Abstract
This study used mice with muscle-specific overexpression of PGC-1α, a transcriptional coactivator that promotes mitochondrial biogenesis, to determine whether increased oxidative potential facilitates metabolic improvements in response to lifestyle modification. MCK-PGC1α mice and nontransgenic (NT) littermates were fed a high-fat diet (HFD) for 10 weeks, followed by stepwise exposures to voluntary wheel running (HFD+Ex) and then 25% caloric restriction with exercise (Ex/CR), each for an additional 10 weeks with continued HFD. Running and CR improved weight and glucose control similarly in MCK-PGC1α and NT mice. Sedentary MCK-PGC1α mice were more susceptible to diet-induced glucose intolerance, and insulin action measured in isolated skeletal muscles remained lower in the transgenic compared with the NT group, even after Ex/CR. Comprehensive profiling of >200 metabolites and lipid intermediates revealed dramatic group-specific responses to the intervention but did not produce a lead candidate that tracked with changes in glucose tolerance irrespective of genotype. Instead, principal components analysis identified a chemically diverse metabolite cluster that correlated with multiple measures of insulin responsiveness. These findings challenge the notion that increased oxidative capacity defends whole-body energy homeostasis and suggest that the interplay between mitochondrial performance, lipotoxicity, and insulin action is more complex than previously proposed.
Introduction
The escalating obesity pandemic presents a serious threat to global health and economic stability. Obesity increases risk of numerous comorbidities, including cardiovascular disease, impaired blood glucose control, and type 2 diabetes (1). Importantly, even moderate levels of weight loss can improve risk factors for these diseases (2). The most effective, noninvasive strategies for achieving weight control are based on lifestyle modifications that promote balanced nutrition and physical activity. Moreover, habitual exercise imparts metabolic benefits independent of weight loss, including improvements in insulin sensitivity, glucose tolerance, and blood lipid profiles. Whereas lifestyle factors clearly influence disease risk, enthusiasm for behavioral modification approaches has been dampened by psychological, economical, or physical barriers to long-term patient compliance.
While the need for novel therapeutics continues to grow, efforts to develop drugs that facilitate weight loss or improve weight-loss maintenance have thus far met limited success. The compelling association between routine physical activity and favorable health outcomes has fueled strong interest in the development of exercise-mimetic compounds. Although the concept of “exercise in a pill” is attractive, the molecular strategy to meet this end remains uncertain because the precise mechanisms responsible for the health benefits of physical activity are poorly understood (3). Prominent among the many molecular and cellular adaptations that occur in response to aerobic exercise training are increased mitochondrial biogenesis and enhanced potential for oxidative metabolism (4). One prevailing view in this field posits that remodeling of skeletal myofibers toward a more oxidative phenotype confers protection against disease by promoting fat catabolism and preventing tissue accumulation of toxic lipid intermediates such as diacylglycerol (DAG), ceramides, and long-chain acyl-CoAs. This model stems from numerous human and animal studies reporting that severe obesity and glucose intolerance associate with increased muscle content of lipid intermediates and reduced respiratory function (5–7). Additionally, investigators have suggested that individuals with an inherently high content of oxidative myofibers fare better during a weight-loss intervention than those with more glycolytic fibers (8–10).
Although a large body of correlational data supports a link between oxidative potential and health outcomes, direct experimental evidence of cause and effect remains sparse (11). The principal goal of the current study was to test the causal relation between respiratory capacity, energy balance, and glucose control using a transgenic mouse model with muscle-specific overexpression of PGC-1α (MCK-PGC1α), a transcriptional coactivator that functions as a master regulator of oxidative metabolism (12). The MCK-PGC1α model was selected as a genetically engineered mimic of exercise training because 1) PGC-1α expression and activity increase with exercise training (13,14), 2) muscle-specific overexpression of this coactivator augments mitochondrial biogenesis, fat oxidation, and exercise endurance (15–17), and 3) activation of PGC-1α is often credited as a unifying mechanism that mediates favorable metabolic responses to various behavioral, pharmacological, and genetic maneuvers (18,19).
Herein, MCK-PGC1α mice and nontransgenic control littermates (NT) (20) were fed a high-fat diet (HFD) administered alone or in combination with weight-loss interventions that encompassed exercise and caloric restriction. The animals were monitored longitudinally, and primary outcomes included body weight, glucose tolerance, and comprehensive metabolic profiling of muscle specimens. Our findings challenge the notion that enhanced oxidative potential protects against metabolic disease and raise new and important questions regarding the roles of mitochondrial function and lipotoxicity in the pathogenesis of obesity and insulin resistance.
Research Design and Methods
Animal studies were approved by the Duke University Institutional Animal Care and Use Committee. Male mice were housed in a temperature-controlled environment with a 12:12 h light:dark cycle and weaned on standard chow (5% fat, 72% carbohydrate) (5001; Harlan Teklad). At 13–16 weeks, the mice were placed on a high-fat/high-sucrose diet (45% fat, 25% sucrose) (D03021303; Research Diets) and water. For voluntary exercise studies, mice were individually housed in cages equipped with running wheels (Columbus Instruments). Mice were fasted 3 h prior to being killed.
Exercise Performance Studies
Mice were acclimated to the treadmill (Exer-3/6 treadmill; Columbus Instruments) 3 days prior to the experiments by running for 5 min/day at 5 m/min and 10 m/min followed by 15 m/min for 1 min. The grade increased by 5% each day, reaching a 10% grade on day three. During the endurance protocol, mice ran for 30 min at 10 m/min. Speed was increased 2 m/min every 15 min until reaching 28 m/min and then every 10 minutes until exhaustion. During the VO2 peak test, mice ran at 6 m/min, and speed was increased 3 m/min every 3 min until exhaustion, defined as remaining on the shock grid for 10 consecutive seconds. Respiratory exchange ratio (RER) and VO2 were monitored using the Oxymax Modular Treadmill System (Columbus Instruments).
Mitochondrial Oxidation
Mitochondria were isolated from the gastrocnemius muscle of MCK-PGC1α and NT mice fed an HFD for 6 weeks (18). Oxidation studies were performed using 0.2 mmol/L [1-14C]palmitate ± 1.0 mmol/L sodium pyruvate. 14CO2 trapped in NaOH and 14C-labeled acid-soluble metabolites (ASMs) were assessed by liquid scintillation counting in Uniscint BD (National Diagnostics) (21).
Citrate Synthase Activity
Ground tibialis anterior muscles were resuspended in CelLytic MT (Sigma-Aldrich). Assays were performed on unclarified homogenates at 25°C in a reaction buffer containing acetyl-CoA, DTNB, and tris-HCl as previously described (22). Reactions were initiated with oxaloacetate. Data were normalized to ground tissue weight.
Glucose Tolerance Tests
Mice were fasted 6 h before an intraperitoneal injection of glucose (1.5 g/kg lean body wt). Plasma glucose levels were measured at the indicated times.
