibiotic therapy through the genetic acquisition of resistance, as well as from the phenotypic and barrier effects of biofilm formation, has led to diminishing returns in infection control using conventional strategies. As with any infection, prevention is the preferred control option, but when there is a risk of biofilm formation, prevention becomes even more critical. While the widespread overuse of antibiotics has led to selection for resistant strains, in implantation cases, where there is even a moderate risk of biofilm infection, the prophylactic application of high concentrations of antibiotics (and possibly combinations of antibiotics with multiple mechanisms of action) delivered directly to the surgical site in order to eradicate the pathogens before they can establish a biofilm could be used more aggressively. Optimizing release kinetics using carriers that are already used in orthopedic surgery, such as synthetic bone grafts and cements, is arguably the strategy that is closest to clinical deployment, and it is hoped that research in this area will translate to the clinic in the next 5 years.
Concurrently, improvements must continue to be made in the ability to diagnose and treat infections. In terms of diagnosis, PCR techniques are raising important – and difficult – questions as to what actually constitutes an infection and when and how to treat when a high number of ‘aseptic loosening’ cases appear to carry periprosthetic pathogens. Growing frustration with the ability of conventional clinical diagnostics to provide useful guidance on how to manage these difficult cases will push research beyond that of developing culture-free pathogen identification techniques to clinical studies correlating long-term outcomes with PCR-based results in order to provide a grounding for more useful clinical interpretations. As these data become available and results begin to emerge from the use of approved PCR-based techniques in other parts of the world, the US FDA will have clearer guidance in terms of the approval process for PCR-based diagnostic tests for orthopedic infections. It seems likely that PCR-based diagnostic platforms will be commonplace in larger clinical microbiology laboratories within the next 5–10 years. PCR (and other molecular diagnostic platforms) will not replace culturing (which is still essential for a providing a complete antibiogram or allowing the study of the pathogen in the laboratory), but will be used to provide rapid information that can be used for initial decision-making. Conventional culture techniques will be enhanced as orthopedic surgeons put growing pressure on clinical microbiology laboratories to perform cultures over longer periods in order to detect slower-growing strains, as well as for the more frequent use of anaerobic culturing. Diagnosis through sonication culturing or microscopic analysis is a useful intermediate step in helping to interpret PCR and conventional clinical culture results, but it is difficult to envisage that these tests will be performed routinely, since they are labor intensive and costly. Medical imaging of biofilms is another area of great potential for clinical translation, but development is needed for biofilm-specific staining. Labeled antibodies have the potential for mapping periprosthetic biofilms (Figure 4). Imaging and rapid diagnostics of tissues will make it possible to better identify the infected margins intraoperatively for more effective debridement. Biofilm infections may come to be seen more like cancers, in that if aggressive steps are not taken to completely remove the biofilm nidus, the infection has a high likelihood of recurring.
Figure 4. Imaging periprosthetic biofilms using quantum dot-labeled antibiofilm antibodies.
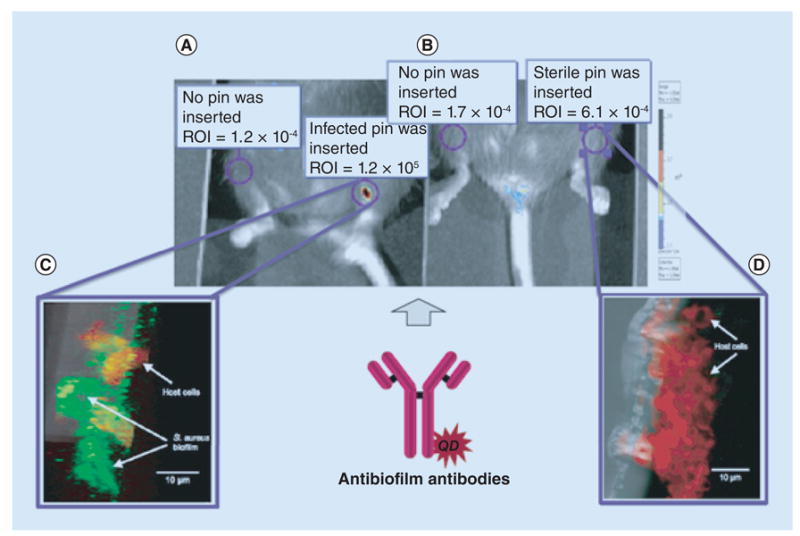
The antibodies were injected via the intravenous route and allowed to localize in mice with (A) Staphylococcus aureus-infected or (B) sterile pins implanted into the left tibia. Live imaging was performed using an IVIS® Lumina Series III imager (PerkinElmer, MA, USA). (C) In order to confirm the results of the live imaging, the pins were extracted and probed with with fluorescein isothiocyanate-labeled universal bacterial probe (green) and a rhodamine-labeled universal eukaryotic cell probe (red) that were specific for bacterial and eukaryotic rRNAs, showing well-developed biofilms. (D) No biofilms were evident in the sterile pins. The relative brightness which was proportional to the amount of biofilm is indicated in the ROI.
ROI: Region of interest.
