Abstract
The influence of autophagy inhibition on radiation sensitivity was studied in human breast, head and neck, and non–small cell lung cancer cell lines, in cell lines that were either wild type or mutant/null in p53, and in cells where p53 was inducible or silenced. Whereas ionizing radiation promoted autophagy in all tumor cell lines studied, pharmacological inhibition of autophagy and/or genetic silencing of autophagy genes failed to influence sensitivity to radiation in p53 mutant Hs578t breast tumor cells, HN6 head and neck tumor cells, and H358 non–small cell lung cancer cells. The requirement for functional p53 in the promotion of cytoprotective autophagy by radiation was confirmed by the observation that radiation-induced autophagy was nonprotective in p53 null H1299 cells but was converted to the cytoprotective form with induction of p53. Conversely, whereas p53 wild-type HN30 head and neck cancer cells did show sensitization to radiation upon autophagy inhibition, HN30 cells in which p53 was knocked down using small hairpin RNA failed to be sensitized by pharmacological autophagy inhibition. Taken together, these findings indicate that radiation-induced autophagy can be either cytoprotective or nonprotective, a functional difference related to the presence or absence of function p53. Alternatively, these findings could be interpreted to suggest that whereas radiation can induce autophagy independent of p53 status, inhibition of autophagy promotes enhanced radiation sensitivity through a mechanism that requires functional p53. These observations are likely to have direct implications with respect to clinical efforts to modulate the response of malignancies to radiation through autophagy inhibition.
Introduction
Virtually all patients with localized cancer are treated with some combination of surgery, radiotherapy, and chemotherapy. Therapy is often successful initially, but disease recurrence is not uncommon and is often associated with resistance to treatment (Fodale et al., 2011). Although there are multiple mechanisms that could contribute to therapeutic resistance to radiation such as enhanced DNA repair capacity and overexpression of select survival signaling pathways, recent work has implicated cytoprotective autophagy as a potential basis for resistance that might be exploited for therapeutic purposes (Gewirtz, 2014a,b).
Studies in both cell culture and animal models have demonstrated the potential for improving the response to therapy by the inhibition of cytoprotective autophagy through either pharmacological intervention using drugs such as chloroquine or genetic silencing of autophagy-related genes (Paglin et al., 2001; Boya et al., 2005; Ito et al., 2005; Kondo et al., 2005; Abedin et al., 2007; Amaravadi et al., 2007; Apel et al., 2008; Qadir et al., 2008; Lomonaco et al., 2009; Carew et al., 2010; Wu et al., 2010; Ding et al., 2011; Lopez et al., 2011; Shi et al., 2011; Tseng et al., 2011; Wilson et al., 2011; Bristol et al., 2012; Godbole et al., 2012; Guo et al., 2012; Liang et al., 2012; Rao et al., 2012). In addition, a number of clinical trials have been initiated to determine whether the chloroquine derivative, hydroxychloroquine, can be used to improve the therapeutic response in a variety of malignancies (Sotelo et al., 2006; Solomon and Lee, 2009).
Although studies in the literature generally support the promotion of cytoprotective autophagy induced in response to either chemotherapy or radiation, it is not clear that autophagy uniformly has a protective function (Gewirtz, 2014a). In a recent report, we demonstrated that chloroquine failed to sensitize 4T1 murine breast tumor cells to radiation either in cell culture or in a syngeneic animal model (Bristol et al., 2013), which was also found to be the case for cisplatin (Maycotte et al., 2012). Furthermore, genetic silencing of autophagy genes failed to sensitize the 4T1 cells to radiation.
This work was designed to evaluate the impact of autophagy inhibition on sensitivity to radiation in human tumor cell lines derived from different tissues, specifically the triple negative Hs578t human breast tumor cell line, HN6 and HN30 head and neck cancer cells, and A549, H460, and H835 non–small cell lung cancer cells. We find both cytoprotective and nonprotective autophagy induced by radiation, with cytoprotective autophagy occurring exclusively in cell lines with functional p53. Consistent with this finding, radiation-induced autophagy was nonprotective in p53 null H1299 non–small cell lung cancer cells but could be converted to the protective form with induction of p53. Conversely, p53 wild-type HN30 head and neck cancer cells were sensitized to radiation upon autophagy inhibition (cytoprotective autophagy), whereas HN30 cells with small hairpin RNA (shRNA)–mediated knockdown of p53 were refractory to such sensitization (nonprotective autophagy).
This work suggests that clinical efforts to sensitize patients to radiation (and possibly chemotherapy) through autophagy inhibition may result in inconsistent and uninterpretable outcomes in the absence of information as to whether the autophagy induced by treatment is cytoprotective or nonprotective, an outcome that may be related to whether the tumor cells are wild type or mutant in p53.
Materials and Methods
T75 culture flasks were obtained from Cellstar (Monroe, NC). Minimum essential medium-α containing l-glutamine was obtained from Invitrogen (Grand Island, NY). Trypsin-EDTA (0.25% trypsin, 0.53 mM EDTA-4Na) and fetal bovine serum (FBS) were purchased from Hyclone Scientific (Logan, UT) or Serum Source International (Charlotte, NC). Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling assay reagents (terminal transferase, reaction buffer, and CoCl2 and fluorescein dUTP) were purchased from Roche (Mannheim, Germany). Annexin phosphatidylinositol and propidium iodide (PI) were purchased from BD Biosciences (San Diego, CA). Trypan blue acetic acid, acridine orange, formaldehyde, and 4′,6-diamidino-2-phenylindole were purchased from Invitrogen (Eugene, OR). Phosphate-buffered saline (PBS), l-glutamine, and penicillin-streptomycin were obtained from Gibco (Life Technologies, NY). 5-Bromo-4-chloro-3-β-d-galactopyranoside) for the β-galactosidase staining assay and M-PER mammalian protein extraction reagent lysis buffer were purchased from Thermo Fisher Scientific/Fermentas (Rockford, IL). Chloroquine, 3-methyladenine (3-MA), NH4Cl, crystal violet and dimethylsulfoxide were obtained from Sigma-Aldrich(St. Louis, MO). The Annexin/apoptosis assay kit was obtained from BD Biosciences. Bradford reagent was obtained from Bio-Rad Laboratories (Hercules, CA).
Cell Lines.
Hs578t mammalian breast tumor cells and A549 non–small cell lung cancer cells were obtained from American Type Culture Collection (Rockville, MD). The HN30, shHN30, and HN6 head and neck cancer cell lines were as previously described (Patel et al., 2000). The H358 and A549 non–small cell lung cancer cell lines were generously provided by Dr. Charles Chalfant from the Virginia Commonwealth University Department of Biochemistry and Molecular Biology. shHN30 and Hs578t shBECN1cells were maintained using (1 µg/ml) puromycin. H1299 null and H1299 cells where p53 was inducible by doxycycline were originally developed by Dr. Constantinos Koumenis (Maecker et al., 2000). All cell lines were stored under liquid nitrogen in 10% dimethylsulfoxide with 10% FBS.
Plasmid Construction and Virus Infection.
Mission shRNA bacterial stocks for ATG5 and BECN1 were purchased from Sigma-Aldrich (TRCN00151963 and TRCN0000299864, respectively). Lentiviruses were produced in HEK 293TN cells cotransfected using Lipofectamine (Invitrogen, Carlsbad, CA) with psPAX2 and pMD2.G packaging constructs (Addgene, Cambridge, MA). Viruses shed into the media were then used to infect H1299 cells. Puromycin (1 μg/ml) was used as the selection marker to enrich the infected cells.
Hs578t were also silenced for the Beclin-1 gene. Lentiviral shRNA constructs from the laboratory of Dr. Hisashi Harada were transfected into 293T cells with lentiviral packaging plasmid. The lentivirus shed into the medium were collected to transfect Hs578t cells.
Cell Culture Conditions.
Hs578t breast tumor cells were grown in minimum essential medium-α supplemented with 20% FBS and penicillin/streptomycin (0.5 ml/100 ml medium). HN30 and HN6 head and neck cancer cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS, 2 mM l-glutamine, and 1% penicillin-streptomycin. A549 non–small cell lung cancer cells were cultured in 50% DMEM/50% of RPMI media and 1% penicillin-streptomycin. H358 non–small cell lung cancer cells were grown in DMEM with 10% FBS and 1% penicillin-streptomycin. H1299 non–small cell lung cancer cells were cultured in DMEM supplemented with 10% FBS and 5% penicillin G/streptomycin; doxycycline at a 1µg/ml concentration was added for induction of p53 expression. Cell cultures were maintained at 37°C under a humidified 5% CO2 atmosphere and examined for bacterial and fungal infections prior to experiments.
Drug and Radiation Treatment.
Chloroquine was prepared as a stock solution of 50 mM in pure water and stored at −20°C. 3-MA was prepared as a stock solution of 100 mM dissolved in PBS with heating and refrigerated. NH4Cl was prepared as a 100-mM stock in PBS. Chloroquine, 3-MA, and NH4Cl were added 3 hours before radiation and cells were maintained in the presence of chloroquine, 3-MA, or NH4Cl until radiation was completed. Media were replenished once treatment was terminated.
Hs578t cells were irradiated with 5 × 2 Gy over a period of 3 days. HN30 and HN6 cells were treated with a single dose of 4 Gy, whereas A549, H358, H1299 (in the absence of doxycycline [−Dox]), and H1299 (in the presence of doxycycline [+Dox]) cells were irradiated with a single 6-Gy dose of radiation. The doses of radiation were chosen to optimize the likelihood of detecting sensitization when autophagy was inhibited.
Assessment of Cell Viability and Clonogenic Survival.