Glucose Oxidation in Isolated Muscle Strips
Whole soleus and extensor digitorum longus (EDL) muscles were dissected from mice and placed in KHB buffer for reoxygenation and then incubated with 5 mmol/L 14C-glucose ± 100 nmol/L porcine insulin for 2 h at 37°C. Reactions were terminated with 70% perchloric acid. 14CO2 was trapped in 1 N NaOH and quantified by liquid scintillation counting in Uniscint BD (National Diagnostics). Flash-frozen muscles were used for measurement of glycogen synthesis (23).
Targeted Proteomics
Proteomic analysis was performed as previously described (24). Briefly, mitochondria isolated from the quadriceps were run on an SDS-PAGE gel and underwent in-gel tryptic digestion. Entire lanes were cut and subjected to selected reaction monitoring on a triple quadrupole mass spectrometer. Five assays were run to measure β-oxidation, tricarboxylic acid (TCA) cycle, and glycolytic and antioxidant proteins. Data were analyzed using the Pinpoint program. Protein levels are expressed as picomoles per 100 micrograms of protein using a BSA standard.
Metabolite Measurements
The fatty acid composition of DAG and triacylglycerol (TAG) in skeletal muscle was analyzed by gas chromatography (25). Acylcarnitines, acyl-CoAs, organic acids, and amino acids were analyzed in skeletal muscle and serum by tandem mass spectrometry (26–28). Ceramides were extracted and analyzed based on published methods using flow-injection mass spectrometry (29).
Statistical Analysis
Data are expressed as means ± SEM. Results were analyzed by Student t test unless otherwise indicated. A P value ≤0.05 was considered statistically significant. Prior to use of principal components analysis (PCA) to reduce a large number of correlated metabolites to a smaller number of uncorrelated factors, the metabolite concentrations were log transformed to approximate normal distribution. PCA was performed using orthogonal varimax rotation, and factors with eigenvalues ≥1.0 were retained (SAS, version 9.3; SAS, Cary, NC). Metabolites with a loading score of ≥|0.4| are reported for each factor. Tests of main effects of genotype, diet, and genotype by diet interactions were performed using two-way ANOVA (SAS PROC GLM). Associations between factor scores and physiological measures are reported as Spearman rank correlation coefficients.
Results
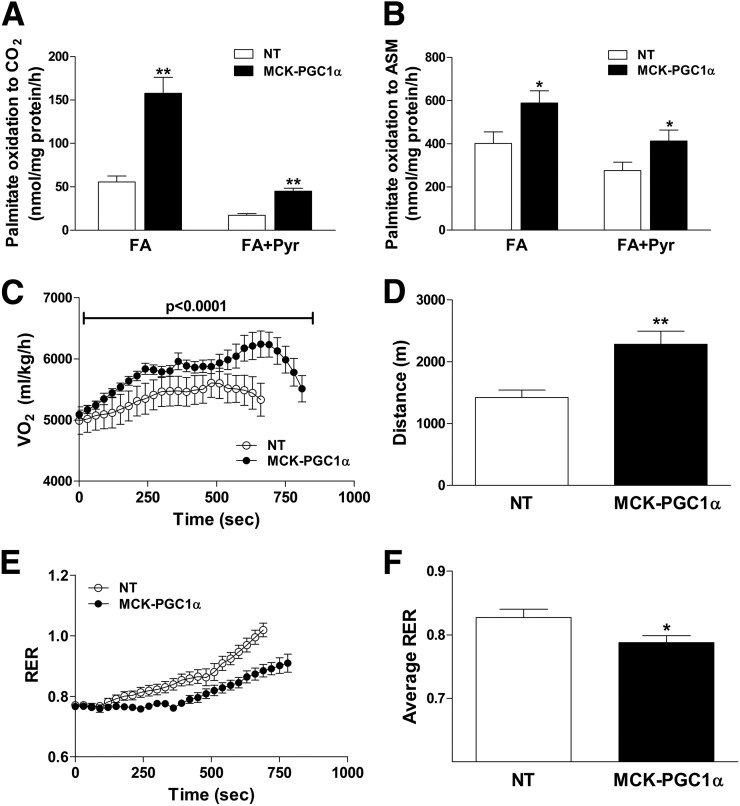
In preliminary experiments, we sought to assess the impact of PGC-1α overexpression on muscle mitochondrial quality and performance in the context of an HFD. Mitochondrial yield from muscle of MCK-PGC1α mice was approximately threefold greater than that in the NT controls, consistent with increased mitochondrial biogenesis (13). A targeted proteomics survey of isolated mitochondria from quadriceps muscles of MCK-PGC1α compared with NT control mice fed an HFD for 6 weeks revealed marked upregulation (two- to threefold) of proteins involved in β-oxidation, the TCA cycle, electron transport chain, energy metabolism, and antioxidant defense (Table 1). Consistent with these results, in isolated mitochondria from MCK-PGC1α mice compared with the NT controls, rates of complete oxidation of [14C]palmitate to CO2 (Fig. 1A) and incomplete oxidation to ASM (Fig. 1B) were likewise increased 3- and 1.5-fold, respectively. Addition of pyruvate as a competing fuel inhibited fat oxidation to a similar degree in both genotypes, although the absolute rates of β-oxidation remained higher in mitochondria from the transgenic mice (Fig. 1A). Together, these findings show that PGC-1α overexpression caused mitochondrial remodeling as well as expansion, resulting in a profound increase in the capacity of the muscle to use lipid substrate.
Table 1.
