Abstract
Dr. Bernard Brodie’s legacy is built on fundamental discoveries in pharmacology and drug metabolism that were then translated to the clinic to improve patient care. Similarly, the development of a novel class of therapeutics termed the soluble epoxide hydrolase (sEH) inhibitors was originally spurred by fundamental research exploring the biochemistry and physiology of the sEH. Here, we present an overview of the history and current state of research on epoxide hydrolases, specifically focusing on sEHs. In doing so, we start with the translational project studying the metabolism of the insect juvenile hormone mimic R-20458 [(E)-6,7-epoxy-1-(4-ethylphenoxy)-3,7-dimethyl-2-octene], which led to the identification of the mammalian sEH. Further investigation of this enzyme and its substrates, including the epoxyeicosatrienoic acids, led to insight into mechanisms of inflammation, chronic and neuropathic pain, angiogenesis, and other physiologic processes. This basic knowledge in turn led to the development of potent inhibitors of the sEH that are promising therapeutics for pain, hypertension, chronic obstructive pulmonary disorder, arthritis, and other disorders.
Introduction
Discovery comes in many colors and flavors. The discovery process is driven by multiple motivations ranging from agency goals to personal quests, with outcomes that can be labeled as basic or applied, fundamental or translational. Our field of xenobiotic metabolism is often seen as an applied field, yet it has repeatedly led to fundamental insight. The work of Dr. Bernard Brodie illustrates that our field was inspired by the need for improved therapeutics (Bickel, 1989; Costa et al., 1989). As improved therapeutics are delivered, we learn more about xenobiotic metabolism, with surprises along the way that provide basic insights into physiologic and biochemical mechanisms. This article follows the metabolic study of a xenobiotic that led to the discovery of an enzyme called the soluble epoxide hydrolase (sEH) (Gill et al., 1972; Morisseau and Hammock, 2013). sEH has proved to be key to the biology of a new group of chemical mediators termed the epoxy fatty acids (EpFAs) (Spector and Norris, 2007; Imig, 2012), which regulate surprisingly diverse aspects of biology and show therapeutic utility ranging from inducing anesthesia to combating inflammation and fibrillation (Table 1). Inhibitors of the sEH are valuable tools in the elucidation of the biologic processes regulated by EpFAs and have evolved to be potential pharmaceuticals (Shen and Hammock, 2012; Morisseau and Hammock, 2013). Exploiting knowledge on EpFAs for therapeutics has required continuing investigation into the metabolism of novel groups of compounds that inhibit the sEH or mimic the EpFA. In this article, a brief review of the history and current state of research on epoxide hydrolases is presented. However, this review focuses on the story of a translational project in xenobiotic metabolism that yielded a fundamental insight that was in turn translated into promising therapeutics (Morisseau and Hammock, 2013). In addition, an unexpected fundamental outcome of this work has been a deeper insight into the mechanism of chronic or neuropathic pain, leading in turn to a promising therapeutic for this often devastating problem.
TABLE 1.
Physiologic roles for EpFAs and sEH
Roles for EpFAs and sEHs have been studied in a number of disease states through the use of sEH inhibitors. References are not exhaustive but are intended to represent original discovery, reviews, or the most current literature.
| Organ System | Physiologic Consequence | Relevant Disease State | References |
|---|---|---|---|
| Circulatory system | Blood pressure regulation | Hypertension | Yu et al., 2000; Imig, 2012; Ulu et al., 2014 |
| Angiogenesis | Cancer, wound healing | Panigrahy et al., 2012; Zhang et al., 2013 | |
| Reduced protein aggregation | Heart failure | Despa et al., 2014 | |
| Reduced fibrosis | Cardiac hypertrophy | Sirish et al., 2013; Li et al., 2014 | |
| Peripheral nervous system | Reduced inflammatory pain | Acute pain | Inceoglu et al., 2006; Schmelzer et al., 2006; Wagner et al., 2011a |
| Reduced neuropathic pain | Chronic pain | Inceoglu et al., 2008; Wagner et al., 2011b, 2014b; Guedes et al., 2013 | |
| Respiratory system | Bronchodilation | Asthma | Lundström et al., 2011, 2012 |
| Chronic obstructive pulmonary disorder | Smith et al., 2005; Wang et al., 2012 | ||
| Pulmonary vascular shunting | Pokreisz et al., 2006; Townsley et al., 2010 | ||
| Kidney | Reduced fibrosis | Kidney failure | Kim et al., 2014 |
| Renal vascular function | Kidney failure | Zhao et al., 2004; Imig, 2012; Kujal et al., 2014 | |
| Liver | Tissue regeneration | Organ damage | Panigrahy et al., 2013 |
| ER stress | Organ damage | Bettaieb et al., 2013 | |
| Central nervous system | Reduced protein aggregation (prion) | Alzheimer’s | Poli et al., 2013 |
| Reduced nerve damage | Parkinsonism | Qin et al., 2014 | |
| Seizure resolution | Inceoglu et al., 2013; Hung et al., 2015 | ||
| Vasodilation | Stroke | Dorrance et al., 2005; Shaik et al., 2013 | |
| Adipose | Adipogenesis | Obesity | De Taeye et al., 2010; Zha et al., 2014 |
| Pancreas | Glucose regulation | Diabetes | Luo et al., 2010; Chen et al., 2013 |
| Tumors | Metastasis | Cancer | Panigrahy et al., 2012; Zhang et al., 2014b |
Although this discussion of the history of epoxide hydrolase discovery is brief, the knowledge on epoxide hydrolase is expansive. There are a number of more comprehensive discussions of epoxide hydrolases in general (Oesch, 1973; Brooks, 1977; Hammock et al., 1997; Arand et al., 2003; Morisseau and Hammock, 2005; Newman et al., 2005; Decker et al., 2009) and the sEH in particular (Harris and Hammock, 2013; Morisseau and Hammock, 2013). These reviews have been written with emphasis on different facets of sEHs, including their enzymatic mechanism (Morisseau and Hammock, 2005; Decker et al., 2009), methods for experimental analysis (Hammock et al., 1985; Wixtrom and Hammock, 1985; Morisseau and Hammock, 2007), and their role in lipid metabolism (Newman et al., 2005; Morisseau, 2013). Likewise, reviews specifically targeting sEH inhibitors (Morisseau and Hammock, 2005; Shen and Hammock, 2012) and enzyme gene structure (Decker et al., 2009; Harris and Hammock, 2013) are available. For more information on the physiologic role of sEHs in various disease states, please refer to reviews on their role in cardiovascular disease (Chiamvimonvat et al., 2007; Imig and Hammock, 2009; Imig, 2012), cancer (Norwood et al., 2010; Panigrahy et al., 2011), and pain (Wagner et al., 2011a, b) as well as citations in Table 1. Finally, for information on the biology associated with the EpFAs, other reviews have focused on their roles as lipid mediators (Spector et al., 2004; Newman et al., 2005; Spector and Norris, 2007; Zhang et al., 2014a; Spector and Kim, 2015).
A History of Epoxide Hydrolases
The discovery and history of mammalian epoxide hydrolases has been directly linked to the ability to synthesize and characterize the appropriate epoxide substrates. The earliest works on microsomal epoxide hydrolase (mEH or EH1, EC 3.3.2.9) were only possible because of simultaneous work done on metabolites of cyclodiene epoxides by Gerry Brooks (Brooks et al., 1970; Morisseau and Hammock, 2008) and arene epoxides in John Daly’s group (Jerina et al., 1968) (Fig. 1; Table 2). Likewise, identification of the sEH (EH2, EC 3.3.2.10) was based on studies by John Casida’s group (Fig. 2) characterizing the metabolism of the juvenile hormone mimic R-20458 [(E)-6,7-epoxy-1-(4-ethylphenoxy)-3,7-dimethyl-2-octene], which showed novel epoxide hydrolase activity in the soluble fraction of liver homogenates (Gill et al., 1972, 1974) (Fig. 3). The mEH and sEH show complementary substrate selectivity and appear to be the major epoxide hydrolases involved in hydrolysis of xenobiotic epoxides, whereas the sEH also appears to be critical for the hydrolysis of the EpFAs. Both the mEH and sEH belong in the superfamily of hydrolases termed α/β-fold hydrolases and share key structural similarities to two other poorly characterized enzymes discussed briefly below, called EH3 and EH4 (Decker et al., 2009). In addition to the epoxide hydrolases linked by genetic similarity, two other mammalian epoxide hydrolases have been identified based on substrate activity. Cholesterol epoxide hydrolase (ChEH) was identified as a unique microsomal epoxide hydrolase that hydrolyzed 5,6-cholesterol epoxide (Oesch et al., 1984; Sevanian and McLeod, 1986; Poirot and Silvente-Poirot, 2013), and leukotriene A4 hydrolase (not discussed here) was identified based on its selectivity toward leukotriene A4 (Maycock et al., 1982; Haeggström et al., 2007).
Fig. 1.
Pioneers in the discovery of mEH. Gerry Brooks (top left) studied the metabolism of cyclodiene insecticides, resulting in the first publication characterizing an epoxide hydrolase found in microsomes. Franz Oesch (bottom left) and Donald Jerina (bottom right), both led by John Daly (top right), studied the metabolism of aromatic hydrocarbons leading to detailed characterization of the mEH.
TABLE 2.
Experimentally important substrates of epoxide hydrolases
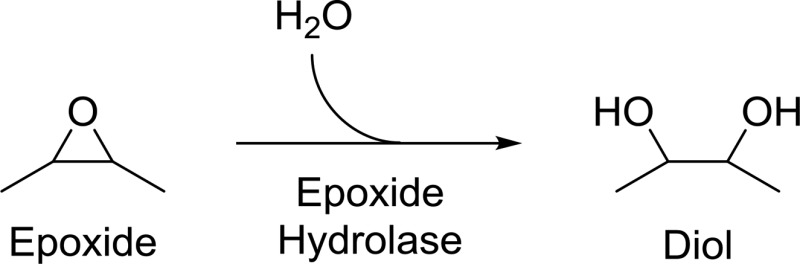
Epoxide hydrolases convert the 3-membered cycle ether of the epoxide in a downhill reaction adding water to generate a 1,2- or vicinal diol. Historically, a number of substrates have been experimentally important for both characterizing epoxide hydrolases and studying their physiologic importance. Kinetic studies using these substrates have primarily been performed only in a few species, so care must be taken when generalizing results across species.
| Name | Structure | Primary Hydrolyzing Enzyme |
|---|---|---|
| Styrene oxide | 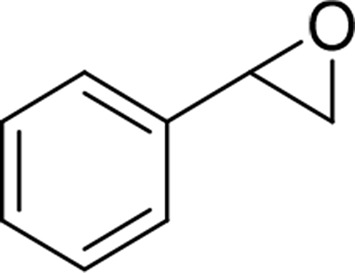 |
mEH and sEH |
| trans-β-ethyl-styrene oxide | 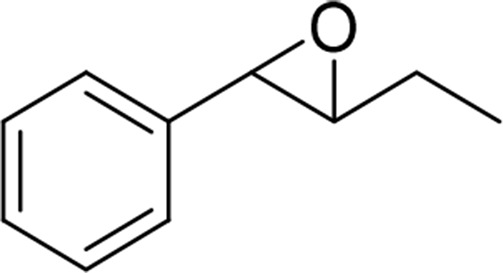 |
sEH |
| Allylbenzene oxide | 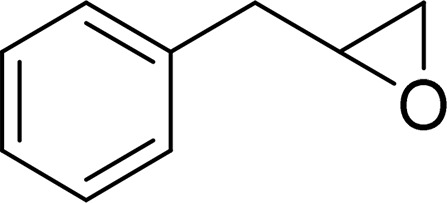 |
mEH and sEH |
| cis-Stilbene oxide (CSO) | 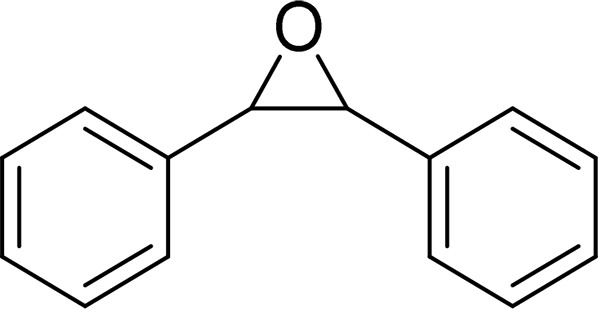 |
mEH |
| trans-Stilbene oxide (TSO) | 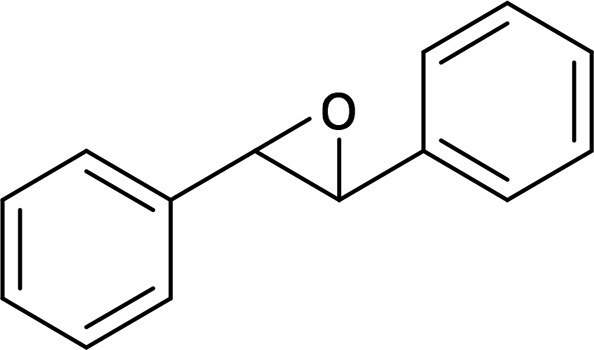 |
sEH |
| trans-Diphenylpropene oxide (t-DPPO) | 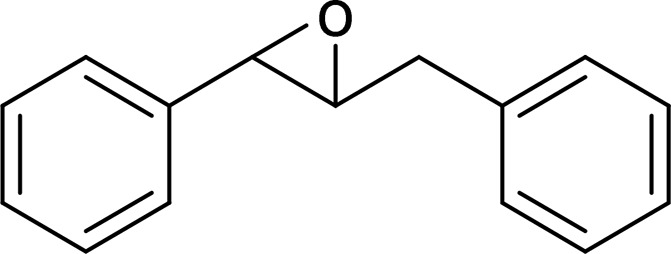 |
sEH |
| Cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl] carbonate (CMNPO) | 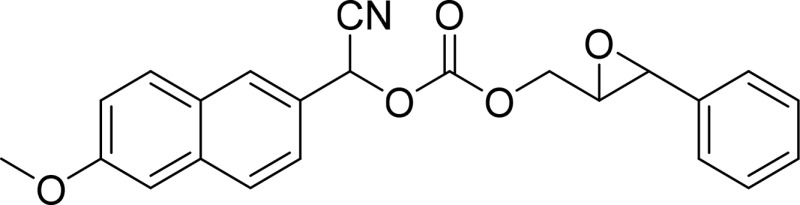 |
sEH |
| Leukotoxin | 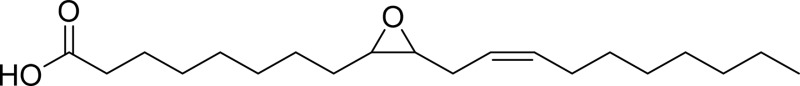 |
sEH |
| 11,12- EET |  |
sEH |
| 19,20-EpDPE | 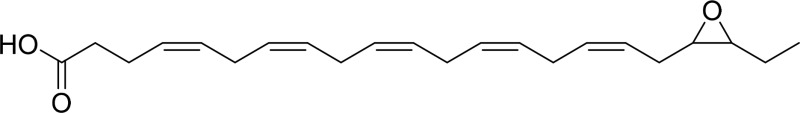 |
sEH |
| Juvenile hormone III (JH3) | 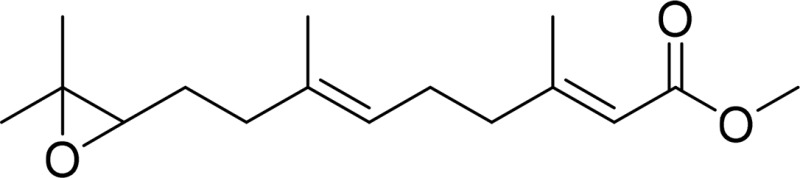 |
Juvenile hormone epoxide hydrolase sEH |
| 5,6-Cholesterol epoxide | 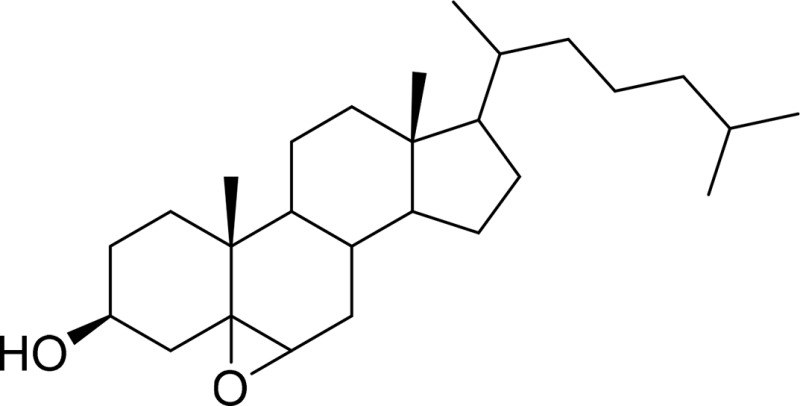 |
ChEH |
Fig. 2.
