Abstract
Herpes simplex virus (HSV) infections can be treated efficiently by the application of antiviral drugs. The herpes family of viruses is responsible for causing a wide variety of diseases in humans. The standard therapy for the management of such infections includes acyclovir (ACV) and penciclovir (PCV) with their respective prodrugs valaciclovir and famciclovir. Though effective, long term prophylaxis with the current drugs leads to development of drug-resistant viral isolates, particularly in immunocompromised patients. Moreover, some drugs are associated with dose-limiting toxicities which limit their further utility. Therefore, there is a need to develop new antiherpetic compounds with different mechanisms of action which will be safe and effective against emerging drug resistant viral isolates. Significant advances have been made towards the design and development of novel antiviral therapeutics during the last decade. As evident by their excellent antiviral activities, pharmaceutical companies are moving forward with several new compounds into various phases of clinical trials. This review provides an overview of structure and life cycle of HSV, progress in the development of new therapies, update on the advances in emerging therapeutics under clinical development and related recent patents for the treatment of Herpes simplex virus infections.
Keywords: Emerging therapeutics, infections life cycle, Herpes simplex virus, recent patents, treatment
Introduction
Herpes family of viruses is responsible for causing a wide variety of diseases in humans. Seroprevalence of many of these diseases is approaching 100% in the first years of life. This family of viruses has been known to establish lifelong latency causing recurrent episodes of the disease. While a few infections are typically mild and self-limiting, others are recurrent at any stage of life. A distinguishing feature shared by all the herpes viruses is their progression to severe form in immunocompromised individuals. Moreover, development of resistance is a major concern in such hosts since the viruses tend to replicate albeit treatment with first line antiviral drugs [1, 2]. Current treatment options for such resistant infections are challenging, because of a few alternate drugs which are potent. Furthermore the second line of therapies is associated with modest potencies, toxicity, and lack of bioavailability [3, 4].
A standard therapy for management of HSV infections includes acyclovir (ACV) and penciclovir (PCV) along with their respective prodrugs valaciclovir and famciclovir. Development of ACV revolutionized the treatment of HSV infections to a great extent. Since its introduction, this drug has become the standard line of therapy for all herpes simplex infections as well as herpes zoster infections. Although effective, delivery is limited by its hydrophilic nature and poor permeability across intestine and corneal tissues, leading to poor oral and ocular bioavailabilities. Moreover, long term prophylaxis with ACV may cause emergence of resistant viral strains. Such development is more prevalent in immunocompromised patients ranging from 3.5-10% [1-2, 5]. Though the exact mechanism of resistance development is not known, it appears to be mediated either by a mutation in thymidine Kinase (TK) (frequent) or mutation in viral DNA polymerase (less common) [5, 6]. Development of new therapies with different mechanism of actions and novel molecular targets should be designed to not only treat resistant infections but also to prevent their recurrence with single or a combination drug therapy [1,7]. Several novel compounds which can suppress viral replication and prevent reactivation in the target population have been identified and are progressing through various phases of clinical trials. Hence, the purpose of this review is to discuss the structure and life cycle of HSV, progress in the development of new therapies, emerging therapeutics under clinical development and finally related recent patents involving the treatment of herpes simplex virus infections.
Structure
Herpes simplex viruses type-1 and 2 (HSV-1 and HSV-2) are human neurotropic viruses belonging to the family Herpesviridae, subfamily Alphaherpesvirinae and the genus Simplexvirus. The term ‘herpes’ originates from Greek and implies to creep or crawl. HSV-1 and HSV-2 are frequent human pathogens causing infections of orofacial, ocular and genital mucosal surfaces. Although HSV-1 and HSV-2 show 70% genetic homology, they differ in terms of few antigenic and biological properties [8, 9]. HSV is a large enveloped DNA virus, approximately 150-200nm diameter. The basic components of a mature viral particle include a core containing linear double-stranded DNA (120 to 230 kbp); an icosadeltahedral capsid consisting of 162 capsomers; an amorphous proteinaceous tegument containing viral proteins and an external trilaminar lipid envelope studded with atleast 12 different glycoproteins Fig. (1) [10-15]. The 152-kbp length HSV genome encodes for 82 different proteins. The genome is composed of two regions, UL (unique long) and US (unique short), covalently linked to each other and flanked by three segments. Each protein encoded by the genome is usually named by its location in UL or US. HSV exhibits three origins of replication (ori); one copy of oriL and two copies of oriS [16-19]. These ori are palindromic sequences and any of the three regions suffice for the replication to initiate [20, 21].
Fig. 1.
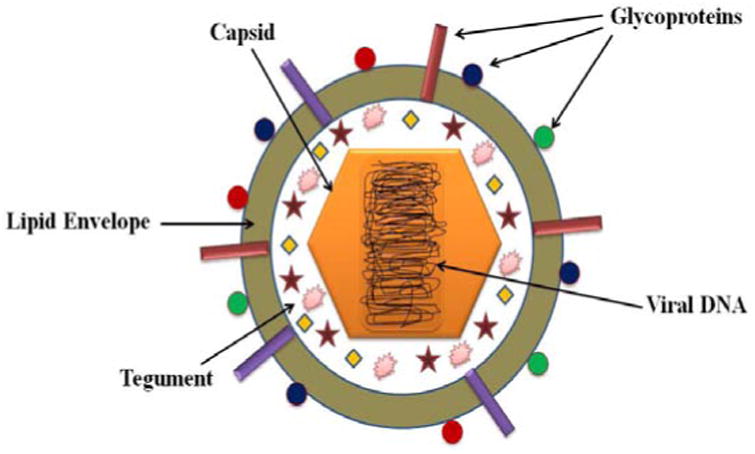
Structure of HSV.
HSV Lytic Cycle
HSV lytic infection involves the following steps: i) viral entry ii) viral replication and iii) viral assembly and egress. Each of these steps is described in detail below.
Viral Entry
HSV can infect a variety of host cells like lymphocytes, epithelial cells, fibroblasts and neurons. Hence, this virus is regarded as ‘broad cell tropic’. The mechanism of entry varies depending on the cell type. Viral entry occurs in two different steps Fig. (2). In the first step, viral glycoproteins bind to the host cell receptors and in the second step, the viral envelope either fuses with the plasma membrane or undergoes endocytosis [22-25]. Viral fusion is a pH-independent process while endocytosis is a pH-dependent process. Although the viral envelope exhibits 12 different glycoproteins, only five of them-glycoprotein C (gC), gB, gD, gH and gL are essential for viral infection. gB functions as a homo-oligomer while gH and gL forms a functional hetero-oligomer [26-28]. The binding of gC to heparan sulfate (HS) initiates virus contact with the host cell [29]. Mutational analysis studies have demonstrated that two hydrophobic residues Ile (142) and Phe(146) play a vital role in maintaining this specific affinity [30, 31]. HSV binding can also occur even in the absence of gC but with reduced infectivity with the help of gB [32, 33]. gC and gB can also bind to C-type lectin dendritic cell-specific ICAM3-grabbing nonintegrin (DC-SIGN), facilitating dendritic cell infection. Apart from HS and DC-SIGN, gB can bind to paired immunoglobulin-like type 2 receptor-α (PILRα), non-muscle myosin heavy chain IIA (NMMHCIIA) and myelin-associated glycoprotein (MAG) [34-37]. Initial tethering followed by viral fusion is facilitated by binding of gD to second receptors such as herpesvirus entry mediator (HVEM/HveA) (member of the tumor necrosis factor superfamily); nectin-1 (HveC) and nectin -2 (HveB) (cell adhesion molecules of the immunoglobulin superfamily); and 3-O-sulphated HS [38-43]. Finally, viral envelope fuses with host cell membrane facilitated by gB, gD and heterodimeric gH/gL [44-45]. Post fusion, viral nucleocapsid and tegument proteins are released into host cytoplasm, from where the proteins are transported into the nucleus by the dynein-dynactin protein complex. This process is aided by viral capsid protein VP26 and tegument protein UL34. The capsids are propelled through the negative end of microtubules and released into nucleus through nuclear pore complexes (NPCs) [46-50].
Fig. 2.
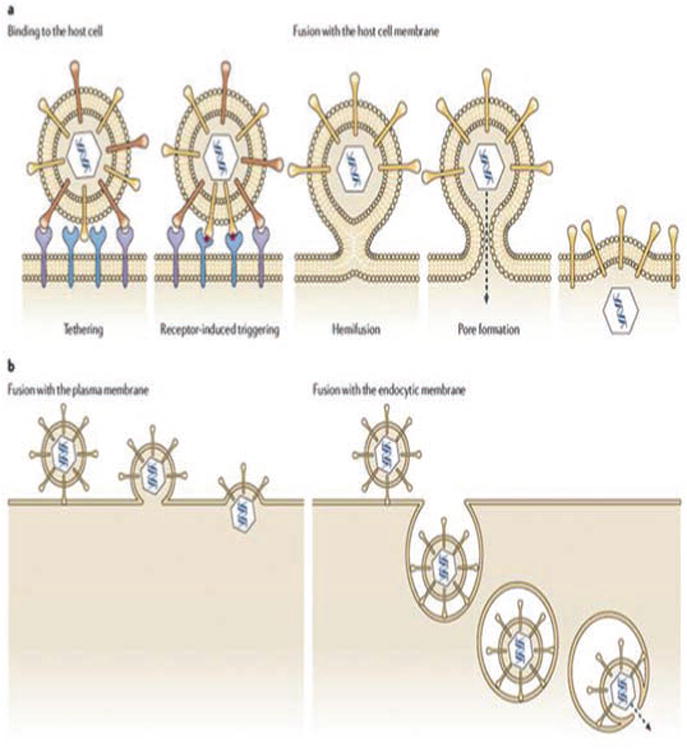
Mechanism of HSV entry into the host cells. Reproduced with permission from [25].