Skeletal muscle mitochondrial proteomics
| Gene | MCK-PGC1α/NT | P |
|---|---|---|
| Housekeeping | ||
| VDAC | 1.31 | 0.024–0.128* |
| β-Oxidation | ||
| Acaa2 | 3.81 | 0.002 |
| Acadl | 3.37 | 0.004 |
| Adacm | 2.42 | 0.003 |
| Acads | 2.54 | 0.010 |
| Acadyl | 3.23 | 0.003 |
| Acot13 | 4.31 | 0.000 |
| Acsl1 | 2.61 | 0.010 |
| Cd36 | 2.46 | 0.001 |
| Cpt1b | 2.38 | 0.005 |
| Crat | 3.33 | 0.003 |
| Cpt2 | 3.33 | 0.009 |
| Dci | 2.39 | 0.000 |
| Decr1 | 2.20 | 0.001 |
| Ech1 | 2.70 | 0.007 |
| Etfa | 2.59 | 0.003 |
| Etfb | 2.66 | 0.009 |
| Fabp3 | 3.00 | 0.000 |
| Hadh | 2.56 | 0.001 |
| Hadha | 2.60 | 0.006 |
| Hadhb | 2.73 | 0.003 |
| Slc25a20 | 3.09 | 0.001 |
| Antioxidant | ||
| Aldh2 | 1.79 | 0.004 |
| Hspa9 | 1.93 | 0.006 |
| Phb | 2.05 | 0.004 |
| Phb2 | 1.85 | 0.030 |
| Prdx3 | 1.80 | 0.020 |
| Prdx5 | 3.57 | 0.004 |
| Sod2 | 2.79 | 0.001 |
| TCA cycle | ||
| Abcd3 | 2.17 | 0.012 |
| Aco2 | 2.04 | 0.002 |
| Cs | 2.13 | 0.008 |
| Dlat | 1.76 | 0.009 |
| Dld | 1.78 | 0.010 |
| Dlst | 2.02 | 0.019 |
| Etfdh | 2.19 | 0.002 |
| Fh | 2.03 | 0.017 |
| Idh3b | 1.79 | 0.012 |
| Mdh1 | 4.14 | 0.013 |
| Mdh2 | 2.29 | 0.013 |
| Ogdh | 1.97 | 0.021 |
| Pdha1 | 1.90 | 0.004 |
| Pdhb | 1.78 | 0.031 |
| Sdha | 1.91 | 0.001 |
| Sdhb | 1.86 | 0.006 |
| Sdhc | 1.86 | 0.000 |
| Sucla2 | 2.17 | 0.018 |
| Suclg1 | 2.22 | 0.025 |
| Tufm | 2.09 | 0.019 |
| Ckmt2 | 3.32 | 0.011 |
| Got2 | 3.19 | 0.007 |
| Aldh2 | 1.79 | 0.004 |
| Hspa9 | 1.93 | 0.006 |
| Phb | 2.05 | 0.004 |
| Phb2 | 1.85 | 0.03 |
| Energy and amino acid metabolism | ||
| Ckmt2 | 3.32 | 0.011 |
| Got2 | 3.19 | 0.007 |
| Slc25a3 | 5.69 | 0.004 |
Mitochondria were isolated from the quadriceps muscles of MCK-PGC1α and NT mice fed an HFD for 6 weeks. Protein abundance was analyzed by a triple quadrupole mass spectrometer using a targeted assay and normalized to a BSA standard. VDAC was measured as a housekeeping protein, and the range of P values from four separate assays is provided.
*Expression levels of VDAC were significant (P < 0.05) in only one of the four assays performed. Data are expressed as a ratio of protein abundance measured in MCK-PGC1α relative to NT mice. Statistical significance was analyzed by Student t test. n = 3–4 per group.
Figure 1.
Muscle-specific overexpression of PGC-1α increased mitochondrial preference for lipid substrate. MCK-PGC1α transgenic mice and NT littermates were fed an HFD for 6 weeks prior to experiments. Mitochondria isolated from gastrocnemius muscles were used to assess oxidation of 200 μmol/L [14C]palmitate to CO2 (A) and ASM (B), measured in the absence (FA) or presence (FA+Pyr) of 1 mmol/L pyruvate. Whole-body energy metabolism and exercise performance were assessed during a graded treadmill test during which oxygen consumption (C), distance to exhaustion (D), RER (E), and average RER (F) were evaluated. Values are means ± SEM for 6–9 mice per group. *P < 0.05 between genotypes, **P < 0.005 between genotypes. FA, fatty acid; Pyr, pyruvate.
To determine whether the effects of mitochondrial reprogramming would manifest at a systemic level, we next evaluated exercise tolerance and whole-body substrate selection after 6 weeks of an HFD. At rest, energy expenditure and RER (22) were similar between genotypes. As expected, MCK-PGC1α mice reached a higher peak VO2 (Fig. 1C) and ran longer (Fig. 1D) during a graded maximal exercise test on an enclosed metabolic treadmill. Compared with the NT group, MCK-PGC1α maintained a lower RER (Fig. 1E and F), indicative of increased muscle fat oxidation during exercise.
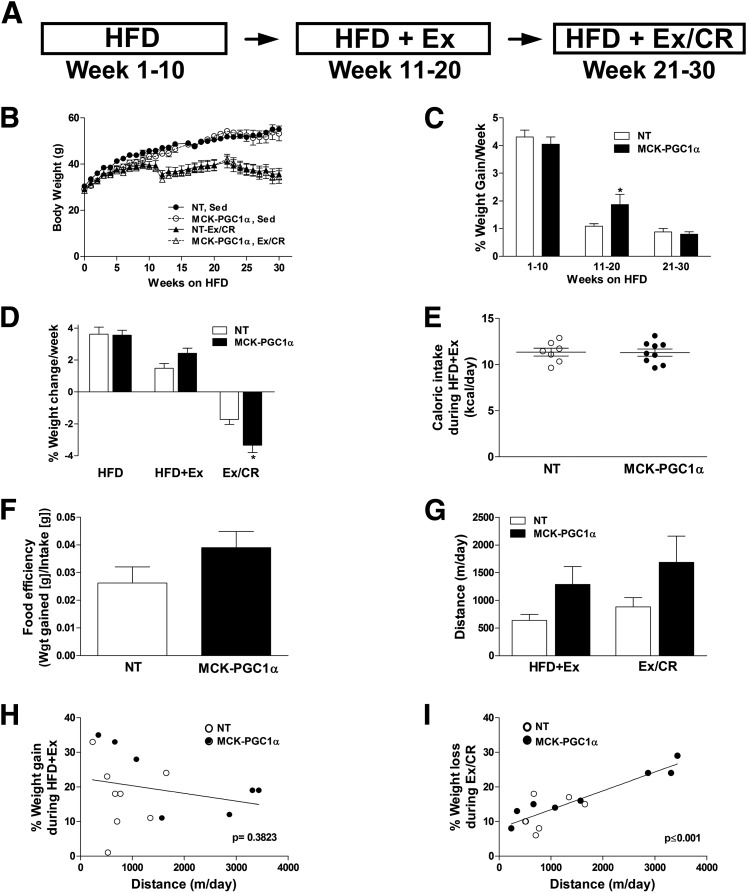
Insulin resistance and impaired glucose control are hallmarks of prediabetes. In a previous study, MCK-PGC1α transgenic mice were found to be more susceptible to insulin resistance when animals were fed a short-term (3-week) HFD (16). Investigators speculated that the health benefits of increased mitochondrial mass might take effect only when mice were permitted to exercise. We therefore questioned whether the foregoing genotype differences in fat oxidation during exercise would confer an advantage during a weight-loss intervention. Accordingly, mice were fed an HFD for 10 weeks in standard cages, followed by stepwise exposure to running wheels (HFD+Ex) and then exercise plus 25% caloric restriction (Ex/CR), each for an additional 10 weeks (Fig. 2A). Weight gain and glucose tolerance were followed longitudinally and compared against a control group that remained sedentary. In general, PGC-1α overexpression did not affect changes in body weight, although weight gain was slightly higher in transgenic mice during weeks 11–20 of the HFD, whereas weight gain was slightly lower in this group during the Ex/CR phase of the intervention (Fig. 2B–D). Daily caloric intake and food efficiency were also similar between NT and MCK-PGC1α mice (Fig. 2E and F). Citrate synthase activity measured in muscle homogenates was 2.3-fold greater in MCK-PGC1α versus NT (362 and 156 μmol/L/min/mg tissue, respectively) but was unaffected by the Ex/CR intervention in both genotypes. Mean running distance was similar between genotypes but highly variable (Fig. 2G). Running distance was unrelated to weight gain when mice were fed ad libitum (Fig. 2H) but correlated strongly with weight loss during the Ex/CR phase of the intervention (r2 = 0.7805, P < 0.001) (Fig. 2I). In a separate experiment, mice were housed with running wheels at the onset of an HFD for 10 weeks. Again, running distance and metabolic outcomes were similar between the NT and MCK-PGC1α mice (Supplementary Fig. 1A–F), and weight gain correlated negatively with running distance but was unaffected by genotype (Supplementary Fig. 1F).