Cell viability was measured by trypan blue exclusion. Cells were incubated with trypsin (0.25% trypsin-EDTA) for 5–10 minutes, stained with trypan blue (0.4% trypan blue), and counted using a hemocytometer with phase-contrast microscopy on the indicated days after radiation. For the clonogenic survival assay, cells were plated in triplicate, typically at a density of 100 cells/well. Ten to 14 days after treatment, cells were washed with PBS, fixed with 100% methanol, air dried, and stained with 0.1% (v/v) crystal violet. Cells in groups of 50 or more were counted as colonies and data were normalized relative to untreated controls.
Detection and Quantification of Autophagic Cells.
Cells were plated in six-well culture dishes, stained with acridine orange at a final dilution of 1:10,000 in DMEM, and allowed to incubate at 37°C for 10 minutes. After two washes with PBS, cells were examined and imaged under an inverted fluorescence microscope (Olympus, Tokyo, Japan). All images were taken at the same magnification. For quantification of autophagic vesicles, cells were trypsinized, harvested, and washed with PBS. Pellets were resuspended in PBS, stained with a 1:10,000 dilution of acridine orange for 10 minutes, and analyzed by BD FACSCanto II using BD FACSDiva software at the Virginia Commonwealth University Flow Cytometry Core Facility. A minimum of 10,000 cells within the gated region were analyzed.
Flow Cytometry Analysis Using Annexin V–PI Staining to Determine Apoptotic and Necrotic Cells.
Treated cells were collected and labeled fluorescently using a fluorescein isothiocyanate (FITC) Annexin V apoptosis detection kit I. Labeling was performed by adding 500 µl of binding buffer, 5 µl Annexin V–FITC, and 5µl propidium iodide to each sample (FITC Annexin V apoptosis detection kit; BD Biosciences, San Jose, CA). Samples were mixed gently and incubated in the dark for 15 minutes. Annexin V–PI labeled cells were measured by flow cytometry and analyzed by BD FACS Canto II and BD DIVA software. A minimum of 10,000 cells were counted within the gated region.
Western Blot Analysis.
After the indicated treatments, cells were scraped from the culture dishes, collected, and lysed using M-PER mammalian protein extraction reagent (Thermo Fisher Scientific, Waltham, MA) containing protease and phosphatase inhibitors. Protein concentrations were determined by the Bradford assay (Bradford, 1976) (Bio-Rad Laboratories). Total protein was then diluted in SDS sample buffer and dry boiled for 10 minutes. Protein samples were subjected to SDS-PAGE, transferred to polyvinylidene difluoride membrane, and blocked in 5% milk/1× PBS/ 0.1% Tween for 1 hour. Primary antibodies used at a 1:1000 dilution with overnight incubation were p62 (BD Transduction), ATG5 (Cell Signaling Technology, Danvers, MA), BECN1 (Cell Signaling Technology), LC3II (Cell Signaling Technology), p53 (BD Pharmingen), and β-actin (Santa Cruz Biotechnology). The membrane was then incubated with secondary antibody of either horseradish peroxidase–conjugated goat anti-rabbit IgG antibody (1:5000; Sigma-Aldrich) or goat anti-mouse (1:5000; Sigma-Aldrich) for 1 hour, followed by extensive washing with Tween-PBS. Blots were developed using Pierce enhanced chemiluminescence reagents and Bio-Max film (Thermo Fisher Scientific).
Statistical Analysis.
Statistical differences were determined by StatView statistical software (SAS Institute, Cary, NC). The data were expressed as means ± S.E. Comparisons were made using one-way analysis of variance followed by the Bonferroni post hoc test. P values <0.05 were taken as statistically significant.
Results
Radiation Induces Autophagy and Prolonged Growth Arrest in Hs578t Breast Tumor Cells.
Previous studies from this laboratory have shown that radiation induces cytoprotective autophagy in MCF-7 and ZR-75 breast tumor cell lines (Wilson et al., 2011; Bristol et al., 2012), both of which are wild type in p53. The first series of experiments presented here involves Hs578t breast tumor cells, which are mutant in p53 (Nieves-Neira and Pommier, 1999). Figure 1A indicates that exposure of Hs578t breast tumor cells, which are triple negative cells (Foulks et al., 2010), to radiation (5 × 2 Gy over a period of 3 days) results primarily in growth arrest; the growth arrest was relatively prolonged as the cells did not recover for at least 11 days after irradiation (data not shown).
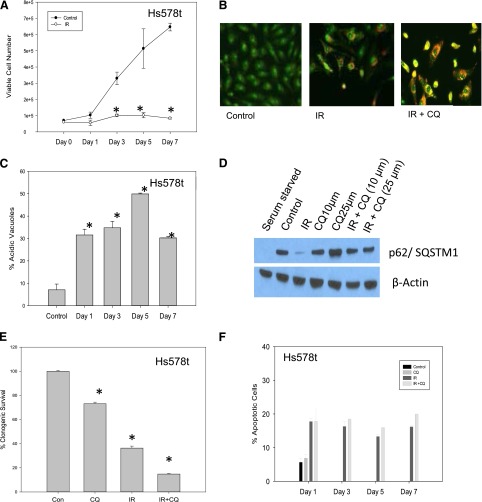
Fig. 1.
Induction of autophagy by radiation in Hs578t breast tumor cells; autophagy inhibition by chloroquine does not result in sensitization. (A) Hs578t cells were exposed to 5 × 2 Gy of radiation over a period of 3 days and the viable cell number was monitored by trypan blue exclusion. Data represent the mean of three experiments ± S.E. (B) Induction of autophagy upon irradiation of Hs578t cells. Acridine orange staining of autophagic vacuoles on day 7 after exposure to 5 × 2 Gy of radiation with and without chloroquine (25 µM). (C) Quantification of autophagy measured by flow cytometry. Data are representative of the mean ± S.E. of three experiments. (D) p62/SQSTM1 degradation indicative of autophagic flux in Hs578t cells on day 1 after radiation. Serum starvation was included as a positive control. The experiment was reproduced three times. (E) Clonogenic survival assay in Hs578t cells. Data represent the average of three experiments plotted with mean ± S.E. (F) Induction of apoptosis in irradiated cells with or without chloroquine. *P < 0.05 compared with control. Con, control; CQ, chloroquine; IR, radiation.
Figure 1B indicates that radiation promoted autophagy in the Hs578t cells based on the detection of puncta by acridine orange staining. Figure 1C (in which the extent of autophagic vesicle formation was assessed by flow cytometry) indicates that once autophagy was induced, its extent was relatively stable over a period of 7 days (with a slight transient increase at day 5).
Evidence That Radiation-Induced Autophagy Does Not Have a Cytoprotective Function in Hs578t Breast Tumors Based on Lack of Sensitization by the Pharmacological Autophagy Inhibitor, Chloroquine.
To determine whether radiation-induced autophagy has a cytoprotective function in Hs578t cells, autophagy was inhibited with the use of three separate pharmacological inhibitors: 3-MA, chloroquine, and NH4Cl. Appropriate concentrations (25 µM for chloroquine, 5 mM for 3-MA, and 10 mM for NH4Cl) were chosen by determining concentrations that were minimally toxic to the tumor cells using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (data not shown). Figure 1B confirms that chloroquine inhibited radiation-induced autophagy based on the change from red/orange to yellow of the autophagosomes, which reflects the fact that chloroquine interferes with the acidification required for fusion of lysosomes with the autophagosome (Bristol et al., 2012). Additional evidence that radiation promotes autophagy in the Hs578t cells, as well as that chloroquine inhibited radiation-induced autophagy, is provided by the observation that chloroquine prevented the radiation-induced degradation of p62/SQSTM1 (Fig. 1D), an indication of radiation-induced autophagic flux (Bjørkøy et al., 2009; He and Klionsky, 2009; Feng et al., 2014). Serum starvation was used as a positive control.
To determine whether autophagy might be playing a cytoprotective (or alternatively a cytotoxic) function in the Hs578t cells, clonogenic survival was assessed in cells exposed to radiation in the absence and presence of chloroquine. As shown in Fig. 1E, only an apparent additive effect of radiation and chloroquine was observed; that is, the reduced clonogenic survival in cells treated with the combination of radiation and chloroquine reflects the combined antiproliferative and/or cytotoxic effects of each of the treatment modalities. Specifically, with exposure of Hs578t cells to fractionated radiation alone, there is an approximately 55% reduction in survival, whereas with chloroquine, cell survival is reduced by approximately 30%. The combination treatment of radiation plus chloroquine reduced survival by approximately 85%, which is what would be anticipated from additive toxicities of the individual treatments.
Blocking Autophagy with Chloroquine Does Not Promote Apoptosis in Irradiated Hs578t Cells.
In classic cytoprotective autophagy, cells use autophagy as a mechanism for cell survival and consequently when autophagy is inhibited, cells die, primarily by apoptosis (Gewirtz et al., 2009). As shown in Fig. 1F, assessment of apoptosis by flow cytometry using Annexin V/PI indicated that only between 15 and 20% of the cell population was undergoing apoptosis after radiation. Furthermore, inhibition of autophagy using chloroquine did not increase the extent of apoptosis, which supports the conclusion that autophagy is unlikely to be cytoprotective in this experimental system.
Chloroquine Fails to Sensitize Hs578t Cells to a Single Dose of Radiation.
Because the 5 × 2 Gy radiation alone produced a relatively pronounced reduction in clonogenic survival, it might be argued that the studies lack sufficient sensitivity to detect sensitization. Consequently, the clonogenic survival studies were repeated using a single radiation dose of 4 Gy. Chloroquine also failed to sensitize the Hs578t cells to the 4 Gy of radiation in that the reduction in clonogenic survival for the combination treatment (approximately 52%) essentially reflected the additive toxic effects of the chloroquine (approximately 13%) and the radiation (approximately 38%) (data not shown).