Viral Replication
Post infection into nucleus, host RNA polymerase II initiates viral gene expression [51-53]. HSV genes are expressed in a temporal regulated manner, in three distinct classes: immediate early (IE/a), early (E/β) and late (L/y) genes Fig. (3). The virion protein VP16 in conjunction with cellular octamer DNA binding protein (Oct-1) induces the expression of five IE proteins (ICP0, ICP4, ICP22, ICP27 and ICP47). This protein synthesis usually occurs within 2-4 hours post infection. All the IE proteins except for ICP47 play a vital role in regulating the expression of E/ βgenes. ICP0 promotes transactivation of all the three classes of genes. Further, it acts as an E3 ubiquitin ligase and degrades several cellular proteins [54-57]. ICP4 helps in negatively regulating the expression of αand βgenes by binding to repressor sequence in its own promoter region, thus promoting their shutdown [58-61]. ICP22 plays an important role in regulating the expression of ICP0 while ICP27 regulates early and late gene transcription [62-64]. ICP47 plays a role in immune system evasion by preventing viral peptides from being presented to major histocompatibility complex (MHC) Class I molecules, evading the recognition by cytotoxic T-cells [65]. α proteins help in the transcription of β proteins, which usually proceeds 5-7 hours post infection. β proteins are mainly required for viral DNA replication [including origin-binding protein (UL9), single-strand DNA-binding protein (SSB/ICP8/UL29), DNA helicase-primase complex (UL5/UL8/UL52), DNA polymerase (UL30/UL42)] and nucleotide metabolism [including thymidine kinase and ribonucleotide reductase] [66-68]. UL9 binds to ori and UL29 stimulates helicase-primase and polymerase activities. Further UL29 negatively regulates the transcription of β proteins post viral DNA replication [69-72]. HSV DNA replicates via a theta mechanism initially and continues via sigma or rolling-circle mechanism [73]. Post DNA replication L/γ genes are transcribed, which mainly include viral structural components [74].
Fig. 3.
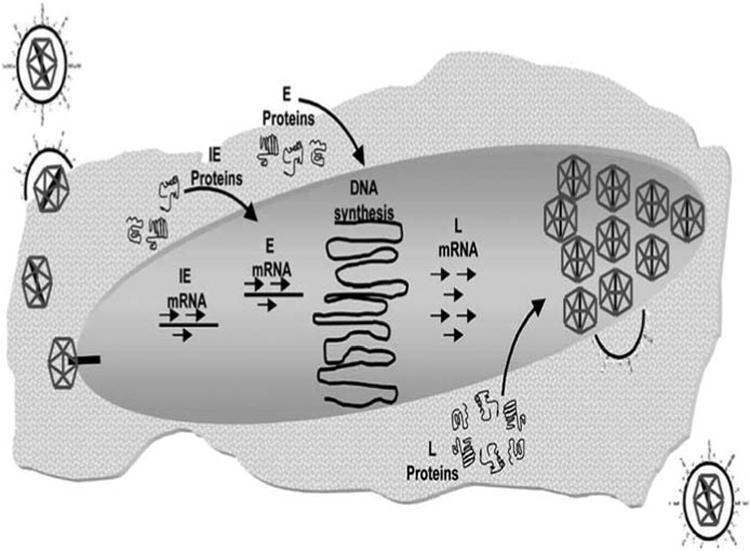
HSV viral replication. Reproduced with permission from [53].
Viral Assembly and Egress
The late proteins are required for capsid assembly and are transported into nucleus via nuclear localization sequences (NLS). A procapsid contains 162 capsomers (150 hexons and 12 pentons) that lie on a capsid floor layer connected by 320 triplexes [75]. The hexons are composed of six molecules of major capsid protein (MCP/VP5) and VP26 joined together [76-78]. All the pentons, except for one (termed portal) show five molecules of VP5. The portal exhibits twelve-fold rotational symmetry with cylindrical shape and is composed of twelve molecules of UL6 protein [79-81]. The portal facilitates DNA entry into capsid during viral assembly. The triplexes are small compact structures composed of one molecule of VP19 and two molecules of VP23 [75, 82]. These proteins hold the capsomers tight during capsid assembly. Another important capsid component includes the C capsid specific component (CCSC) having one molecule of each UL17 and UL25. This rod shaped structure appears at each capsid vertex, supporting the capsid against pressure gradient during DNA packing [83, 84]. The procapsid is assembled inside the nucleus and packaged with viral DNA to form a mature capsid. The re-envelopment model for viral egress proposes that a mature capsid initially fuses with inner nuclear membrane (primary envelopment) to form an enveloped particle and gain fuses with outer nuclear membrane (ONM) (de-envelopment) to release the capsids into cytoplasm. In the cytoplasm, capsids re-envelope (secondary envelopment), by budding into the Golgi compartment and are finally secreted from the infected cells Fig. (4) [85-87].
Fig. 4.
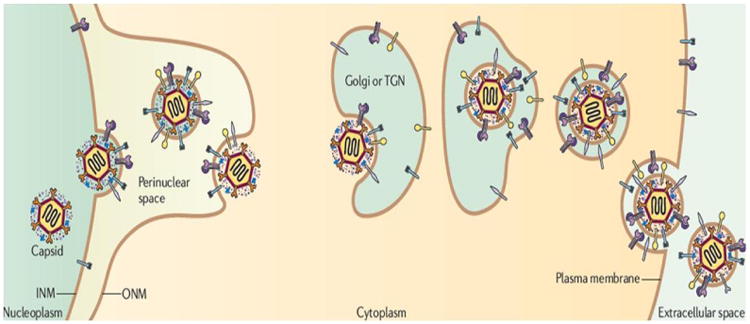
HSV viral egress. Reproduced with permission from [85].
HSV Latent Cycle
A major hallmark of herpes viruses is their ability to undergo latentiation in the hosts for lifetime. HSV-1 can undergo latency in the trigeminal or cervical ganglia while the major site for HSV-2 is sacral ganglia. Following primary infection at oral or genital mucosal surfaces, the virion finds its entry into the innervating neuronal axon terminals Fig. (5). The capsid containing viral DNA undergoes retrograde transport along the axon via an active process occurring at an estimated rate of 0.5-3 (im/s [88]. Within the neuronal cell body, viral DNA is circularized and loaded with histone proteins to form nucleosomes and remains as extra unintegrated DNA. This arrangement facilitates latency for longer periods [89-90]. During latency, viral transcription is shutdown except for an 8.3kb latency associated transcript (LAT). This polyadenylated primary transcript is unstable and rapidly processed into two major stable introns (1.5kb and 2kb) with extended half-lives [91-94]. Although the exact function of these transcripts is unknown, it has been shown that they act as anti-apoptotic proteins protecting infected neurons [95-96]. Upon reactivation by proper stimuli including immunosupression, intercurrent illness, exposure to UV and/or stress, these viruses re-initiate the lytic cycle and cause various diseases [97].
Fig. 5.
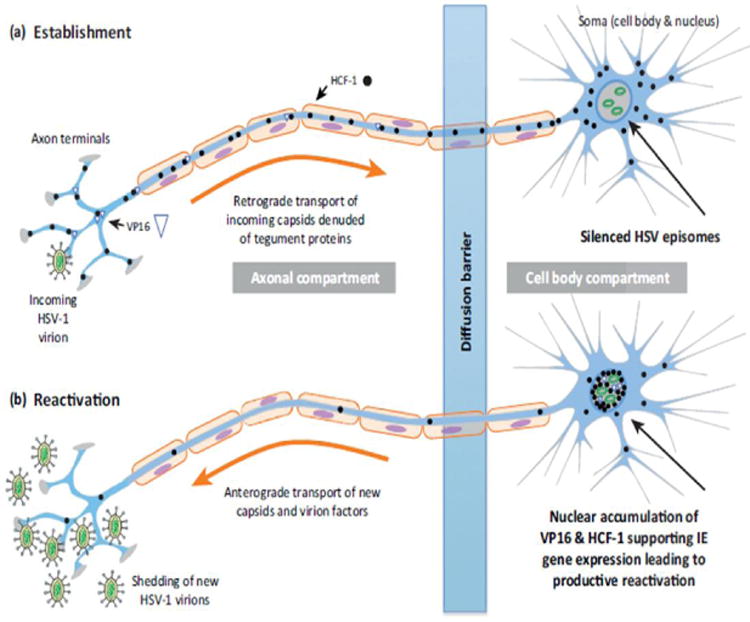
HSV latent cycle. Reproduced with permission from [89].