Finally, with respect to the treatment of mature biofilms, while there are many exciting strategies being developed in the research environment, only a few of these have been assessed for cytotoxicity and progressed to animal trials. Those that have tend to be in rodent models and are assessed relatively short-term studies. It is unlikely that vaccinations will come to the market within a 10-year time frame. However, agents already in medical use for other indications, such as DNase I (the active ingredient of Pulmozyme® (Genentech Inc., CA, USA), a synthetic DNase I supplied as 1 mg ml-1solution, which is used to reduce the viscosity of sputum in cystic fibrosis patients by targeting the break-up of human DNA) and gaseous nitric oxide (used as a vasodilator), which have been shown to act as biofilm-dispersing agents, may make a more rapid clinical translation for the treatment of biofilm infections. While biofilms continue to reveal themselves as formidable foes, there has never been so much research directed towards their prevention, diagnosis and treatment. Currently, academic research has given little in the way of providing orthopedic surgeons with specific weapons to help in the management of biofilm infections. There is utility through knowledge, however, and the biofilm paradigm does provide the surgeon (and patient) with a science-based understanding of why an infection might be so difficult to manage. We expect that in due time, biofilm-specific therapies will begin to trickle from the bench to the clinic and enable a meaningful reduction in biofilm infections in orthopedics and other medical specialties.
Executive Summary.
Periprosthetic infection in total joint arthroplasties & fixation devices
The number of arthroplasties and implanted fixation devices continues to rise, and while infection rates may decline, the absolute number of infections is expected to increase.
These infections are now recognized to largely derive from bacterial biofilms residing on and around the implanted prostheses.
Adequate prevention, diagnosis and treatment strategies for biofilm-based prosthetic joint infections (PJIs) and, more generally, periprosthetic infections are still lacking.
Formation of biofilms
Planktonic bacteria attach to implanted prostheses as well as periprosthetic tissue.
The bacteria produce an extracellular polymeric slime (EPS) matrix that holds the biofilm together and to the surface.
Chemical gradients in the biofilm cause the generation of highly localized regions that facilitate the bacteria within to adopt specialized phenotypes, such as resistant slow-growing or dormant persister states.
The close proximity of cells within the biofilm enables cell signaling communication mechanisms to coordinate behavior.
Biofilms provide a reservoir from which bacteria can periodically disperse into surrounding tissues and joint fluid.
The EPS limits the ability of antibodies and phagocytic cells to penetrate the biofilm.
Clinical complications due to bacterial growth in a biofilm
Biofilm PJIs are often subclinical and culture negative, confounding diagnosis and treatment.
The infection may only fully declare itself when the released cells revert to the rapidly growing planktonic phenotype and cause acute-on-chronic exacerbations of symptoms.
Biofilm bacteria are more highly resistant to antibiotics than when rapidly growing in the planktonic phenotype, making prevention or early treatment paramount.
Direct microscopic examination and indirect techniques (i.e., culturing following hardware sonication) support the hypothesis that biofilm formation is a major cause of PJIs.
Guidelines for immunological, microbiological and clinical findings have been drawn up in order to construct a diagnostic decision tree for addressing issues regarding culture ambiguity.
Future directions in the prevention & treatment of biofilms in orthopedics
Surface-tethered antibiotics and metal oxide nanoparticle prosthetic coatings show potential for the prevention of biofilm formation.
Local antibiotic delivery from absorbable bone cements provides good release kinetics with no permanent surface for biofilm formation.
Vaccines targeting biofilm-specific components, such as the EPS, have shown promise in laboratory studies.
Dispersal agents as antibiotic adjuvants that attack the EPS matrix, breaking up biofilms and releasing bacterial cells, have shown promise in in vitro and animal studies.
Quorum sensing inhibitors impede the ability of bacteria to communicate with each other, thereby interrupting biofilm development.
Attacking the biofilm persister/dormant state by activating proteases inside dormant cells causes them to ‘self-digest’.
Footnotes
Financial & competing interests disclosure: P Stoodley and JH Calhoun have had funding from Biocomposites Ltd (Keele, UK) and Biocomposites Inc. (NC, USA), respectively, to assess their Stimulan® bone void filler for preventing and treating biofilms in laboratory tests. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest.