Early discovery of the sEH. Under the guidance of John Casida (right), Sarjeet Gill (left) and Bruce Hammock found a novel epoxide hydrolase in the soluble fraction of liver homogenate that hydrolyzed R-20458.
Fig. 3.
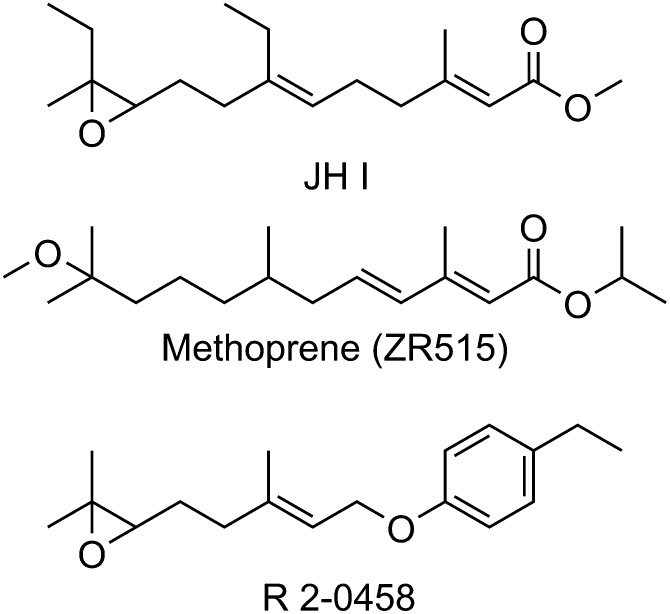
Juvenoid hormone and juvenile hormone mimics methoprene and R-20458. A study of the insect hormone led to commercial hormone juvenile mimics such as methoprene and to the discovery of the sEH during the study of the juvenoid hormone mimic R-20458. JHI, juvenoid hormone.
Identification of the Microsomal Epoxide Hydrolase Involved with Xenobiotic Metabolism.
The first report of an epoxide hydrolase was published in 1970, when Gerry Brooks was working on the metabolism of insecticides in mammals and insects. At the time, he knew that oxidation of cyclodiene insecticides produced stable and highly toxic epoxides, and it had been demonstrated that hydration of the epoxide of dieldrin could occur in vivo. Jerina et al. (1968) had previously shown that hydration of 1,2-naphthalene oxide occurred in the rat liver microsomes; however, characterization of the enzyme responsible (mEH) had not yet been performed. In his seminal article, Brooks showed that hydration of the chlorinated cyclodiene epoxides occurred through microsomal enzymes (Brooks et al., 1970). This in turn led to Brooks’ development of the hypothesis that inhibiting mEHs could lead to synergism (Brooks, 1973; Morisseau and Hammock, 2008). Epoxide-containing cyclodiene insecticides were first used in his work. However, the rates of hydration were so slow for these insecticides with hindered epoxides that they were of marginal value for characterizing the enzyme. Brooks and his team then introduced a less hindered and biodegradable cyclodiene epoxide. This substrate, 1,2,3,4,9,9-hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-1,4-methanonaphthalene (HEOM), was hydrolyzed more quickly by the mEH and could be detected by gas-liquid chromatography, allowing characterization of the mEH and development of the first inhibitors, including the noncompetitive inhibitor 1,1,1-trichloropropane-2,3-epoxide (Brooks, 1973).
While Brooks’ characterized the epoxide hydrolysis in chlorinated insecticides, Daly worked with Jerina and Oesch to determine the metabolism of aromatic hydrocarbons. For Brooks, discovery of the mEH was slowed by the fact that the epoxides he studied were exceptionally stable. By contrast, the work of the National Institutes of Health group under Daly was hindered by the aromatic epoxides being ephemeral in aqueous environments. During this period, Jerina identified and worked out the mechanism for the “NIH shift,” the intramolecular migration of a hydrogen atom upon hydroxylation (Jerina et al., 1967, 1968). At the same time, he demonstrated conversion of arene oxides to a series of metabolites including premercapturic derivatives and dihydrodiols (Jerina et al., 1968). Several researchers had suggested the formation of dihydroxy metabolites from naphthalene or similar aromatic compounds (Young, 1947; Smith et al., 1950); however, Jerina et al. (1968) were the first to suggest an epoxide intermediate and the enzymatic conversion of epoxides to diols. Jerina would later extend his studies to benzo[a]pyrene, synthesizing all of the potential metabolites to determine which intermediate was the ultimate carcinogen. His work resulted in the identification of diol epoxides as highly mutagenic metabolites (Levin et al., 1976) and the development of the bay region theory, which identified the metabolites that had diol epoxides in the “bay region” of the molecule as those with increased chemical reactivity resulting in the highest mutagenic potency (Lehr et al., 1985).
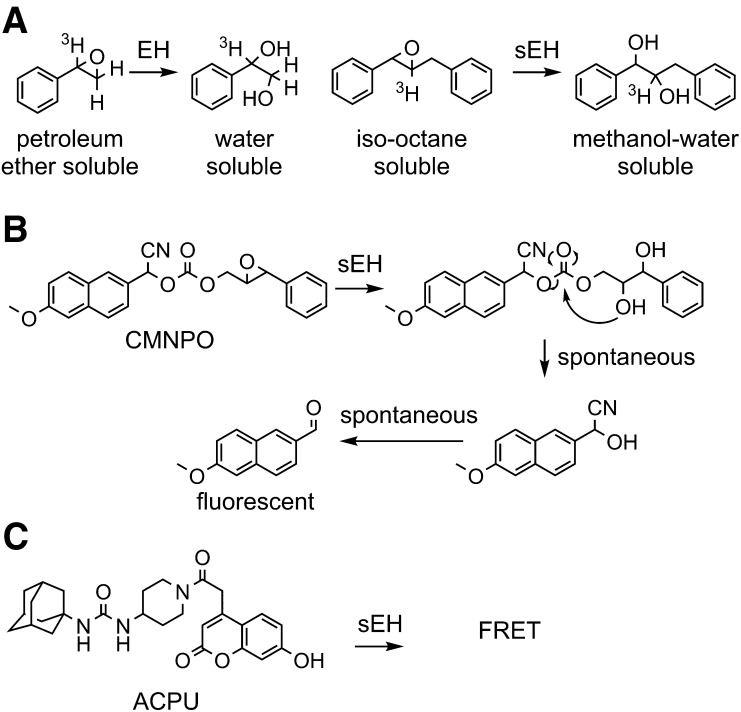
While Jerina worked out the mechanism of toxicity for the arene epoxides, Oesch performed the early work on identifying a series of substrates and inhibitors (Oesch et al., 1971b; Oesch, 1973; Oesch et al., 1973). Just as Brooks’ success relied on developing a rapid gas-liquid chromatography–based mEH assay with HEOM, Oesch’s success was largely due to the synthesis of a radiolabeled styrene oxide, which turned out to be an excellent substrate for an epoxide hydrolase in the microsomal fraction (Oesch et al., 1971a). Although this epoxide of styrene was relatively volatile, tended to polymerize, reacted with thiols, and was quite unstable at neutral and acidic pHs, it was far easier to use for routine experiments than arene oxides. Oesch took advantage of the difference between the petroleum ether–soluble styrene oxide and the water-soluble diol product to develop a simple assay for the mEH (Fig. 4A) that has since advanced extensive studies on this enzyme (Oesch and Daly, 1971). Unfortunately, the relatively basic pH that was optimum for the mEH delayed the discovery of epoxide hydrolase activity in the soluble fraction, as did inhibition of sEH by styrene oxide.
Fig. 4.
Methods for measuring epoxide hydrolase activity. Several substrates have been generated for rapid analysis of epoxide hydrolase enzymes and more sophisticated kinetic treatments. (A) The radioactive [7-3H] styrene oxide developed by Oesch et al. (1971a) used water solubility of the diol to monitor the reaction kinetics, and a similar partition method was used for trans-β-ethyl styrene oxide (Table 2) (Mullin and Hammock, 1980), trans-stilbene oxide (Table 2) (Gill et al., 1983), and trans-diphenylpropene oxide (Borhan et al., 1995). (B) More recent substrates rely on cyclization of the epoxide hydration product to release a chromophore. In the case of CMNPO, the prochromophore is a nonfluorescent cyanohydrin that spontaneously hydrolyzes to yield CN−, for which there are sensitive reagents, and an intensely fluorescent, red-shifted methoxynapthaldehyde (Jones et al., 2005). (C) There are a variety of techniques for high-throughput analysis of binding of a fluorescent molecule, such as ACPU designed for the sEH. In this case, it was used to determine a Ki value for the enzyme and a kinetic off rate to give an estimation of substrate occupancy of the catalytic site (Lee et al., 2013). ACPU, 1-(adamantan-1-yl)-3-(1-(2-(7-hydroxy-2-oxo-2H-chromen-4-yl)acetyl)piperidin-4-yl)urea; CMNPO, cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl] carbonate; EH, epoxide hydrolase; FRET, Förster resonance energy transfer.
Development of Juvenile Hormone Analogs or Juvenoids.
The cyclodiene insecticides studied by Brooks proved valuable and were widely used, but many of them were broad spectrum, persistent, and toxic environmental contaminants. The publication of Silent Spring by Rachel Carson led to a search for environmentally softer pesticides that avoided the environmental problems of persistent organochlorines and the off-target toxicity of organophosphates (Carson, 1962). Carroll Williams coined the term “third generation pesticides” as materials meeting this goal and urged development of mimics of juvenoid hormone, the insect terpenoid hormone controlling, among other things, growth and metamorphosis (Williams, 1967) (Fig. 3). Like the mammalian EpFAs, these juvenile hormones (Fig. 3) were too expensive and unstable to use effectively as insecticides. The epoxide of juvenile hormone was seen as a liability by some groups, and it was mimicked by a variety of pharmacophores, including amides, aziridines, esters, ureas, and alkoxides (Henrick et al., 1973; Hammock et al., 1974), in two of the early scaffolds developed. One of these alkoxide mimics from Zoecon Corp. (now Wellmark), ZR 515 [1-methylethyl (E,E)-11- methoxy-3,7,11-trimethyl-2,4-dodecadienoate] or methoprene, continues to be a major agent for the control of fleas, mosquitoes, and other insects. Another mimic from Stauffer Chemical Company retained the epoxide. It was during the study of this insect growth regulator that the sEH was discovered (Gill et al., 1972; Hammock and Quistad, 1976).
Discovery of the sEH.