Evidence That Radiation-Induced Autophagy Does Not Have a Cytoprotective Function in Hs578t Breast Tumor Cells Based on Lack of Sensitization by the Pharmacological Autophagy Inhibitors, Ammonium Chloride and 3-MA.
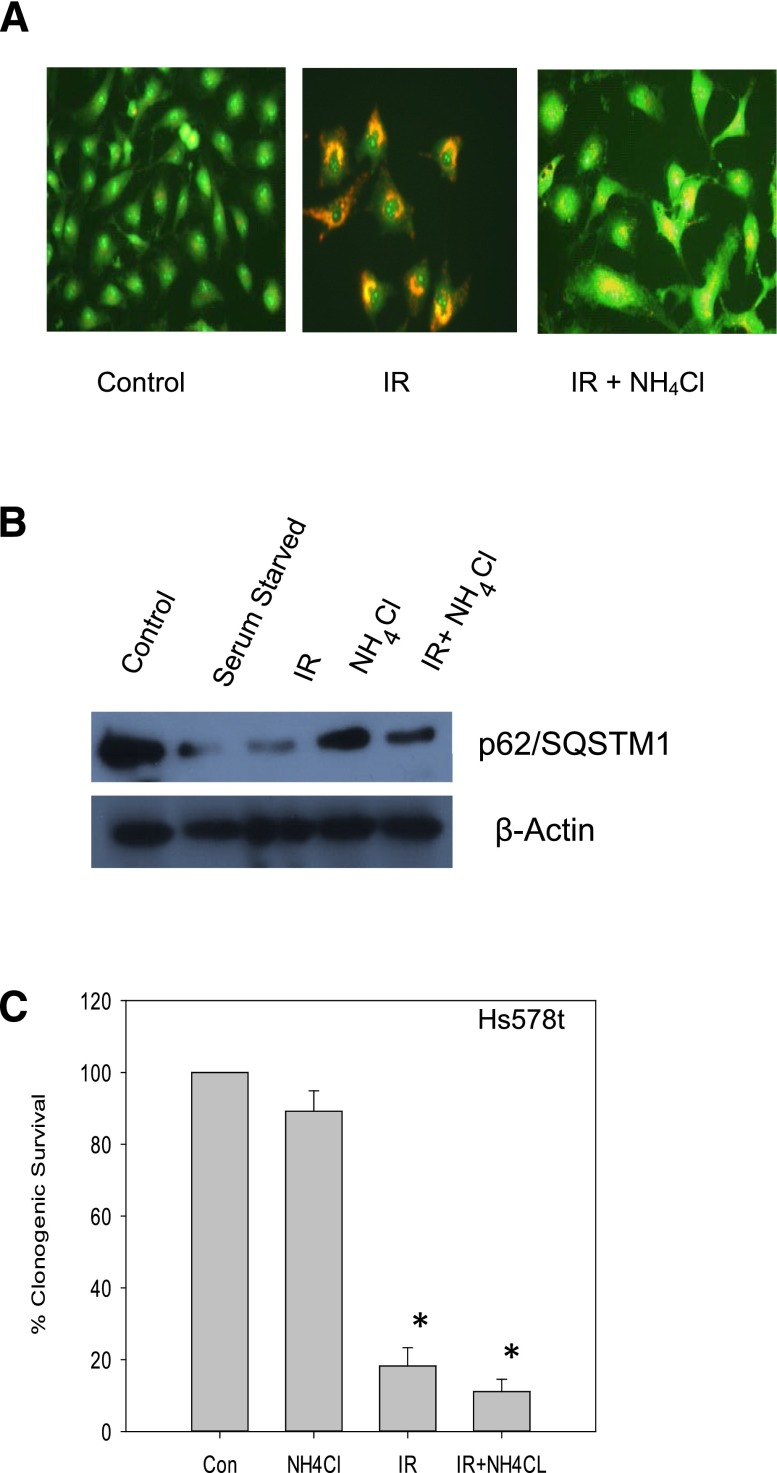
Figure 2 presents studies that confirm that radiation promotes a nonprotective form of autophagy in Hs578t breast tumor cells by assessing the influence of another late stage autophagy inhibitor, NH4Cl (Ikeda et al., 2013), on sensitivity to radiation. Figure 2A shows the promotion of autophagy by radiation and that NH4Cl inhibits radiation-induced autophagy based on the change from the reddish-orange color of the acridine orange–stained vesicles to a yellowish color (again indicative of interference with the acidification step required for completion of autophagy). Figure 2B again indicates that radiation reduced p62/SQSTM1 levels, and that NH4Cl interfered with the degradation of p62. In the clonogenic survival assay presented in Fig. 2C, the combination of an autophagy inhibitor, NH4Cl, with radiation showed a modest but insignificantly greater effect than radiation alone that again, appears to reflect the additive antiproliferative effects of radiation and NH4Cl.
Fig. 2.
Inhibition of radiation-induced autophagy in Hs578t cells using NH4Cl does not result in sensitization. (A) Influence of ammonium chloride (10 mM) on autophagic vacuole formation based on acridine orange staining (day 4 after radiation). (B) p62/SQSMT1 degradation indicative of autophagic flux in Hs578t cells (day 1 after radiation). The experiment was repeated three times with similar results. (C) Clonogenic survival assay indicating that inhibition of autophagy by NH4Cl failed to sensitize Hs578t cells to radiation. Data are representative of three experiments plotted with mean ± S.E. *P < 0.05 compared with control. Con, control; IR, radiation.
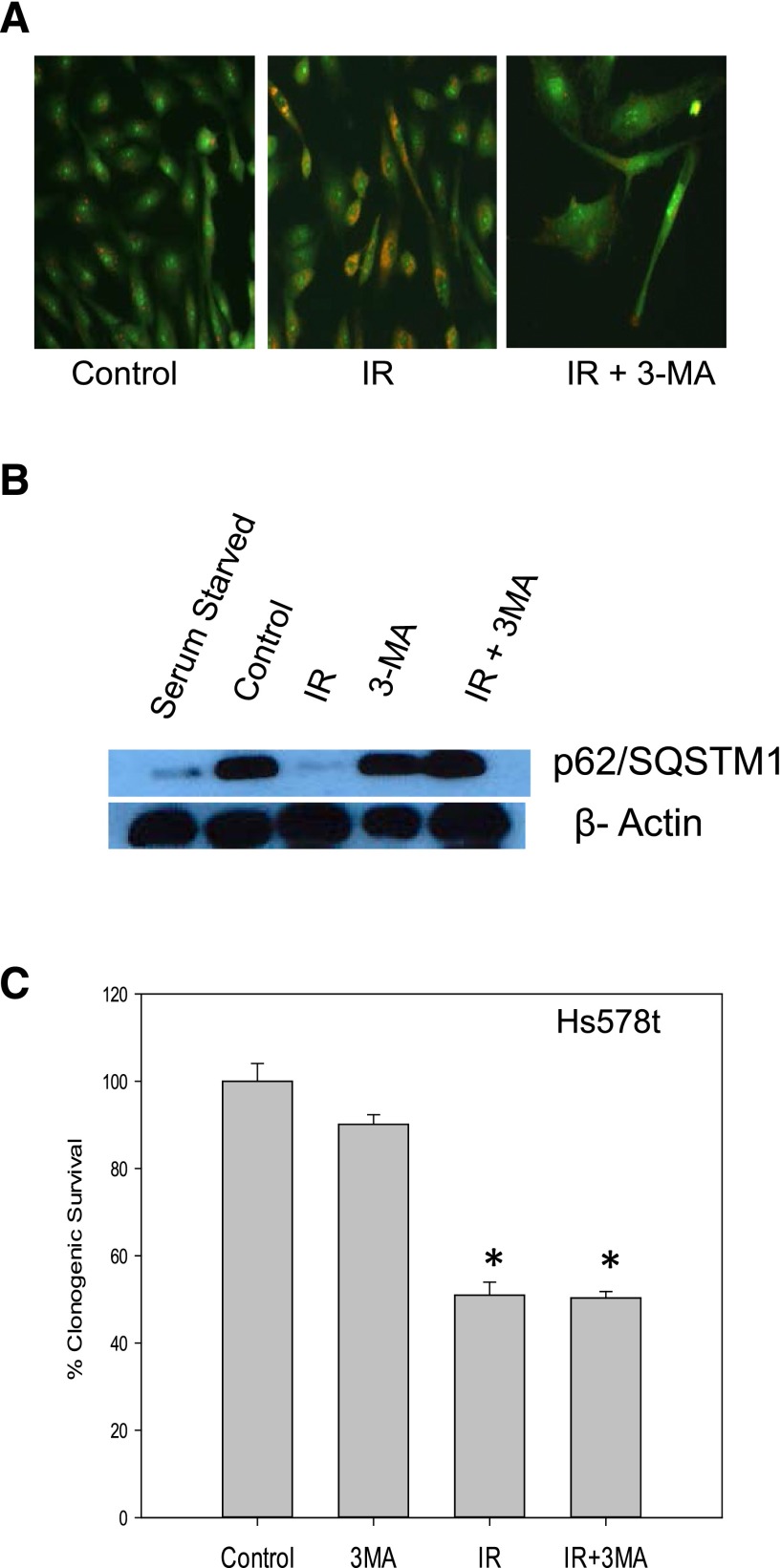
Additional studies were performed using the early stage autophagy inhibitor, 3-MA (Boya et al., 2005). Figure 3A demonstrates that, as would be expected, there is a decline in autophagic vesicle formation in Hs578t cells treated with 3-MA and radiation (in contrast with the accumulation of vacuoles in the studies with the late stage autophagy inhibitors chloroquine and ammonium chloride). The autophagic flux in irradiated cells is shown by the degradation of p62/SQSMT1 in Fig. 3B, which is prevented by 3-MA. The clonogenic survival assay presented in Fig. 3C indicates that 3-MA failed to alter sensitivity to radiation, confirming the findings when autophagy was inhibited using either chloroquine or NH4Cl.