New Anti-HSV Drugs
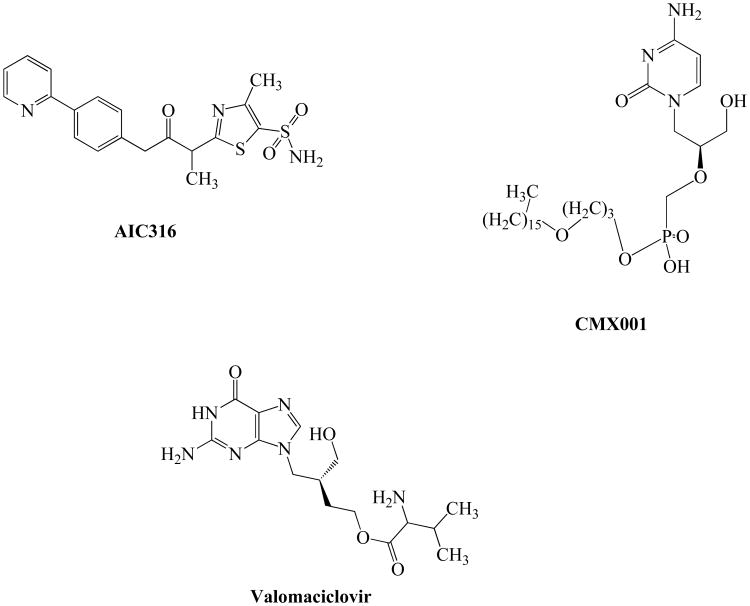
AIC316
AIC316, also known as BAY 57-1293 (N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl) phenyl]acetamide) represents a new class of potent inhibitors of HSV which target the virus helicase primase complex Fig. (6). This novel non-nucleosidic inhibitor has shown excellent antiviral activity against HSV-1 and HSV-2, bovine herpesvirus and pseudorabies virus [98-100]. This compound possess the ability to inhibit the helicase primase complex encoded by HSV genes (UL5, UL52 and UL8) which results in the inhibition of viral DNA synthesis. BAY 57-1293 is effective against HSV both in vitro (Vero cells) and in vivo (mice model) [101]. AIC316 exposure confers resistance due to mutations in UL5 and UL52 genes [102]. A distinct mechanism of action enables it to be active against ACV-resistant HSV isolates suggesting potential utility of this agent in the treatment of resistant infections [103]. In addition, this compound may be used in combination with other drugs to overcome resistance in high-risk immunocompromised individuals [7]. Also, a recent Phase II clinical trial reported that this compound is well tolerated and highly effective.
Fig. 6.
Inhibitors of HSV virus replication currently in clinical development.
CMX001
Alkoxyalkyl esters of cidofovir (CDV) such as hexadecy-loxypropyl CDV (CMX001) were developed to improve oral bioavailability of CDV Fig. (6) [104-105]. This compound was effective against clinical isolates of HSV as well as ACV, CDV, GCV and FOS-resistant isolates of HSV. More importantly, this lipid ester analog was more active than CDV itself against HSV, VZV, CMV, EBV, HHV-6, and HHV-8 in vitro, suggesting its potential utility for treatment of HSV infections [106-107]. In virus infected cells, CMX001 metabolizes to release CDV which inhibits viral DNA polymerase and in turn arrests viral replication [105]. CMX001 is 300-400 fold more active in vitro against HSV replication than ACV or CDV [108]. Furthermore, this compound is also efficacious in BALB/c mice inoculated with HSV-1 or 2 [109]. Also, CMX001 potentiates the efficacy of ACV and this combination synergistically inhibits the replication of HSV both in vitro and in vivo. Results from this study suggested that CMX001 may be effective in the treatment of ACV-resistant HSV infections and can be administered as an adjunct therapy in individuals with poor clinical response to ACV [110]. Phase I studies reported that this compound is well tolerated with significantly reduced kidney accumulation. CMX001 avoided the nephrotoxicity associated with CDV [109,111].
Valomaciclovir
Valomaciclovir (EPB-348) is the prodrug of H2G (R-9[4-hydroxy-2(hydroxymethyl) butyl] guanine), a guanosine analog with excellent activity against HSV-1, HSV-2, EBV and VZV Fig. (6) [112]. This compound was found to be less active or inactive against HHV-6A, HHV-6B, HHV-7, HHV-8 or HCMV [113]. EPB-348 is phosphorylated by viral TK homologs encoded by susceptible viruses. It forms triphosphate metabolite which acts as a competitive inhibitor of the viral DNA polymerase, thus interfering with viral DNA chain elongation. Although the in vitro potency of this compound is greater against VZV than ACV, development of resistance has been reported due to mutations in the TK gene [114,115]. Preclinical studies have shown that this compound is well tolerated. It is currently being evaluated for infectious mononucleosis and shingles in Phase II studies. A recent randomized, double-blinded, placebo-controlled non-inferiority study reported that once-daily valomaciclovir is safe and effective in immunocompetent patients when administered within 72 hr of onset of the acute rash of herpes zoster. However, further studies are warranted to optimize the doses and conditions necessary to develop this agent for acute herpes zoster therapy [116].
Entry and Fusion Peptidic Inhibitors
Entry of HSV-1 into host cells occurs via fusion of the viral envelope with the plasma membrane. However, this mechanism is very complex and the interactions between viral and cellular proteins are poorly understood. The most common approach is to employ specific inhibitors to dissect/hinder the function of gB, gH, and gD in the entry process [117]. Bultmann et al. have previously demonstrated that the EB peptide consisting of the RRKK tetramer attached to the 16-amino-acid FGF4 signal peptide, significantly inhibited HSV-1 entry and blocks viral infection during cell-to-cell spreading. Inhibition of HSV-1 entry and plaque formation was found to be dependent on virus concentrations and presence of serum, with 50% inhibitory concentrations typically ranging from 1-10 μM [117].
Also, a library of 138 overlapping peptides homologous to the 773-residue ectodomain of HSV-1 gB were synthesized and screened for the ability of the peptides to inhibit viral infection. Among these, seven 15-mer peptides significantly inhibited HSV-1 infection by more than 50% at a concentration of 100 μM [118]. The EC50 values of three peptides (gB94, gB122, and gB131) were below 20 μM. The gB131 peptide was a specific entry inhibitor with an EC50 value of approximately 12 μM. The gB122 peptide blocked viral entry (EC50, ≈ 18 μM), protected cells from infection (EC50, ≈ 72 μM), and inactivated virions in solution (EC50, ≈ 138 μM) [118].
HSV membrane fusion appears to be an attractive target for anti-HSV therapy. Galdiero et al. have investigated different membranotropic domains of HSV-1 gH envelope glycoprotein to examine the structural basis of HSV membrane fusion and identify novel targets for inhibition. Five fusion peptides (gH220-262, gH381-420, gH493-537, gH493-512 and gH626-644) were synthesized and screened for their effect on inhibition of HSV infectivity [119]. Peptides gH493-537 and gH626-644 were found to inhibit the entry of HSV by 50-60% at 250 mM and 60–70% at 500 mM. However, peptides gH220-262 and gH381-420 did not exhibit antiviral activity up to 500 mM. Interestingly, the shorter peptide gH493-512, corresponding only to the N-terminus of gH493-537, effectively inhibited HSV entry with approximately 60% inhibition observed at 250 mM and 90% inhibition at 500 mM. Peptides gH626–644 and gH493–512 demonstrated the strongest inhibitory effects of all peptides modeled on HSV-1 fusion glycoproteins till date. Their inhibitory effect could probably be due to their ability to partition into membranes and aggregate within them. Furthermore, the investigators hypothesized that these peptides could sterically hinder their relative domain, either in a pre-fusogenic or in an intermediate conformation. Such conformations could prevent the complete and functional interaction between gH and the membrane to fuse [120, 121].
This peptidic strategy could lead to the identification of functionally important regions of various glycoproteins or other membrane proteins and subsequently aid in the identification of novel inhibitors of HSV-1 entry and membrane fusion. Selected examples of antiviral peptides have been summarized in Table 1.
Table 1. Selected examples of antiviral peptides effective towards HSV.
| Peptide | Sequence | Reference |
|---|---|---|
| EB | RRKKAAVALLPAVLLALLAP | [117] |
| gB94 | KTTSSIEFARLQFTY | [118] |
| gB122 | GHRRYFTFGGGYVYF | |
| gB131 | HEVVPLEVYTRHEIK | |
| gH220–262 | TWLATRGLLRSPGRYVYFSPSASTWPVGIWTTGELVLGCDAAL | [119] |
| gH381–420 | RLTGLLATSGFAFVNAAHANGAVCLSDLLGFLAHSRALAG | |
| gH493–537 | AAHLIDALYAEFLGGRVLTTPVVHRALFYASAVLRQPFLAGVPSA | |
| gH493–512 | AAHLIDALYAEFLGGRVLTT | |
| gH626–644 | GLASTLTRWAHYNALIRAF | |
| Defensin HNP-2 | CYCRIPACIAGERRYGTCIYQGRLWAFCC | [146] |
| Brevinin-1 | FLPVLAGIAAKVVPALFCKITKKC | |
| Tachyplesin | KWCFRVCYRGICYRRCR | [147] |
| Magainin-2 | GIGKFLHSAKKFGKAFVGEIMNS | [148] |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ | |
| Dermaseptin S4 | ALWMTLLKKVLKAAAKAALNAVLVGANA | [149] |
| Bovine Lactoferricin | FKCRRWQWRMKKLGAPSITCVRRAF | [150] |
| Indolicidin | ILPWKWPWWPWRR | [151] |
Other Novel Therapies with Promising Results
Shinji Nakama et al. recently reported anti-HSV activity of Bidens pilosa (B. pilosa), a tropical weed, in tissue culture cells and a cutaneous mouse HSV-1 infection model. B. pilosa extract demonstrated potent virucidal activity, inhibited plaque formation and suppressed virus yield in both Vero and RAW 264.7 cells infected with HSV-1 and HSV-2. This extract blocked the binding of virus to host cells and viral cell penetration. Interestingly, B. pilosa extract is effective against TK-deficient and phosphonoacetate-resistant HSV-1 strains. Furthermore, treatment with B. pilosa raised the survival rate of HSV-infected mice and stopped the development of skin lesions [122].