In the early 1970s, John Casida assigned two students, Sarjeet Gill and Bruce Hammock, to examine the metabolism of the Stauffer juvenile hormone mimic or juvenoid R-20458. After its radiosynthesis, the juvenoid was found to be metabolized in insects in part by an microsomal epoxide hydrolase that later became known as the juvenile hormone epoxide hydrolase. It has since been found that insect juvenile hormones are regulated as much by degradation as by biosynthesis (Hammock, 1985), an observation that was important in later studies on regulation of EpFA in mammals. This and work on the metabolism of the natural insect hormones led to synthesis of many compounds with epoxide mimics. One of these, as previously mentioned, remains as the commercial product termed methoprene (Fig. 3). Lessons from these isosteres are now being used to generate mimics of EpFAs.
As one would anticipate, the mammalian oxidative metabolism in vivo and in vitro of R-20458 led to numerous metabolites generated from combinations of ω hydroxylation, allylic hydroxylation, cyclization, and other reactions. However, in liver homogenate preparations lacking NADPH, a single diol metabolite was found in the microsomal fraction and largely in the soluble fraction of liver homogenates (Gill et al., 1972). Since epoxide hydration was thought to be due to one enzyme found in the microsomal fraction (Oesch and Daly, 1972), it was surprising to find that most of this catalysis was in the 100,000g soluble fraction. In fact, it was later discovered that the small amount of hydrolysis of R-20458 in the microsomal fraction was actually due to traces of the sEH bound to the endoplasmic reticulum (ER) (Guenthner et al., 1981). In the end, it was the resistance of R-20458 to glutathione conjugation and high selectivity of sEH for this trisubstituted epoxide that led to the discovery of a new epoxide hydrolase distinct from mEH.
The reasons that sEH was previously overlooked are covered in several articles (Ota and Hammock, 1980; Morisseau and Hammock, 2008). Gill, Hammock, and Casida assumed incorrectly that the sEH had been overlooked because it showed such a high preference for trisubstituted epoxides as substrates (Gill et al., 1974; Hammock et al., 1976). This was based in part on careful reading of articles from E.J. Corey’s laboratory at Harvard University, which provided evidence for an enzyme hydrolyzing trisubstituted squalene oxides and related compounds (Dean et al., 1967). On the basis of this work, it could be argued that the Corey laboratory actually first reported the sEH in S-9 preparations, but further observations on the sEH from Harvard University ended when the group switched to pure microsomal preparations (Morisseau and Hammock, 2008). It was not until the work of Mumby and Hammock (1979) that it was observed that the sEH actually turns over most monosubstituted epoxides faster than the mEH and seems to prefer cis and trans-1,2-disubstituted epoxides as substrates over trisubstituted and tetrasubstituted epoxides. Ota and Hammock (1980) found the discrepancy was not from errors in the early work on the mEH, where the enzyme in rat liver was found with cyclic epoxides and styrene oxide as substrates and a basic pH to be microsomal, but instead from errors in later reviews that extrapolated from the original data on mEH to multiple species, substrates and assay conditions (Oesch and Daly, 1972; Gill and Hammock, 1980; Hammock et al., 1980a; Ota and Hammock, 1980). Early studies used the rat liver as a model and the laboratory rat has by far the lowest level of hepatic sEH of any mammalian species studied (Gill and Hammock, 1980; Fornage et al., 2002). The sEH does not hydrolyze epoxides on cyclic systems (Moghaddam et al., 1997) and thus would not have been seen with these substrates. Although the sEH turns over styrene oxide rapidly, it is actually inhibited by the substrate and also would have been overlooked using this tool. Ironically, closely related and more chemically stable substrates such as allyl benzene oxide or trans-β-ethyl-styrene oxide are rapidly metabolized by the sEH (Table 2) (Ota and Hammock, 1980). Since the mEH activity is optimum at basic pH and such a pH is needed to increase stability of styrene oxide, these conditions were commonly used. Inadvertently, these conditions minimize the relative activity of the sEH. Simplistically, both the sEH and mEH have broad and partially overlapping substrate selectivities, with the mEH preferring epoxides on cyclic systems and monosubstituted epoxides and the sEH preferring 1,2-disubstituted epoxides.
A Second Mammalian Microsomal Epoxide Hydrolase: ChEH.
The biology and chemistry of ChEH was previously reviewed (Silvente-Poirot and Poirot, 2012; Poirot and Silvente-Poirot, 2013), so the history of ChEH is described here only briefly. Before the identification of ChEH, 5,6-cholesterol epoxide had been shown to be enzymatically converted to cholestanetriol by several groups (Black and Lenger, 1979; Sevanian et al., 1980; Watabe et al., 1980). The enzyme responsible for 5,6-cholesterol epoxide hydrolysis was eventually identified as a microsomal EH that was determined to be distinct from mEH based on antigenicity (Oesch et al., 1984) and inhibitor selectivity (Sevanian and McLeod, 1986). Despite many studies characterizing enzymatic activity and tissue distribution (Aström et al., 1986) of ChEH, only recently has the molecular entity been characterized. Surprisingly, the molecular entity responsible for the ChEH activity was in fact the antiestrogen binding site, a multidomain protein that has high affinity for the pharmaceutical tamoxifen, which is composed of two enzymes, 3β-hydroxysterol-Δ7-Δ8-isomerase and 3β-hydroxysterol-Δ7-reductase, involved in cholesterol synthesis (de Medina et al., 2010). The Ki for tamoxifen inhibition of ChEH is 34 nM. Since effective doses of tamoxifen are with the range of ChEH inhibition, cholesterol epoxides could potentially contribute to the biological activity of tamoxifen.
For years, it had been believed that 5,6-cholesterol epoxides had a role in carcinogenesis (Petrakis, 1986; Poirot and Silvente-Poirot, 2013). Although early studies indicated that 5,6-cholesterol epoxide binds covalently to nucleophilic DNA (Blackburn et al., 1979; Sevanian and Peterson, 1984), 5,6-cholesterol epoxide is relatively unreactive with nucleophiles, only forming adducts with mercaptoethanol and aminoethanol under catalytic conditions (Paillasse et al., 2012). Despite this lack of chemical reactivity, an undescribed enzyme was found to catalyze the addition of histamine to 5,6α-cholesterol epoxide, resulting in Dendrogenin A (DDA). This compound is a potent tumor suppressor that induces cell differentiation and is also a potent, selective inhibitor of ChEH (de Medina et al., 2009, 2013). DDA has been found in mammalian tissue with levels significantly reduced in breast cancer tissues. It was shown to significantly enhance survival time of mice implanted with tumors (de Medina et al., 2013). Given that nondiol downstream metabolites of 5,6α-cholesterol epoxide have been implicated in enhanced tumor survival, it has been suggested that tamoxifen and DDA could both reduce cancer severity through a common mechanism of ChEH inhibition (de Medina et al., 2013). Targeting ChEH may therefore represent a new therapeutic approach with similar efficacy to tamoxifen but with reduced off-target effects.
EpFAs Are Chemical Mediators and Endogenous Substrates of sEH
Based on the distribution and properties of sEH, Gill and Hammock correctly hypothesized that it had an endogenous role in regulating chemical mediators. Early on, they thought that it might be a scavenger enzyme that hydrolyzed terpene epoxides such as squalene dioxide and lanosterol epoxide (Dean et al., 1967; Hammock et al., 1980b). Involvement of sEH in these reactions has not ruled out, but the discovery by Mumby and Hammock (1979) that 1,2-disubstituted epoxides were turned over far faster than trisubstituted and tetrasubstituted epoxides led to the hypothesis that fatty acid oxides were endogenous substrates, which was quickly confirmed by Gill and Hammock (1979). Furthermore, Chacos et al. (1983) demonstrated the epoxyeicosatrienoic acids (EETs), epoxides of arachidonic acid, were substrates as well. Later work would identify epoxides of lineolate, termed leukotoxins, as substrates that become bioactivated to inflammatory diols by the sEH (Moghaddam et al., 1997). Although sEH inhibition is primarily discussed in the context of biologically active EpFAs, the leukotoxin-diols remain important toxic mediators for acute respiratory distress syndrome and possibly endogenous mediators regulating vascular permeability and inflammation (Zheng et al., 2001).
There were hints of biologic roles for EpFAs in the literature for years; however, technical issues, including the wide abundance of the sEH in tissues, rapid metabolism of EpFAs by sEH, the general lack of availability of these epoxides, and dearth of good analytical methods, slowed the field. However, critical breakthroughs toward understanding the biologic role of EpFAs were made after the development of sEH inhibitors. There were multiple attempts to develop potent inhibitors of the sEH as well as the mEH, with most inhibitors being either alternate substrates or substrates that were slowly turned over (Morisseau and Hammock, 2005). However, the discovery that carbamates, amides, and ureas as linear and heterocyclic systems are competitive transition state inhibitors of the enzyme led to compounds that were generally useful for demonstrating the biologic effects of EpFAs in vivo (Morisseau et al., 1999; Kim et al., 2004, 2005). These inhibitors, combined with the later development of knockout animals, fostered multiple discoveries regarding the diverse biologic actions of EpFAs and led to the general appreciation that there is a third cytochrome P450 (P450) branch of the arachidonic acid cascade.
Development of Transition State Competitive Inhibitors of the sEH
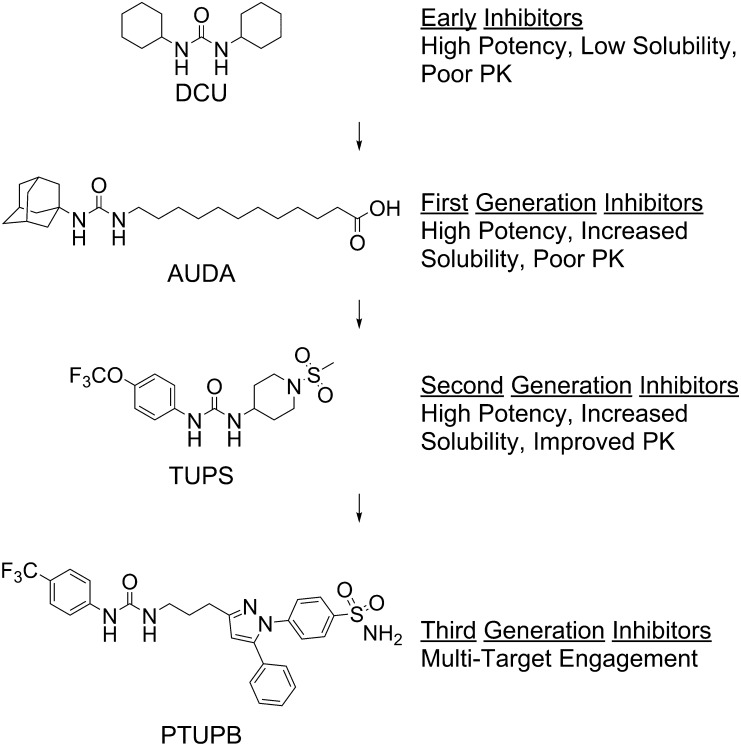
Early noncompetitive inhibitors of the sEH were too unstable for effective use in vivo (Mullin and Hammock, 1982). The first potent competitive inhibitors of the sEH included materials such as the industrial byproduct dicyclohexylurea (Fig. 5) and a variety of diphenyl ureas (Morisseau et al., 1999). Although they are quite potent, these and related compounds like 1-cyclohexyl-3-dodecyl urea were difficult to use in vivo and, as expected of lipophilic high-melting solids, difficult to formulate. However, the availability of inhibitors allowed for crystal structures demonstrating hydrogen bonding between urea inhibitors and the enzyme active site residues (Argiriadi et al., 2000), confirming previous kinetic evidence demonstrating competitive inhibition determined using the high-affinity model substrate trans-diphenylpropene oxide (Morisseau et al., 1999). In a latter study, Morisseau et al. (2010) determined the Vm, Km, and kcat of several EpFAs and found that, like trans-diphenylpropene oxide, they have a high specificity ratio (Vm/Km). This work indicates that high target occupancy of an inhibitor will be critical for effective inhibition of the enzyme in vivo. An advance was made when 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) was prepared as a mimic of the 14,15-epoxide of arachidonic acid. Within the molecule, a dodecanoic acid was used to mimic the α end of arachidonic acid, a 1,3-urea was used to mimic the epoxide and its transition state, and an adamantane was used to mimic the ω hydrophobic tail (Morisseau et al., 2002). The adamantane was a fortunate selection, since it provided high sensitivity for detection on liquid chromatography/mass spectrometry, so that only a few microliters of blood were required to follow in vivo levels and thus allowed for optimization of future structures for pharmacokinetics (PK)–absorption, distribution, metabolism, and excretion (ADME) (Watanabe et al., 2006). AUDA remains among the most potent sEH inhibitors with significantly greater potency than the Investigational New Drug (IND) candidate AR9281 [1-(1-acetyl-piperidin-4-yl)-3-adamantan-1-yl-urea] described below. However, its physical properties and rapid metabolism make it challenging to use. In addition, its activity as a moderate peroxisome proliferator-activated receptor-α agonist (Fang et al., 2005) and as a reasonably potent mimic of EETs (Olearczyk et al., 2006) has made it experimentally unfavorable.
Fig. 5.
Evolution of sEH inhibitors. Over the past decade, sEH inhibitors have evolved with increased potency, increased PK-ADME, and multiple target engagement. From this development, AUDA has been investigated in human clinical trials (Tran et al., 2012) and second-generation inhibitors have been optimized to IND candidates. PTUPB, 4-(5-phenyl-3-{3-[3-(4-trifluoromethyl-phenyl)-ureido]-propyl}-pyrazol-1-yl)benzenesulfonamide; TUPS, 1-(1-methanesulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)urea.