Figure 2.
Muscle-specific overexpression of PGC-1α does not defend against diet-induced obesity or promote weight loss in response to exercise and caloric restriction. A: NT and MCK-PGC1α mice were fed a 45% HFD for 10 weeks prior to 10 weeks of voluntary wheel running (HFD+Ex) followed by an additional 10 weeks of wheel running combined with 25% caloric restriction (Ex/CR) with continued high-fat feeding. B: Body weight measurements taken throughout the course of this study for both sedentary (Sed) and Ex/CR groups. Rates of weight change per week during the three phases of the study for the sedentary (C) and Ex/CR (D) group. E: Caloric intake measured every 2 days during the HFD+Ex phase. F: Food efficiency, an estimate of how much food ingested is converted to body mass (weight gain [g] per week/food ingested [g] per week) during the HFD+Ex phase of the study. G: Average daily running distance during HFD+Ex and Ex/CR. The relationships between running distance and weight gain (H) or weight loss (I) during HFD+Ex and Ex/CR, respectively. Values are means ± SEM for 6–8 mice per group. *P < 0.05 between genotypes. Wgt, weight.
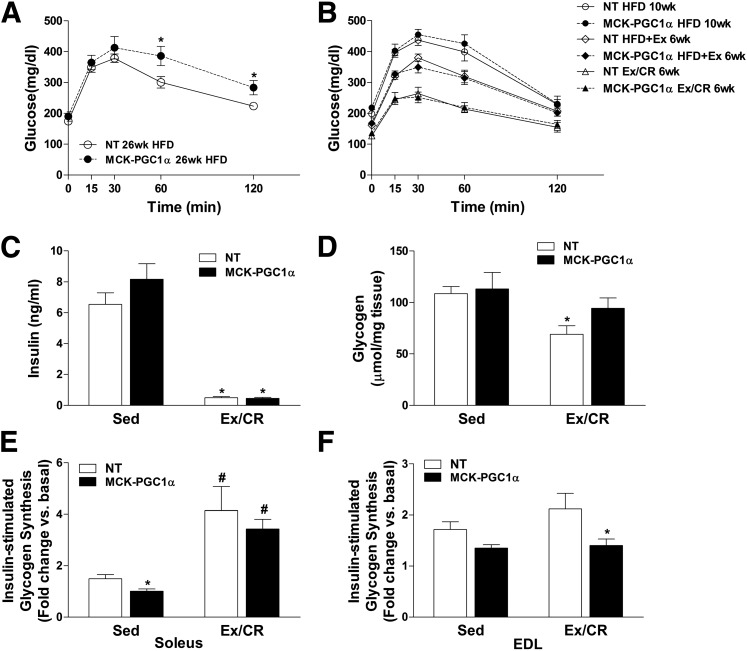
Figure 3A shows results of a glucose tolerance test performed in the age-matched sedentary control groups after 26 weeks of HFD. At this time point, which corresponds with the 6-week Ex/CR group in Fig. 3B, diet-induced glucose intolerance was more severe in the transgenic compared with NT mice. Similar results were observed after only 2 weeks of HFD (not shown). Fig. 3B shows a series of glucose tolerance tests performed longitudinally after 10 weeks of HFD and then at the 6-week time point of each intervention arm. Glucose tolerance tests were similar between genotypes at the 10-week time point. Exercise alone elicited comparable improvements in glucose clearance in NT and MCK-PGC1α mice (Fig. 3B), and the combination of Ex/CR further enhanced glucose tolerance in both groups (Fig. 3B). In both the NT and MCK-PGC1α mice, the Ex/CR intervention resulted in a dramatic 80% decline in fasting plasma insulin levels compared with the sedentary groups, indicative of profound improvements in whole-body insulin sensitivity (Fig. 3C). The Ex/CR regimen decreased muscle glycogen levels in both genotypes (Fig. 3D), consistent with negative energy balance. Muscle insulin sensitivity per se was evaluated by measuring insulin-stimulated glycogen synthesis in isolated soleus and EDL muscles, which are comprised predominantly of red/oxidative and white/glycolytic fiber types, respectively. When mice were fed an HFD and remained sedentary, basal rates of glycogen synthesis were similar in both genotypes, but PGC-1α overexpression decreased rates of insulin-stimulated glycogen synthesis in soleus muscle by 35% (Fig. 3E). A similar trend in rates of insulin-stimulated glycogen synthesis was evident in the EDL (Fig. 3F). In both genotypes, Ex/CR improved insulin responsiveness in soleus muscles from ∼1.3-fold in the sedentary state to fourfold over the basal condition in the intervention groups. By contrast, insulin sensitivity in isolated EDL muscles, which are only minimally recruited during wheel running, was not improved in either group (Fig. 3D).
Figure 3.
Muscle-specific overexpression of PGC-1α does not defend against glucose intolerance or enhance glucose control in response to exercise and caloric restriction. Mice were fed an HFD for 26 weeks, and measures of insulin action were made at the designated time points. Intraperitoneal glucose tolerance tests (1.5 mg/kg lean body wt) were performed on age-matched cohorts of NT and MCK-PGC1α mice that remained sedentary for the duration of the HFD (A) or were given access to running wheels (HFD+Ex) (B) during weeks 11–20, followed by an additional 10 weeks of exercise combined with 25% caloric restriction (Ex/CR). Blood and tissues were harvested at 30 weeks and used for analysis of fasting insulin levels (C) and glycogen content (D) in gastrocnemius muscles. Insulin-stimulated glycogen synthesis, expressed as fold change relative to basal rates assessed in the contralateral muscles, was measured in isolated soleus (E) and isolated EDL (F). Data were analyzed by two-way ANOVA. A main effect of Ex/CR on insulin, glycogen, and insulin-stimulated glycogen synthesis in the soleus was detected, but symbols were excluded for simplicity. Data represent means ± SEM for 6–8 mice per group. *P < 0.05 between genotypes. #P < 0.05 within a genotype between treatment conditions (sedentary versus Ex/CR). Sed, sedentary; wk, week.