Fig. 3.
Inhibition of radiation-induced autophagy in Hs578t cells using 3-MA does not result in sensitization. (A) Influence of 3-MA (5 mM) on autophagic vacuole formation based on acridine orange staining (day 1 after radiation). (B) p62/SQSMT1 degradation indicative of autophagic flux in Hs578t cells (day 1 after radiation). The experiment was repeated three times with similar results. (C) Clonogenic survival assay indicating that inhibition of autophagy by 3-MA failed to sensitize Hs578t cells to radiation. Data are the average of three experiments plotted with mean ± S.E. *P < 0.05 compared with control. IR, radiation.
Evidence That Radiation-Induced Autophagy Does Not Have a Cytoprotective Function in Hs578t Breast Tumors Based on Lack of Sensitization by Genetic Autophagy Inhibition.
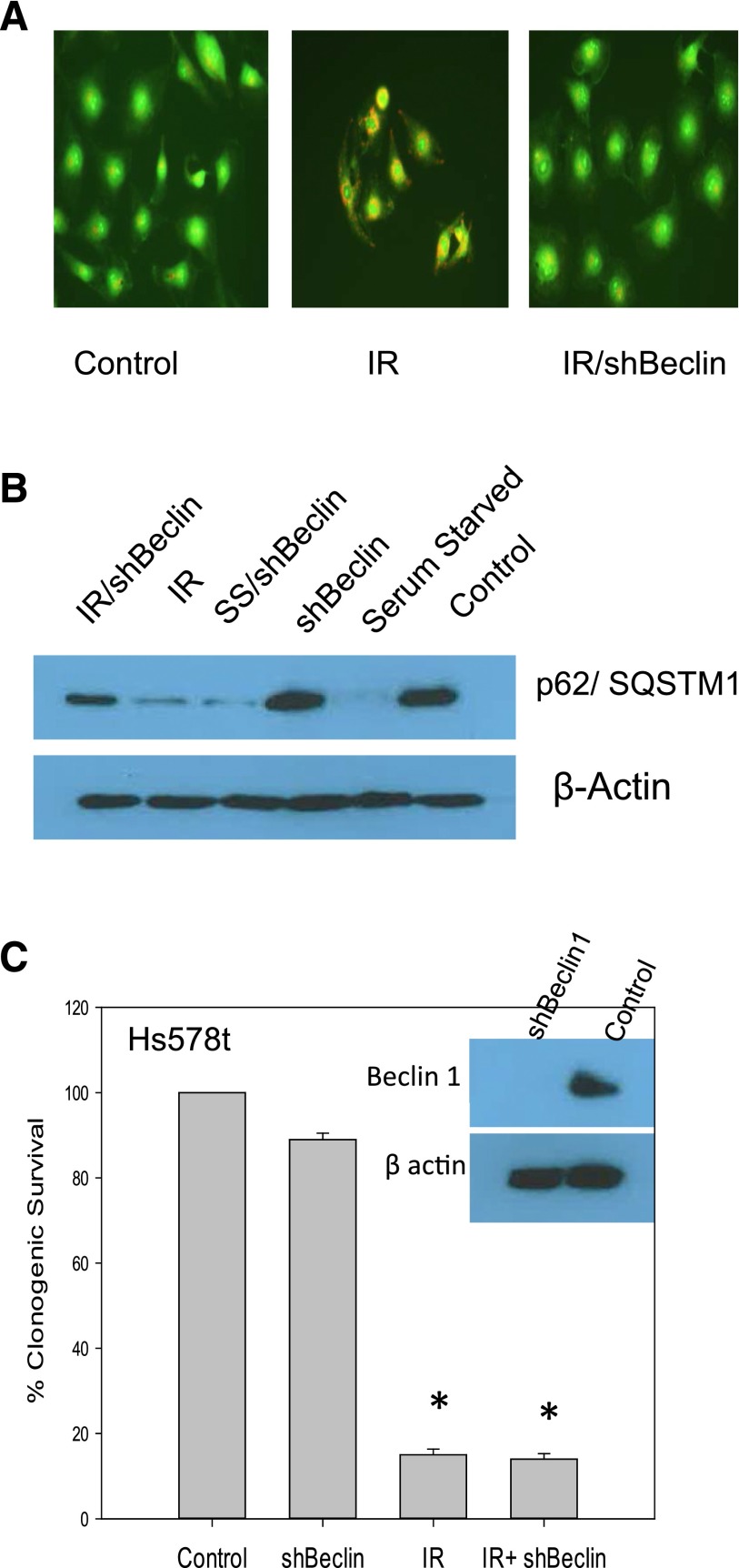
Although the results generated using three separate pharmacological autophagy inhibitors were consistent in failing to demonstrate significant sensitization to radiation, it was nevertheless necessary to confirm these observations through genetic inhibition of autophagy. The inset in Fig. 4C indicates that Beclin-1, often considered as an essential gene in the autophagy pathway (Kang et al., 2011), was knocked down using an shRNA strategy. Figure 4A shows that induction of autophagy by radiation was suppressed in the Beclin-1–silenced cells based on acridine orange vacuole staining. Figure 4B further demonstrates that the silencing of Beclin-1 interfered with the radiation-induced degradation of p62/SQSMT1. Somewhat disconcertingly, silencing of Beclin-1 had only a modest effect on degradation of p62/SQSMT1 under conditions of serum starvation, suggesting that autophagy under these conditions may not be entirely Beclin-1 dependent. Figure 4C shows that when Hs578t cells were silenced for Beclin-1, radiation sensitivity was identical to that in the Hs578t cells without Beclin-1 silencing. These studies appear to confirm the conclusion that autophagy induced by radiation in Hs578t cells is not protective in function.
Fig. 4.
Genetic inhibition of radiation-induced autophagy in Hs578t cells does not result in sensitization. (A) Autophagic vacuole formation based on acridine orange staining in irradiated Hs578t cells in which Beclin-1 was silenced (day 1 after radiation). (B) p62/SQSMT1 degradation indicative of autophagic flux in Hs578t cells (day 1 after radiation). The experiment was repeated three times with similar results. (C) Clonogenic survival assay indicating that silencing of Beclin-1 failed to sensitize Hs578t cells to radiation. The inset shows Beclin-1 silencing. Data represent the average of three experiments plotted with mean ± S.E. *P < 0.05 compared with control. IR, radiation.
Radiation-Induced Autophagy Is Cytoprotective in p53 Wild-Type HN30 Head and Neck Cancer Cells and Nonprotective in p53 Mutant HN6 Head and Neck Cancer Cells.
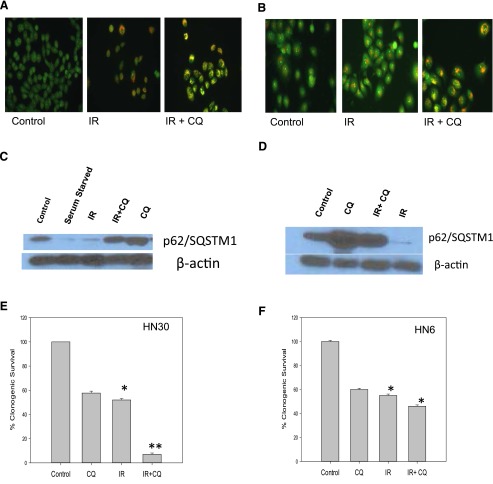
We previously reported on the cytoprotective autophagy induced by radiation in MCF-7 and ZR-75 breast tumor cell lines (Wilson et al., 2011; Bristol et al., 2012) and the data presented above establish that autophagy induced by radiation can likewise be nonprotective in breast tumor cells. To extend these findings to tumor cells derived from another tissue source, studies were conducted in p53 wild-type HN30 and p53 mutant HN6 head and neck tumor cells, a disease in which radiation is also a conventional therapy (Zackrisson et al., 2003). Figure 5A again shows radiation-induced autophagy in the HN30 cells based on acridine orange staining and the capacity of chloroquine to alter the nature of the staining, indicative of a blockade to the acidification step that is necessary for autophagosome-lysosome fusion. Figure 5C confirms that radiation is promoting degradation of p62/SQSTM1 and that this degradation is essentially abrogated by chloroquine. Figure 5E presents clonogenic survival in the HN30 cells with radiation alone and radiation plus chloroquine. Although the clonogenic survival studies presented in Fig. 5E are suggestive of sensitization, we recognize that it could be argued that the data in are merely showing additive toxicities of the combination treatment. To demonstrate that chloroquine actually sensitized the HN30 cells to radiation, temporal response studies were performed. As shown in Supplemental Fig. 1, the combination of chloroquine with radiation produces a sustained growth arrest that is not seen for either radiation or chloroquine treatment alone.
Fig. 5.
Radiation-induced autophagy in head and neck cancer cells. Inhibition of radiation-induced autophagy sensitizes p53 wild-type HN30 but not p53 mutant HN6 head and neck cancer cells. (A and B) Acridine orange staining of autophagic vacuoles (day 1 after exposure to 4 Gy of radiation) in HN30 cells (A) and HN6 cells (B) with and without chloroquine (10 µM). (C and D) p62/SQSMT1 degradation indicative of autophagic flux in HN30 cells (C) and HN6 cells (D) day 1 after radiation. (E) Clonogenic survival assay indicating sensitization upon inhibition of radiation-induced autophagy in HN30 cells. Data represent the average of three experiments plotted with mean ± S.E. (F) Clonogenic survival assay indicating lack of sensitization upon inhibition of radiation-induced autophagy in HN6 cells. *P < 0.05 compared with control; **P < 0.05 compared with radiation. CQ, chloroquine; IR, radiation.