- 1.Vergidis P, Patel R. Novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect Dis Clin N Am. 2012;26(1):173–186. doi: 10.1016/j.idc.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen EN, Zmistowski B, Parvizi J. Periprosthetic joint infection: what is on the horizon? Int J Artific Organs. 2012;35(10):935–950. doi: 10.5301/ijao.5000145. [DOI] [PubMed] [Google Scholar]
- 3.Peel TN, Buising KL, Choong PF. Diagnosis and management of prosthetic joint infection. Curr Opin Infect Dis. 2012;25(6):670–676. doi: 10.1097/QCO.0b013e32835915db. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65(2):158–168. doi: 10.1111/j.1574-695X.2012.00938.x. [DOI] [PubMed] [Google Scholar]
- 5.Romano CL, Toscano M, Romano D, Drago L. Antibiofilm agents and implant-related infections in orthopedics: where are we? J Chemother. 2013;25(2):67–80. doi: 10.1179/1973947812Y.0000000045. [DOI] [PubMed] [Google Scholar]
- 6.Levy PY, Fenollar F. The role of molecular diagnostics in implant-associated bone and joint infection. Clin Microbiol Infect. 2012;18(12):1168–1175. doi: 10.1111/1469-0691.12020. [DOI] [PubMed] [Google Scholar]
- 7.Esteban J, Sorli L, Alentorn-Geli E, Puig L, Horcajada JP. Conventional and molecular diagnostic strategies for prosthetic joint infections. Expert Rev Mol Diagnost. 2014;14(1):83–96. doi: 10.1586/14737159.2014.861327. [DOI] [PubMed] [Google Scholar]
- 8.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 9.Game FL. Osteomyelitis in the diabetic foot: diagnosis and management. Med Clin N Am. 2013;97(5):947–956. doi: 10.1016/j.mcna.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Choong PF, Dowsey MM, Carr D, Daffy J, Stanley P. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampinbased regimen. Acta Orthop. 2007;78(6):755–765. doi: 10.1080/17453670710014527. [DOI] [PubMed] [Google Scholar]
- 11.Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38–47. doi: 10.2106/JBJS.G.01686. [DOI] [PubMed] [Google Scholar]
- 12.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthoped Relat Res. 2001;392:15–23. [PubMed] [Google Scholar]
- 13.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88(7):943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 14.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthoped Relat Res. 2008;466(7):1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene LR. Guide to the elimination of orthopedic surgery surgical site infections: an executive summary of the Association for Professionals in Infection Control and Epidemiology elimination guide. Am J Infect Control. 2012;40(4):384–386. doi: 10.1016/j.ajic.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 17.Parvizi J, Gehrke T, Chen AF. Proceedings of the international consensus on periprosthetic joint infection. Bone Joint J. 2013;95-B(11):1450–1452. doi: 10.1302/0301-620X.95B11.33135. [DOI] [PubMed] [Google Scholar]
- 18.Willenegger H, Roth B. Treatment tactics and late results in early infection following osteosynthesis. Unfallchirurgie. 1986;12(5):241–246. doi: 10.1007/BF02586085. [DOI] [PubMed] [Google Scholar]
- 19.Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570–574. doi: 10.1007/s004020000174. [DOI] [PubMed] [Google Scholar]
- 20.Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol. 2010;48(4):1208–1214. doi: 10.1128/JCM.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis. 2006;43(8):961–967. doi: 10.1086/507633. [DOI] [PubMed] [Google Scholar]
- 22.Von Ohle C, Gieseke A, Nistico L, Decker EM, Debeer D, Stoodley P. Real-time microsensor measurement of local metabolic activities in ex vivo dental biofilms exposed to sucrose and treated with chlorhexidine. Appl Environ Microbiol. 2010;76(7):2326–2334. doi: 10.1128/AEM.02090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Stoodley P, Conti SF, Demeo PJ, et al. Characterization of a mixed MRSA/MRSE biofilm in an explanted total ankle arthroplasty. FEMS Immunol Med Microbiol. 2011;62(1):66–74. doi: 10.1111/j.1574-695X.2011.00793.x. First report combining a nontargeted PCR approach, using Ibis T5000™ technology, and confocal microscopy as a culture-free corrobrative method to identify specific pathogens present as a biofilm in a failed joint arthroplasty. [DOI] [PubMed] [Google Scholar]
- 24.Stoodley P, Nistico L, Johnson S, et al. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty A case report. J Bone Joint Surg Am. 2008;90(8):1751–1758. doi: 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houry A, Briandet R, Aymerich S, Gohar M. Involvement of motility and flagella in Bacillus cereus biofilm formation. Microbiology. 2010;156(Pt 4):1009–1018. doi: 10.1099/mic.0.034827-0. [DOI] [PubMed] [Google Scholar]
- 26.Vuong C, Voyich JM, Fischer ER, et al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004;6(3):269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 27.Wood TK, Gonzalez Barrios AF, Herzberg M, Lee J. Motility influences biofilm architecture in Escherichia coli. Appl Microbiol Biotechnol. 2006;72(2):361–367. doi: 10.1007/s00253-005-0263-8. [DOI] [PubMed] [Google Scholar]