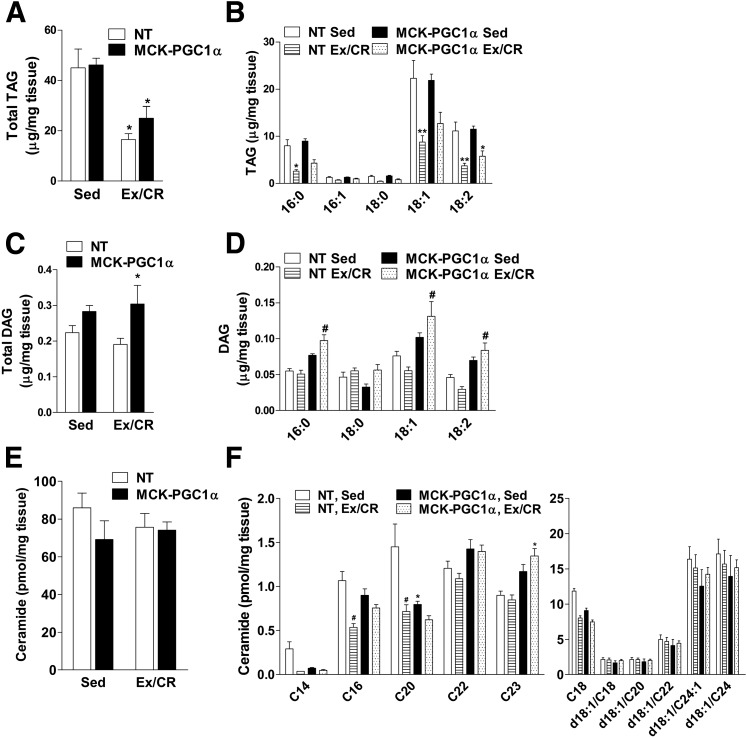
Using a targeted metabolomics approach, we proceeded to evaluate a comprehensive set of lipid molecules and metabolic intermediates that have been linked to the pathogenesis of insulin resistance. Intramuscular TAG (IMTG) levels were similar between genotypes in sedentary mice but decreased 60% and 45% in response to Ex/CR in NT and MCK-PGC1α mice, respectively (Fig. 4A and B). Total DAG levels and most individual DAG species (Fig. 4C and D) were modestly elevated in MCK-PGC1α compared with NT mice. Whereas Ex/CR tended to decrease the 18:1 and 18:2 DAG species in NT mice, the opposite response was observed in MCK-PGC1α mice. Total muscle ceramide content was unaffected by genotype and the Ex/CR intervention, whereas specific species (C16 and C20) were decreased in response to Ex/CR but only in the NT mice (Fig. 4E and F).
Figure 4.
Effects of exercise and caloric restriction on muscle content of TAG, DAG, and ceramides in NT and MCK-PGC1α mice fed an HFD. Lipid metabolites were measured in gastrocnemius muscles of NT and MCK-PGC1α mice after 30 weeks of high-fat feeding without or with exercise and caloric restriction (Ex/CR). Total TAG (A) and individual TAG species (B). Total DAG (C) and individual DAG species (D). Total ceramides (E) and individual ceramide species (F). Two-way ANOVA revealed an interaction between genotype and TAG and DAG levels. Data represent means ± SEM for 6–8 mice per group. Statistical differences were analyzed by two-way ANOVA. *P < 0.05 between genotypes, **P < 0.01 between genotypes, #P < 0.05 within a genotype between treatment conditions (sedentary [Sed] versus Ex/CR).
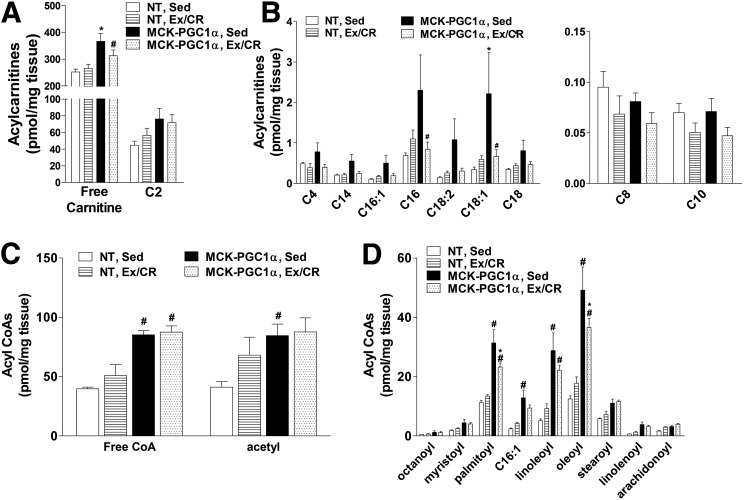
Acylcarnitine metabolites have emerged as strong biomarkers of mitochondrial stress and/or nutrient load. These metabolites are generated by a family of mitochondrial localized acyltransferase enzymes that convert acyl-CoA intermediates of glucose, fatty acid, and amino acid catabolism to their cognate carnitine esters (23,28). Evaluation of muscles from HFD-fed, sedentary mice revealed robust increases in several carnitine and CoA species as a result of PGC-1α overexpression (Fig. 5A–D), including free carnitine, free CoA, and multiple even-chain acyl moieties. Most even-chain acyl groups represent partially oxidized intermediates of fatty acid β-oxidation; thus, this metabolite profile aligns with the mitochondrial phenotype presented in Fig. 1. In the MCK-PGC1α group, the Ex/CR intervention lowered several fatty acid–derived mitochondrial intermediates, implying that excessive nutrient load as well as increased mitochondrial mass contributed to the foregoing accumulation of metabolites. By contrast, in the NT control group, the same intermediates were largely unchanged or modestly increased by Ex/CR (Fig. 5B–D).
Figure 5.
Effects of exercise and caloric restriction on lipid-derived acylcarnitine and acyl-CoA metabolites in NT and MCK-PGC1α mice fed an HFD. Lipid metabolites were measured by tandem mass spectrometry using gastrocnemius muscles from NT and MCK-PGC1α mice after 30 weeks of high-fat feeding without or with exercise and caloric restriction (Ex/CR). A: Free carnitine and acetylcarnitine. B: Even-chain acylcarnitines. C: Free CoA and acetyl-CoA. D: Even-chain acyl-CoAs. Data represent means ± SEM for 6–8 mice per group. Statistical differences were analyzed by two-way ANOVA. There was an effect of treatment group on free carnitine, acylcarnitine, and acyl-CoA levels. *P < 0.05 between genotypes, #P < 0.05 within a genotype between treatment conditions (sedentary [Sed] versus Ex/CR).