Antiviral activity of Bifidobacterium spp. against HSV-1 was recently studied. Efficacy of B. adolescentis SPM 0214 was tested using the plaque reduction and yield reduction assays against HSV-1. HSV-1 infected Vero cells treated with high concentration of B. adolescentis SPM 0214 allowed formation of very few plaques. However, at lower concentration of B. adolescentis SPM 0214, many plaques were formed. B. adolescentis SPM 0214 appears to be a potential new therapeutic tool against HSV-1, although the mechanism of the antiviral action of Bifidobacterium spp. is unknown [123].
Recently, non-nucleoside anti-HSV compounds have received significant attention. 1,6-Naphthyridines are a class of heterocyclic compounds exhibiting broad spectrum of biological activities such as inhibitor of HIV-1 integrase, HCMV, FGF receptor-1 tyrosine kinase, and enzyme acetyl-cholinesterase. A series of compounds were tested against HSV-1 and 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines were found to be more effective inhibitors than their corresponding 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines. Among all the active compounds, 6-chloro-3-phenyl-9-fluoro-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine reduced the virus yield by 91% at 50 μM and exhibited a low cyto-toxicity (CC50=600 μM) [124].
Notoginsenoside ST-4 was investigated for its antiviral activity against HSV-1 and 2 in vitro. EC50 values determined by plaque reduction assay were 16.47 ± 0.67 and 19.44 ± 1.16 μM for HSV-1 and HSV-2, respectively. Antiviral activity of notoginsenoside ST-4 is presumably due to penetration inhibition effects, which was further confirmed by fluorescence microscopy which demonstrated that notoginsenoside ST-4 blocked the viral penetration [125].
Ren et al. recently reported in vitro antiviral activity of total alkaloids extracted from Tripterygium hypoglaucum against HSV-1. A crude total alkaloids extract prepared from the roots of T. hypoglaucum was examined against HSV-1 infected Vero cells by plaque reduction assays. The alkaloids significantly reduced plaque formation at concentrations of 6.25-12.5 g/mL, the plaque reduction ratio reached 55-75% which was about 35% higher than that of ACV at the same concentration [126]. Monophosphorylated ACV prodrug derivatives (ACV ProTides) were developed and tested for their ability to suppress both HIV-1 and HSV-2. ACV ProTides exhibited efficacy in the sub-micromolar range in ex vivo lymphoid and cervicovaginal human tissues and EC50 between 3-12 μM in CD4+ T cells. Also, ACV ProTides retained activity against ACV-resistant HSV-2 viral strains [127].
Palem et al. recently investigated the putative inhibitory effect of manzamine A on HSV-1 infection. This compound effectively inhibited viral replication and infection on SIRC, a corneal cell line at 1 μM; while ACV showed a comparable activity at 50 μM. Plaque reduction assays demonstrated that manzamine A reduced the release of infectious virus by approximately 10-fold. The investigators suggested that manzamines could reduce potent viral infections in corneal cells and prevent HSV-1 induced keratitis [128].
Recent Patents For Treatment Of Herpes Simplex Virus Infections
A US patent by Mitra discloses dipeptide and tripeptide ester derivatives of ACV and its analogs for the treatment of herpes virus infections of the eye [129]. Transporter targeted prodrug approach has gained significant attention in drug delivery [130, 131]. This patent provides methods for the synthesis of di- and tri-peptide mono- and di-esters of ACV and GCV and their derivatives. The esters described herein were reported to possess sufficient hydrophilic-lipophilic balance to be formulated into pharmacologically active compositions, such as aqueous eye drops. The compounds described in this patent were designed to target oligopeptide transporters for delivery into intraocular tissues. These prodrugs effectively reach the anterior segment and/or the vitreo-retinal region when administered either topically or systemically. Interestingly, these conjugates exhibited excellent antiviral activity against HSV as well as cytomegaloviruses. Although effective, dipeptide prodrugs are rapidly metabolized to parent drug resulting in limited bioavailability. For effective absorption at the blood ocular barrier (BOB), prolonged residence of intact prodrug is required. To overcome such limitation, specific stereoisomers of di-peptidyl esters of ACV and mono- and di-esters of GCV were designed.
These prodrugs were fairly stable with enhanced enzymatic stability and demonstrated efficient transporter translocation across ocular barriers. This patent disclosed prodrugs with excellent solution stability. The patents also disclose solubility facilitating formulation of stable eye drops [132].
Another recent patent by Mitra and Samanta discloses a novel prodrug strategy which is more lipophilic and at the same time site specific thereby providing a dual approach to improve cellular absorption of ACV [133]. This patent describes conjugated compounds comprising a therapeutic or diagnostic agent linked to a substrate for a cell membrane transporter or receptor through a lipophilic linker. The inventors employed a lipid raft that is conjugated to ACV to impart lipophilicity and a targeting moiety (biotin) which can be recognized by a specific transporter/receptor (SMVT) on the other terminal of the lipid raft. It is well known that lipophilic prodrugs readily diffuse across the cell membrane by facilitated diffusion whereas transporter/receptor targeted prodrugs translocate compounds across the cell membrane via active transport via molecular recognition. Only a marginal improvement in cellular uptake was evident from each of the two approaches. However, this patent disclosed a novel approach which combines both lipid and transporter/receptor targeted delivery to generate synergistic effect. Compared to ACV, the uptake of targeted lipid prodrugs (B-R-ACV and B-12HS-ACV) increased by 10 and 8.3 times respectively, whereas the uptake of B-ACV, R-ACV and 12HS-ACV was higher by 3.5, 1.4 and 1.3 times respectively in Caco-2 cells. The targeted lipid prodrugs B-R-ACV and B-12HSACV exhibited much higher cellular accumulation than B-ACV, R-ACV and 12HS-ACV. Both the targeted lipid prodrugs B-R-ACV and B-12HS-ACV demonstrated higher affinity towards SMVT than B-ACV. Such enhanced affinity may be attributed to the presence of lipid raft which facilitates enhanced interaction of prodrug with membrane transporters/receptors thereby assisting docking of the targeted ligand into the binding domain of transporter/receptor protein. The net effect observed was rapid translocation of the cargo across cell membrane. This novel prodrug design may also allow for enhanced plasma membrane uptake of hydrophilic therapeutic agents such as genes, siRNA, nucleosides, nucleotides, oligonucleotides or antisense oligonucleotides, peptides and proteins [134].
A recent US patent application by Hsu discusses compositions and methods for treating HSV infections [135]. This application reports therapeutic utility of green tea polyphenol compositions including (−)- epigallocatechin-3-gallate as well as green tea polyphenols with one on more ester-linked fatty acids. The green tea polyphenols of the present inventions include, but not limited to (-)-epigallocatechin-3-gallate, (-)-epicatechin, (-)-epigallocatechin, and (-)-epicatechin-3-gallate. Furthermore, proanthocyanidins, their enantiomers, isomers, pharmaceutically acceptable salts, and prodrugs of green tea polyphenols are included. Green tea is made from the plant named “Camellia sinensis” plant. It is rich in catechin polyphenols, in particular, epigallocatechin gallate, successfully inhibited HSV infection in a concentration dependent manner. Since epigallocatechin gallate is highly unstable and rapidly oxidized, it loses its antiviral abilities long before it is applied. Therefore, lipophilic tea polyphenols having an ester-linked C1 to C30 hydrocarbon chain were prepared by catalytic esterification of green tea polyphenols to enhance stability and permeability of the green tea polyphenols. Antiviral screening studies indicated that epigallocatechin gallate inhibits HSV-1 by 95% and modified ester of epigallocatechin gallate inhibits HSV-1 by 99.46% [136, 137]. These compounds act by interfering with the virion envelope glycoproteins or by interfering with viral compounds for viral adsorption and cell entry. Though the modified ester of epigallocatechin gallate has demonstrated greater potency compared to epigallocatechin gallate against HSV-1 infections in vitro, further studies need to be conducted in order to completely delineate the exact mechanism of action in humans. Nevertheless, natural products could improve the lives of many patients and offer a better health.
A US patent by Boyd discusses nucleoside analogs in combination for the treatment of herpes simplex infections [138]. This application discloses a pharmaceutical product comprising a nucleoside analogue active against HSV such as ACV/valaciclovir or peniclovir/famciclovir, and an im-munosuppressant such as cyclosporine A. These combined preparations are useful for simultaneous, separate or sequential therapy in the treatment and/or prevention of HSV infections. This patent reports that coadministration of penci-clovir/famciclovir with an immunosuppressant is particularly useful for the treatment of severe and/or prolonged HSV infections [139].
A recent US patent application by Whitten discusses therapeutic compositions for treating HSV infections. This therapeutic composition comprises a mixture of ACV, penci-clovir, and 2-Deoxy-D-Glucose. The inventor claims that these therapeutic compositions will meet a long felt need in the art of providing a treatment for lesions that result from HSV that significantly reduce the duration of a cold sore when vesicles have already emerged and a treatment that will prevent the outbreak of a lesion and vesicle formation when applied in the prodromal stage [140].