- 28.Hoiby N, Ciofu O, Johansen HK, et al. The clinical impact of bacterial biofilms. Int J Oral Sci. 2011;3(2):55–65. doi: 10.4248/IJOS11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30(11):513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 30•.Rice KC, Mann EE, Endres JL, et al. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci USA. 2007;104(19):8113–8118. doi: 10.1073/pnas.0610226104. Demonstration that extracellular DNA (eDNA) release in Staphylococcus is genetically regulated, elucidtaing a new target for biofilm dispersal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gloag ES, Turnbull L, Huang A, et al. Self-organization of bacterial biofilms is facilitated by extracellular DNA. Proc Natl Acad Sci USA. 2013;110(28):11541–11546. doi: 10.1073/pnas.1218898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Goodman SD, Obergfell KP, Jurcisek JA, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 2011;4(6):625–637. doi: 10.1038/mi.2011.27. Discovery that eDNA binding proteins can provide a structural framework for eDNA in biofilm extracellular polymeric slime, and that their disruption can cause biofilm dispersal. [DOI] [PubMed] [Google Scholar]
- 33.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12(10):3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lina G, Jarraud S, Ji G, et al. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol Microbiol. 1998;28(3):655–662. doi: 10.1046/j.1365-2958.1998.00830.x. [DOI] [PubMed] [Google Scholar]
- 35.Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4(4):e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Otto M. Staphylococcus epidermidis – the ‘accidental’ pathogen. Nat Rev Microbiol. 2009;7(8):555–567. doi: 10.1038/nrmicro2182. Review article discussing the link between small peptide cell signaling, dispersal and the inverse relationship between secreted virulence factors and biofilm accumulation in Staphylococcus epidermidis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galloway WR, Hodgkinson JT, Bowden S, Welch M, Spring DR. Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria. Trends Microbiol. 2012;20(9):449–458. doi: 10.1016/j.tim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Wood TK, Knabel SJ, Kwan BW. Bacterial persister cell formation and dormancy. Appl Environ Microbiol. 2013;79(23):7116–7121. doi: 10.1128/AEM.02636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barraud N, Schleheck D, Klebensberger J, et al. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J Bacteriol. 2009;191(23):7333–7342. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amari DT, Marques CN, Davies DG. The putative enoyl-coenzyme A hydratase DspI is required for production of the Pseudomonas aeruginosa biofilm dispersion autoinducer cis-2-decenoic acid. J Bacteriol. 2013;195(20):4600–4610. doi: 10.1128/JB.00707-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang CT, Yu FP, Mcfeters GA, Stewart PS. Nonuniform spatial patterns of respiratory activity within biofilms during disinfection. Appl Environ Microbiol. 1995;61(6):2252–2256. doi: 10.1128/aem.61.6.2252-2256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoiby N. Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Med. 2011;9:32. doi: 10.1186/1741-7015-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jefferson KK, Goldmann DA, Pier GB. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2005;49(6):2467–2473. doi: 10.1128/AAC.49.6.2467-2473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anwar H, Costerton JW. Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990;34(9):1666–1671. doi: 10.1128/aac.34.9.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furustrand Tafin U, Majic I, Zalila Belkhodja C, et al. Gentamicin improves the activities of daptomycin and vancomycin against Enterococcus faecalis in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2011;55(10):4821–4827. doi: 10.1128/AAC.00141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moskowitz SM, Foster JM, Emerson J, Burns JL. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J Clin Microbiol. 2004;42(5):1915–1922. doi: 10.1128/JCM.42.5.1915-1922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 48•.Fux CA, Wilson S, Stoodley P. Detachment characteristics and oxacillin resistance of Staphyloccocus aureus biofilm emboli in an in vitro catheter infection model. J Bacteriol. 2004;186(14):4486–4491. doi: 10.1128/JB.186.14.4486-4491.2004. Demonstration that aggregates of detached biofilms from a methecilin-sensitive Staphylococcus aureus biofilm were as tolerant of oxacilin as a methecilin-resistant S. aureus strain, and the tolerance could be attributed to starvation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Ciofu O, Bagge N, Hoiby N. Antibodies against beta-lactamase can improve ceftazidime treatment of lung infection with beta-lactam-resistant Pseudomonas aeruginosa in a rat model of chronic lung infection. APMIS. 2002;110(12):881–891. doi: 10.1034/j.1600-0463.2002.1101207.x. [DOI] [PubMed] [Google Scholar]
- 51.Jensen PO, Givskov M, Bjarnsholt T, Moser C. The immune system vs Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol. 2010;59(3):292–305. doi: 10.1111/j.1574-695X.2010.00706.x. [DOI] [PubMed] [Google Scholar]
- 52.Zimmerli W, Sendi P. Pathogenesis of implant-associated infection: the role of the host. Sem Immunopathol. 2011;33(3):295–306. doi: 10.1007/s00281-011-0275-7. [DOI] [PubMed] [Google Scholar]
