Abstract
Membrane preparations from developing soybean (var. Prize) cotyledon tissue, at the time of synthesis of storage glycoproteins, catalyze the sequential assembly of lipid-linked oligosaccharides from uridine-5′-diphospho-N-acetyl-d-[6-3H] glucosamine and guanosine-5′diphospho-d-[U-14C]mannose. The maximum size of lipid-linked oligosaccharide that accumulates contains the equivalent of 10 saccharide units on the basis of Bio-Gel P-2 gel filtration studies. These lipid-linked oligosaccharides show similar characteristics to polyisoprenyl diphosphate derivatives on diethylaminoethyl-cellulose chromatography and are potential intermediates in glycoprotein biosynthesis in this tissue. These glycolipids do not appear to turn over in pulse-chase experiments and no completed storage glycoproteins were detected among the products of these incubations.
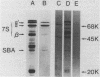
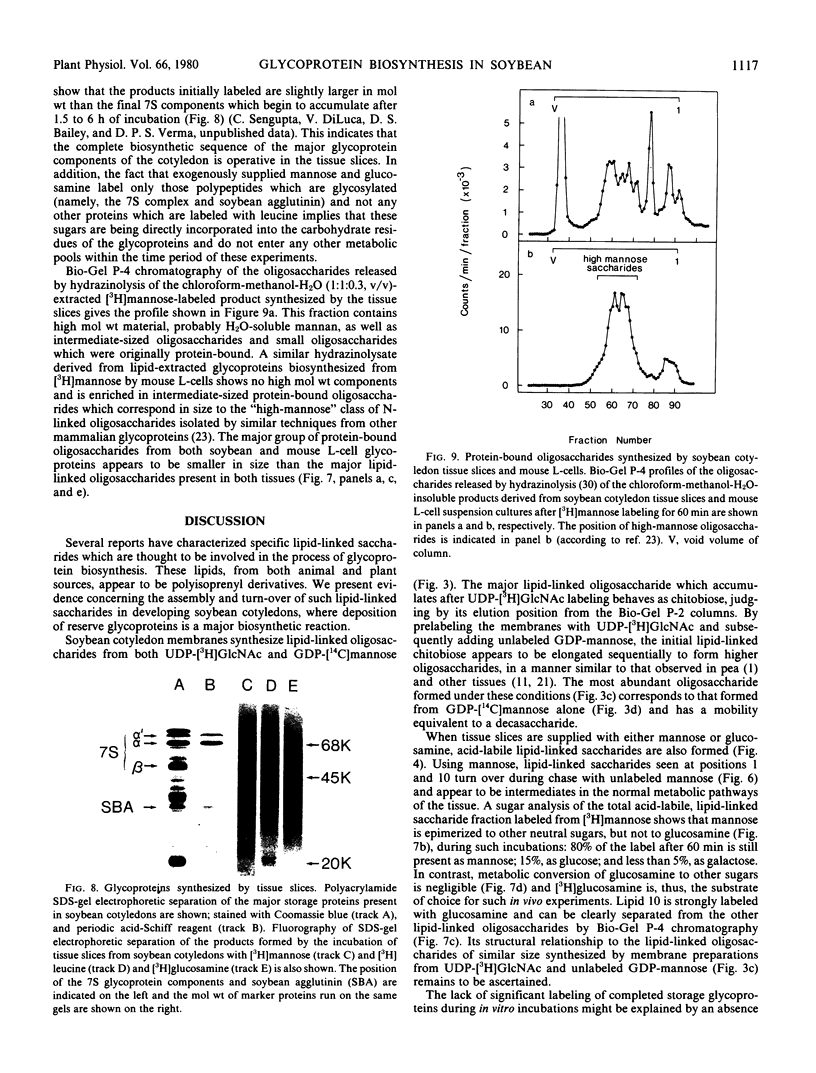
Tissue slices from cotyledons at the same stage of development synthesize lipid-linked oligosaccharides from [3H]mannose and [3H]glucosamine with sizes equivalent to 1, 7, 10, and approximately 15 saccharide units. In pulse-chase experiments, the lipid-linked saccharides with the equivalent of 1 and 10 units rapidly turnover, whereas those with 7 and 15 units do not. Examination of the higher oligosaccharide peaks (10 and 15) by Bio-Gel P-4 gel filtration shows them to comprise 2 distinct subsets of oligosaccharides containing different proportions of glucosamine and mannose units. Tissue slices synthesize products which resemble the completed 7S storage glycoproteins as judged by similarity of molecular weight and precipitation with specific antisera. Analysis of the oligosaccharides obtained by hydrazinolysis of glycoproteins shows the presence of a similar size “high-mannose” type N-linked oligosaccharides as in other glycoproteins from animal and plant cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey D. S., Dürr M., Burke J., Maclachlan G. The assembly of lipid-linked oligosaccharides in plant and animal membranes. J Supramol Struct. 1979;11(2):123–138. doi: 10.1002/jss.400110203. [DOI] [PubMed] [Google Scholar]
- Beevers L., Mense R. M. Glycoprotein Biosynthesis in Cotyledons of Pisum sativum L: Involvement of Lipid-linked Intermediates. Plant Physiol. 1977 Nov;60(5):703–708. doi: 10.1104/pp.60.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Poulson R. Protein Synthesis in Cotyledons of Pisum sativum L: I. Changes in Cell-Free Amino Acid Incorporation Capacity during Seed Development and Maturation. Plant Physiol. 1972 Apr;49(4):476–481. doi: 10.1104/pp.49.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brett C. T., Leloir L. F. Dolichyl monophosphate and its sugar derivatives in plants. Biochem J. 1977 Jan 1;161(1):93–101. doi: 10.1042/bj1610093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUMPTON M. J. Identification of amino sugars. Biochem J. 1959 Jul;72:479–486. doi: 10.1042/bj0720479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A., Trowbridge I. S., Hyman R., Kornfeld S. Structure of the lipid-linked oligosaccharides that accumulate in class E Thy-1-negative mutant lymphomas. Cell. 1979 Jul;17(3):509–515. doi: 10.1016/0092-8674(79)90259-9. [DOI] [PubMed] [Google Scholar]