Burrell recently disclosed a therapeutic composition of glycyrrhizic acid, slippery elm, zinc oxide, allantoin, lysine monohydrochloride, L-carnitine, lipoic acid, salicylic Acid, citric Acid, vitamin E acetate, wheat germ oil, and shea butter for the treatment of HSV-1, HSV-2, canker sores, shingles, and other epidermal and oral ailments [141]. The present invention is directed to generate synergistic effects by combination of compounds designed to inhibit/treat the virus itself as well as to alleviate the symptoms and triggers associated with HSV-1, HSV-2 and herpes zoster. The combination is prepared by mixing the ingredients until they are evenly distributed throughout the solution. The preferred composition of this combination include 1% Glycyrhizic acid, 0.05% Zinc Oxide, USP, 2% allantoin, 2% slippery elm, 2% Lysine (L) Monohydrochloride, USP, 2% Carnitine (L), 1% Lipoic Acid, DL-alpha (DL-thiotic acid, 0.025% Salicylic acid, USP crystalline poder, 0.5% citric acid, USP Hydrous powder, 16.25% wheat germ oil (cold pressed), 0.5% stevioside (90% extract), 4% Raspberry, 68.675% Shea butter.
A recent patent by Bornmann and Kalman disclosed the use of kinase inhibitors to inhibit kinases involved in pathogen-host cell interactions that are associated with or cause pathogenic infections including HSV [142]. This invention is directed towards development and identification of compounds that modulate/alter the way in which diverse viral pathogens interact with the host, so as to block or limit disease caused by these viruses and allow the host immune system to clear the viral pathogens. Several kinase inhibitors have been screened against herpes viruses. Some of the compounds tested such as ApCK103, Apck-43, LG2-55, and LG2-71 proved effective in treating infections caused by herpes virus. Also, the inventors claim that one or more of the kinase inhibitors can be selected in combination with antiviral agents such as CDV. Such combinations would lower the dosage requirements and thereby minimize toxic effects of this nucleoside analogue.
Novel hydroxybenzoic acid ester and analogues have been patented by Shenghua Guangzhou Pharmaceuticals [143]. Several compounds including 4-hydroxybenzoic acid ester; 2, 4-dihydroxybenzoic acid ester; 3, 4- dihydroxybenzoic acid ester; 2, 3, 4- trihydroxybenzoic acid ester; 3, 4, 5-trihydroxybenzoic acid ester or 3, 4, 6- trihydroxybenzoic acid ester have been synthesized. In vitro antiviral studies demonstrated that hydroxybenzoic acid esters and analogues are more potent than hydroxybenzoic acid and analogues. For example, propyl gallate was more stable than gallic acid, especially when it is in weak alkaline condition of the plasma and tissues (pH 7.4) or intestinal alkaline condition (pH 8.6). The antiviral activity of a hydroxybenzoic acid ester (propyl gallate) is higher than its corresponding acid (gallic acid).
A recent US patent application by Tkachuk describes the utility of RNA molecules because of their ability to adopt a wide variety of conformations thereby performing a range of cellular functions [144]. A new antiviral compound i.e, modified highly purified yeast RNA displayed pronounced multiple antiviral activities in a wide range of concentrations. This modified yeast RNA is capable of inhibiting the viral replication from several viruses including ortho-myxoviridae, paramyxovirus, hepatitis, herpesviridae families, enterovirus, adenovirus, influenza viruses, hepatitis C virus, genital herpes, human immunodeficiency virus and Coxsackie B virus. The antiherpetic activity of RNA-M was studied in a model of murine herpetic meningoencephalitis caused by HSV-1, as well as in a model of genital herpetic infection in guinea pigs infected by HSV-2. Results from this study demonstrated that this RNA-based drug has potent antiherpetic action and is effective in the treatment of herpetic diseases, especially genitals herpes. Since viral infections are frequently associated with several strains of viruses, antiviral agents with multiple mechanisms of action are warranted to ameliorate the rapidly escalating resistance to antiviral agents.
Cantin et al. in a recent US patent application discusses the use of compositions comprising pooled immunoglobulin for the treatment and/or prophylaxis of herpes infection and its associated disorders [145]. Human Immunoglobulin (IVIG) consisting of the polyclonal IgG fractions from thousands of donors was pooled. Consequently, poly reactive natural antibodies and antibodies specific for allotypic antigens must also be represented in the pool to suppress antibody-mediated autoimmune disease, chronic or acute inflammatory states in which damage is caused by activated leukocytes. The active component of IVIG includes sialylated IgG species which is prepared from human plasma or serum. In particular, the immunoglobulin comprised of sialylated IgG domains, is a monomeric Fc domain which is preferred for the treatment and prophylaxis of herpes infections including HSV-1 infection and its associated encephalitis and herpes stromal keratitis. The inhibitory activity of IVIG has been evaluated by studying HSV infection (B6- and 129-Rag/E strains) and studying suppression of inflammation and virus replication.
Current & Future Developments
The herpes family of viruses is responsible for causing a wide variety of infections in humans. With escalating sero-prevalence rates, treatment of herpes infections still remains a challenging task, regardless of the introduction of several therapeutics agents with excellent intrinsic antiviral activities. Current treatment options for the treatment of HSV infections are reasonably safe and fairly effective. However, long term therapy with these agents is often associated with toxicities which limit their utility and ultimate druggability. Emergence of resistance and development of drug-resistant viral isolates have been observed especially in immunocom-promised patients, who are treated with antiviral drugs for longer periods of time. Hence, there is an apparent need to develop newer therapeutics with a novel mechanism of action, providing superior efficacy and diminished potential for adverse effects. All the new anti-herpetic compounds summarized in this review appear to be promising and have the potential to significantly enhance therapies for HSV infections. Development of novel compounds with enhanced efficacy and less potential for toxicity is obviously essential. Since many new compounds are currently in clinical development, it would be better if those compounds will be screened not only for their antiviral potency but also for their potential use in combination with other antivirals as multidrug regimens. Hopefully, some of these new molecules which are being developed would lead to block buster drugs in the near future and management of herpes infections would be less complicated.
Acknowledgments
The authors would like to acknowledge NIH grants R01EY09171 and R01EY010659 for financial support.
Footnotes
Conflict of Interest: The authors confirm that this article content has no conflicts of interest.
References
- 1.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55(2):459–72. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt-Rauf PW, Andrews LR, Schwarz-Miller J. Sick-hospital syndrome. J Occup Med. 1991;33(6):737–9. doi: 10.1097/00043764-199106000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010;23(4):689–712. doi: 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strasfeld L, Chou S. Antiviral drug resistance: mechanisms and clinical implications. Infect Dis Clin North Am. 2010;24(2):413–37. doi: 10.1016/j.idc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauerbrei A, Deinhardt S, Zell R, Wutzler P. Testing of herpes simplex virus for resistance to antiviral drugs. Virulence. 2010;1(6):555–7. doi: 10.4161/viru.1.6.13806. [DOI] [PubMed] [Google Scholar]
- 6.Duan R, de Vries RD, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198(5):659–63. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- 7.Price NB, Prichard MN. Progress in the development of new therapies for herpesvirus infections. Curr Opin Virol. 2011;1(6):548–54. doi: 10.1016/j.coviro.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamora MR. DNA viruses (CMV, EBV, and the herpesviruses) Semin Respir Crit Care Med. 2011;32(4):454–70. doi: 10.1055/s-0031-1283285. [DOI] [PubMed] [Google Scholar]
- 9.Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: Herpes simplex and varicella-zoster. Lancet Neurol. 2007;6(11):1015–28. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- 10.Brown JC, Newcomb WW. Herpesvirus capsid assembly: Insights from structural analysis. Curr Opin Virol. 2011;1(2):142–9. doi: 10.1016/j.coviro.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor TJ, Brockman MA, McNamee EE, Knipe DM. Herpes simplex virus. Front Biosci. 2002;7:d752–64. doi: 10.2741/taylor. [DOI] [PubMed] [Google Scholar]
- 12.Kieff ED, Bachenheimer SL, Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971;8(2):125–32. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker Y, Dym H, Sarov I. Herpes simplex virus DNA. Virology. 1968;36(2):184–92. doi: 10.1016/0042-6822(68)90135-9. [DOI] [PubMed] [Google Scholar]
- 14.Wildy P, Russell WC, Horne RW. The morphology of herpes virus. Virology. 1960;12:204–22. doi: 10.1016/0042-6822(60)90195-1. [DOI] [PubMed] [Google Scholar]