Subsequent work in this and other series of compounds has led to compounds of improved physical properties but also improved potency and PK-ADME (Morisseau and Hammock, 2013). Generally, most of these compounds retain the urea pharmacophore; however, several contain carbamate or amide groups that form strong transition state interactions with the enzyme active site. Given similarities in the sEH active site and the active sites of other α/β-fold enzymes, it seems plausible that other α/β-fold hydrolase inhibitors, including inhibitors of fatty acid amide hydrolase, may inhibit sEH. Although physical properties have improved slightly, many of the inhibitors have high melting points and low water solubilities. Generally, increasing water solubility and decreasing the melting point of sEH inhibitors are important goals. However, it is relatively easy to formulate water-soluble materials regardless of melting point; even with increasing lipophilicity, formulation remains relatively easy as long as the melting point is low or the potency is high. However, for these high-melting-point lipophilic compounds, effective biologic experiments have relied on careful formulation. Fortunately, as one improves potency and PK-ADME, the required dose drops and formulation are simplified (Shen and Hammock, 2012). The development of high-throughput techniques for measuring potency has been necessary for testing structure-activity relationships to optimize potency, solubility, and melting point. The availability of recombinant enzymes and a high-turnover fluorescent-based substrate [cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl] carbonate] synthesized by Jones et al. (2005) has allowed for the development of rapid assays for testing inhibitor potency (Fig. 4B). More recently, inhibitors have also been tested using a fluorescent probe [1-(adamantan-1-yl)-3-(1-(2-(7-hydroxy-2-oxo-2H-chromen-4-yl)acetyl)piperidin-4-yl)urea] that engages in Förster resonance energy transfer with neighboring tryptophan residues in the sEH active site (Fig. 4C). This tool decreases the lower limit for determining potency and can be used for measuring koff, the rate at which inhibitors leave the active site (Lee et al., 2013, 2014). High enzyme target occupancy, as determined by koff, appears particularly important in predicting the in vivo efficacy of sEH inhibitors because the inhibitors are competing with low abundance, but biologically powerful substrates with high affinity for the enzyme (Morisseau et al., 1999; Schebb et al., 2011).” Along with increased potency, the PK has shifted from highly aliphatic and quickly metabolized inhibitors to compounds with long half-lives in a variety of species, including dogs and nonhuman primates (Tsai et al., 2010; Ulu et al., 2012). Inhibitors have also evolved for multitarget engagement toward cyclooxygenase (COX) (Hwang et al., 2011) and lipoxygenase (Meirer et al., 2013). Dual sEH/COX inhibition results in synergism that dramatically improves the potency of compounds (Zhang et al., 2014b). In the process of optimizing the medicinal chemistry, a number of these compounds have been evaluated as clinical candidates (Fig. 6).
Fig. 6.
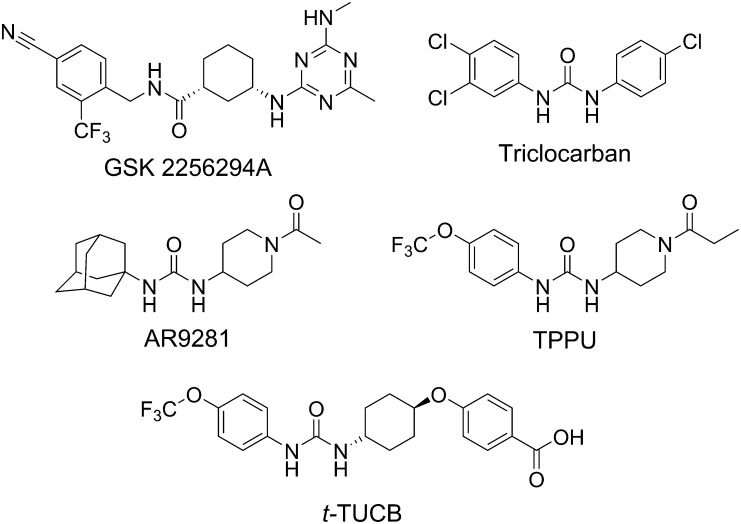
IND and development candidates among sEH inhibitors. GSK2256294 is a potent inhibitor that has demonstrated efficacy in vivo and is in the clinic (Podolin et al., 2013). Triclocarban is a commonly used antimicrobial that also inhibits the human sEH (Schebb et al., 2011) and has proved successful as a topical analgesic when used with diclofenac in a double-blind clinical trial for diabetic neuropathic pain. AR9281 (UC11A53, APAU), originally reported by Jones et al. (2006), was taken through phase 2A for hypertension and metabolic syndrome (Anandan et al., 2011). AR9281 is more potent on the rat recombinant sEH than the human sEH, has poor target occupancy of the human sEH, and has a short half-life due to rapid P450 metabolism of the adamantane moiety. TPPU (UC1770) shows high potency in rodent and primate species and has been provided to many laboratories as a model sEH inhibitor (Ulu et al., 2012). t-TUCB (UC1728) is in trials for the neuropathic pain of equine laminitis (Guedes et al., 2013) and is being evaluated for canine arthritis and feline joint pain. TPPU, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea; t-TUCB, trans-4-{4-[3-(4-trifluoromethyoxy-phenyl)-ureido]-cyclohexyloxy}benzoic acid.
Discovering Physiologic Roles for EpFAs
Although the role of sEH in EpFA hydrolysis was discovered relatively early, it has not been until the past 15 years that a physiologic role for this enzyme has been generally realized. EET generated by oxidation of arachidonic acid was previously shown to regulate vasodilation (Carroll et al., 1987) and Ca2+ uptake (Kutsky et al., 1983). Although other metabolic pathways have been described, including β-oxidation, chain elongation, and glutathione conjugation, the contribution of these pathways is minor except when sEH is inhibited. For example, the specific activity for glutathione S-transferase on enzymatic conjugation of EET is approximately 100-fold less than hydration of EET by sEH. (Spearman et al., 1985; Morisseau et al., 2010). Thus, in tissues in which expression of these two enzymes is comparable, glutathione conjugates are significantly less abundant than diol metabolites. Yu et al. (2000) were the first to show that inhibition of sEH could regulate a biologic process by demonstrating that sEH inhibitors caused a marked reduction in hypertension. Since then, sEH inhibition has been investigated in numerous disease states in multiple organ systems (Table 1). Generally, a few physiologic mechanisms, including resolution of inflammation, vasodilation, and enhanced tissue repair, account for most of the biologic activities observed; however, there are several exceptions. Increasingly, reduction of and resistance to reactive oxygen species–induced ER stress appears to be an underlying mechanism (Bettaieb et al., 2013).
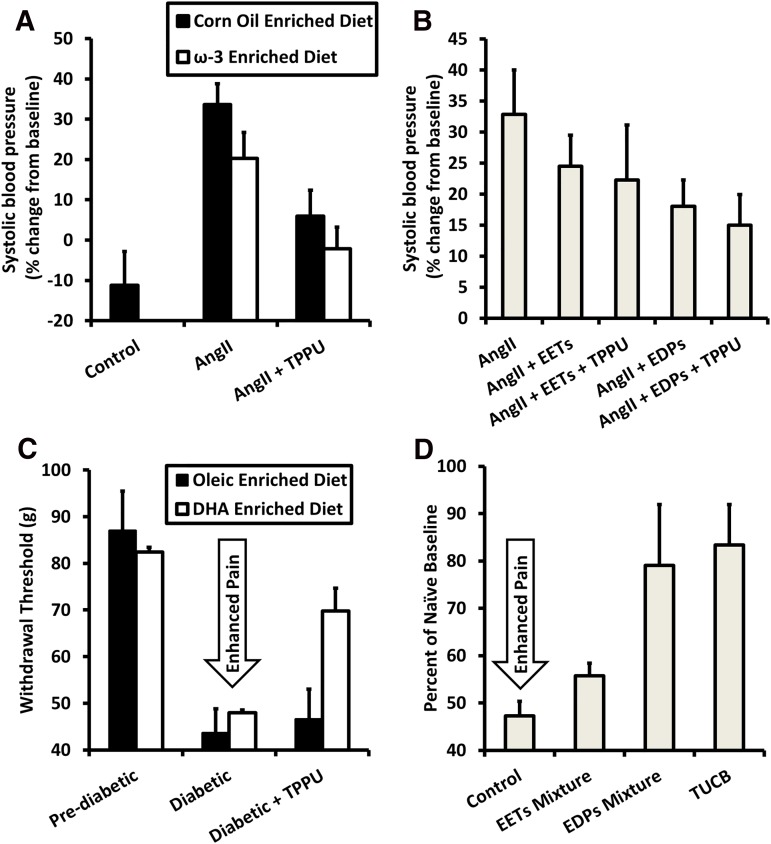
Several of the effects seen with EETs have been observed with the epoxide derivatives of ω-3 fatty acids, including epoxyeicosatetraenoic acid, an epoxide of eicosapentaenoic acid, and epoxydocosapentaenoic acid (EpDPE), an epoxide of docosahexaenoic acid (Morisseau et al., 2010; Ulu et al., 2014). In the case of angiotensin II (AngII)–induced hypertension, the connection between an increase in EpDPEs caused by dietary ω-3 fatty acid consumption and sEH inhibitor treatment has been clearly demonstrated. When directly comparing the effects of the EpDPEs against EETs with coadministration of the sEH inhibitor, EpDPEs are more efficacious at reducing AngII-induced hypertension (Fig. 7B). If the amount of EpDPEs generated relative to EETs is modified by increased ω-3 fatty acids in the diet, the effects of the sEH inhibitor are improved. By coadministering the ω-3 fatty acid diet with sEH inhibitors, the dose of sEH inhibitors needed may be reduced (Fig. 7A). However, this also emphasizes the need for understanding diet–drug interactions to reduce unwanted side effects.
Fig. 7.
Efficacy of sEH inhibitors may be improved by supplementing ω-3 fatty acids in the diet by producing EpDPEs. (A) Either sEH inhibition or an ω-3–rich diet alone partially reduce increase in blood pressure 7 days after AngII injection, while giving both simultaneously reduces blood pressure close to control levels. Data are replotted from Ulu et al. (2013). (B) EpDPEs are likely the metabolites responsible for the improved efficacy of simultaneous administration of the ω-3 diet and sEH inhibitors. Slow release of EpDPEs into the bloodstream achieved by surgical implantation of osmotic minipumps resulted in decreased blood pressure 6 days after AngII injection and increasing half-life of EpDPEs by inhibiting sEH increased efficacy. By contrast, EETs with sEH inhibitors were not as potent toward reducing blood pressure. Data are replotted from Ulu et al. (2014). (C) In a rat model of streptozocin-induced diabetic neuropathy, an ω-3–rich diet had minimal effect at increasing pain thresholds relative to those on an oleic oil–based diet. However, when administering subtherapeutic levels (0.3 mg/kg) of sEH inhibitors, rats fed ω-3–rich food had an increased threshold relative to those on a control diet. Data are replotted from Wagner et al. (2014a). (D) As seen in hypertension, EpDPEs are likely responsible for the improved efficacy when both an ω-3 diet and sEH inhibitors are used to treat diabetic neuropathy. EpDPEs administered by intraperitoneal injection (1 mg/kg) in a mouse model of diabetic neuropathy were more efficacious at reducing pain than EETs and had a response similar to the sEH inhibitor t-TUCB (10 mg/kg). Data are replotted from Wagner et al. (2014a). DHA, docosahexaenoic acid; EDP, Epoxydocosapentaenoic Acid; TPPU, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea; t-TUCB, trans-4-{4-[3-(4-trifluoromethyoxy-phenyl)-ureido]-cyclohexyloxy}benzoic acid.
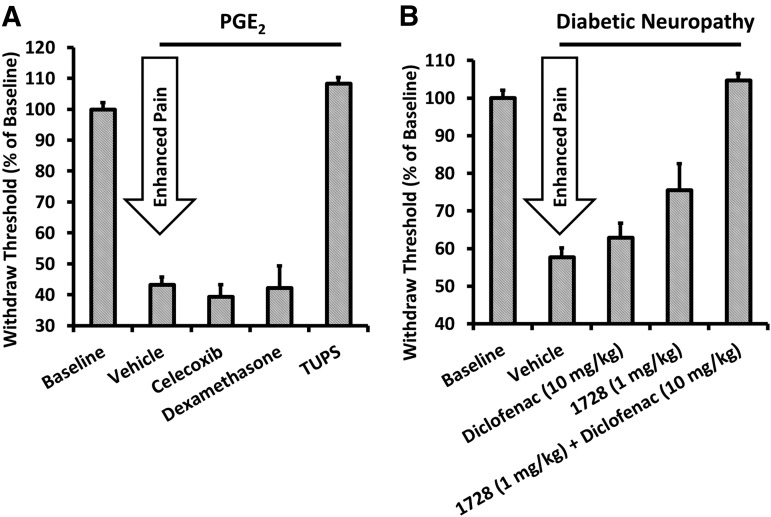
One of the most interesting and exciting, but still poorly understood, therapeutic applications for sEH inhibitors is the regulation of pain. Early on, it was found that inhibition of sEH could prevent acute inflammation (Schmelzer et al., 2005). As expected, the reduction in inflammation through sEH inhibition led to analgesia from inflammatory pain (Inceoglu et al., 2006). Reduced inflammatory pain is observed even when pain is induced by prostaglandin E2, indicating that anti-inflammation occurs downstream or independently of COX signaling (Fig. 8A) (Inceoglu et al., 2011). Unexpectedly, other forms of pain that are not inflammation based, such as traumatic nerve injury or diabetic neuropathic pain, have also been prevented by sEH inhibition (Inceoglu et al., 2012). Neuropathic pain has been traditionally difficult to treat because of the low efficacy of nonsteroidal anti-inflammatory drugs (NSAIDs) and undesirable side effects of other therapies including morphine and gabapentin (Inceoglu et al., 2012). Since there are only three Food and Drug Administration–approved therapeutics for neuropathic pain and no therapy fully treats neuropathic pain, development of an sEH inhibitor for neuropathic pain would serve an unmet medical need. Many NSAIDs, including diclofenac, sometimes can enhance neuropathic pain. Interestingly, the sEH inhibitors synergize with diclofenac to reduce pain in a streptozocin-induced model of diabetic neuropathy, indicating that dual COX/sEH inhibition may prove to be an effective strategy for reducing neuropathic pain while keeping side effects low (Fig. 8B) (Hammock et al., 2011). In addition to NSAIDs, the sEH inhibitors synergize with phosphodiesterase inhibitors (Inceoglu et al., 2011), which may be an alternative approach toward improving potency. The efficacy of EETs in the presence of increased cAMP, in part, explains the analgesic efficacy of sEH inhibitors in the presence, but not the absence, of pain. The mechanisms by which sEH inhibitors reduce neuropathic pain are still being investigated; however, components of cannabinoid (Wagner et al., 2011b), opioid (Terashvili et al., 2008; Conroy et al., 2010), and neurosteroid signaling (Wang et al., 2006; Inceoglu et al., 2008) have been implicated in the pain-relieving effects of EETs and other EpFA.