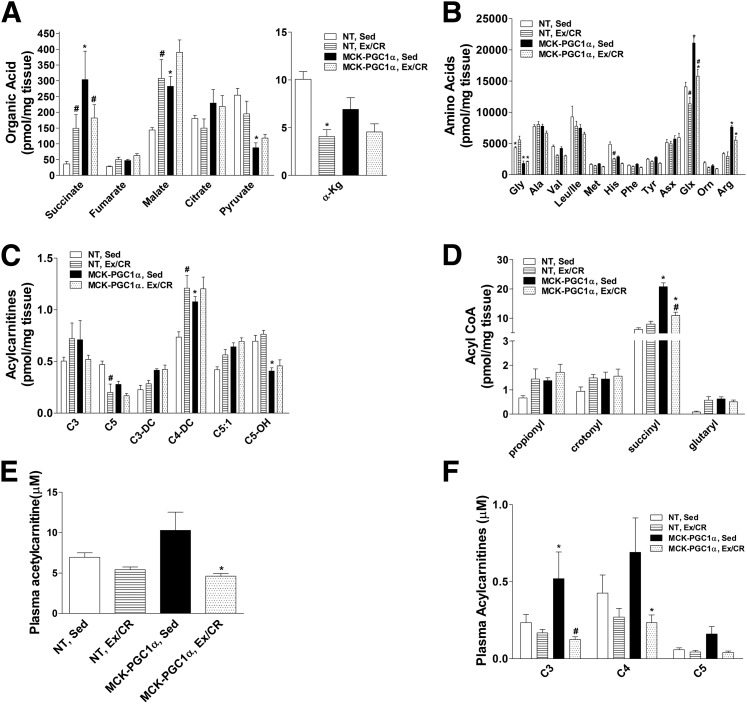
In the sedentary, HFD condition, muscle concentrations of the TCA cycle intermediates, succinate, fumarate, and malate, were elevated in MCK-PGC1α compared with NT mice, whereas levels of pyruvate, the end product of glycolysis, were lower. With the exception of α-ketoglutarate, which decreased uniformly with Ex/CR, the effects of the intervention differed by genotype. Most notably, succinate increased by 75% in NT but decreased by 40% in MCK-PGC1α mice. We also examined a cluster of amino acids that have been identified as strong predictors of diabetes risk in humans, including the branched-chain amino acids (BCAAs) (leucine, isoleucine, and valine) as well as tyrosine and phenylalanine (30). PGC-1α overexpression did not affect muscle levels of these amino acids but did cause dramatic increases in glutamate/glutamine (Glx) and arginine, along with a marked reduction in glycine levels. In general, the Ex/CR intervention tended to lower most amino acids in both genotypes. Catabolism of amino acids gives rise to the odd-chain species of acylcarnitines and acyl-CoAs. With the exception of isovalerylcarnitine (C5), these metabolites were elevated in sedentary MCK-PGC1α mice (Fig. 6C and D). Only succinyl-CoA declined upon Ex/CR in the transgenic animals. By contrast, the intervention increased most of these metabolites in NT mice, with the notable exception of C5, which declined in both genotypes (Fig. 6C).
Figure 6.
Effects of exercise and caloric restriction on muscle organic and amino acid and plasma acylcarnitine metabolites in NT and MCK-PGC1α mice fed an HFD. Metabolites were measured by tandem mass spectrometry using gastrocnemius muscles from NT and MCK-PGC1α mice after 30 weeks of high-fat feeding without or with exercise and caloric restriction (Ex/CR). A: Organic acids. B: Amino acids. Acylcarnitine (C) and acyl-CoA intermediates (D) of amino acid catabolism. E: Plasma acetylcarnitine (C2). F: Plasma short-chain acylcarnitines. Data represent means ± SEM for 6–8 mice per group. Statistical differences were analyzed by two-way ANOVA. There was an effect of treatment group on organic acid, amino acid, and their acylcarnitine and acyl-CoA metabolites. *P < 0.05 between genotypes, #P < 0.05 within a genotype between treatment conditions (sedentary [Sed] versus Ex/CR).
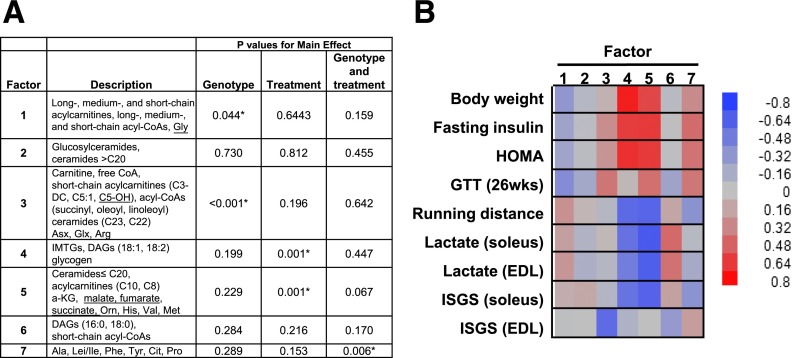
The conversion of acyl-CoAs to their membrane-permeant carnitine esters permits acyl group efflux from the mitochondrial matrix to the general circulation, which typically occurs when substrate provision exceeds flux. Accordingly, the plasma acylcarnitine profile provides a systemic view of mitochondrial metabolism. Compared with the NT control group, C2–C5 plasma carnitine species were elevated in sedentary MCK-PGC1α mice and decreased in response to Ex/CR in both genotypes (Fig. 6E and F). PCA was used as an unbiased data-reduction strategy to examine the relationship between muscle metabolites and several physiologic outcomes (Fig. 7). Factor 1 emerged as a large group of mitochondrial-derived intermediates (Fig. 7A). This factor was affected by genotype but unrelated to changes in body weight and insulin action (Fig. 7B). Factor 2, representing a large cluster of ceramide metabolites, was not affected by genotype or the Ex/CR treatment and was unrelated to glucose tolerance. Factor 4, consisting of muscle glycogen, several IMTG species, and the 18:1 and 18:2 DAG species, was affected by the Ex/CR intervention and correlated with body weight, running distance fasting insulin, and insulin-stimulated glycogen synthesis in soleus muscle. Factor 5, which was also robustly influenced by Ex/CR, emerged as the most compelling correlate of glucose control evidenced by strong associations with multiple measures of muscle and whole-body insulin action (Fig. 7B). Interestingly, this factor was heavily weighted by an eclectic group of metabolites that included specific species of ceramides, acylcarnitines, amino acids, and TCA cycle intermediates. Factor 7, consisting of a sizable cluster of amino acids, was the only factor influenced by an interaction between genotype and treatment and was modestly associated with fasting insulin and whole-body glucose tolerance.
Figure 7.
PCA and factor associations with functional outcomes. PCA was used as a data reduction strategy for exploratory purposes. Factors comprised of strongly corrected metabolites were surveyed for potential relationships with measures of energy and glucose homeostasis (see research design and methods). A: Key metabolites in PCA factors 1–7 and the effect of genotype and treatment (Ex/CR) on each factor. Key metabolites within each retained factor (i.e., metabolites with factor load ≥|0.4|) and an overall description of each factor are presented. Underlined metabolites had a negative load score. *P > 0.05. DC, dicarboxylic, OH, hydroxyl. B: Heat map illustrating the positive (red) and negative (blue) associations between factors 1–7 and physiologic outcome measures. Glucose tolerance test (GTT) (26 weeks [wks]): blood glucose levels measured 120 min after an intraperitoneal glucose injection. ISGS, insulin-stimulated glycogen synthesis.