- 15.Jenssen H. Therapeutic Approaches Using Host Defence Peptides to Tackle Herpes Virus Infections. Viruses. 2009;1(3):939–64. doi: 10.3390/v1030939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockshon D, Galloway DA. Cloning and characterization of oriL2, a large palindromic DNA replication origin of herpes simplex virus type 2. J Virol. 1986;58(2):513–21. doi: 10.1128/jvi.58.2.513-521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weller SK, Spadaro A, Schaffer JE, Murray AW, Maxam AM, Schaffer PA. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol Cell Biol. 1985;5(5):930–42. doi: 10.1128/mcb.5.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stow ND, McMonagle EC. Characterization of the TRS/IRS origin of DNA replication of herpes simplex virus type 1. Virology. 1983;130(2):427–38. doi: 10.1016/0042-6822(83)90097-1. [DOI] [PubMed] [Google Scholar]
- 19.Stow ND. Localization of an origin of DNA replication within the TRS/IRS repeated region of the herpes simplex virus type 1 genome. EMBO J. 1982;1(7):863–7. doi: 10.1002/j.1460-2075.1982.tb01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igarashi K, Fawl R, Roller RJ, Roizman B. Construction and properties of a recombinant herpes simplex virus 1 lacking both S-component origins of DNA synthesis. J Virol. 1993;67(4):2123–32. doi: 10.1128/jvi.67.4.2123-2132.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polvino-Bodnar M, Orberg PK, Schaffer PA. Herpes simplex virus type 1 oriL is not required for virus replication or for the establishment and reactivation of latent infection in mice. J Virol. 1987;61(11):3528–35. doi: 10.1128/jvi.61.11.3528-3535.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78(14):7508–17. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77(9):5324–32. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spear PG. Entry of alphaherpesviruses into cells. Seminars Virol. 1993;4(3):167–80. [Google Scholar]
- 25.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: A structural view of the herpesvirus entry machinery. Nat Rev Microbiol. 2011;9(5):369–81. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westra DF, Glazenburg KL, Harmsen MC, Tiran A, Jan Scheffer A, Welling GW, et al. Glycoprotein H of herpes simplex virus type 1 requires glycoprotein L for transport to the surfaces of insect cells. J Virol. 1997;71(3):2285–91. doi: 10.1128/jvi.71.3.2285-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laquerre S, Person S, Glorioso JC. Glycoprotein B of herpes simplex virus type 1 oligomerizes through the intermolecular interaction of a 28-amino-acid domain. J Virol. 1996;70(3):1640–50. doi: 10.1128/jvi.70.3.1640-1650.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Highlander SL, Goins WF, Person S, Holland TC, Levine M, Glorioso JC. Oligomer formation of the gB glycoprotein of herpes simplex virus type 1. J Virol. 1991;65(8):4275–83. doi: 10.1128/jvi.65.8.4275-4283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trybala E, Roth A, Johansson M, Liljeqvist JA, Rekabdar E, Larm O, et al. Glycosaminoglycan-binding ability is a feature of wild-type strains of herpes simplex virus type 1. Virology. 2002;302(2):413–9. doi: 10.1006/viro.2002.1639. [DOI] [PubMed] [Google Scholar]
- 30.Trybala E, Olofsson S, Mardberg K, Svennerholm B, Umemoto K, Glorioso JC, et al. Structural and functional features of the polycationic peptide required for inhibition of herpes simplex virus invasion of cells. Antiviral Res. 2004;62(3):125–34. doi: 10.1016/j.antiviral.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, Tufaro F, et al. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69(7):4471–83. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108(4):503–10. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herold BC, WuDunn D, Soltys N, Spear PG. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65(3):1090–8. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arii J, Goto H, Suenaga T, Oyama M, Kozuka-Hata H, Imai T, et al. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature. 2010;467(7317):859–62. doi: 10.1038/nature09420. [DOI] [PubMed] [Google Scholar]
- 35.Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci U S A. 2010;107(2):866–71. doi: 10.1073/pnas.0913351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Jong MA, de Witte L, Bolmstedt A, van Kooyk Y, Geijtenbeek TB. Dendritic cells mediate herpes simplex virus infection and transmission through the C-type lectin DC-SIGN. J Gen Virol. 2008;89(Pt 10):2398–409. doi: 10.1099/vir.0.2008/003129-0. [DOI] [PubMed] [Google Scholar]
- 37.Satoh T, Arii J, Suenaga T, Wang J, Kogure A, Uehori J, et al. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132(6):935–44. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon H, Bai Q, Baek HJ, Felmet K, Burton EA, Goins WF, et al. Soluble V domain of Nectin-1/HveC enables entry of herpes simplex virus type 1 (HSV-1) into HSV-resistant cells by binding to viral glycoprotein D. J Virol. 2006;80(1):138–48. doi: 10.1128/JVI.80.1.138-148.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Donnell CD, Tiwari V, Oh MJ, Shukla D. A role for heparan sulfate 3-O-sulfotransferase isoform 2 in herpes simplex virus type 1 entry and spread. Virology. 2006;346(2):452–9. doi: 10.1016/j.virol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, Eisenberg RJ, et al. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 2005;24(23):4144–53. doi: 10.1038/sj.emboj.7600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Eisenberg RJ, Cohen GH. Structure-based analysis of the herpes simplex virus glycoprotein D binding site present on herpesvirus entry mediator HveA (HVEM) J Virol. 2002;76(21):10894–904. doi: 10.1128/JVI.76.21.10894-10904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280(5369):1618–20. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 43.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8(1):21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 44.Subramanian RP, Geraghty RJ. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc Natl Acad Sci USA. 2007;104(8):2903–8. doi: 10.1073/pnas.0608374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72(1):873–5. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Douglas MW, Diefenbach RJ, Homa FL, Miranda-Saksena M, Rixon FJ, Vittone V, et al. Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J Biol Chem. 2004;279(27):28522–30. doi: 10.1074/jbc.M311671200. [DOI] [PubMed] [Google Scholar]
- 47.Marozin S, Prank U, Sodeik B. Herpes simplex virus type 1 infection of polarized epithelial cells requires microtubules and access to receptors present at cell-cell contact sites. J Gen Virol. 2004;85(Pt 4):775–86. doi: 10.1099/vir.0.19530-0. [DOI] [PubMed] [Google Scholar]
- 48.Garner JA. Herpes simplex virion entry into and intracellular transport within mammalian cells. Adv Drug Deliv Rev. 2003;55(11):1497–513. doi: 10.1016/j.addr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Dohner K, Wolfstein A, Prank U, Echeverri C, Dujardin D, Vallee R, et al. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol Biol Cell. 2002;13(8):2795–809. doi: 10.1091/mbc.01-07-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye GJ, Vaughan KT, Vallee RB, Roizman B. The herpes simplex virus 1 U(L)34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J Virol. 2000;74(3):1355–63. doi: 10.1128/jvi.74.3.1355-1363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costanzo F, Campadelli-Fiume G, Foa-Tomasi L, Cassai E. Evidence that herpes simplex virus DNA is transcribed by cellular RNA polymerase B. J Virol. 1977;21(3):996–1001. doi: 10.1128/jvi.21.3.996-1001.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alwine JC, Steinhart WL, Hill CW. Transcription of herpes simplex type 1 DNA in nuclei isolated from infected HEp-2 and KB cells. Virology. 1974;60(1):302–7. doi: 10.1016/0042-6822(74)90390-0. [DOI] [PubMed] [Google Scholar]
- 53.Mohr I. To replicate or not to replicate: Achieving selective oncolytic virus replication in cancer cells through translational control. Oncogene. 2005;24(52):7697–709. doi: 10.1038/sj.onc.1209053. [DOI] [PubMed] [Google Scholar]
- 54.Van Sant C, Hagglund R, Lopez P, Roizman B. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin-conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc Natl Acad Sci USA. 2001;98(15):8815–20. doi: 10.1073/pnas.161283098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stow ND, Stow EC. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986;67(Pt 12):2571–85. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 56.Gelman IH, Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci USA. 1985;82(16):5265–9. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quinlan MP, Knipe DM. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol Cell Biol. 1985;5(5):957–63. doi: 10.1128/mcb.5.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leopardi R, Michael N, Roizman B. Repression of the herpes simplex virus 1 alpha 4 gene by its gene product (ICP4) within the context of the viral genome is conditioned by the distance and stereoaxial alignment of the ICP4 DNA binding site relative to the TATA box. J Virol. 1995;69(5):3042–8. doi: 10.1128/jvi.69.5.3042-3048.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muller MT. Binding of the herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J Virol. 1987;61(3):858–65. doi: 10.1128/jvi.61.3.858-865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faber SW, Wilcox KW. Association of the herpes simplex virus regulatory protein ICP4 with specific nucleotide sequences in DNA. Nucleic Acids Res. 1986;14(15):6067–83. doi: 10.1093/nar/14.15.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristie TM, Roizman B. Separation of sequences defining basal expression from those conferring alpha gene recognition within the regulatory domains of herpes simplex virus 1 alpha genes. Proc Natl Acad Sci U S A. 1984;81(13):4065–9. doi: 10.1073/pnas.81.13.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jean S, LeVan KM, Song B, Levine M, Knipe DM. Herpes simplex virus 1 ICP27 is required for transcription of two viral late (gamma 2) genes in infected cells. Virology. 2001;283(2):273–84. doi: 10.1006/viro.2001.0902. [DOI] [PubMed] [Google Scholar]