Fig. 8.
sEH inhibitors reduce pain downstream of COX metabolism and can synergize with COX inhibitors. (A) Withdrawal threshold was measured 45 minutes after PGE2 was injected subcutaneously with TUPS, celecoxib (COX-2 inhibitor), or dexamethasone (corticosteroid). Celecoxib and dexamethasone act upstream of PGE2 formation and therefore do not prevent PGE2-induced pain. However, sEH inhibitors do inhibit PGE2 induced pain, indicating that they act downstream of PGE2 formation. Data are replotted from Inceoglu et al. (2011) (B) sEH inhibitors synergize with diclofenac, a COX inhibitor, to reduce neuropathic pain in a streptozocin-induced model of diabetic neuropathy. Increased EETs are associated with a reduction in blood clotting caused by COX-2 selective inhibitors such as rofecoxib and the gastric erosion from COX-1 inhibitors. Data are replotted from Hammock et al. (2011). PGE2, prostaglandin E2; TUPS, 1-(1-methanesulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)urea.
Aside from analgesic properties, the epoxidized fatty acids also regulate angiogenesis and metastasis. Unlike inflammation and analgesia, in which the effects of ω-3 and ω-6 metabolites are similar, the ω-3 metabolites inhibit vascular endothelial growth factor–mediated angiogenesis and metastasis (Zhang et al., 2013), whereas the ω-6 metabolites promote angiogenesis and metastasis (Panigrahy et al., 2012). Compounds that reduce angiogenesis have previously been investigated therapeutically to prevent cancer recurrence. On the other hand, angiogenesis is essential for organ growth and tissue regeneration and its promotion could be used to improve wound healing. Therefore, modulation of these metabolites by sEH inhibition could be therapeutically beneficial for both inhibiting cancer recurrence and for promoting would healing, but such therapeutic approaches will likely require careful regulation of ω-3 and ω-6 metabolites through dietary intervention.
Other physiologic effects of the EpFAs that could lead to therapeutic uses have been identified in the past several years; however, further research is needed to fully understand their mechanisms and overall benefit. Inhibition of sEH attenuates the normal ER stress response, which could have implications for metabolic disorders (Bettaieb et al., 2013). In addition, the ER stress response has been linked to a number of neurodegenerative disorders but no studies have investigated sEH inhibition on these end points.
Developing a Path to the Clinic
There are many possible therapeutic uses of sEH inhibitors, EpFAs, and their mimics, ranging from quite obvious indications such as hypertension and pain to more obscure uses. Potential clinical trials for uses such as cardiac ischemia or atrial fibrillation appear relatively straightforward, whereas clinical trials for stroke, atherosclerosis, or sepsis would be more difficult. Numerous factors go into decisions regarding a clinical path for development, as discussed in the context of sEH inhibitors by Shen and Hammock (2012). These decisions go beyond chemical properties of the molecule and target efficacy to include such things as budgetary constraints, developer expertise, and the patent landscape.
A recently explored clinical application for sEH inhibitors as therapeutics has been the reduction in inflammatory and neuropathic pain. They are as effective in animal models of inflammatory disease as NSAIDs and COX inhibitors (Schmelzer et al., 2006; Wagner et al., 2011b) and reduce the gastrointestinal erosion and cardiovascular effects associated with the use of COX inhibitors. However, the clinical trials required for using sEH inhibitors to treat inflammatory pain are long and the current therapies are sufficiently effective if doses are monitored carefully, making a pathway to the clinic difficult in humans. On the other hand, neuropathic or chronic pain remains an unmet medical need and sEH inhibitors have proven efficacious in rodent models and equine laminitis patients (Guedes et al., 2013). sEH inhibitors are particularly attractive because they are nonopiate and non-NSAID compounds that do not impair cognition, mobility, or coordination in animal trials. In the chronic pain field, it is notoriously difficult to extrapolate from rodent models to humans, but this difficulty potentially arises as much from using disease models rather than patients as it does from differences between chronic pain in humans and other animals. Most rodent studies involve pain reduction in mouse or rat experimental models of pain. By contrast, treating patients for clinical pain often requires addressing other complex disease factors not considered in a rodent model. EicOsis Animal Health has already begun trials to treat inflammatory and neuropathic pain in companion animals, a market that primarily consists of dogs and cats. Its sister company, EicOsis Human Health, is working on chronic pain in humans. A third company, Sphaera, has also run a successful human clinical trial using a topical application of an sEH inhibitor to treat diabetic peripheral neuropathy. Thus, some confidence in ultimate clinical success with sEH inhibitors to treat pain comes from pain reduction in five different animal orders. In addition to pain, several studies suggest that sEH inhibitors could be broadly effective in a variety of diseases involving neuroinflammation (Hung et al., 2015).
Other clinical applications, including reduction in hypertension, myocardial infarction, and diabetes, were previously covered by Shen and Hammock (2012) but are worth briefly discussing. The first biologic application in which efficacy was determined in vivo and the first clinical target by Arête Therapeutics was reduction in hypertension. The sEH inhibitors were clearly active, resulting in decreased blood pressure in AngII-induced rodent models (Jung et al., 2005) and a dramatic reduction in hypertension for horses with severe laminitis (Guedes et al., 2013). Unfortunately, Arête Therapeutics was unsuccessful at developing AR9281 (Fig. 6), an IND candidate, into a marketed drug largely due to mediocre efficacy and short half-life. sEH inhibitors have also been investigated for the improvement of cardiac function and reduction of cardiac fibrosis after myocardial infarction in a mouse model (Sirish et al., 2013). Trials for cardiac hypertrophy caused by fibrosis are notoriously expensive and long; however, there is a serious need for drugs that will attenuate hypertrophy. In addition, numerous comorbidities associated with diabetes are effectively controlled in animal models by sEH inhibitors, but the effectiveness on diabetes itself has varied with the animal model evaluated (Luria et al., 2011; Chen et al., 2013). ER stress (a component of many disease states, including diabetes) appears to be significantly reduced by giving sEH inhibitors, which represents an additional approach toward getting to the market (Bettaieb et al., 2013). In fact, in mice with enhanced levels of ω-3 fatty acids, sEH inhibitors reduce the symptoms of diabetes (López-Vicario et al., 2015).
The profile of EpFA associated with sepsis suggests that sEH inhibitors should also be very effective at treating sepsis-related mortality (Zheng et al., 2001; Schmelzer et al., 2005; Liu et al., 2009). In particular, the high level of plasma linoleate diol or leukotoxin suggests a role for sEH in therapy (Moghaddam et al., 1997). A variety of studies using rodent models have shown that many of the symptoms associated with sepsis can be blocked by sEH inhibitors, resulting in reduced mortality. One of the most dramatic effects of sEH inhibitors in sepsis has been in reducing morbid hypotension (Liu et al., 2009). Since clinical treatment of hypotension is exceptionally difficult, the sEH inhibitors would offer an attractive new approach. However, predicting which patients will develop acute respiratory distress syndrome, multiorgan failure, or sepsis will make execution of a clinical trial difficult. Compassionate use treatments for serious viral infections, such as those associated with swine flu or Ebola, could potentially provide a clinical path for using sEH inhibitors to reduce sepsis. The Arête Therapeutics IND candidate AR9281, for example, represents a new approach with an IND on file with the Food and Drug Administration that could be evaluated for sepsis.
Finally, a promising candidate produced by GlaxoSmithKline, GSK2256294, has gone through phase I trials including measurement of forearm blood flow in moderately obese smokers (ClinicalTrials.gov NCT01762774; http://clinicaltrials.gov/ct2/show/NCT01762774). A phase II trial is planned for the treatment of chronic obstructive pulmonary disease (COPD). Chronic obstructive pulmonary disorder is common among chronic smokers and was estimated to affect 5.1% of the population in 2009 (Akinbami and Liu, 2011), making it a good indication for potential entry into the market for sEH inhibitors. GSK2256294 demonstrates picomolar activity toward sEH, effectively increases in vivo EET/DHET and epoxyoctadecenoic acid/dihydroxyoctadecenoic acid ratios, and reduces cell counts of macrophages, neutrophils, and keratinocyte chemoattractant cells of smoke-exposed rodents (Podolin et al., 2013). These observations are consistent with several studies demonstrating the efficacy of sEH inhibitors at reducing inflammation and general lung injury from smoke exposure (Smith et al., 2005; Wang et al., 2012) and asthma (Yang et al., 2015).
Future Challenges in Epoxide Hydrolases
Thus far, discovery in the field of epoxide hydrolases has led to insight on the roles of important lipid mediators called EpFAs as well as development of potential pharmaceuticals based on potent inhibition of sEH with optimized PK-ADME. Yet there are hints that opportunity is still available for novel discoveries in the epoxide hydrolase field.
Each monomer of the sEH antiparallel homodimer consists of two globular proteins linked by a proline-rich bridge. The role of the N-terminal phosphatase domain, first characterized a decade ago, remains poorly understood. The phosphatase activity was identified and characterized using the artificial substrates 4-nitrophenyl phosphate and 4-methylumbelliferyl phosphate (Cronin et al., 2003) as well as a number of dihydroxy lipid phosphates (Newman et al., 2003). Unfortunately, these early lipid phosphates were not detected in biologic samples. Lysophosphatidic acids were recently identified as substrates for the phosphatase domain of sEH, with activity higher than the lipid phosphate phosphatases believed to be responsible for their hydrolysis (Oguro and Imaoka, 2012; Morisseau et al., 2013). There is additional evidence that the phosphatase domain regulates cholesterol levels (EnayetAllah et al., 2008) and endothelial nitric oxide synthase activity (Hou et al., 2012); however, it is unclear what the exact substrates are for the phosphatase domain. The simultaneous inhibition of both the epoxide hydrolase and phosphatase activities of sEH by the thrombolysis drug Stachybotrys microspora triprenylphenol-7 (SMTP-7) suggests a possible clinical role for dual inhibitors (Matsumoto et al., 2014). In nematodes, homologs of the phosphatase domain are separate enzymes (Harris et al., 2008) as they are in plants. However, the conservation of the phosphatase as well as the epoxide hydrolase protein from the prokaryotic through eukaryotic species suggests fundamental biologic roles.
A number of epoxide hydrolases whose activity has not been linked to physiologic relevance or a mediating substrate have recently been described. Two of these, the EH3 and EH4, were identified as theoretical epoxide hydrolases by comparing structural homology with mEH and sEH. Thus far, only EH3 has been successfully expressed recombinantly and neither protein has a demonstrated role in vivo (Decker et al., 2012). EH3 has been reported to hydrolyze EpFAs (Decker et al., 2012), but there is no indication that this activity is related to its endogenous function. In addition to the EH3 and EH4, cystic fibrosis transmembrane conductance regulator inhibitory factor is a virulence factor with epoxide hydrolase activity produced by the bacterium Pseudomonas aeruginosa, which contributes to the progression of cystic fibrosis. It has been reported to hydrolyze the sEH substrate cis-stilbene oxide (Bahl et al., 2010), but it has not been reported to hydrolyze a natural substrate and the connection between epoxide hydrolase activity and disease progression is poorly understood. Certainly, epoxide hydrolases are widespread in prokaryotic and eukaryotic species with physiologic roles yet to be determined. Aside from these novel epoxide hydrolases, there is the possibility that the mEH, typically studied in the context of xenobiotic metabolism, has a role in endogenous epoxide metabolism. Many articles have minimized the contributions of mEH on EET metabolism due to the low activity relative to sEH and relatively low abundance, but the contribution of mEH toward hydrolysis of EETs and other EpFAs may be significant in cell types with low sEH abundance, including some neurons (Marowsky et al., 2009). Similar to how the identification of juvenile hormones and EpFAs was important toward understanding the role of the juvenile hormone epoxide hydrolase and sEH, the essential step toward understanding the physiologic role of mEH and these novel epoxide hydrolases will be the identification of the novel epoxides or diols that act as chemical mediators.