Similar studies performed in the HN6 cells show the induction of autophagy by radiation and interference with autophagy by chloroquine (Fig. 5B). Furthermore, chloroquine is again shown to prevent the degradation of p62 induced by radiation (Fig. 5D). However, in contrast with the findings with the HN30 cells, Fig. 5F shows a lack of radiation sensitization by chloroquine in the HN6 cells by the clonogenic survival assay. This lack of sensitization was confirmed by the temporal response study presented in Supplemental Fig. 1B.
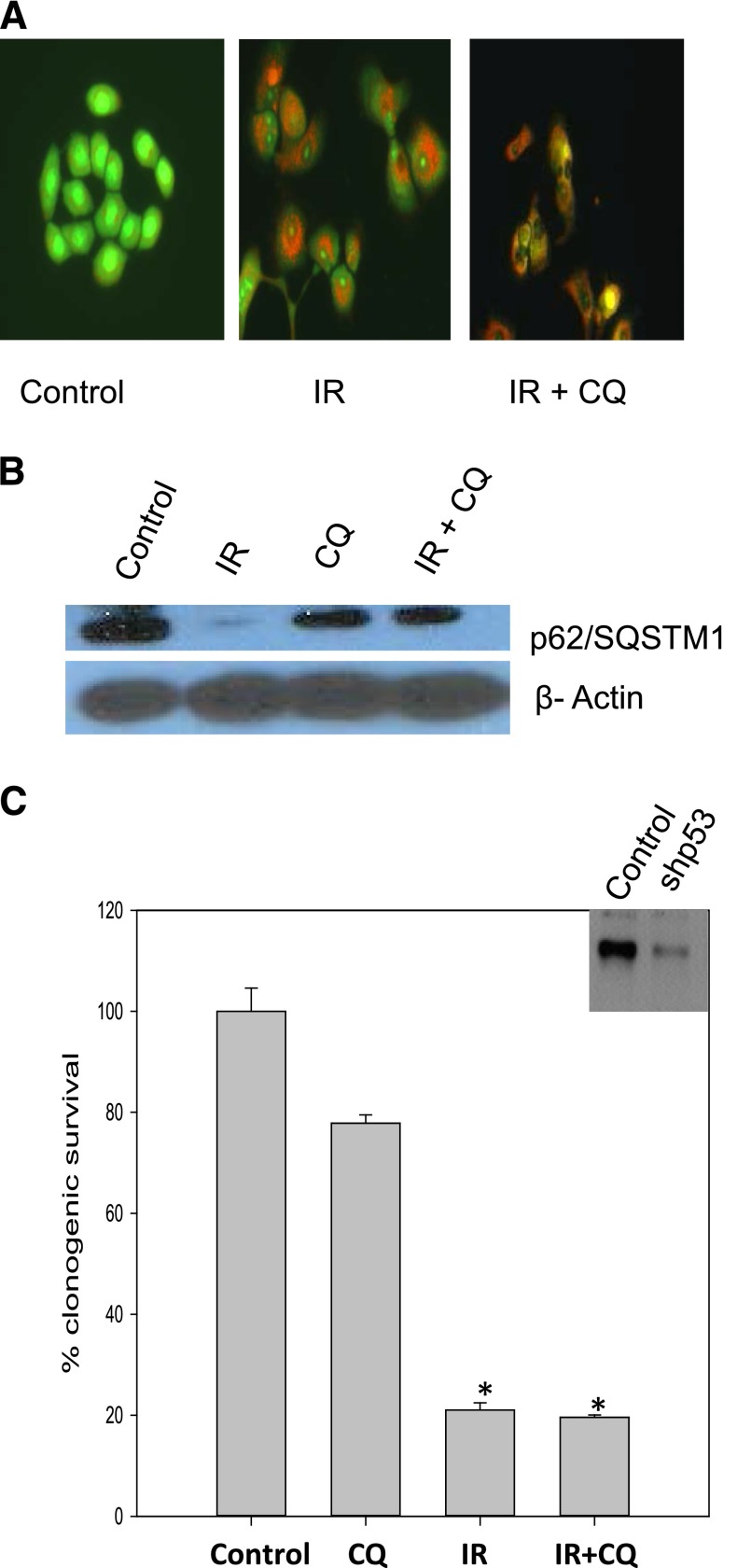
Autophagy Inhibition with Chloroquine Fails to Sensitize HN30 Cells with Knockdown of p53.
To this point, all of the data presented are essentially circumstantial in terms of implicating a requirement for functional p53 in the capacity of ionizing radiation to promote the cytoprotective form of autophagy. To more directly address this question, p53 was knocked down in the p53 wild-type HN30 head and neck tumor cells (shown in the inset in Fig. 6C). Supplemental Figure 2B again shows sensitization in the clonogenic survival assay in which autophagy in the HN30 cells was inhibited by chloroquine (as supported by the acridine orange staining in Fig. 5C and Supplemental Fig. 2B). This study had to be repeated for comparative purposes with the p53 silenced cells since the experiments presented in Fig. 5 had been performed months previously. By contrast, the shp53 HN30 cells failed to be sensitized to radiation upon autophagy inhibition (Fig. 6C). Again, the inhibition of autophagy was confirmed based on acridine orange staining and interference with the degradation of p62/SMQST1 (Fig. 6, A and B).
Fig. 6.
Evidence for p53 dependence of cytoprotective autophagy; lack of sensitization by chloroquine in HN30 cells with knockdown of p53. (A) Acridine orange staining in shp53 HN30 shows induction of autophagy and inhibition of autophagy by chloroquine. (B) p62/SQSMT1 degradation indicative of autophagic flux in shp53HN30 cells (day 1 after radiation). (C) Clonogenic survival assay in shp53 HN30 cells indicating lack of sensitization by autophagy inhibition. CQ, chloroquine; IR, radiation. *P < 0.05 compared with control.
Radiation-Induced Autophagy Is Protective in p53 Wild-Type A549 Non–Small Cell Lung Cancer Cells and Nonprotective in p53 Mutant H358 Non–Small Cell Lung Cancer Cells.
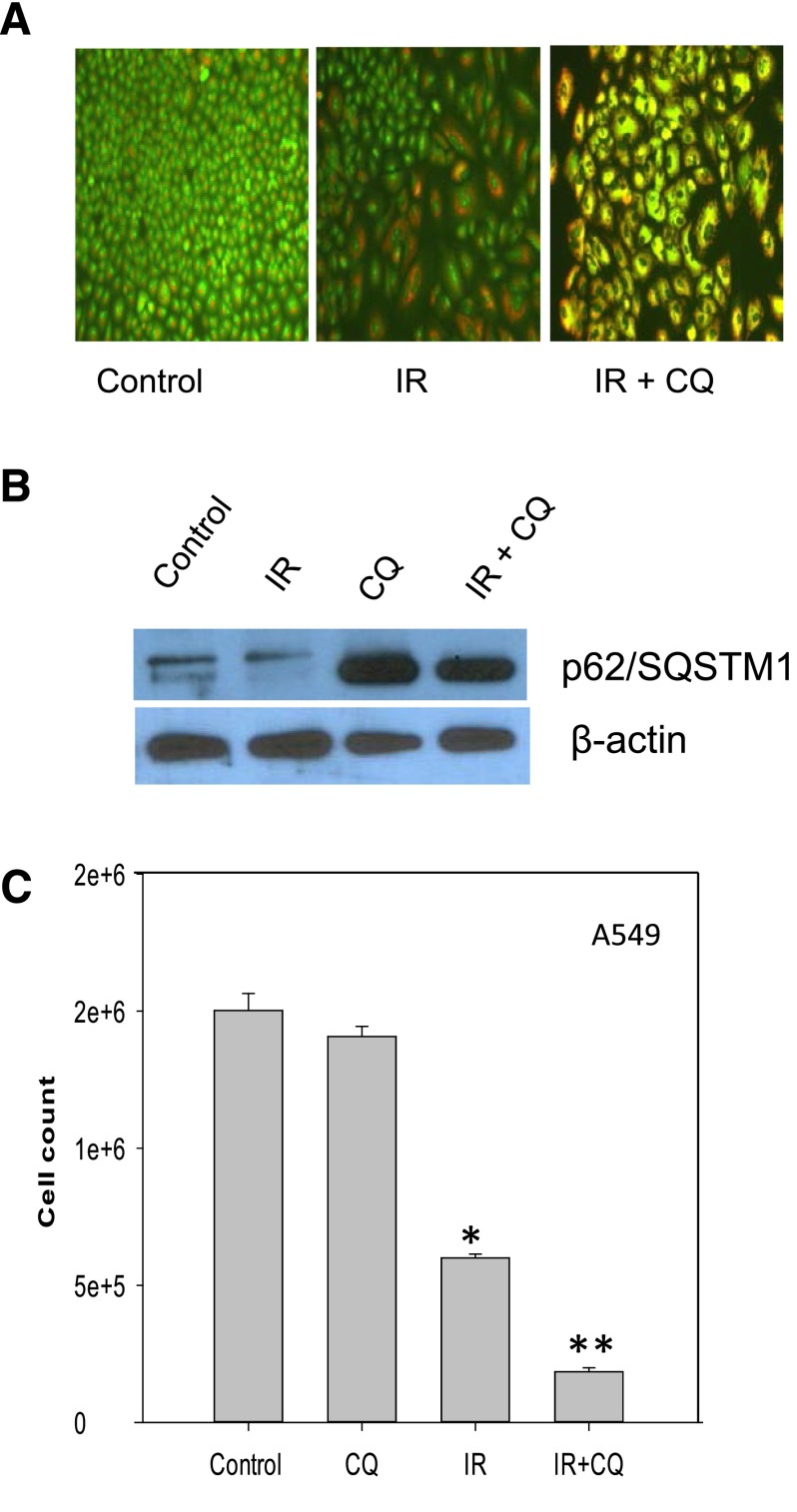
In a recent report, radiation was shown to promote cytoprotective autophagy in A549 and H460 non–small cell lung cancer cells (Ko et al., 2014), both of which are wild type in p53. We have confirmed these findings in A549 cells (H460 cells not shown). Figure 7A shows the induction of autophagy by ionizing radiation and interference with autophagy by chloroquine based on the yellow color that reflects suppression of acidification and autophagosome-lysosome fusion. Figure 7B shows that the p62/SQSTM1 degradation is compromised by the chloroquine treatment. Figure 7C demonstrates sensitization to radiation by the chloroquine based on a count of viable cells.