- 63.Uprichard SL, Knipe DM. Herpes simplex ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes. J Virol. 1996;70(3):1969–80. doi: 10.1128/jvi.70.3.1969-1980.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carter KL, Roizman B. The promoter and transcriptional unit of a novel herpes simplex virus 1 alpha gene are contained in, and encode a protein in frame with, the open reading frame of the alpha 22 gene. J Virol. 1996;70(1):172–8. doi: 10.1128/jvi.70.1.172-178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, et al. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375(6530):411–5. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 66.McGeoch DJ, Dalrymple MA, Dolan A, McNab D, Perry LJ, Taylor P, et al. Structures of herpes simplex virus type 1 genes required for replication of virus DNA. J Virol. 1988;62(2):444–53. doi: 10.1128/jvi.62.2.444-453.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu CA, Nelson NJ, McGeoch DJ, Challberg MD. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988;62(2):435–43. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Challberg MD. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci USA. 1986;83(23):9094–8. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elias P, O'Donnell ME, Mocarski ES, Lehman IR. A DNA binding protein specific for an origin of replication of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1986;83(17):6322–6. doi: 10.1073/pnas.83.17.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Godowski PJ, Knipe DM. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc Natl Acad Sci U S A. 1986;83(2):256–60. doi: 10.1073/pnas.83.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conley AJ, Knipe DM, Jones PC, Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Purifoy DJ, Lewis RB, Powell KL. Identification of the herpes simplex virus DNA polymerase gene. Nature. 1977;269(5629):621–3. doi: 10.1038/269621a0. [DOI] [PubMed] [Google Scholar]
- 73.Weller SK, Coen DM. Herpes simplex viruses: Mechanisms of DNA replication. Cold Spring Harb Perspect Biol. 2012;4(9):a013011. doi: 10.1101/cshperspect.a013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kibler PK, Duncan J, Keith BD, Hupel T, Smiley JR. Regulation of herpes simplex virus true late gene expression: Sequences downstream from the US11 TATA box inhibit expression from an unreplicated template. J Virol. 1991;65(12):6749–60. doi: 10.1128/jvi.65.12.6749-6760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newcomb WW, Trus BL, Booy FP, Steven AC, Wall JS, Brown JC. Structure of the herpes simplex virus capsid. Molecular composition of the pentons and the triplexes. J Mol Biol. 1993;232(2):499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- 76.Wingfield PT, Stahl SJ, Thomsen DR, Homa FL, Booy FP, Trus BL, et al. Hexon only binding of VP26 reflects differences between the hexon and penton conformations of VP5, the major capsid protein of herpes simplex virus. J Virol. 1997;71(12):8955–61. doi: 10.1128/jvi.71.12.8955-8961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou ZH, He J, Jakana J, Tatman JD, Rixon FJ, Chiu W. Assembly of VP26 in herpes simplex virus-1 inferred from structures of wild-type and recombinant capsids. Nat Struct Biol. 1995;2(11):1026–30. doi: 10.1038/nsb1195-1026. [DOI] [PubMed] [Google Scholar]
- 78.Booy FP, Trus BL, Newcomb WW, Brown JC, Conway JF, Steven AC. Finding a needle in a haystack: detection of a small protein (the 12-kDa VP26) in a large complex (the 200-MDa capsid of herpes simplex virus) Proc Natl Acad Sci USA. 1994;91(12):5652–6. doi: 10.1073/pnas.91.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rochat RH, Liu X, Murata K, Nagayama K, Rixon FJ, Chiu W. Seeing the portal in herpes simplex virus type 1 B capsids. J Virol. 2011;85(4):1871–4. doi: 10.1128/JVI.01663-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cardone G, Winkler DC, Trus BL, Cheng N, Heuser JE, Newcomb WW, et al. Visualization of the herpes simplex virus portal in situ by cryo-electron tomography. Virology. 2007;361(2):426–34. doi: 10.1016/j.virol.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trus BL, Cheng N, Newcomb WW, Homa FL, Brown JC, Steven AC. Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1. J Virol. 2004;78(22):12668–71. doi: 10.1128/JVI.78.22.12668-12671.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spencer JV, Newcomb WW, Thomsen DR, Homa FL, Brown JC. Assembly of the herpes simplex virus capsid: Preformed triplexes bind to the nascent capsid. J Virol. 1998;72(5):3944–51. doi: 10.1128/jvi.72.5.3944-3951.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Conway JF, Cockrell SK, Copeland AM, Newcomb WW, Brown JC, Homa FL. Labeling and localization of the herpes simplex virus capsid protein UL25 and its interaction with the two triplexes closest to the penton. J Mol Biol. 2010;397(2):575–86. doi: 10.1016/j.jmb.2010.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trus BL, Newcomb WW, Cheng N, Cardone G, Marekov L, Homa FL, et al. Allosteric signaling and a nuclear exit strategy: Binding of UL25/UL17 heterodimers to DNA-Filled HSV-1 capsids. Mol Cell. 2007;26(4):479–89. doi: 10.1016/j.molcel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9(5):382–94. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- 86.Mettenleiter TC, Minson T. Egress of alphaherpesviruses. J Virol. 2006;80(3):1610–1. doi: 10.1128/JVI.80.3.1610-1612.2006. author reply 1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mettenleiter TC. Herpesvirus assembly and egress. J Virol. 2002;76(4):1537–47. doi: 10.1128/JVI.76.4.1537-1547.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith GA, Gross SP, Enquist LW. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci U S A. 2001;98(6):3466–70. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilson AC, Mohr I. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol. 2012;20(12):604–11. doi: 10.1016/j.tim.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deshmane SL, Fraser NW. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J Virol. 1989;63(2):943–7. doi: 10.1128/jvi.63.2.943-947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zabolotny JM, Krummenacher C, Fraser NW. The herpes simplex virus type 1 2.0-kilobase latency-associated transcript is a stable intron which branches at a guanosine. J Virol. 1997;71(6):4199–208. doi: 10.1128/jvi.71.6.4199-4208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zwaagstra JC, Ghiasi H, Slanina SM, Nesburn AB, Wheatley SC, Lillycrop K, et al. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J Virol. 1990;64(10):5019–28. doi: 10.1128/jvi.64.10.5019-5028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitchell WJ, Lirette RP, Fraser NW. Mapping of low abundance latency-associated RNA in the trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J Gen Virol. 1990;71(Pt 1):125–32. doi: 10.1099/0022-1317-71-1-125. [DOI] [PubMed] [Google Scholar]
- 94.Wagner EK, Flanagan WM, Devi-Rao G, Zhang YF, Hill JM, Anderson KP, et al. The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J Virol. 1988;62(12):4577–85. doi: 10.1128/jvi.62.12.4577-4585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kent JR, Kang W, Miller CG, Fraser NW. Herpes simplex virus latency-associated transcript gene function. J Neurovirol. 2003;9(3):285–90. doi: 10.1080/13550280390200994. [DOI] [PubMed] [Google Scholar]
- 96.Perng GC, Jones C, Ciacci-Zanella J, Stone M, Henderson G, Yukht A, et al. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287(5457):1500–3. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 97.Lachmann R. Herpes simplex virus latency. Expert Rev Mol Med. 2003;5(29):1–14. doi: 10.1017/S1462399403006975. [DOI] [PubMed] [Google Scholar]
- 98.Biswas S, Field HJ. Antiviral Drug Strategies. Wiley-VCH Verlag GmbH & Co; KGaA: 2011. Helicase–Primase Inhibitors: A New Approach to Combat Herpes Simplex Virus and Varicella Zoster Virus; pp. 129–46. [Google Scholar]
- 99.Baumeister J, Fischer R, Eckenberg P, Henninger K, Ruebsamen-Waigmann H, Kleymann G. Superior efficacy of helicase-primase inhibitor BAY 57-1293 for herpes infection and latency in the guinea pig model of human genital herpes disease. Antivir Chem Chemother. 2007;18(1):35–48. doi: 10.1177/095632020701800104. [DOI] [PubMed] [Google Scholar]
- 100.Biswas S, Jennens L, Field HJ. The helicase primase inhibitor, BAY 57-1293 shows potent therapeutic antiviral activity superior to famciclovir in BALB/c mice infected with herpes simplex virus type 1. Antiviral Res. 2007;75(1):30–5. doi: 10.1016/j.antiviral.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 101.Kleymann G, Fischer R, Betz UA, Hendrix M, Bender W, Schneider U, et al. New helicase-primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nat Med. 2002;8(4):392–8. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- 102.Biswas S, Swift M, Field HJ. High frequency of spontaneous helicase-primase inhibitor (BAY 57-1293) drug-resistant variants in certain laboratory isolates of HSV-1. Antivir Chem Chemother. 2007;18(1):13–23. doi: 10.1177/095632020701800102. [DOI] [PubMed] [Google Scholar]