In addition to the epoxide hydrolases discussed here in mammals and insects, epoxide hydrolases have been identified in a range of organisms, including plants (Newman et al., 2005), bacteria (Arand et al., 2003), the nematode Caenorhabditis elegans (Harris et al., 2008), and other animals including chickens (Harris et al., 2006) and amphibian Xenopus (Purba et al., 2014). To accommodate new discoveries in the epoxide hydrolase field, it will be necessary to adopt a naming system similar to that generated for the P450 superfamily (Nebert et al., 1987; Nelson, 2006), which currently has at least 20,000 members representing both prokaryotic and eukaryotic organisms (Nelson, 2009). For P450s, identified P450s are divided into numerical families based on 40% sequence identity and can be further organized into alphabetical subfamilies if enzymes maintain at least 55% sequence identity. Successful implementation of an epoxide hydrolase nomenclature would allow systematic comparisons of epoxide hydrolase substrate selectivity, activity, and physiologic relevance across species. As more epoxide hydrolase enzymes are described, the importance of such a systematic nomenclature system will increase.
Conclusion
It appears that most xenobiotic epoxides are hydrolyzed by either the mEH or sEH, the former enzyme being responsible for epoxides on most cyclic systems. An investigation into the mammalian metabolism of an early insect growth regulator led to the discovery of the sEH, which degrades some xenobiotics but is largely responsible for the hydration of EpFAs. Many of these EpFAs are potent chemical mediators formed in the P450 branch of the arachidonate cascade. EpFA generally can be considered as having the opposite effects of mediators from the better studied COX and lipoxygenase branches of the cascade, which are largely but not exclusively hypertensive, proinflammatory, and pain-inducing mediators.
A variety of authors demonstrated that in insects, the hydrolysis of the terpenoid insect juvenile hormone by α/β-fold esterases and epoxide hydrolases is as important in regulating its biology as it is the biosynthesis of the hormone (Hammock and Quistad, 1976). Similarly, with EpFA such as EETs and EpDPEs, the regulation of the titer of these chemical mediators by degradation seems as important as biosynthesis. Fang et al. (2004) showed that the sEH is the major enzyme involved in the degradation of EETs, and its inhibition increases the plasma and presumably tissue levels of a variety of these generally beneficial EpFA chemical mediators.
Thus, an applied study of the metabolism of a xenobiotic for use in agriculture and vector control led to the discovery of the sEH. Addressing fundamental questions regarding the enzyme’s biochemistry, catalytic mechanism and physiology led to potent inhibitors, which, as physiologic probes, demonstrated endogenous roles for the natural EpFA chemical mediators. These same inhibitors now appear promising for the control of a variety of illnesses in humans and in companion animals. This is particularly true for the largely unmet medical need of neuropathic pain and a variety of acute pain indications. The sEH inhibitors as well as the EpFAs and their mimics offer the possibility of non-NSAID, nonopiate therapeutics for inflammatory and neuropathic pain. To date, the principle value of sEH inhibitors has been in demonstrating endogenous roles for the EpFA chemical mediators and in determining their mechanism of action. In doing so, the sEH inhibitors illustrate a possible therapeutic role for mimics of EpFAs just as the chemical mimics of insect juvenile hormone have proven to be valuable tools in the control of disease vectors and agricultural pests. The epoxide hydrolase field, as well as several others, illustrates that the study of xenobiotic metabolism is an essential component of translational science in drug development. However, it also offers new biologic insight, which in this case is being translated into novel therapeutics.
Acknowledgments
The authors thank Arzu Ulu, Karen Wagner, Bora Inceoglu, Todd Harris, and Christophe Morisseau for helpful comments in the preparation of this article.
Abbreviations
- ADME
absorption, distribution, metabolism, and excretion
- AngII
angiotensin II
- AR9281
1-(1-acetyl-piperidin-4-yl)-3-adamantan-1-yl-urea
- AUDA
12-(3-adamantan-1-yl-ureido)-dodecanoic acid
- ChEH
cholesterol epoxide hydrolase
- COX
cyclooxygenase
- DDA
Dendrogenin A
- EET
epoxyeicosatrienoic acid
- EpDPE
epoxydocosapentaenoic acid
- EpFA
epoxy fatty acid
- ER
endoplasmic reticulum
- GSK2256294
(1R, 3S)-(cis)-N-(4-cyano-2-(trifluoromethyl)benzyl)-3-((4-methyl-6-(methylamino)-1,3,5-triazin-2-yl)amino)cyclohexanecarboxamide
- HEOM
1,2,3,4,9,9-hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-1,4-methanonaphthalene
- IND
Investigational New Drug
- mEH
microsomal epoxide hydrolase
- NSAID
nonsteroidal anti-inflammatory drug
- P450
cytochrome P450
- PK
pharmacokinetics
- R-20458
(E)-6,7-epoxy-1-(4-ethylphenoxy)-3,7-dimethyl-2-octene
- sEH
soluble epoxide hydrolase
- ZR 515
1-methylethyl (E,E)-11- methoxy-3,7,11-trimethyl-2,4-dodecadienoate
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Kodani, Hammock.
Footnotes
This research was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grants R01-ES002170 and P42-ES004699], the National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grant R21-AR062866], and the Research Investments in the Science and Engineering Program at the University of California, Davis. B.D.H. is a cofounder of EicOsis LLC, and he has several patents on soluble epoxide hydrolase technology.
References
- Akinbami LJ, Liu X. (2011) Chronic Obstructive Pulmonary Disease Among Adults Aged 18 and Over in the United States, 1998-2009, National Center for Health Statistics, Hyattsville, MD. [PubMed] [Google Scholar]
- Anandan SK, Webb HK, Chen D, Wang YX, Aavula BR, Cases S, Cheng Y, Do ZN, Mehra U, Tran V, et al. (2011) 1-(1-acetyl-piperidin-4-yl)-3-adamantan-1-yl-urea (AR9281) as a potent, selective, and orally available soluble epoxide hydrolase inhibitor with efficacy in rodent models of hypertension and dysglycemia. Bioorg Med Chem Lett 21:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arand M, Cronin A, Oesch F, Mowbray SL, Jones TA. (2003) The telltale structures of epoxide hydrolases. Drug Metab Rev 35:365–383. [DOI] [PubMed] [Google Scholar]
- Argiriadi MA, Morisseau C, Goodrow MH, Dowdy DL, Hammock BD, Christianson DW. (2000) Binding of alkylurea inhibitors to epoxide hydrolase implicates active site tyrosines in substrate activation. J Biol Chem 275:15265–15270. [DOI] [PubMed] [Google Scholar]
- Aström A, Eriksson M, Eriksson LC, Birberg W, Pilotti A, DePierre JW. (1986) Subcellular and organ distribution of cholesterol epoxide hydrolase in the rat. Biochim Biophys Acta 882:359–366. [DOI] [PubMed] [Google Scholar]
- Bahl CD, Morisseau C, Bomberger JM, Stanton BA, Hammock BD, O’Toole GA, Madden DR. (2010) Crystal structure of the cystic fibrosis transmembrane conductance regulator inhibitory factor Cif reveals novel active-site features of an epoxide hydrolase virulence factor. J Bacteriol 192:1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettaieb A, Nagata N, AbouBechara D, Chahed S, Morisseau C, Hammock BD, Haj FG. (2013) Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem 288:14189–14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel MH. (1989) In memoriam: Bernard B. Brodie. Pharmacol Toxicol 65:241–244. [Google Scholar]
- Black HS, Lenger WA. (1979) A practical radiochromatographic assay for cholesterol epoxide hydrase. Anal Biochem 94:383–385. [DOI] [PubMed] [Google Scholar]
- Blackburn GM, Rashid A, Thompson MH. (1979) Interaction of 5α,6α-cholesterol oxide with DNA and other nucleophiles. J Chem Soc Chem Commun 420–421. [Google Scholar]
- Borhan B, Mebrahtu T, Nazarian S, Kurth MJ, Hammock BD. (1995) Improved radiolabeled substrates for soluble epoxide hydrolase. Anal Biochem 231:188–200. [DOI] [PubMed] [Google Scholar]
- Brooks GT. (1973) Insect epoxide hydrase inhibition by juvenile hormone analogues and metabolic inhibitors. Nature 245:382–384. [Google Scholar]
- Brooks GT. (1977) Epoxide hydratase as a modifier of biotransformation and biological activity. Gen Pharmacol 8:221–226. [DOI] [PubMed] [Google Scholar]
- Brooks GT, Harrison A, Lewis SE. (1970) Cyclodiene epoxide ring hydration by microsomes from mammalian liver and houseflies. Biochem Pharmacol 19:255–273. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Schwartzman M, Capdevila J, Falck JR, McGiff JC. (1987) Vasoactivity of arachidonic acid epoxides. Eur J Pharmacol 138:281–283. [DOI] [PubMed] [Google Scholar]
- Carson R. (1962) Silent Spring, Houghton Mifflin, Boston. [Google Scholar]
- Chacos N, Capdevila J, Falck JR, Manna S, Martin-Wixtrom C, Gill SS, Hammock BD, Estabrook RW. (1983) The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys 223:639–648. [DOI] [PubMed] [Google Scholar]
- Chen L, Fan C, Zhang Y, Bakri M, Dong H, Morisseau C, Maddipati KR, Luo P, Wang CY, Hammock BD, et al. (2013) Beneficial effects of inhibition of soluble epoxide hydrolase on glucose homeostasis and islet damage in a streptozotocin-induced diabetic mouse model. Prostaglandins Other Lipid Mediat 104-105:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. (2007) The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol 50:225–237. [DOI] [PubMed] [Google Scholar]
- Conroy JL, Fang C, Gu J, Zeitlin SO, Yang W, Yang J, VanAlstine MA, Nalwalk JW, Albrecht PJ, Mazurkiewicz JE, et al. (2010) Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nat Neurosci 13:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Karczmar AG, Vesell ES. (1989) Bernard B. Brodie and the rise of chemical pharmacology. Annu Rev Pharmacol Toxicol 29:1–21. [DOI] [PubMed] [Google Scholar]
- Cronin A, Mowbray S, Dürk H, Homburg S, Fleming I, Fisslthaler B, Oesch F, Arand M. (2003) The N-terminal domain of mammalian soluble epoxide hydrolase is a phosphatase. Proc Natl Acad Sci USA 100:1552–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medina P, Paillasse MR, Payré B, Silvente-Poirot S, Poirot M. (2009) Synthesis of new alkylaminooxysterols with potent cell differentiating activities: identification of leads for the treatment of cancer and neurodegenerative diseases. J Med Chem 52:7765–7777. [DOI] [PubMed] [Google Scholar]
- de Medina P, Paillasse MR, Segala G, Poirot M, Silvente-Poirot S. (2010) Identification and pharmacological characterization of cholesterol-5,6-epoxide hydrolase as a target for tamoxifen and AEBS ligands. Proc Natl Acad Sci USA 107:13520–13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medina P, Paillasse MR, Segala G, Voisin M, Mhamdi L, Dalenc F, Lacroix-Triki M, Filleron T, Pont F, Saati TA, et al. (2013) Dendrogenin A arises from cholesterol and histamine metabolism and shows cell differentiation and anti-tumour properties. Nat Commun 4:1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Taeye BM, Morisseau C, Coyle J, Covington JW, Luria A, Yang J, Murphy SB, Friedman DB, Hammock BB, Vaughan DE. (2010) Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity (Silver Spring) 18:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean PDG, Ortiz de Montellano PR, Bloch K, Corey EJ. (1967) A soluble 2,3-oxidosqualene sterol cyclase. J Biol Chem 242:3014–3015. [PubMed] [Google Scholar]
- Decker M, Adamska M, Cronin A, Di Giallonardo F, Burgener J, Marowsky A, Falck JR, Morisseau C, Hammock BD, Gruzdev A, et al. (2012) EH3 (ABHD9): the first member of a new epoxide hydrolase family with high activity for fatty acid epoxides. J Lipid Res 53:2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker M, Arand M, Cronin A. (2009) Mammalian epoxide hydrolases in xenobiotic metabolism and signalling. Arch Toxicol 83:297–318. [DOI] [PubMed] [Google Scholar]
- Despa S, Sharma S, Harris TR, Dong H, Li N, Chiamvimonvat N, Taegtmeyer H, Margulies KB, Hammock BD, Despa F. (2014) Cardioprotection by controlling hyperamylinemia in a “humanized” diabetic rat model. J Am Heart Assoc 3:e001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. (2005) An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol 46:842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EnayetAllah AE, Luria A, Luo B, Tsai HJ, Sura P, Hammock BD, Grant DF. (2008) Opposite regulation of cholesterol levels by the phosphatase and hydrolase domains of soluble epoxide hydrolase. J Biol Chem 283:36592–36598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Hu S, Watanabe T, Weintraub NL, Snyder GD, Yao J, Liu Y, Shyy JY, Hammock BD, Spector AA. (2005) Activation of peroxisome proliferator-activated receptor alpha by substituted urea-derived soluble epoxide hydrolase inhibitors. J Pharmacol Exp Ther 314:260–270. [DOI] [PubMed] [Google Scholar]
- Fang X, Weintraub NL, McCaw RB, Hu S, Harmon SD, Rice JB, Hammock BD, Spector AA. (2004) Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol 287:H2412–H2420. [DOI] [PubMed] [Google Scholar]
- Fornage M, Hinojos CA, Nurowska BW, Boerwinkle E, Hammock BD, Morisseau CHP, Doris PA. (2002) Polymorphism in soluble epoxide hydrolase and blood pressure in spontaneously hypertensive rats. Hypertension 40:485–490. [DOI] [PubMed] [Google Scholar]
- Gill SS, Hammock BD. (1979) Hydration of cis- and trans-epoxymethyl stearates by the cytosolic epoxide hydrase of mouse liver. Biochem Biophys Res Commun 89:965–971. [DOI] [PubMed] [Google Scholar]
- Gill SS, Hammock BD. (1980) Distribution and properties of a mammalian soluble epoxide hydrase. Biochem Pharmacol 29:389–395. [DOI] [PubMed] [Google Scholar]
- Gill SS, Hammock BD, Casida JE. (1974) Mammalian metabolism and environmental degradation of the juvenoid 1-(4′-ethylphenoxy)-3,7-dimethyl-6,7-epoxy-trans-2-octene and related compounds. J Agric Food Chem 22:386–395. [DOI] [PubMed] [Google Scholar]