Fig. 7.
Inhibition of radiation-induced autophagy sensitizes A549 non–small cell lung cancer cells. (A) Induction of autophagy upon radiation in A549 cells. Acridine orange staining of autophagic vacuoles (day 1 after exposure to 6 Gy of radiation) with and without chloroquine (10 µM). (B) p62/SMQST1 degradation indicative of autophagic flux in A549 cells. The experiment was repeated three times with similar results. (C) Clonogenic survival studies indicative of sensitization. Data represent the average of three experiments plotted with mean ± S.E. *P < 0.05 compared with control; **P < 0.05 compared with radiation alone. CQ, chloroquine; IR, radiation.
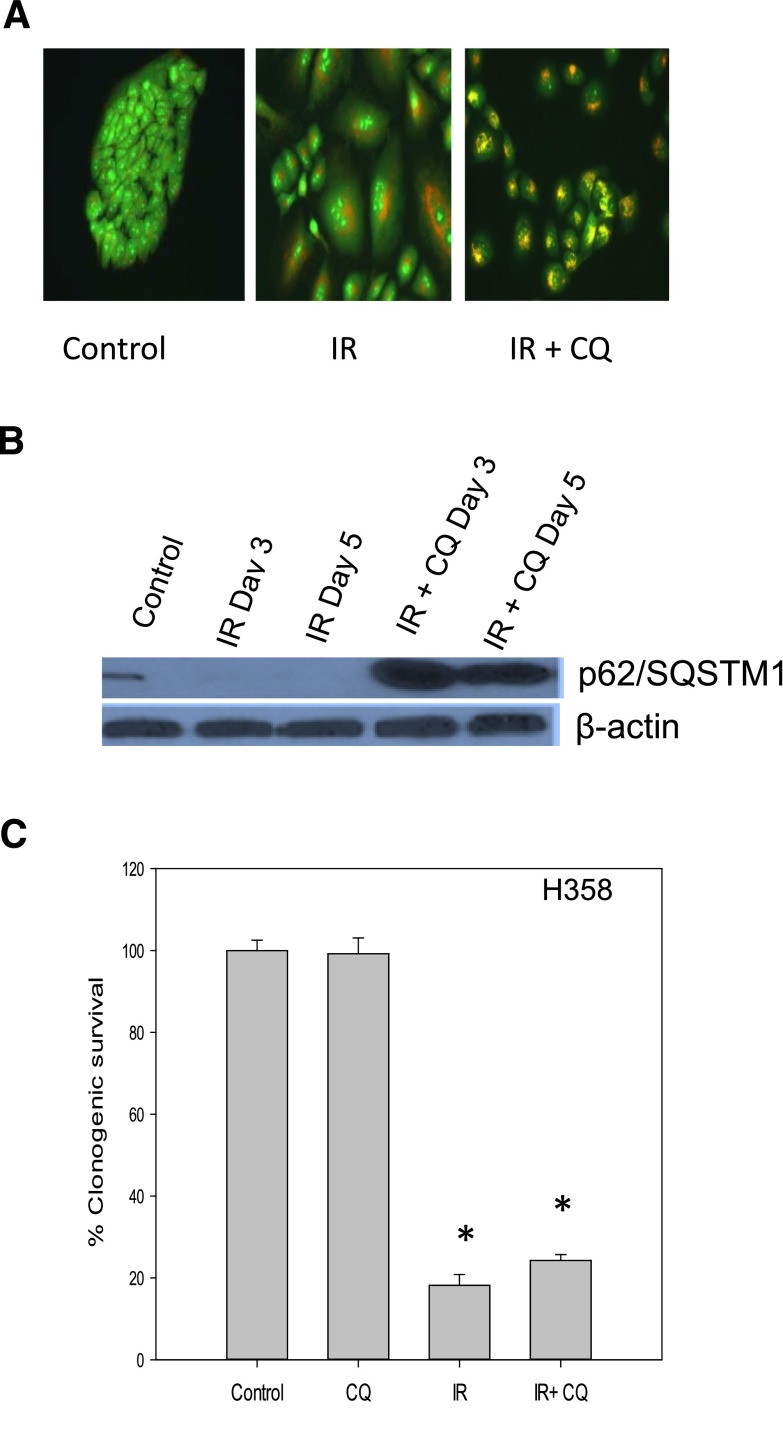
When similar studies were performed using the (p53 mutant) H358 non–small cell lung cancer cell line, autophagy and autophagic flux were again induced by radiation and were inhibited by chloroquine (Fig. 8, A and B). However, in contrast with the results observed in the A549 and H460 cells, chloroquine failed to alter radiation sensitivity in the H358 cells (Fig. 8C).
Fig. 8.
Inhibition of radiation-induced autophagy fails to sensitize H358 non–small cell lung cancer cells. (A) Induction of autophagy upon irradiation. Acridine orange staining of autophagic vacuoles (day 1 after exposure to 6 Gy of radiation) with and without chloroquine (10 µM). (B) p62/SQSMT1 degradation indicative of autophagic flux in H358 cells. (C) Clonogenic survival studies indicative of lack of sensitization. Data represent the average of three experiments with mean ± S.E. CQ, chloroquine; IR, radiation. *P < 0.05 compared with control.
Radiation-Induced Autophagy Is Nonprotective in p53 Null H1299 Non–Small Cell Lung Cancer Cells but Is Converted to Protective Autophagy with Induction of p53.
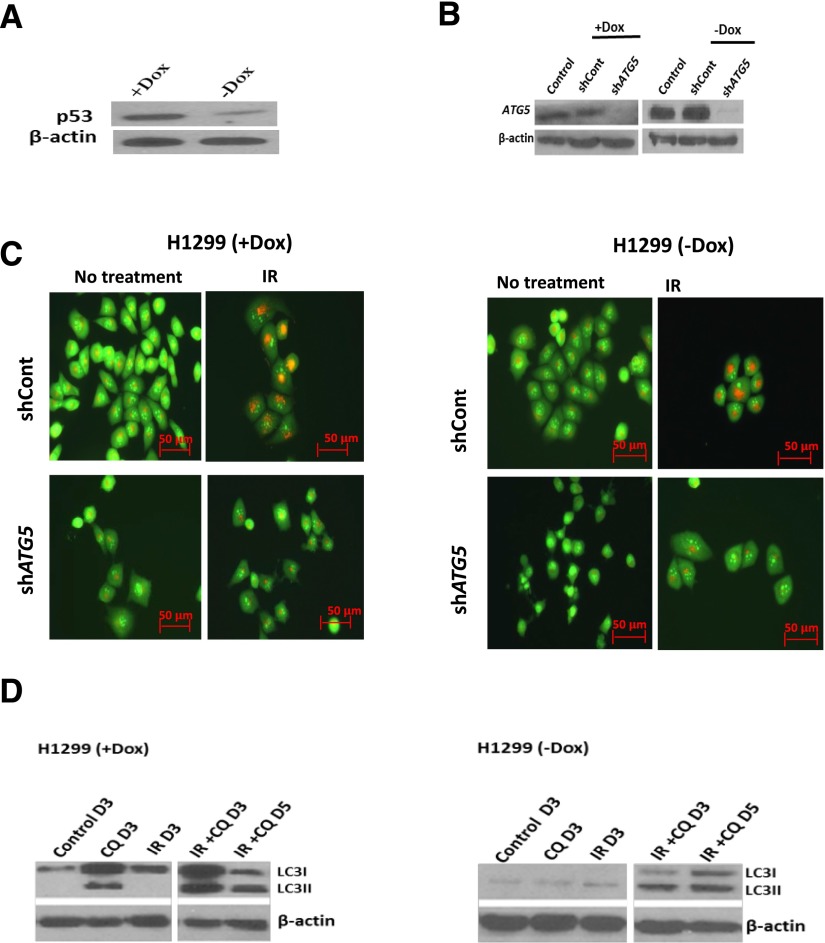
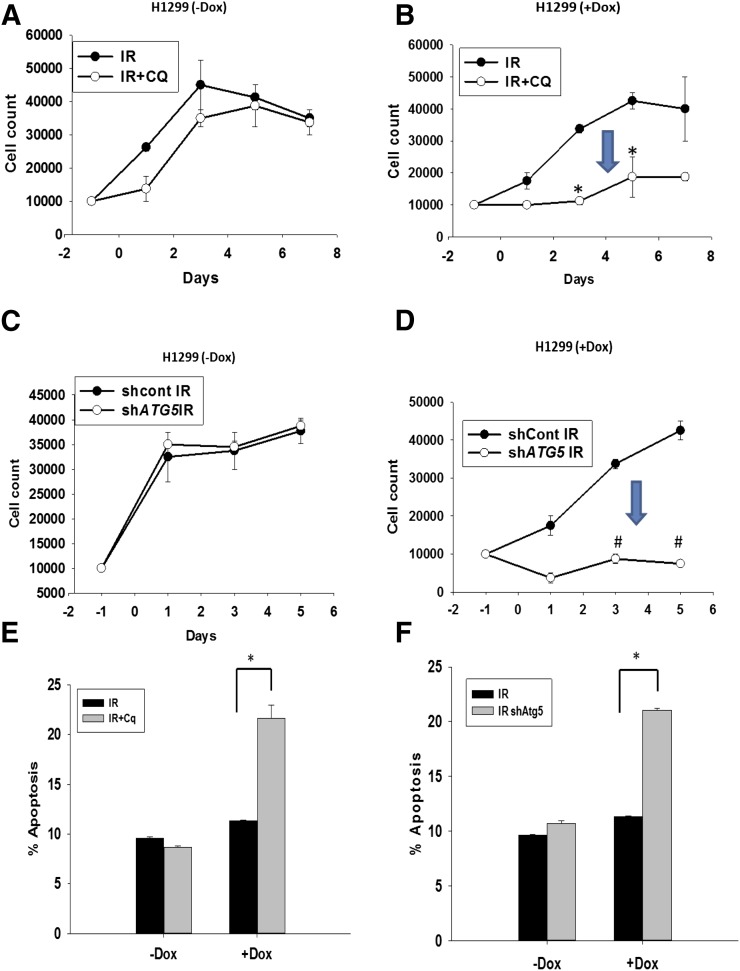
Taken together, these findings are clearly suggestive of p53 dependence for radiation-induced cytoprotective autophagy since in this and previous work, protective autophagy was observed in cells with functional p53 (MCF-7, ZR-75, A549, H460, and HN30) but not in cells null or mutant in p53 (4T1, Hs578t, H358, and HN6). This conclusion is supported by the findings in the HN30 cells with knockdown of p53, shown above. To more convincingly establish this conclusion, additional studies were designed to assess the impact of interfering with radiation-induced autophagy in p53 null H1299 non–small cell lung cancer cells and their isogenic counterparts in which p53 was induced by doxycycline. Figure 9C indicates that radiation induced autophagy in both the p53 null cells and the cells where p53 was induced by doxycycline (the Western blot in Fig. 9A indicates p53 induction). Figure 9, C and D, present evidence that autophagy has been inhibited based on a decrease in acidic vesicle formation when ATG5 is knocked down (knockdown indicated in Fig. 9B) or an accumulation of LC3II with exposure to chloroquine (Fig. 9D). Radiation sensitivity was essentially unaltered when radiation-induced autophagy in H1299 cells that are null in p53 was inhibited (either by silencing of ATG5 or chloroquine treatment) (Fig. 10, A and C), with the exception of a small transient increase on day 1 (shown in Fig. 10A). In quite dramatic contrast, both ATG5 silencing and treatment with chloroquine resulted in pronounced sensitization to radiation in the H1299 cells when p53 was induced by doxycycline (Fig. 10, B and D). Control growth and growth in the presence of chloroquine alone were purposely omitted from Fig. 10, A–D, to limit the range of the y-axis and allow focus on the effects (or lack thereof) of autophagy inhibition. Chloroquine alone had minimal impact on cell growth. Baseline apoptosis and apoptosis in the presence of chloroquine are also not shown in Fig. 10, E and F, since apoptosis was essentially undetectable in the absence of irradiation of the cells. Furthermore, induction of apoptosis was observed in H1299 (with induction by doxycycline [+Dox]) cells but not the H1299 (in the absence of doxycycline [−Dox]) cells upon inhibition of autophagy by either chloroquine or genetic knockdown of ATG5 as shown in Fig. 10, E and F.
Fig. 9.
Evidence for p53 dependence of cytoprotective autophagy; induction of p53 results in sensitization to radiation when autophagy is inhibited in H1299 non–small cell lung cancer cells. (A) Induction of p53 under the influence of doxycycline in H1299 cells. (B) Knockdown of ATG5 in both H1299 null (−Dox) and p53 inducible (+Dox) cell lines. (C) Induction of autophagy (upper panel) as assessed by acridine orange staining in H1299 cells and inhibition of autophagy (lower panel) when the ATG5 gene is knocked down as shown by reduction in acidic vesicle formation. (D) Accumulation of LC3II when the autophagy inhibitor chloroquine is used in combination with radiation indicative of inhibition of autophagic flux in both H1299 (−Dox) and (+Dox) cells. CQ, chloroquine; Dox, doxycycline; IR, radiation; shCont, scrambled control. Original magnification, 20×.
Fig. 10.
Evidence for p53 dependence of cytoprotective autophagy; induction of p53 results in sensitization to radiation when autophagy is inhibited in H1299 non–small cell lung cancer cells. (A and C) H1299 non–small cell lung carcinoma cells null in p53 do not show any change in sensitivity to radiation when autophagy is blocked using chloroquine or genetic knockdown of ATG5. (B and D) Induction of p53 under the influence of doxycycline results in sensitization to radiation when autophagy is blocked using chloroquine or knockdown of ATG5. (E and F) Induction of apoptosis in H1299 (+Dox) cells when autophagy is blocked using chloroquine or genetic knockdown of ATG5 (n = 3, mean ± S.E.). *P < 0.05; #P < 0.001. CQ, chloroquine; Dox, doxycycline; IR, radiation; shCont, scrambled control.
Discussion