- 53.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70(11):6339–6345. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmerli W, Lew PD, Waldvogel FA. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984;73(4):1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tashiro Y, Nomura N, Nakao R, et al. Opr86 is essential for viability and is a potential candidate for a protective antigen against biofilm formation by Pseudomonas aeruginosa. J Bacteriol. 2008;190(11):3969–3978. doi: 10.1128/JB.02004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]
- 57.Elek SD, Conen PE. The virulence of Staphylococcus pyogenes for man; a study of the problems of wound infection. Br J Exp Pathol. 1957;38(6):573–586. [PMC free article] [PubMed] [Google Scholar]
- 58.Lam J, Chan R, Lam K, Costerton JW. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Beer D, Stoodley P, Lewandowski Z. Measurement of local diffusion coefficients in biofilms by microinjection and confocal microscopy. Biotechnol Bioengin. 1997;53(2):151–158. doi: 10.1002/(SICI)1097-0290(19970120)53:2<151::AID-BIT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 60.Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun. 2006;74(8):4849–4855. doi: 10.1128/IAI.00230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010;5(11):1663–1674. doi: 10.2217/fmb.10.125. [DOI] [PubMed] [Google Scholar]
- 62.Benakanakere M, Kinane DF. Innate cellular responses to the periodontal biofilm. Front Oral Biol. 2012;15:41–55. doi: 10.1159/000329670. [DOI] [PubMed] [Google Scholar]
- 63.Widmer AF. New developments in diagnosis and treatment of infection in orthopedic implants. Clin Infect Dis. 2001;33(Suppl. 2):S94–S106. doi: 10.1086/321863. [DOI] [PubMed] [Google Scholar]
- 64.Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296(2):202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65••.Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplast. 2011;26(8):1136–1138. doi: 10.1016/j.arth.2011.09.026. Comprehensive review of the academic and clinical literature used to establish a consensus statement for the more reliable diagnosis of a periprosthetic joint infection. [DOI] [PubMed] [Google Scholar]
- 66.Tetreault MW, Wetters NG, Aggarwal VK, et al. Should draining wounds and sinuses associated with hip and knee arthroplasties be cultured? J Arthroplast. 2013;28(8 Suppl):133–136. doi: 10.1016/j.arth.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 67.Tigges S, Stiles RG, Roberson JR. Appearance of septic hip prostheses on plain radiographs. AJR Am J Roentgenol. 1994;163(2):377–380. doi: 10.2214/ajr.163.2.8037035. [DOI] [PubMed] [Google Scholar]
- 68.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006;2(2):102–113. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levy PY, Fournier PE, Fenollar F, Raoult D. Systematic PCR detection in culture-negative osteoarticular infections. Am J Med. 2013;126(12):1143.e25–e33. doi: 10.1016/j.amjmed.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 70.Palmer MP, Altman DT, Altman GT, et al. Can we trust intraoperative culture results in nonunions? J Orthped Trauma. 2014;28(7):384–390. doi: 10.1097/BOT.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 71.Guembe M, Marin M, Martin-Rabadan P, et al. Use of universal 16S rRNA gene PCR as a diagnostic tool for venous access port-related bloodstream infections. J Clin Microbiol. 2013;51(3):799–804. doi: 10.1128/JCM.02414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72••.Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247–2254. doi: 10.2106/JBJS.L.00210. Demonstration that a novel, nontargeted PCR-based Ibis T5000 technology was more sensitive in the detection and identifcation of pathogens in synovial fluid than clinical culture in periprosthetic joint infection in a study of 23 patients. [DOI] [PubMed] [Google Scholar]
- 73.Trampuz A, Steckelberg JM, Osmon DR, et al. Advances in the laboratory diagnosis of prosthetic joint infection. Rev Med Microbiol. 2003;14(1):1–14. [Google Scholar]
- 74••.Trampuz A, Piper KE, Jacobson MJ, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357(7):654–663. doi: 10.1056/NEJMoa061588. Large (331-patient) study demonstrating that sonication of retrieved hardware increased the sensitivty of culture for the detection of pathogens and also provides indirect evidence that the recovered bacteria were previously adhered. [DOI] [PubMed] [Google Scholar]
- 75.Sorli L, Puig L, Torres-Claramunt R, et al. The relationship between microbiology results in the second of a two-stage exchange procedure using cement spacers and the outcome after revision total joint replacement for infection: the use of sonication to aid bacteriological analysis. J Bone Joint Surg Br. 2012;94(2):249–253. doi: 10.1302/0301-620X.94B2.27779. [DOI] [PubMed] [Google Scholar]
- 76.Zaat S, Broekhuizen C, Riool M. Host tissue as a niche for biomaterial-associated infection. Future Microbiol. 2010;8:1149–1151. doi: 10.2217/fmb.10.89. [DOI] [PubMed] [Google Scholar]