- Gill SS, Hammock BD, Yamamoto I, Casida JE. (1972) Preliminary chromatographic studies on the metabolites and photodecomposition products of the juvenoid 1-(4'-ethylphenoxy)-6,7-epoxy-3,7-dimethyl-2-octene, in Insect Juvenile Hormones: Chemistry and Action (Menn JJ, Beroza M. eds), pp 177–189, Academic Press, New York. [Google Scholar]
- Gill SS, Ota K, Hammock BD. (1983) Radiometric assays for mammalian epoxide hydrolases and glutathione S-transferase. Anal Biochem 131:273–282. [DOI] [PubMed] [Google Scholar]
- Guedes AG, Morisseau C, Sole A, Soares JH, Ulu A, Dong H, Hammock BD. (2013) Use of a soluble epoxide hydrolase inhibitor as an adjunctive analgesic in a horse with laminitis. Vet Anaesth Analg 40:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenthner TM, Hammock BD, Vogel U, Oesch F. (1981) Cytosolic and microsomal epoxide hydrolases are immunologically distinguishable from each other in the rat and mouse. J Biol Chem 256:3163–3166. [PubMed] [Google Scholar]
- Haeggström JZ, Tholander F, Wetterholm A. (2007) Structure and catalytic mechanisms of leukotriene A4 hydrolase. Prostaglandins Other Lipid Mediat 83:198–202. [DOI] [PubMed] [Google Scholar]
- Hammock BD. (1985) Regulation of juvenile hormone titer: degradation, in Comprehensive Insect Physiology Biochemistry and Pharmacology (Kerkut GA, Gilbert LI. eds) pp 431–472, Pergamon Press, New York. [Google Scholar]
- Hammock BD, El Tantawy M, Gill SS, Hasagawa L, Mullin CA, Ota K. (1980a) Extramicrosomal epoxide hydration, in Microsomes, Drug Oxidations, and Chemical Carcinogenesis (Coon MJ, Conney AH, Estabrook RW, Gelboin HV, Gillette JR, O'Brien PJ. eds), pp 655–658, Academic Press, Ann Arbor, MI. [Google Scholar]
- Hammock BD, Gill SS, Casida JE. (1974) Synthesis and morphogenetic activity of derivaties and analogs of aryl geranyl ether juvenoids. J Agric Food Chem 22:379–385. [DOI] [PubMed] [Google Scholar]
- Hammock BD, Gill SS, Mumby SM, Ota K. (1980b) Comparison of epoxide hydrases in the soluble and microsomal fractions of mammalian liver, in Molecular Basis of Environmental Toxicology (Bhatnagar RS. ed), pp 229–272, Ann Arbor Science Publishers Inc., Ann Arbor, MI. [Google Scholar]
- Hammock BD, Gill SS, Stamoudis V, Gilbert LI. (1976) Soluble mammalian epoxide hydratase: action on juvenile hormone and other terpenoid epoxides. Comp Biochem Physiol B 53:263–265. [DOI] [PubMed] [Google Scholar]
- Hammock BD, Moody DE, Sevanian A. (1985) Epoxide hydrolases in the catabolism of sterols and isoprenoids. Methods Enzymol 111:303–311. [DOI] [PubMed] [Google Scholar]
- Hammock BD, Quistad GB. (1976) The degradative metabolism of juvenoids by insects, in The Juvenile Hormones (Gilbert LE. ed) pp 374–393, Plenum Press, New York. [Google Scholar]
- Hammock BD, Storms DH, Grant DF. (1997) Epoxide hydrolases, in Comprehensive Toxicology (Guengerich FP. ed) pp 283–305, Pergamon, Oxford, UK. [Google Scholar]
- Hammock BD, Wagner K, Inceoglu B. (2011) The soluble epoxide hydrolase as a pharmaceutical target for pain management. Pain Manag 1:383–386. [DOI] [PubMed] [Google Scholar]
- Harris TR, Aronov PA, Jones PD, Tanaka H, Arand M, Hammock BD. (2008) Identification of two epoxide hydrolases in Caenorhabditis elegans that metabolize mammalian lipid signaling molecules. Arch Biochem Biophys 472:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TR, Hammock BD. (2013) Soluble epoxide hydrolase: gene structure, expression and deletion. Gene 526:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TR, Morisseau C, Walzem RL, Ma SJ, Hammock BD. (2006) The cloning and characterization of a soluble epoxide hydrolase in chicken. Poult Sci 85:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrick CA, Staal GB, Siddall JB. (1973) Alkyl 3,7,11-trimethyl-2,4-dodecadienoates, a new class of potent insect growth regulators with juvenile hormone activity. J Agric Food Chem 21:354–359. [DOI] [PubMed] [Google Scholar]
- Hou HH, Hammock BD, Su KH, Morisseau C, Kou YR, Imaoka S, Oguro A, Shyue SK, Zhao JF, Lee TS. (2012) N-terminal domain of soluble epoxide hydrolase negatively regulates the VEGF-mediated activation of endothelial nitric oxide synthase. Cardiovasc Res 93:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YW, Hung SW, Wu YC, Wong LK, Lai MT, Shih YH, Lee TS, Lin YY. (2015) Soluble epoxide hydrolase activity regulates inflammatory responses and seizure generation in two mouse models of temporal lobe epilepsy. Brain Behav Immun 43:118–129. [DOI] [PubMed] [Google Scholar]
- Hwang SH, Wagner KM, Morisseau C, Liu JY, Dong H, Wecksler AT, Hammock BD. (2011) Synthesis and structure-activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J Med Chem 54:3037–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD. (2012) Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev 92:101–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD, Hammock BD. (2009) Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov 8:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. (2006) Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci 79:2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. (2008) Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci USA 105:18901–18906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, Hegedus C, Rose T, Brosnan R, Hammock BD. (2011) Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci USA 108:5093–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Wagner KM, Yang J, Bettaieb A, Schebb NH, Hwang SH, Morisseau C, Haj FG, Hammock BD. (2012) Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes. Proc Natl Acad Sci USA 109:11390–11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Zolkowska D, Yoo HJ, Wagner KM, Yang J, Hackett E, Hwang SH, Lee KS, Rogawski MA, Morisseau C, et al. (2013) Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PLoS ONE 8:e80922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerina D, Daly J, Witkop B, Zaltzman-Nirenberg P, Udenfriend S. (1968) Role of the arene oxide-oxepin system in the metabolism of aromatic substrates. I. In vitro conversion of benzene oxide to a premercapturic acid and a dihydrodiol. Arch Biochem Biophys 128:176–183. [Google Scholar]
- Jerina DM, Daly JW, Landis WR, Witkop B, Udenfriend S. (1967) Intramolecular migration of tritium and deuterium during nonenzymatic aromatic hydroxylation. J Am Chem Soc 89:3347–3349. [Google Scholar]
- Jones PD, Tsai HJ, Do ZN, Morisseau C, Hammock BD. (2006) Synthesis and SAR of conformationally restricted inhibitors of soluble epoxide hydrolase. Bioorg Med Chem Lett 16:5212–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PD, Wolf NM, Morisseau C, Whetstone P, Hock B, Hammock BD. (2005) Fluorescent substrates for soluble epoxide hydrolase and application to inhibition studies. Anal Biochem 343:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Busse R, Fleming I. (2005) Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension 45:759–765. [DOI] [PubMed] [Google Scholar]
- Kim IH, Heirtzler FR, Morisseau C, Nishi K, Tsai HJ, Hammock BD. (2005) Optimization of amide-based inhibitors of soluble epoxide hydrolase with improved water solubility. J Med Chem 48:3621–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IH, Morisseau C, Watanabe T, Hammock BD. (2004) Design, synthesis, and biological activity of 1,3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility. J Med Chem 47:2110–2122. [DOI] [PubMed] [Google Scholar]
- Kim J, Imig JD, Yang J, Hammock BD, Padanilam BJ. (2014) Inhibition of soluble epoxide hydrolase prevents renal interstitial fibrosis and inflammation. Am J Physiol Renal Physiol 307:F971–F980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujal P, Čertíková Chábová V, Škaroupková P, Husková Z, Vernerová Z, Kramer HJ, Walkowska A, Kompanowska-Jezierska E, Sadowski J, Kitada K, et al. (2014) Inhibition of soluble epoxide hydrolase is renoprotective in 5/6 nephrectomized Ren-2 transgenic hypertensive rats. Clin Exp Pharmacol Physiol 41:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsky P, Falck JR, Weiss GB, Manna S, Chacos N, Capdevila J. (1983) Effects of newly reported arachidonic acid metabolites on microsomal Ca++ binding, uptake and release. Prostaglandins 26:13–21. [DOI] [PubMed] [Google Scholar]
- Lee KS, Liu JY, Wagner KM, Pakhomova S, Dong H, Morisseau C, Fu SH, Yang J, Wang P, Ulu A, et al. (2014) Optimized inhibitors of soluble epoxide hydrolase improve in vitro target residence time and in vivo efficacy. J Med Chem 57:7016–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Morisseau C, Yang J, Wang P, Hwang SH, Hammock BD. (2013) Förster resonance energy transfer competitive displacement assay for human soluble epoxide hydrolase. Anal Biochem 434:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr RE, Kumar S, Levin W, Wood AW, Chang RL, Conney AH, Yagi H, Sayer JM, Jerina DM. (1985) The bay region theory of polycyclic aromatic hydrocarbon carcinogenesis, in Polycyclic Hydrocarbons and Carcinogenesis (Harvey RG. ed) pp 63–84, University of Chicago, Chicago. [Google Scholar]
- Levin W, Wood AW, Yagi H, Jerina DM, Conney AH. (1976) (+/-)-Trans-7,8-dihydroxy-7,8-dihydrobenzo (a)pyrene: a potent skin carcinogen when applied topically to mice. Proc Natl Acad Sci USA 73:3867–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li N, Pang W, Zhang X, Hammock BD, Ai D, Zhu Y. (2014) Opposite effects of gene deficiency and pharmacological inhibition of soluble epoxide hydrolase on cardiac fibrosis. PLoS ONE 9:e94092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Tsai HJ, Hwang SH, Jones PD, Morisseau C, Hammock BD. (2009) Pharmacokinetic optimization of four soluble epoxide hydrolase inhibitors for use in a murine model of inflammation. Br J Pharmacol 156:284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Vicario C, Alcaraz-Quiles J, García-Alonso V, Rius B, Hwang SH, Titos E, Lopategi A, Hammock BD, Arroyo V, Clària J. (2015) Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci USA 112:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström SL, Levänen B, Nording M, Klepczynska-Nyström A, Sköld M, Haeggström JZ, Grunewald J, Svartengren M, Hammock BD, Larsson BM, et al. (2011) Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure. PLoS ONE 6:e23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström SL, Yang J, Källberg HJ, Thunberg S, Gafvelin G, Haeggström JZ, Grönneberg R, Grunewald J, van Hage M, Hammock BD, et al. (2012) Allergic asthmatics show divergent lipid mediator profiles from healthy controls both at baseline and following birch pollen provocation. PLoS ONE 7:e33780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Chang HH, Zhou Y, Zhang S, Hwang SH, Morisseau C, Wang CY, Inscho EW, Hammock BD, Wang MH. (2010) Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J Pharmacol Exp Ther 334:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, Hammock BD. (2011) Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA 108:9038–9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M. (2009) Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience 163:646–661. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Suzuki E, Ishikawa M, Shirafuji T, Hasumi K. (2014) Soluble epoxide hydrolase as an anti-inflammatory target of the thrombolytic stroke drug SMTP-7. J Biol Chem 289:35826–35838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycock AL, Anderson MS, DeSousa DM, Kuehl FA., Jr (1982) Leukotriene A4: preparation and enzymatic conversion in a cell-free system to leukotriene B4. J Biol Chem 257:13911–13914. [PubMed] [Google Scholar]
- Meirer K, Rödl CB, Wisniewska JM, George S, Häfner AK, Buscató EL, Klingler FM, Hahn S, Berressem D, Wittmann SK, et al. (2013) Synthesis and structure-activity relationship studies of novel dual inhibitors of soluble epoxide hydrolase and 5-lipoxygenase. J Med Chem 56:1777–1781. [DOI] [PubMed] [Google Scholar]
- Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. (1997) Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med 3:562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C. (2013) Role of epoxide hydrolases in lipid metabolism. Biochimie 95:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. (1999) Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA 96:8849–8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Goodrow MH, Newman JW, Wheelock CE, Dowdy DL, Hammock BD. (2002) Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem Pharmacol 63:1599–1608. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. (2005) Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol 45:311–333. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. (2007) Measurement of soluble epoxide hydrolase (sEH) activity. Curr Protoc Toxicol Chapter 4:23. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. (2008) Gerry Brooks and epoxide hydrolases: four decades to a pharmaceutical. Pest Manag Sci 64:594–609. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. (2013) Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol 53:37–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. (2010) Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res 51:3481–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Sahdeo S, Cortopassi G, Hammock BD. (2013) Development of an HTS assay for EPHX2 phosphatase activity and screening of nontargeted libraries. Anal Biochem 434:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin CA, Hammock BD. (1980) A rapid radiometric assay for mammalian cytosolic epoxide hydrolase. Anal Biochem 106:476–485. [DOI] [PubMed] [Google Scholar]
- Mullin CA, Hammock BD. (1982) Chalcone oxides—potent selective inhibitors of cytosolic epoxide hydrolase. Arch Biochem Biophys 216:423–439. [DOI] [PubMed] [Google Scholar]