It is generally thought that when chemotherapy and radiation promote autophagy in tumor cells, the autophagy has a cytoprotective function that can be inhibited to sensitize the cells to treatment. We and our collaborators recently demonstrated lack of sensitization to radiation as well as to cisplatin in the 4T1 murine breast cancer model upon autophagy inhibition, indicating the existence of a nonprotective function of autophagy in tumor cells (Maycotte et al., 2012; Bristol et al., 2013).
In this work, we demonstrate a lack of sensitization to radiation in Hs578t breast cancer cells upon inhibition of radiation-induced autophagy utilizing both multiple pharmacological approaches as well as confirming these results with genetic silencing of Beclin-1. The concept that inhibition of autophagy fails to sensitize Hs578t cells to radiation therapy is supported by the lack of increased apoptosis when autophagy is inhibited, which supports the absence of cytoprotective autophagy. At the same time, the autophagy does not appear to demonstrate a cytotoxic or growth inhibitory function in this model system since there is no reduction in radiation sensitivity when autophagy is inhibited. Parallel studies demonstrate the existence of both cytoprotective and nonprotective autophagy, respectively, in the A549/H460 and H358 non–small cell lung cancer cells and in the HN30 and HN6 head and neck cancer cells. Thus, the concept that radiation can produce either cytoprotective or nonprotective autophagy is supported by studies in human tumor cells derived from different tissues. One caveat to these findings is that both the induction and inhibition of autophagy in a number of the studies presented was limited to experiments involving acridine orange staining and p62/SMQST1 degradation.
Other than functional studies in which autophagy is inhibited, there is no a priori approach for distinguishing between cytoprotective autophagy and the nonprotective form (Gewirtz, 2014a). It might have been predicted that nonprotective autophagy occurs when autophagy fails to go to completion; however, it is evident from the studies involving p62/SMQST1 degradation that radiation is inducing autophagic flux even when the autophagy is nonprotective.
It is interesting that Hs578t, HN6, and H358 cells, in which autophagy was nonprotective, are all p53 mutant cell lines, which generally respond quite poorly to conventional cancer treatments. By contrast, in our previous work with MCF-7 and ZR-75 breast tumor cells as well as the current studies with HN30 head and neck tumor cells and A549/H460 non–small cell lung cancer cells are all wild type in p53. These data suggest that nonprotective autophagy is related to the absence of functional p53 since nonprotective autophagy induced by radiation was also shown in the 4T1 breast tumor cells (Bristol et al., 2012), which are null in p53.
To confirm this conclusion, we have demonstrated that nonprotective autophagy is converted to protective autophagy when p53 is induced in p53 null H1299 cells and that conversely protective autophagy is converted to nonprotective autophagy when p53 is silenced in p53 wild-type HN30 cells. The apparent requirement for functional p53 in radiation-induced cytoprotective autophagy was also recently observed by Cheng et al. (2013), who showed that the relatively nonspecific pharmacological autophagy inhibitor, 3-MA, sensitized p53-induced H1299 cells to radiation but not the H1299 cells that are null in p53. These findings strongly support our own studies in which both pharmacological and genetic inhibition of autophagy enhanced radiation sensitivity only in the H1299 cells where wild-type p53 function was restored.
An alternative interpretation of the findings presented in this article is that whereas radiation can induce autophagy independent of p53 status, inhibition of autophagy enhances radiation sensitivity through a mechanism that requires functional p53. Consequently, we cannot completely rule out the existence of an autophagy-independent but mutant p53-dependent pathway that might be promoting cell survival. For instance, mutant p53 could be responsible for cell survival by interfering with apoptosis or senescence. This would be consistent with the fact that historically mutant p53 cell lines have been shown to be more radioresistant than p53 wild-type cells.
Specific morphologic or biochemical characteristics that might be used to distinguish between autophagy that is cytoprotective or nonprotective (or, for that matter, cytotoxic or cytostatic) have not yet been identified (Gewirtz, 2014a). Furthermore, the extent of autophagy has not been shown to differ when its function is altered. Consequently, it is likely that key determinants as to whether autophagy will be protective or nonprotective in the case of radiation could involve signaling pathways acting downstream of p53. Considering the multiplicity of p53 targets, identifying these pathways is likely to prove quite challenging; however, ongoing studies in our laboratory are designed to address this question using a number of experimental strategies including analysis of known autophagy signaling pathways as well as analysis of the phosphoproteomic profile associated with radiation-induced cytoprotective and nonprotective autophagy in isogenic tumor cell lines.
The potential importance of this work relates, in large part, to the fact clinical trials are in progress to study whether sensitivity to chemotherapy or radiation treatment can be increased by using the autophagy inhibitors chloroquine or hydroxychloroquine. Our findings indicate that the fact that radiation can induce autophagy in a given tumor cell does not obligatorily mean that this autophagy is protective. Consequently, if the strategy of autophagy inhibition is to potentially be successful as a therapeutic strategy, it will likely be necessary to stratify patients in terms of whether the autophagy being induced is cytoprotective in function.