- 77•.Tunney MM, Patrick S, Curran MD, et al. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999;37(10):3281–3290. doi: 10.1128/jcm.37.10.3281-3290.1999. One of the first studies using sonication of retrieved orthopedic hardware in order to demonstrate that, despite conventional clinical culture negativity, bacteria were frequently present as biofilm aggregates using culture-independent methods in a 120-patient study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borens O, Yusuf E, Steinrucken J, Trampuz A. Accurate and early diagnosis of orthopedic device-related infection by microbial heat production and sonication. J Orthped Res. 2013;31(11):1700–1703. doi: 10.1002/jor.22419. [DOI] [PubMed] [Google Scholar]
- 79.Kathju S, Nistico L, Hall-Stoodley L, Post JC, Ehrlich GD, Stoodley P. Chronic surgical site infection due to suture-associated polymicrobial biofilm. Surg Infect (Larchmt) 2009;10(5):457–461. doi: 10.1089/sur.2008.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trampuz A, Osmon DR, Hanssen AD, Steckelberg JM, Patel R. Molecular and antibiofilm approaches to prosthetic joint infection. Clin Orthoped Relat Res. 2003;414:69–88. doi: 10.1097/01.blo.0000087324.60612.93. [DOI] [PubMed] [Google Scholar]
- 81.Levy PY, Ollivier M, Drancourt M, Raoult D, Argenson JN. Relation between nasal carriage of Staphylococcus aureus and surgical site infection in orthopedic surgery: the role of nasal contamination. A systematic literature review and meta-analysis. Orthoped Traumatol Surg Res. 2013;99(6):645–651. doi: 10.1016/j.otsr.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 82.Marculescu CE, Osmon DR. Antibiotic prophylaxis in orthopedic prosthetic surgery. Infect Dis Clin N Am. 2005;19(4):931–946. doi: 10.1016/j.idc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 83.Widmer AF. Infections and sepsis from intravascular catheters. Internist (Berl) 2005;46(6):643–651. doi: 10.1007/s00108-005-1399-4. [DOI] [PubMed] [Google Scholar]
- 84.Cordero J, Munuera L, Folgueira MD. The influence of the chemical composition and surface of the implant on infection. Injury. 1996;27(Suppl. 3):SC34–SC37. doi: 10.1016/0020-1383(96)89030-9. [DOI] [PubMed] [Google Scholar]
- 85.Cordero J, Munuera L, Folgueira MD. Influence of metal implants on infection. An experimental study in rabbits. J Bone Joint Surg Br. 1994;76(5):717–720. [PubMed] [Google Scholar]
- 86.Deligianni DD, Katsala N, Ladas S, Sotiropoulou D, Amedee J, Missirlis YF. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials. 2001;22(11):1241–1251. doi: 10.1016/s0142-9612(00)00274-x. [DOI] [PubMed] [Google Scholar]
- 87.Gomez-Barrena E, Esteban J, Medel F, et al. Bacterial adherence to separated modular components in joint prosthesis: a clinical study. J Orthped Res. 2012;30(10):1634–1639. doi: 10.1002/jor.22114. [DOI] [PubMed] [Google Scholar]
- 88.Swartz RD, Messana JM, Boyer CJ, Lunde NM, Weitzel WF, Hartman TL. Successful use of cuffed central venous hemodialysis catheters inserted percutaneously. J Am Soc Nephrol. 1994;4(9):1719–1725. doi: 10.1681/ASN.V491719. [DOI] [PubMed] [Google Scholar]
- 89.Lund GB, Trerotola SO, Scheel PF, Jr, et al. Outcome of tunneled hemodialysis catheters placed by radiologists. Radiology. 1996;198(2):467–472. doi: 10.1148/radiology.198.2.8596851. [DOI] [PubMed] [Google Scholar]
- 90.Marr KA, Sexton DJ, Conlon PJ, Corey GR, Schwab SJ, Kirkland KB. Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Ann Intern Med. 1997;127(4):275–280. doi: 10.7326/0003-4819-127-4-199708150-00003. [DOI] [PubMed] [Google Scholar]
- 91.Saad TF. Bacteremia associated with tunneled, cuffed hemodialysis catheters. Am J Kidney Dis. 1999;34(6):1114–1124. doi: 10.1016/S0272-6386(99)70018-1. [DOI] [PubMed] [Google Scholar]
- 92.Pourchez T, Moriniere P, Fournier A, Pietri J. Use of Permcath (Quinton) catheter in uraemic patients in whom the creation of conventional vascular access for haemodialysis is difficult. Nephron. 1989;53(4):297–302. doi: 10.1159/000185771. [DOI] [PubMed] [Google Scholar]
- 93.Callaghan JJ, Katz RP, Johnston RC. One-stage revision surgery of the infected hip. A minimum 10-year followup study. Clin Orthoped Relat Res. 1999;369:139–143. doi: 10.1097/00003086-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 94.Hope PG, Kristinsson KG, Norman P, Elson RA. Deep infection of cemented total hip arthroplasties caused by coagulase-negative staphylococci. J Bone Joint Surg Br. 1989;71(5):851–855. doi: 10.1302/0301-620X.71B5.2584258. [DOI] [PubMed] [Google Scholar]
- 95.Raut VV, Siney PD, Wroblewski BM. One-stage revision of infected total hip replacements with discharging sinuses. J Bone Joint Surg Br. 1994;76(5):721–724. [PubMed] [Google Scholar]
- 96.Ure KJ, Amstutz HC, Nasser S, Schmalzried TP. Direct-exchange arthroplasty for the treatment of infection after total hip replacement. An average ten-year follow-up. J Bone Joint Surg Am. 1998;80(7):961–968. doi: 10.2106/00004623-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 97.Colyer RA, Capello WN. Surgical treatment of the infected hip implant. Two-stage reimplantation with a one-month interval. Clin Orthoped Relat Res. 1994;298:75–79. [PubMed] [Google Scholar]
- 98.Langlais F. Can we improve the results of revision arthroplasty for infected total hip replacement? J Bone Joint Surg Br. 2003;85(5):637–640. [PubMed] [Google Scholar]
- 99.Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Two-stage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72(2):272–278. [PubMed] [Google Scholar]
- 100.Zimmerli W, Ochsner PE. Management of infection associated with prosthetic joints. Infection. 2003;31(2):99–108. doi: 10.1007/s15010-002-3079-9. [DOI] [PubMed] [Google Scholar]