- Mumby SM, Hammock BD. (1979) Substrate selectivity and stereochemistry of enzymatic epoxide hydration in the soluble fraction of mouse liver. Pestic Biochem Physiol 11:275–284. [Google Scholar]
- Nebert DW, Adesnik M, Coon MJ, Estabrook RW, Gonzalez FJ, Guengerich FP, Gunsalus IC, Johnson EF, Kemper B, Levin W, et al. (1987) The P450 gene superfamily: recommended nomenclature. DNA 6:1–11. [DOI] [PubMed] [Google Scholar]
- Nelson DR. (2006) Cytochrome P450 nomenclature, 2004. Methods Mol Biol 320:1–10. [DOI] [PubMed] [Google Scholar]
- Nelson DR. (2009) The cytochrome p450 homepage. Hum Genomics 4:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JW, Morisseau C, Hammock BD. (2005) Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res 44:1–51. [DOI] [PubMed] [Google Scholar]
- Newman JW, Morisseau C, Harris TR, Hammock BD. (2003) The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA 100:1558–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood S, Liao J, Hammock BD, Yang GY. (2010) Epoxyeicosatrienoic acids and soluble epoxide hydrolase: potential therapeutic targets for inflammation and its induced carcinogenesis. Am J Transl Res 2:447–457. [PMC free article] [PubMed] [Google Scholar]
- Oesch F. (1973) Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica 3:305–340. [DOI] [PubMed] [Google Scholar]
- Oesch F, Daly J. (1971) Solubilization, purification, and properties of a hepatic epoxide hydrase. Biochim Biophys Acta 227:692–697. [DOI] [PubMed] [Google Scholar]
- Oesch F, Daly J. (1972) Conversion of naphthalene to trans-naphthalene dihydrodiol: evidence for the presence of a coupled aryl monooxygenase-epoxide hydrase system in hepatic microsomes. Biochem Biophys Res Commun 46:1713–1720. [DOI] [PubMed] [Google Scholar]
- Oesch E, Jerina DM, Daly J. (1971a) A radiometric assay for hepatic epoxide hydrase activity with [7-3H] styrene oxide. Biochim Biophys Acta 227:685–691. [DOI] [PubMed] [Google Scholar]
- Oesch F, Jerina DM, Daly JW, Rice JM. (1973) Induction, activation and inhibition of epoxide hydrase: an anomalous prevention of chlorobenzene-induced hepatotoxicity by an inhibitor of epoxide hydrase. Chem Biol Interact 6:189–202. [DOI] [PubMed] [Google Scholar]
- Oesch F, Kaubisch N, Jerina DM, Daly JW. (1971b) Hepatic epoxide hydrase. Structure-activity relationships for substrates and inhibitors. Biochemistry 10:4858–4866. [DOI] [PubMed] [Google Scholar]
- Oesch F, Timms CW, Walker CH, Guenthner TM, Sparrow A, Watabe T, Wolf CR. (1984) Existence of multiple forms of microsomal epoxide hydrolases with radically different substrate specificities. Carcinogenesis 5:7–9. [DOI] [PubMed] [Google Scholar]
- Oguro A, Imaoka S. (2012) Lysophosphatidic acids are new substrates for the phosphatase domain of soluble epoxide hydrolase. J Lipid Res 53:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olearczyk JJ, Field MB, Kim IH, Morisseau C, Hammock BD, Imig JD. (2006) Substituted adamantyl-urea inhibitors of the soluble epoxide hydrolase dilate mesenteric resistance vessels. J Pharmacol Exp Ther 318:1307–1314. [DOI] [PubMed] [Google Scholar]
- Ota K, Hammock BD. (1980) Cytosolic and microsomal epoxide hydrolases: differential properties in mammalian liver. Science 207:1479–1481. [DOI] [PubMed] [Google Scholar]
- Paillasse MR, Saffon N, Gornitzka H, Silvente-Poirot S, Poirot M, de Medina P. (2012) Surprising unreactivity of cholesterol-5,6-epoxides towards nucleophiles. J Lipid Res 53:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, Barnés CM, Mammoto A, Mammoto T, Luria A, et al. (2012) Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest 122:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. (2011) EET signaling in cancer. Cancer Metastasis Rev 30:525–540. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Panigrahy D, Kalish BT, Huang S, Bielenberg DR, Le HD, Yang J, Edin ML, Lee CR, Benny O, Mudge DK, et al. (2013) Epoxyeicosanoids promote organ and tissue regeneration. Proc Natl Acad Sci USA 110:13528–13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis NL. (1986) Physiologic, biochemical, and cytologic aspects of nipple aspirate fluid. Breast Cancer Res Treat 8:7–19. [DOI] [PubMed] [Google Scholar]
- Podolin PL, Bolognese BJ, Foley JF, Long E, 3rd, Peck B, Umbrecht S, Zhang X, Zhu P, Schwartz B, Xie W, et al. (2013) In vitro and in vivo characterization of a novel soluble epoxide hydrolase inhibitor. Prostaglandins Other Lipid Mediat 104-105:25–31. [DOI] [PubMed] [Google Scholar]
- Poirot M, Silvente-Poirot S. (2013) Cholesterol-5,6-epoxides: chemistry, biochemistry, metabolic fate and cancer. Biochimie 95:622–631. [DOI] [PubMed] [Google Scholar]
- Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, Hammock BD, Kim IH, Szelid Z, Vermeersch P, et al. (2006) Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension 47:762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Corda E, Martino PA, Dall’ara P, Bareggi SR, Bondiolotti G, Iulini B, Mazza M, Casalone C, Hwang SH, et al. (2013) Therapeutic activity of inhibition of the soluble epoxide hydrolase in a mouse model of scrapie. Life Sci 92:1145–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purba ER, Oguro A, Imaoka S. (2014) Isolation and characterization of Xenopus soluble epoxide hydrolase. Biochim Biophys Acta 1841:954–962. [DOI] [PubMed] [Google Scholar]
- Qin X, Wu Q, Lin L, Sun A, Liu S, Li X, Cao X, Gao T, Luo P, Zhu X, et al. (2014) Soluble epoxide hydrolase deficiency or inhibition attenuates MPTP-induced parkinsonism. Mol Neurobiol DOI: 10.1007/s12035-014-8833-3 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Schebb NH, Inceoglu B, Ahn KC, Morisseau C, Gee SJ, Hammock BD. (2011) Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ Sci Technol 45:3109–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, Hammock BD. (2006) Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA 103:13646–13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. (2005) Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA 102:9772–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanian A, McLeod LL. (1986) Catalytic properties and inhibition of hepatic cholesterol-epoxide hydrolase. J Biol Chem 261:54–59. [PubMed] [Google Scholar]
- Sevanian A, Peterson AR. (1984) Cholesterol epoxide is a direct-acting mutagen. Proc Natl Acad Sci USA 81:4198–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanian A, Stein RA, Mead JF. (1980) Lipid epoxide hydrolase in rat lung preparations. Biochim Biophys Acta 614:489–500. [DOI] [PubMed] [Google Scholar]
- Shaik JS, Ahmad M, Li W, Rose ME, Foley LM, Hitchens TK, Graham SH, Hwang SH, Hammock BD, Poloyac SM. (2013) Soluble epoxide hydrolase inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is neuroprotective in rat model of ischemic stroke. Am J Physiol Heart Circ Physiol 305:H1605–H1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC, Hammock BD. (2012) Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J Med Chem 55:1789–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvente-Poirot S, Poirot M. (2012) Cholesterol epoxide hydrolase and cancer. Curr Opin Pharmacol 12:696–703. [DOI] [PubMed] [Google Scholar]
- Sirish P, Li N, Liu JY, Lee KSS, Hwang SH, Qiu H, Zhao C, Ma SM, López JE, Hammock BD, et al. (2013) Unique mechanistic insights into the beneficial effects of soluble epoxide hydrolase inhibitors in the prevention of cardiac fibrosis. Proc Natl Acad Sci USA 110:5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JN, Spencer B, Williams RT. (1950) Studies in detoxication; the metabolism of chlorobenzene in the rabbit; isolation of dihydrodihydroxychlorobenzene, p-chlorophenylglucuronide, 4-chlorocatechol glucuronide and p-chlorophenylmercapturic acid. Biochem J 47:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. (2005) Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci USA 102:2186–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman ME, Prough RA, Estabrook RW, Falck JR, Manna S, Leibman KC, Murphy RC, Capdevila J. (1985) Novel glutathione conjugates formed from epoxyeicosatrienoic acids (EETs). Arch Biochem Biophys 242:225–230. [DOI] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. (2004) Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 43:55–90. [DOI] [PubMed] [Google Scholar]
- Spector AA, Kim HY. (2015) Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim Biophys Acta 1851:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, Norris AW. (2007) Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol 292:C996–C1012. [DOI] [PubMed] [Google Scholar]
- Terashvili M, Tseng LF, Wu HE, Narayanan J, Hart LM, Falck JR, Pratt PF, Harder DR. (2008) Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Ther 326:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley MI, Morisseau C, Hammock B, King JA. (2010) Impact of epoxyeicosatrienoic acids in lung ischemia-reperfusion injury. Microcirculation 17:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Kompa AR, Wang BH, Krum H. (2012) Evaluation of the effects of urotensin II and soluble epoxide hydrolase inhibitor on skin microvessel tone in healthy controls and heart failure patients. Cardiovasc Ther 30:295–300. [DOI] [PubMed] [Google Scholar]
- Tsai HJ, Hwang SH, Morisseau C, Yang J, Jones PD, Kasagami T, Kim IH, Hammock BD. (2010) Pharmacokinetic screening of soluble epoxide hydrolase inhibitors in dogs. Eur J Pharm Sci 40:222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulu A, Appt S, Morisseau C, Hwang SH, Jones PD, Rose TE, Dong H, Lango J, Yang J, Tsai HJ, et al. (2012) Pharmacokinetics and in vivo potency of soluble epoxide hydrolase inhibitors in cynomolgus monkeys. Br J Pharmacol 165:1401–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulu A, Harris TR, Morisseau C, Miyabe C, Inoue H, Schuster G, Dong H, Iosif AM, Liu JY, Weiss RH, et al. (2013) Anti-inflammatory effects of ω-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol 62:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulu A, Stephen Lee KS, Miyabe C, Yang J, Hammock BG, Dong H, Hammock BD. (2014) An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol 64:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Inceoglu B, Gill SS, Hammock BD. (2011a) Epoxygenated fatty acids and soluble epoxide hydrolase inhibition: novel mediators of pain reduction. J Agric Food Chem 59:2816–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Inceoglu B, Hammock BD. (2011b) Soluble epoxide hydrolase inhibition, epoxygenated fatty acids and nociception. Prostaglandins Other Lipid Mediat 96:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Vito S, Inceoglu B, Hammock BD. (2014a) The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat 113-115C:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Yang J, Inceoglu B, Hammock BD. (2014b) Soluble epoxide hydrolase inhibition is antinociceptive in a mouse model of diabetic neuropathy. J Pain 15:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yang J, Guo L, Uyeminami D, Dong H, Hammock BD, Pinkerton KE. (2012) Use of a soluble epoxide hydrolase inhibitor in smoke-induced chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 46:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Shen CL, Dyson MT, Yin X, Schiffer RB, Grammas P, Stocco DM. (2006) The involvement of epoxygenase metabolites of arachidonic acid in cAMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J Endocrinol 190:871–878. [DOI] [PubMed] [Google Scholar]
- Watabe T, Kanai M, Isobe M, Ozawa N. (1980) Cholesterol α- and β-epoxides as obligatory intermediates in the hepatic microsomal metabolism of cholesterol to cholestanetriol. Biochim Biophys Acta 619:414–419. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Schulz D, Morisseau C, Hammock BD. (2006) High-throughput pharmacokinetic method: cassette dosing in mice associated with minuscule serial bleedings and LC/MS/MS analysis. Anal Chim Acta 559:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM. (1967) Third-generation pesticides. Sci Am 217:13–17. [DOI] [PubMed] [Google Scholar]
- Wixtrom RN, Hammock BD.(1985) Membrane-bound and soluble-fraction epoxide hydrolase: methodological aspects, in Biochemical Pharmacology and Toxicology, Volume 1: Methodological Aspects of Drug Metabolizing Enzymes (Zakim D, Vessey DA. eds), pp 1–93, John Wiley & Sons, New York. [Google Scholar]
- Yang J, Bratt J, Franzi L, Liu JY, Zhang G, Zeki AA, Vogel CF, Williams K, Dong H, Lin Y, et al. (2015) Soluble epoxide hydrolase inhibitor attenuates inflammation and airway hyperresponsiveness in mice. Am J Respir Cell Mol Biol 52:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. (1947) The metabolic conversion of naphthalene to 1:2-dihydronaphthalene-1:2-diol. Biochem J 41:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, et al. (2000) Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87:992–998. [DOI] [PubMed] [Google Scholar]
- Zha W, Edin ML, Vendrov KC, Schuck RN, Lih FB, Jat JL, Bradbury JA, DeGraff LM, Hua K, Tomer KB, et al. (2014) Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J Lipid Res 55:2124–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Kodani S, Hammock BD. (2014a) Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog Lipid Res 53:108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Panigrahy D, Hwang SH, Yang J, Mahakian LM, Wettersten HI, Liu JY, Wang Y, Ingham ES, Tam S, et al. (2014b) Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis. Proc Natl Acad Sci USA 111:11127–11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, et al. (2013) Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci USA 110:6530–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. (2004) Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol 15:1244–1253. [PubMed] [Google Scholar]
- Zheng J, Plopper CG, Lakritz J, Storms DH, Hammock BD. (2001) Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome. Am J Respir Cell Mol Biol 25:434–438. [DOI] [PubMed] [Google Scholar]