Discussion
This study was designed to test the idea that elevated mitochondrial mass and oxidative potential in skeletal muscle afford protection against metabolic disease or facilitate metabolic improvements in response to lifestyle modification. The results offer two important outcomes regarding the relationship between mitochondrial function, intramuscular lipid balance, and whole-body energy homeostasis. First, PGC-1α–mediated enhancement of oxidative potential did not prove beneficial for preventing or treating obesity and glucose intolerance. Secondly, comprehensive metabolic profiling of several leading candidate mediators of muscle insulin resistance failed to reveal a compelling front-runner that tracked with changes in glucose tolerance irrespective of genotype. Instead, PCA identified a chemically diverse cluster of metabolites (factor 5) that correlated with multiple measures of muscle and whole-body insulin responsiveness. Together, these findings show that the interplay between mitochondrial performance, lipotoxicity, and insulin action is more complex than previously proposed (31).
In the current study, as well as in a previous report (16), diet-induced weight gain was similar between controls and MCK-PGC1α mice despite markedly augmented capacity for lipid oxidation in the latter group. These findings argue against the possibility that increasing muscle mitochondrial density raises resting energy expenditure by elevating uncoupled respiration due to inherent mitochondrial leak. Because PGC-1α transgenic mice had a lower RER during treadmill running, we questioned whether this exercise-induced augmentation of fat oxidation would result in diminished weight gain or enhanced weight loss when animals were given access to running wheels. As anticipated, exercise and caloric restriction improved metabolic homeostasis in both groups. Interestingly, in two separate experiments, running distance had a stronger influence on body weight and glucose homeostasis than genotype. In aggregate, these findings suggest that changes in adiposity depended on the energetic costs of muscle contraction rather than muscle mitochondrial content or substrate selection. By contrast, an earlier report found that MCK-PGC1α transgenic mice had enhanced weight loss and glucose metabolism in response to an exercise intervention comprised of graded high-intensity treadmill running performed three times weekly for the final 3.5 weeks of a 6-week HFD (32). Although these results imply that increased mitochondrial biogenesis might benefit weight loss during a high-intensity exercise intervention, the lower-intensity running wheel model better represents lifestyle modification programs that are likely to be maintained in human populations.
When animals remained sedentary, diet-induced glucose intolerance was actually more severe in the MCK-PGC1α mice. This phenotype was originally reported in a study that confirmed muscle insulin resistance by hyperinsulinemic-euglycemic clamp after 3 weeks of high-fat feeding (16). Investigators attributed the diabetic nature of MCK-PGC1α mice to elevated intramuscular TAG and DAG and consequent activation of serine kinases that interfere with insulin signaling. In the current study, muscle levels of total TAG, DAG, and ceramide were similar between genotypes after a prolonged (30-week) HFD, whereas a subset of specific DAG species was modestly increased in the MCK-PGC1α mice. These results are similar to a study wherein intramuscular DAG species were elevated in trained athletes (33). The metabolite clusters that best discriminated the MCK-PGC1α from NT mice were those of mitochondrial origin, including acetyl-CoA, acetylcarnitine, long-chain acylcarnitines, long-chain acyl-CoAs, succinate, and succinyl-CoA. Thus, raising mitochondrial content in the context of caloric excess and physical inactivity elevated muscle production and/or accumulation of mitochondrial intermediates without changing energy expenditure. A strong indication that nutrient load contributed to this signature comes from the observation that muscle concentrations of many of these metabolites decreased in MCK-PGC1α mice in response to the Ex/CR intervention. Whether this form of nutrient-induced mitochondrial stress contributed to adverse physiological outcomes remains uncertain at this stage.
The current study afforded the opportunity to query muscle tissues for metabolic signatures that associate with improved glucose tolerance in response to a weight-loss intervention. We interrogated a comprehensive array of lipids and other metabolites previously implicated as chief culprits of insulin resistance. These included multiple species of IMTG, DAG, ceramides, acylcarnitines, and acyl-CoAs, as well as the BCAAs.
The most consistent response to the weight-loss intervention was a marked decline in muscle energy reserves. Thus, in both the NT and MCK-PGC1α groups the Ex/CR intervention lowered muscle levels of glycogen and IMTG, likely reflecting a local shift from positive to negative energy balance. In general, these macromolecular fuel reservoirs are considered innocuous, whereas the foregoing metabolic intermediates harness more potential as bioactive signaling molecules. Remarkably, however, few of the >200 metabolic intermediates measured changed uniformly in the NT and transgenic mice when the sedentary was compared with the Ex/CR condition. For example, Ex/CR caused marked reductions in muscle concentrations of several mitochondrial-derived intermediates in MCK-PGC1α mice, but these same metabolites were either unchanged or modestly increased by the intervention in the control group. Likewise, gross changes in muscle levels of DAGs, ceramides, and BCAAs failed to explain disparities in glucose tolerance across genotypes and treatments. Interestingly, Ex/CR lowered circulating levels of short-chain acylcarnitines in both genotypes. Although circulating metabolites originate from multiple tissues, the observation that several serum acylcarnitines were increased in MCK-PGC1α compared with NT mice suggests that the systemic pool of these analytes can be derived from and report on muscle energy metabolism. Thus, the foregoing reduction in serum short-chain acylcarnitines could be reflecting diminished nutrient load on mitochondria residing in skeletal muscle as well as other tissues.
Interestingly, an unbiased PCA identified a single factor (factor 5) that correlated with changes in energy balance and running distance as well as multiple measures of muscle insulin sensitivity and whole-body glucose tolerance. This factor was heavily weighted by a diverse group of metabolites comprised of specific species of ceramides, acylcarnitines, amino acids, and organic acids. The simplest interpretation of these data is that intermediates arising from multiple nutrient sources and metabolic pathways influence insulin action. Alternatively, flux of these metabolites might be coupled to the generation of common regulatory molecules that were not evaluated. It is also possible that metabolite concentrations measured in whole tissues do not reflect the specific pool(s) that interact with the insulin signaling pathway.
Lastly, it is important to consider the utility and caveats of the MCK-PGC1α transgenic mouse model. We selected this model because when fed a standard chow diet, the transgenic mice phenocopy an exercise-trained state in nearly every physiological parameter examined. They outperform their NT counterparts during an exercise challenge, have improved metabolic and cognitive function upon aging, and live longer (15,17,32,34–36). Although it could be argued that the ∼2.5-fold induction of mitochondrial content measured in PGC-1α transgenic muscles lacks physiological relevance, this is true only when considering the adaptive impact of an exercise intervention in a given individual. Alternatively, when mitochondrial content in muscles of individuals at far ends of the aging, disease, or fitness spectrums was compared, a threefold difference did fall within a physiological range (37–39). A caveat of the model is that PGC-1α targets other metabolic processes in addition to mitochondrial biogenesis and oxidative capacity, including nutrient delivery and storage (40). Nonetheless, these same processes are upregulated by exercise training (41,42). Additionally, both exercise and PGC-1α overexpression have been shown to regulate muscle production and secretion of myokines that act both centrally and peripherally to regulate energy metabolism (43). Thus, intermittent versus constitutive production of these factors could yield different outcomes. Three other genetically engineered mouse models of increased mitochondrial biogenesis in skeletal muscle have been described (20,44–46). Skeletal muscle-specific overexpression of either ERRγ (44) or PPARδ (20,45) failed to confer protection against diet-induced metabolic derangement, whereas mice overexpressing constitutively active PPARδ resisted both diet-induced obesity and glucose intolerance. Notably, overexpression of constitutively active PPARδ caused a dramatic increase in whole-body energy expenditure that coincided with improvements in glucose control (20), further underscoring the tight connection between glucose homeostasis and energy balance.