- 103.Field HJ, Biswas S. Antiviral drug resistance and helicase-primase inhibitors of herpes simplex virus. Drug Resist Updat. 2011;14(1):45–51. doi: 10.1016/j.drup.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 104.Vadlapudi AD, Vadlapatla RK, Mitra AK. Current and emerging antivirals for the treatment of cytomegalovirus (CMV) retinitis: An update on recent patents. Recent Pat Antiinfect Drug Discov. 2012;7(1):8–18. doi: 10.2174/157489112799829765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 2009;82(2):A84–98. doi: 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hostetler KY. Synthesis and early development of hexadecyloxypropylcidofovir: an oral antipoxvirus nucleoside phosphonate. Viruses. 2010;2(10):2213–25. doi: 10.3390/v2102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams-Aziz SL, Hartline CB, Harden EA, Daily SL, Prichard MN, Kushner NL, et al. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob Agents Chemother. 2005;49(9):3724–33. doi: 10.1128/AAC.49.9.3724-3733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dropulic LK, Cohen JI. Update on new antivirals under development for the treatment of double-stranded DNA virus infections. Clin Pharmacol Ther. 2010;88(5):610–9. doi: 10.1038/clpt.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quenelle DC, Lampert B, Collins DJ, Rice TL, Painter GR, Kern ER. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J Infect Dis. 2010;202(10):1492–9. doi: 10.1086/656717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prichard MN, Kern ER, Hartline CB, Lanier ER, Quenelle DC. CMX001 potentiates the efficacy of acyclovir in herpes simplex virus infections. Antimicrob Agents Chemother. 2011;55(10):4728–34. doi: 10.1128/AAC.00545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ciesla SL, Trahan J, Wan WB, Beadle JR, Aldern KA, Painter GR, et al. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 2003;59(3):163–71. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 112.Lowe DM, Alderton WK, Ellis MR, Parmar V, Miller WH, Roberts GB, et al. Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses. Antimicrob Agents Chemother. 1995;39(8):1802–8. doi: 10.1128/aac.39.8.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Clercq E. Guanosine analogues as anti-herpesvirus agents. Nucleosides Nucleotides Nucleic Acids. 2000;19(10-12):1531–41. doi: 10.1080/15257770008045444. [DOI] [PubMed] [Google Scholar]
- 114.Ng TI, Shi Y, Huffaker HJ, Kati W, Liu Y, Chen CM, et al. Selection and characterization of varicella-zoster virus variants resistant to (R)-9-[4-hydroxy-2-(hydroxymethy)butyl]guanine. Antimicrob Agents Chemother. 2001;45(6):1629–36. doi: 10.1128/AAC.45.6.1629-1636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Andrei G, Snoeck R, Reymen D, Liesnard C, Goubau P, Desmyter J, et al. Comparative activity of selected antiviral compounds against clinical isolates of varicella-zoster virus. Eur J Clin Microbiol Infect Dis. 1995;14(4):318–29. doi: 10.1007/BF02116525. [DOI] [PubMed] [Google Scholar]
- 116.Tyring SK, Plunkett S, Scribner AR, Broker RE, Herrod JN, Handke LT, et al. Valomaciclovir versus valacyclovir for the treatment of acute herpes zoster in immunocompetent adults: A randomized, double-blind, active-controlled trial. J Med Virol. 2012;84(8):1224–32. doi: 10.1002/jmv.23329. [DOI] [PubMed] [Google Scholar]
- 117.Bultmann H, Busse JS, Brandt CR. Modified FGF4 signal peptide inhibits entry of herpes simplex virus type 1. J Virol. 2001;75(6):2634–45. doi: 10.1128/JVI.75.6.2634-2645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Akkarawongsa R, Pocaro NE, Case G, Kolb AW, Brandt CR. Multiple peptides homologous to herpes simplex virus type 1 glycoprotein B inhibit viral infection. Antimicrob Agents Chemother. 2009;53(3):987–96. doi: 10.1128/AAC.00793-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Galdiero S, Falanga A, Vitiello M, D'Isanto M, Cantisani M, Kampanaraki A, et al. Peptides containing membrane-interacting motifs inhibit herpes simplex virus type 1 infectivity. Peptides. 2008;29(9):1461–71. doi: 10.1016/j.peptides.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galdiero S, Falanga A, Vitiello M, D'Isanto M, Collins C, Orrei V, et al. Evidence for a role of the membrane-proximal region of herpes simplex virus type 1 glycoprotein H in membrane fusion and virus inhibition. Chembiochem. 2007;8(8):885–95. doi: 10.1002/cbic.200700044. [DOI] [PubMed] [Google Scholar]
- 121.Galdiero S, Falanga A, Vitiello M, Browne H, Pedone C, Galdiero M. Fusogenic domains in herpes simplex virus type 1 glycoprotein H. J Biol Chem. 2005;280(31):28632–43. doi: 10.1074/jbc.M505196200. [DOI] [PubMed] [Google Scholar]
- 122.Nakama S, Tamaki K, Ishikawa C, Tadano M, Mori N. Efficacy of Bidens pilosa extract against herpes simplex virus infection in vitro and in vivo. Evid Based Complement Alternat Med. 2012;2012:413453. doi: 10.1155/2012/413453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.An HM, Lee do K, Kim JR, Lee SW, Cha MK, Lee KO, et al. Antiviral activity of Bifidobacterium adolescentis SPM 0214 against herpes simplex virus type 1. Arch Pharm Res. 2012;35(9):1665–71. doi: 10.1007/s12272-012-0918-9. [DOI] [PubMed] [Google Scholar]
- 124.Bernardino AM, Azevedo AR, Pinheiro LC, Borges JC, Paixao IC, Mesquita M, et al. Synthesis and anti-HSV-1 evaluation of new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines. Org Med Chem Lett. 2012;2(1):3. doi: 10.1186/2191-2858-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pei Y, Du Q, Liao PY, Chen ZP, Wang D, Yang CR, et al. Notoginsenoside ST-4 inhibits virus penetration of herpes simplex virus in vitro. J Asian Nat Prod Res. 2011;13(6):498–504. doi: 10.1080/10286020.2011.571645. [DOI] [PubMed] [Google Scholar]
- 126.Ren Z, Zhang CH, Wang LJ, Cui YX, Qi RB, Yang CR, et al. In vitro anti-viral activity of the total alkaloids from Tripterygium hypoglaucum against herpes simplex virus type 1. Virol Sin. 2010;25(2):107–14. doi: 10.1007/s12250-010-3092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vanpouille C, Lisco A, Derudas M, Saba E, Grivel JC, Brichacek B, et al. A new class of dual-targeted antivirals: monophosphory-lated acyclovir prodrug derivatives suppress both human immunodeficiency virus type 1 and herpes simplex virus type 2. J Infect Dis. 2010;201(4):635–43. doi: 10.1086/650343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Palem JR, Bedadala GR, El Sayed KA, Hsia SC. Manzamine A as a novel inhibitor of herpes simplex virus type-1 replication in cultured corneal cells. Planta Med. 2011;77(1):46–51. doi: 10.1055/s-0030-1250093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mitra AK. US7825086. Acyclovir-peptide analogs. 2010
- 130.Majumdar S, Duvvuri S, Mitra AK. Membrane transporter/receptor-targeted prodrug design: Strategies for human and veterinary drug development. Adva Drug Deliv Rev. 2004;56(10):1437–52. doi: 10.1016/j.addr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 131.Yang C, Tirucherai GS, Mitra AK. Prodrug based optimal drug delivery via membrane transporter/receptor. Expert opinion on biological therapy. 2001;1(2):159–75. doi: 10.1517/14712598.1.2.159. [DOI] [PubMed] [Google Scholar]
- 132.Mitra AK, Samanta SK, Talluri RS. US7951774. Stereochemically defined dipeptide esters of antiviral agents for enhanced ocular treatment. 2011
- 133.Mitra AK, Samanta SK. US20110105417. Drug conjugates. 2011
- 134.Vadlapudi AD, Vadlapatla RK, Kwatra D, Earla R, Samanta SK, Pal D, et al. Targeted lipid based drug conjugates: a novel strategy for drug delivery. Int J Pharmaceut. 2012;434(1-2):315–24. doi: 10.1016/j.ijpharm.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsu SD. US20120172423. Compositions and methods for treating herpes simplex virus. 2012
- 136.Williamson MP, McCormick TG, Nance CL, Shearer WT. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy. The Journal of allergy and clinical immunology. 2006;118(6):1369–74. doi: 10.1016/j.jaci.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 137.Song JM, Lee KH, Seong BL. Antiviral effect of catechins in green tea on influenza virus. Antiviral research. 2005;68(2):66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 138.Boyd MR. US20080287389. Nucleoside analogs in combination therapy of herpes simplex infections patent. 2008
- 139.Boyd MR. US20100323952. Nucleoside analogs in combination therapy of herpes simplex infections patent. 2010
- 140.Whitten K. US20110065655. Therapeutic composition to treat lesions caused by herpes simplex virus. 2011
- 141.Burrell JD. US20110229584. Compound for the control of herpes simplex virus using glycyrrhizic acid, lipoic acid, allantoin, and slippery elm. 2011
- 142.Kalman D, Bornmann W. US8268809. Kinase inhibitors for preventing or treating pathogen infection and method of use thereof. 2012
- 143.Qin W. EP1930002A1. Use of hydroxybenzoic acid ester and analogues for the manufacture of a mendicament for the prevention and treatment of virus infection patent. 2008
- 144.Tkachuk Z. US20120232129. Multiantivirus compound, composition and method for treatment of virus diseases. 2012
- 145.Cantin EM, Chandran R, Lundberg P. US20100040601. Compositions And Methods For Treating Herpes Simplex Virus Infections And Related Diseases patent. 2010
- 146.Yasin B, Pang M, Turner JS, Cho Y, Dinh NN, Waring AJ, et al. Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis. 2000;19(3):187–94. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]
- 147.Murakami T, Niwa M, Tokunaga F, Miyata T, Iwanaga S. Direct virus inactivation of tachyplesin I and its isopeptides from horseshoe crab hemocytes. Chemotherapy. 1991;37(5):327–34. doi: 10.1159/000238875. [DOI] [PubMed] [Google Scholar]
- 148.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19(3):491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mor A, Hani K, Nicolas P. The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J Biol Chem. 1994;269(50):31635–41. [PubMed] [Google Scholar]
- 150.Gifford JL, Hunter HN, Vogel HJ. Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell Mol Life Sci. 2005;62(22):2588–98. doi: 10.1007/s00018-005-5373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chan DI, Prenner EJ, Vogel HJ. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta. 2006;1758(9):1184–202. doi: 10.1016/j.bbamem.2006.04.006. [DOI] [PubMed] [Google Scholar]