- 101.Westrich G, Salvati EA, Brause B. Postoperative infection. In: Bono JV, McCarthy JC, Thornhill TS, et al., editors. Revision Total Hip Arthroplasty. Springer; NY, USA: 1999. pp. 371–390. [Google Scholar]
- 102.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33(26):5967–5982. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 103.Rodrigues LR. Inhibition of bacterial adhesion on medical devices. Adv Exp Med Biol. 2011;715:351–367. doi: 10.1007/978-94-007-0940-9_22. [DOI] [PubMed] [Google Scholar]
- 104.Bryers JD. Medical biofilms. Biotechnol Bioengin. 2008;100(1):1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35(9):780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 106.Gottenbos B, Grijpma DW, Van Der Mei HC, Feijen J, Busscher HJ. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J Antimicrob Chemother. 2001;48(1):7–13. doi: 10.1093/jac/48.1.7. [DOI] [PubMed] [Google Scholar]
- 107.Arciola CR, Caramazza R, Pizzoferrato A. In vitro adhesion of Staphylococcus epidermidis on heparin-surface-modified intraocular lenses. J Cataract Refract Surg. 1994;20(2):158–161. doi: 10.1016/s0886-3350(13)80157-5. [DOI] [PubMed] [Google Scholar]
- 108.Arciola CR, Radin L, Alvergna P, Cenni E, Pizzoferrato A. Heparin surface treatment of poly(methylmethacrylate) alters adhesion of a Staphylococcus aureus strain: utility of bacterial fatty acid analysis. Biomaterials. 1993;14(15):1161–1164. doi: 10.1016/0142-9612(93)90161-t. [DOI] [PubMed] [Google Scholar]
- 109.Nagaoka S, Kawakami H. Inhibition of bacterial adhesion and biofilm formation by a heparinized hydrophilic polymer. ASAIO J. 1995;41(3):M365–M368. doi: 10.1097/00002480-199507000-00032. [DOI] [PubMed] [Google Scholar]
- 110.Ruggieri MR, Hanno PM, Levin RM. Reduction of bacterial adherence to catheter surface with heparin. J Urol. 1987;138(2):423–426. doi: 10.1016/s0022-5347(17)43177-6. [DOI] [PubMed] [Google Scholar]
- 111.Clement JL, Jarrett PS. Antibacterial silver. Met Based Drugs. 1994;1(5–6):467–482. doi: 10.1155/MBD.1994.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Flemming CA, Ferris FG, Beveridge TJ, Bailey GW. Remobilization of toxic heavy metals adsorbed to bacterial wall-clay composites. Appl Environ Microbiol. 1990;56(10):3191–3203. doi: 10.1128/aem.56.10.3191-3203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liau SY, Read DC, Pugh WJ, Furr JR, Russell AD. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett Appl Microbiol. 1997;25(4):279–283. doi: 10.1046/j.1472-765x.1997.00219.x. [DOI] [PubMed] [Google Scholar]
- 114.Matsumura Y, Yoshikata K, Kunisaki S, Tsuchido T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microbiol. 2003;69(7):4278–4281. doi: 10.1128/AEM.69.7.4278-4281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against Gram-positive and Gram-negative bacteria. Nanomedicine. 2010;6(1):103–109. doi: 10.1016/j.nano.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 116.Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet JB. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J Antimicrob Chemother. 2008;61(4):869–876. doi: 10.1093/jac/dkn034. [DOI] [PubMed] [Google Scholar]
- 117.Secinti KD, Ozalp H, Attar A, Sargon MF. Nanoparticle silver ion coatings inhibit biofilm formation on titanium implants. J Clin Neurosci. 2011;18(3):391–395. doi: 10.1016/j.jocn.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 118.Huang Z, Zheng X, Yan D, et al. Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir. 2008;24(8):4140–4144. doi: 10.1021/la7035949. [DOI] [PubMed] [Google Scholar]
- 119.Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011;77(7):2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raghupathi KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27(7):4020–4028. doi: 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- 121.Seil JT, Webster TJ. Reduced Staphylococcus aureus proliferation and biofilm formation on zinc oxide nanoparticle PVC composite surfaces. Acta Biomater. 2011;7(6):2579–2584. doi: 10.1016/j.actbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 122.Aydin Sevinc B, Hanley L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J Biomed Mater Res B Appl Biomater. 2010;94(1):22–31. doi: 10.1002/jbm.b.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim JS, Adamcakova-Dodd A, O’Shaughnessy PT, Grassian VH, Thorne PS. Effects of copper nanoparticle exposure on host defense in a murine pulmonary infection model. Part Fibre Toxicol. 2011;8:29. doi: 10.1186/1743-8977-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kruszewski KM, Nistico L, Longwell MJ, et al. Reducing Staphylococcus aureus biofilm formation on stainless steel 316L using functionalized self-assembled monolayers. Materials Sci engineering C, Mat Biol Appl. 2013;33(4):2059–2069. doi: 10.1016/j.msec.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406–1415. doi: 10.2106/JBJS.K.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patti BN, Lindeque BG. Antibiotic-loaded acrylic bone cement in the revision of septic arthroplasty: where's the evidence? Orthopedics. 2011;34(3):210. doi: 10.3928/01477447-20110124-22. [DOI] [PubMed] [Google Scholar]