In summary, exercise-induced mitochondrial adaptations in skeletal muscle are known to play a key role in mediating improvements in fitness and athletic performance; however, their presumed role in combating metabolic disease is based largely on circumstantial evidence. The MCK-PGC1α transgenic mouse model provides a tractable experimental tool for proof-of-concept studies aimed at understanding the health benefits of increased mitochondrial biogenesis in the absence of exercise training. The findings reported here raise doubt that pharmacological exercise mimetics that increase oxidative capacity have high potential as antiobesity and/or antidiabetic agents. Instead, the evidence suggests that the salutary metabolic effects of habitual physical activity depend on the energetic costs of muscle contraction and that lifestyle factors, such as diet and exercise, induce local shifts in intramyocellular energy balance, which in turn impacts a broad network of metabolic intermediates involved in nutrient sensing and insulin action.
Supplementary Material
Article Information
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants 1F32-DK-094573 (to K.E.W.), R01-DK-082803 (to C.L.K.), R01-DK-089312 (to D.M.M.), and 2P01-DK-058398 (to D.M.M.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.E.W. conducted research, designed experiments, and wrote the manuscript. C.R.M. performed statistical analysis and data interpretation and reviewed and edited the manuscript. D.H.S., S.E.S., and K.L.D. executed metabolic experiments. O.R.I. performed mass spectrometry measurements. K.I.C. performed mass spectrometry measurements and measured muscle lipid content with gas chromatography. M.T.K. performed the proteomic analysis. C.L.K. reviewed and edited the manuscript and conducted research. R.D.S. performed mass spectrometry analysis and reviewed the manuscript. D.M.M. designed experiments and wrote the manuscript. D.M.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0827/-/DC1.
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–787 [DOI] [PubMed] [Google Scholar]
- 2.Astrup A. Healthy lifestyles in Europe: prevention of obesity and type II diabetes by diet and physical activity. Public Health Nutr 2001;4:499–515 [DOI] [PubMed] [Google Scholar]
- 3.Goodyear LJ. The exercise pill--too good to be true? N Engl J Med 2008;359:1842–1844 [DOI] [PubMed] [Google Scholar]
- 4.Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol 2003;88:99–107 [DOI] [PubMed] [Google Scholar]
- 5.Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev 2007;65:S39–S46 [DOI] [PubMed] [Google Scholar]
- 6.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 2006;55(Suppl. 2):S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol 1999;276:E1–E18 [DOI] [PubMed] [Google Scholar]
- 8.Kriketos AD, Pan DA, Lillioja S, et al. Interrelationships between muscle morphology, insulin action, and adiposity. Am J Physiol 1996;270:R1332–R1339 [DOI] [PubMed] [Google Scholar]
- 9.Lillioja S, Young AA, Culter CL, et al. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 1987;80:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanner CJ, Barakat HA, Dohm GL, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 2002;282:E1191–E1196 [DOI] [PubMed] [Google Scholar]
- 11.Hulver MW, Berggren JR, Cortright RN, et al. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 2003;284:E741–E747 [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002;418:797–801 [DOI] [PubMed] [Google Scholar]
- 13.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 2003;546:851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem 2011;286:10605–10617 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Calvo JA, Daniels TG, Wang X, et al. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol (1985) 2008;104:1304–1312 [DOI] [PubMed] [Google Scholar]
- 16.Choi CS, Befroy DE, Codella R, et al. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA 2008;105:19926–19931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev 2007;21:770–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koves TR, Noland RC, Bates AL, Henes ST, Muoio DM, Cortright RN. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol 2005;288:C1074–C1082 [DOI] [PubMed] [Google Scholar]
- 19.Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Appl Physiol Nutr Metab 2007;32:874–883 [DOI] [PubMed]
- 20.Wang YX, Zhang CL, Yu RT, et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2004;2:e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JY, Koves TR, Yu GS, et al. Evidence of a malonyl-CoA-insensitive carnitine palmitoyltransferase I activity in red skeletal muscle. Am J Physiol Endocrinol Metab 2002;282:E1014–E1022 [DOI] [PubMed] [Google Scholar]
- 22.Srere PA. Citrate synthase. Methods Enzymol 1969;13:3–1111265473 [Google Scholar]
- 23.Noland RC, Koves TR, Seiler SE, et al. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 2009;284:22840–22852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinter CS, Lundie JM, Patel H, Rindler PM, Szweda LI, Kinter M. A quantitative proteomic profile of the Nrf2-mediated antioxidant response of macrophages to oxidized LDL determined by multiplexed selected reaction monitoring. PLoS ONE 2012;7:e50016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kien CL, Everingham KI, D Stevens R, Fukagawa NK, Muoio DM. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity (Silver Spring) 2011;19:305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 2004;10:268–274 [DOI] [PubMed] [Google Scholar]
- 27.Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis 1990;13:321–324 [DOI] [PubMed] [Google Scholar]
- 28.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 29.Merrill AH Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005;36:207–224 [DOI] [PubMed] [Google Scholar]
- 30.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012;148:852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Summermatter S, Shui G, Maag D, Santos G, Wenk MR, Handschin C. PGC-1α improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes 2013;62:85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amati F, Dubé JJ, Alvarez-Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 2011;60:2588–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandri M, Lin J, Handschin C, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 2006;103:16260–16265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenz T, Diaz F, Spiegelman BM, Moraes CT. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab 2008;8:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci USA 2009;106:20405–20410 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol 2008;59(Suppl. 7):5–18 [PubMed]
- 38.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 2005;54:8–14 [DOI] [PubMed] [Google Scholar]
- 39.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 2005;102:5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koves TR, Sparks LM, Kovalik JP, et al. PPARγ coactivator-1α contributes to exercise-induced regulation of intramuscular lipid droplet programming in mice and humans. J Lipid Res 2013;54:522–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab 2008;294:E882–E888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001;86:5755–5761 [DOI] [PubMed] [Google Scholar]
- 43.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012;481:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rangwala SM, Wang X, Calvo JA, et al. Estrogen-related receptor gamma is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem 2010;285:22619–22629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luquet S, Lopez-Soriano J, Holst D, et al. Roles of peroxisome proliferator-activated receptor delta (PPARdelta) in the control of fatty acid catabolism. A new target for the treatment of metabolic syndrome. Biochimie 2004;86:833–837 [DOI] [PubMed] [Google Scholar]
- 46.Narkar VA, Fan W, Downes M, et al. Exercise and PGC-1α-independent synchronization of type I muscle metabolism and vasculature by ERRγ. Cell Metab 2011;13:283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.