Supplementary Material
Acknowledgments
The authors thank the Virginia Commonwealth University Flow Cytometry and Imaging Shared Resource Facility for conducting quantification of PI staining for cell cycle analysis, Annexin V–PI staining, and acridine orange staining.
Abbreviations
- 3-MA
3-methyladenine
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- PBS
phosphate-buffered saline
- PI
propidium iodide
- shRNA
small hairpin RNA
Authorship Contributions
Participated in research design: Chakradeo, Sharma, Alhaddad, Bakhshwin, Le, Harada, Nakajima, Yeudall, Gewirtz.
Conducted experiments: Chakradeo, Sharma, Alhaddad, Bakhshwin, Le, Nakajima.
Contributed new reagents or analytic tools: S. V. Torti, F. M. Torti.
Performed data analysis: Chakradeo, Sharma, Alhaddad, Bakhshwin, Le, Harada, Nakajima, Yeudall, Gewirtz.
Wrote or contributed to the writing of the manuscript: Chakradeo, Sharma, Alhaddad, Bakhshwin, Le, Harada, Yeudall, Gewirtz.
Footnotes
This research was supported in part by the National Institutes of Health National Cancer Institute [Grants R01-CA171101 (to S.V.T. and F.M.T.) and 5R21-CA171974 (to D.A.G.)] and the National Institutes of Health National Center for Advancing Translational Sciences [Award UL1-TR000058 (to D.A.G.)]. The Virginia Commonwealth University Flow Cytometry and Imaging Shared Resource Facility is supported in part by the National Institutes of Health National Cancer Institute [Grant P30-CA16059]. D.A.G. is also supported by the American Cancer Society [Institutional Research Grant].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A. (2007) Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ 14:500–510. [DOI] [PubMed] [Google Scholar]
- Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. (2007) Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest 117:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. (2008) Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res 68:1485–1494. [DOI] [PubMed] [Google Scholar]
- Bjørkøy G, Lamark T, Pankiv S, Øvervatn A, Brech A, Johansen T. (2009) Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol 452:181–197. [DOI] [PubMed] [Google Scholar]
- Boya P, González-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D, Souquere S, Yoshimori T, et al. (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25:1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. [DOI] [PubMed] [Google Scholar]
- Bristol ML, Di X, Beckman MJ, Wilson EN, Henderson SC, Maiti A, Fan Z, Gewirtz DA. (2012) Dual functions of autophagy in the response of breast tumor cells to radiation: cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D 3. Autophagy 8:739–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol ML, Emery SM, Maycotte P, Thorburn A, Chakradeo S, Gewirtz DA. (2013) Autophagy inhibition for chemosensitization and radiosensitization in cancer: do the preclinical data support this therapeutic strategy? J Pharmacol Exp Ther 344:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew JS, Medina EC, Esquivel JA, 2nd, Mahalingam D, Swords R, Kelly K, Zhang H, Huang P, Mita AC, Mita MM, et al. (2010) Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation. J Cell Mol Med 14:2448–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Kong D, Hou X, Liang B, He M, Liang N, Ma S, Liu X. (2013) The tumor suppressor, p53, contributes to radiosensitivity of lung cancer cells by regulating autophagy and apoptosis. Cancer Biother Radiopharm 28:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZB, Hui B, Shi YH, Zhou J, Peng YF, Gu CY, Yang H, Shi GM, Ke AW, Wang XY, et al. (2011) Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation. Clin Cancer Res 17:6229–6238. [DOI] [PubMed] [Google Scholar]
- Feng Y, He D, Yao Z, Klionsky DJ. (2014) The machinery of macroautophagy. Cell Res 24:24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodale V, Pierobon M, Liotta L, Petricoin E. (2011) Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer J 17:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulks WD, Smith IE, Reis-Filho JS. (2010) Triple-negative breast cancer. N Engl J Med 363:1938– 1948. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. (2014a) The four faces of autophagy: implications for cancer therapy. Cancer Res 74:647–651. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. (2014b) The autophagic response to radiation: relevance for radiation sensitization in cancer therapy. Radiat Res 182:363–367. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA, Hilliker ML, Wilson EN. (2009) Promotion of autophagy as a mechanism for radiation sensitization of breast tumor cells. Radiother Oncol 92:323–328. [DOI] [PubMed] [Google Scholar]
- Godbole AM, Purushottamachar P, Martin MS, Daskalakis C, Njar VC. (2012) Autophagy inhibition synergistically enhances anticancer efficacy of RAMBA, VN/12-1 in SKBR-3 cells, and tumor xenografts. Mol Cancer Ther 11:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo XL, Li D, Hu F, Song JR, Zhang SS, Deng WJ, Sun K, Zhao QD, Xie XQ, Song YJ, et al. (2012) Targeting autophagy potentiates chemotherapy-induced apoptosis and proliferation inhibition in hepatocarcinoma cells. Cancer Lett 320:171–179. [DOI] [PubMed] [Google Scholar]
- He C, Klionsky DJ. (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Ishii KA, Saito Y, Miura M, Otagiri A, Kawakami Y, Shimano H, Hara H, Takekoshi K. (2013) Inhibition of autophagy enhances sunitinib-induced cytotoxicity in rat pheochromocytoma PC12 cells. J Pharmacol Sci 121:67–73. [DOI] [PubMed] [Google Scholar]
- Ito H, Daido S, Kanzawa T, Kondo S, Kondo Y. (2005) Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol 26:1401–1410. [PubMed] [Google Scholar]
- Kang R, Zeh HJ, Lotze MT, Tang D. (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko A, Kanehisa A, Martins I, Senovilla L, Chargari C, Dugue D, Mariño G, Kepp O, Michaud M, Perfettini JL, et al. (2014) Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ 21:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. (2005) The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5:726–734. [DOI] [PubMed] [Google Scholar]
- Liang X, De Vera ME, Buchser WJ, Romo de Vivar Chavez A, Loughran P, Beer Stolz D, Basse P, Wang T, Van Houten B, Zeh HJ, 3rd, et al. (2012) Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Cancer Res 72:2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonaco SL, Finniss S, Xiang C, Decarvalho A, Umansky F, Kalkanis SN, Mikkelsen T, Brodie C. (2009) The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int J Cancer 125:717–722. [DOI] [PubMed] [Google Scholar]
- Lopez G, Torres K, Lev D. (2011) Autophagy blockade enhances HDAC inhibitors’ pro-apoptotic effects: potential implications for the treatment of a therapeutic-resistant malignancy. Autophagy 7:440–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker HL, Koumenis C, Giaccia AJ. (2000) p53 promotes selection for Fas-mediated apoptotic resistance. Cancer Res 60:4638–4644. [PubMed] [Google Scholar]
- Maycotte P, Aryal S, Cummings CT, Thorburn J, Morgan MJ, Thorburn A. (2012) Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy 8:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves-Neira W, Pommier Y. (1999) Apoptotic response to camptothecin and 7-hydroxystaurosporine (UCN-01) in the 8 human breast cancer cell lines of the NCI Anticancer Drug Screen: multifactorial relationships with topoisomerase I, protein kinase C, Bcl-2, p53, MDM-2 and caspase pathways. Int J Cancer 82:396–404. [DOI] [PubMed] [Google Scholar]
- Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. (2001) A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res 61:439–444. [PubMed] [Google Scholar]
- Patel V, Ensley JF, Gutkind JS, Yeudall WA. (2000) Induction of apoptosis in head-and-neck squamous carcinoma cells by γ-irradiation and bleomycin is p53-independent. Int J Cancer 88:737–743. [DOI] [PubMed] [Google Scholar]
- Qadir MA, Kwok B, Dragowska WH, To KH, Le D, Bally MB, Gorski SM. (2008) Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res Treat 112:389–403. [DOI] [PubMed] [Google Scholar]
- Rao R, Balusu R, Fiskus W, Mudunuru U, Venkannagari S, Chauhan L, Smith JE, Hembruff SL, Ha K, Atadja P, et al. (2012) Combination of pan-histone deacetylase inhibitor and autophagy inhibitor exerts superior efficacy against triple-negative human breast cancer cells. Mol Cancer Ther 11:973–983. [DOI] [PubMed] [Google Scholar]
- Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW, Wang XY, Dai Z, Peng YF, Gu CY, et al. (2011) Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy 7:1159–1172. [DOI] [PubMed] [Google Scholar]
- Solomon VR, Lee H. (2009) Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol 625:220–233. [DOI] [PubMed] [Google Scholar]
- Sotelo J, Briceño E, López-González MA. (2006) Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 144:337–343. [DOI] [PubMed] [Google Scholar]
- Tseng HC, Liu WS, Tyan YS, Chiang HC, Kuo WH, Chou FP. (2011) Sensitizing effect of 3-methyladenine on radiation-induced cytotoxicity in radio-resistant HepG2 cells in vitro and in tumor xenografts. Chem Biol Interact 192:201–208. [DOI] [PubMed] [Google Scholar]
- Wilson EN, Bristol ML, Di X, Maltese WA, Koterba K, Beckman MJ, Gewirtz DA. (2011) A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Horm Cancer 2:272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Chang PC, Yang JC, Chu CY, Wang LY, Chen NT, Ma AH, Desai SJ, Lo SH, Evans CP, et al. (2010) Autophagy blockade sensitizes prostate cancer cells towards Src family kinase inhibitors. Genes Cancer 1:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackrisson B, Mercke C, Strander H, Wennerberg J, Cavallin-Ståhl E. (2003) A systematic overview of radiation therapy effects in head and neck cancer. Acta Oncol 42:443–461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.