- 127.Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9) doi: 10.3928/01477447-20090728-20. pii: orthosupersite.com/view.asp?rID=42849. [DOI] [PubMed] [Google Scholar]
- 128.Lee GH, Khoury JG, Bell JE, Buckwalter JA. Adverse reactions to OsteoSet bone graft substitute, the incidence in a consecutive series. Iowa Orthped J. 2002;22:35–38. [PMC free article] [PubMed] [Google Scholar]
- 129.Mousset B, Benoit MA, Delloye C, Bouillet R, Gillard J. Biodegradable implants for potential use in bone infection. An in vitro study of antibiotic-loaded calcium sulphate. Int Orthoped. 1995;19(3):157–161. doi: 10.1007/BF00181861. [DOI] [PubMed] [Google Scholar]
- 130.Kanellakopoulou K, Galanopoulos I, Soranoglou V, et al. Treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with a synthetic carrier of calcium sulphate (Stimulan) releasing moxifloxacin. Int J Antimicrob Agents. 2009;33(4):354–359. doi: 10.1016/j.ijantimicag.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 131.Roberts R, McConoughey SJ, Calhoun JH. Size and composition of synthetic calcium sulfate beads influence dissolution and elution rates in vitro. J Biomed Mater Res B Appl Biomater. 2013;102(4):667–673. doi: 10.1002/jbm.b.33045. [DOI] [PubMed] [Google Scholar]
- 132.Kluin OS, Van Der Mei HC, Busscher HJ, Neut D. Biodegradable vs non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin Drug Deliv. 2013;10(3):341–351. doi: 10.1517/17425247.2013.751371. [DOI] [PubMed] [Google Scholar]
- 133.Josefsson E, Hartford O, O’Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis. 2001;184(12):1572–1580. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- 134.Kuklin NA, Clark DJ, Secore S, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun. 2006;74(4):2215–2223. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135••.Brady RA, O’May GA, Leid JG, Prior ML, Costerton JW, Shirtliff ME. Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment. Infect Immun. 2011;79(4):1797–1803. doi: 10.1128/IAI.00451-10. Use of biofilm-specific proteins as vaccine targets shows promise in treating S. aureus osteomyelitis in a mouse model and also has potential for treating periprosthetic infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peterson BW, Van Der Mei HC, Sjollema J, Busscher HJ, Sharma PK. A distinguishable role of eDNA in the viscoelastic relaxation of biofilms. MBio. 2013;4(5):e00497–13. doi: 10.1128/mBio.00497-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kiedrowski MR, Kavanaugh JS, Malone CL, et al. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS ONE. 2011;6(11):e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138•.Kaplan JB. Therapeutic potential of biofilm-dispersing enzymes. Int J Artific Organs. 2009;32(9):545–554. doi: 10.1177/039139880903200903. Discussion of the use of enzymes specifically targeted to extracellular polymeric slime components in order to break up biofilms as an adjuvunct to antibiotic therapy. [DOI] [PubMed] [Google Scholar]
- 139.Hall-Stoodley L, Nistico L, Sambanthamoorthy K, et al. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 2008;8:173. doi: 10.1186/1471-2180-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tetz GV, Artemenko NK, Tetz VV. Effect of DNase and antibiotics on biofilm characteristics. Antimicrob Agents Chemother. 2009;53(3):1204–1209. doi: 10.1128/AAC.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tetz VV, Tetz GV. Effect of extracellular DNA destruction by DNase I on characteristics of forming biofilms. DNA Cell Biol. 2010;29(8):399–405. doi: 10.1089/dna.2009.1011. [DOI] [PubMed] [Google Scholar]
- 142.Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB combination. J Antimicrob Chemother. 2009;64(1):88–93. doi: 10.1093/jac/dkp158. [DOI] [PubMed] [Google Scholar]
- 143.Donelli G, Francolini I, Romoli D, et al. Synergistic activity of dispersin B and cefamandole nafate in inhibition of staphylococcal biofilm growth on polyurethanes. Antimicrob Agents Chemother. 2007;51(8):2733–2740. doi: 10.1128/AAC.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hentzer M, Wu H, Andersen JB, et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22(15):3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu H, Song Z, Hentzer M, et al. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J Antimicrob Chemother. 2004;53(6):1054–1061. doi: 10.1093/jac/dkh223. [DOI] [PubMed] [Google Scholar]
- 146.Papaioannou E, Wahjudi M, Nadal-Jimenez P, Koch G, Setroikromo R, Quax WJ. Quorum-quenching acylase reduces the virulence of Pseudomonas aeruginosa in a Caenorhabditis elegans infection model. Antimicrob Agents Chemother. 2009;53(11):4891–4897. doi: 10.1128/AAC.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jennings JA, Courtney HS, Haggard WO. Cis-2-decenoic acid inhibits S. aureus growth and biofilm in vitro: a pilot study. Clin Orthoped Relat Res. 2012;470(10):2663–2670. doi: 10.1007/s11999-012-2388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148•.Conlon BP, Nakayasu ES, Fleck LE, et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature. 2013;503(7476):365–370. doi: 10.1038/nature12790. Discusses of how exogenous activation of proteases in S. aureus persister cells might be used to cause these highly antimicrobial-tolearant phenotypes, which develop in biofilms, to ‘self-digest’. [DOI] [PMC free article] [PubMed] [Google Scholar]