- Delmer D. P., Kulow C., Ericson M. C. Glycoprotein Synthesis in Plants: II. Structure of the Mannolipid Intermediate. Plant Physiol. 1978 Jan;61(1):25–29. doi: 10.1104/pp.61.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr M., Bailey D. S., MacLachlan G. Subcellular distribution of membrane-bound glycosyltransferases from pea stems. Eur J Biochem. 1979 Jul;97(2):445–453. doi: 10.1111/j.1432-1033.1979.tb13132.x. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Delmer D. P. Glycoprotein Synthesis in Plants: III. Interaction between UDP-N-Acetylglucosamine and GDP-Mannose as Substrates. Plant Physiol. 1978 May;61(5):819–823. doi: 10.1104/pp.61.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. C., Delmer D. P. Glycoprotein synthesis in plants: I. Role of lipid intermediates. Plant Physiol. 1977 Mar;59(3):341–347. doi: 10.1104/pp.59.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of Soybean Seeds: II. Accumulation of the Major Protein Components during Seed Development and Maturation. Plant Physiol. 1974 May;53(5):747–751. doi: 10.1104/pp.53.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lezica R. P., Brett C. T., Martinez P. R., Dankert M. A. A glucose acceptor in plants with the properties of an alpha-saturated polyprenyl-monophosphate. Biochem Biophys Res Commun. 1975 Oct 6;66(3):980–987. doi: 10.1016/0006-291x(75)90736-6. [DOI] [PubMed] [Google Scholar]
- Li E., Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. I. Structure of the lipid-linked oligosaccharide precursor of the complex-type oligosaccharides of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7762–7770. [PubMed] [Google Scholar]
- Lis H., Sharon N. Soybean agglutinin--a plant glycoprotein. Structure of the carboxydrate unit. J Biol Chem. 1978 May 25;253(10):3468–3476. [PubMed] [Google Scholar]
- Millerd A., Simon M., Stern H. Legumin Synthesis in Developing Cotyledons of Vicia faba L. Plant Physiol. 1971 Oct;48(4):419–425. doi: 10.1104/pp.48.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. J., Leloir L. F. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim Biophys Acta. 1979 Apr 23;559(1):1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Thanh V. H., Shibasaki K. Heterogeneity of beta-conglycinin. Biochim Biophys Acta. 1976 Aug 9;439(2):326–338. doi: 10.1016/0005-2795(76)90068-4. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R. Abnormal lipid-linked oligosaccharides in class E Thy-1-negative mutant lymphomas. Cell. 1979 Jul;17(3):503–508. doi: 10.1016/0092-8674(79)90258-7. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]
- Yosizawa Z., Sato T., Schmid K. Hydrazinolysis of alpha-1-acid glycoprotein. Biochim Biophys Acta. 1966 Jun 29;121(2):417–420. doi: 10.1016/0304-4165(66)90134-6. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Ceccarini C., Atkinson P. H. Labeling complex carbohydrates of animal cells with monosaccharides. Methods Enzymol. 1978;50:175–204. doi: 10.1016/0076-6879(78)50019-0. [DOI] [PubMed] [Google Scholar]