Abstract
Our laboratory previously developed a novel neuropathic and inflammatory facial pain model for mice referred to as the Trigeminal Inflammatory Compression (TIC) model. Rather than inducing whole nerve ischemia and neuronal loss, this injury induces only slight peripheral nerve demyelination triggering long-term mechanical allodynia and cold hypersensitivity on the ipsilateral whisker pad. The aim of the present study is to further characterize the phenotype of the TIC injury model using specific behavioral assays (i.e. light-dark box, open field exploratory activity, and elevated plus maze) to explore pain- and anxiety-like behaviors associated with this model. Our findings determined that the TIC injury produces hypersensitivity 100% of the time after surgery that persists at least 21 weeks post injury (until the animals are euthanized). Three receptive field sensitivity pattern variations in mice with TIC injury are specified. Animals with TIC injury begin displaying anxiety-like behavior in the light-dark box preference and open field exploratory tests at week 8 post injury as compared to sham and naïve animals. Panic anxiety-like behavior was shown in the elevated plus maze in mice with TIC injury if the test was preceded with acoustic startle. Thus, in addition to mechanical and cold hypersensitivity, the present study identified significant anxiety-like behaviors in mice with TIC injury which resembling the clinical symptomatology and psychosocial impairments of patients with chronic facial pain. Overall, the TIC injury model’s chronicity, reproducibility, and reliability in producing pain- and anxiety-like behaviors demonstrate its usefulness as a chronic neuropathic facial pain model.
Keywords: chronic orofacial pain, stress-induced analgesia, acoustic startle, mouse model, operant tests, nerve injury
Approximately 22% of the US population suffers from facial and headache pain. Patients with trigeminal neuropathic pain, one type of chronic facial pain, frequently report experiencing a continuous aching and burning pain sensation that may be accompanied by intermittent electrical shock-like pain. Patients with this type of facial pain also report mechanical allodynia and cold hypersensitivity (Baron et al., 2010; Zakrzewska, 2013a,b). While dental procedures or trauma are known causes of peripheral trigeminal nerve injury and inflammation, in some cases, no clear causes are identified for the origin and maintenance of trigeminal neuropathic pain (Porto et al., 2011, Renton and Yilmaz, 2011).
There are, however, a limited number of models of such pain conditions available for use in laboratory experiments. Historically, one model of neuropathic, facial pain frequently used in rats is known as the chronic constriction injury of the infraorbital nerve (CCI-ION) (Vos et al., 1994). This model has been adapted for use in mice and is referred to as the partial CCI-ION (Xu et al., 2008). Both models involve tying chromic gut suture around the ION, a branch of the maxillary nerve which innervates the whisker pad of rodents, which causes mechanical hypersensitivity in the whisker pad region. However, tying this suture causes deformation of the ION and constricts blood flow thus inducing partial nerve ischemia and loss (Bennett and Xie, 1988, Kim and Chung, 1992, Kawamura et al., 1997), features not consistent with the clinical symptomatology of patients suffering from trigeminal neuropathic pain. To address these issues, a novel chronic facial neuropathic pain model in mice, named the Trigeminal Inflammatory Compression (TIC) injury model, was developed in our laboratory to more closely mimic the clinical characteristics of trigeminal neuropathic pain (Ma et al., 2012). As previously reported, the TIC injury model is produced by inserting chromic gut suture between the infraorbital nerve and the maxillary bone. This placement alongside the nerve, rather than constriction of the nerve, has been successful in limiting whole nerve ischemia and demyelination in mice but promoting long-term whisker pad hypersensitivity (Ma et al., 2012).
The present studies were performed by another surgeon and other testers than in the original study to validate the method. Due to its novelty, reliability, and relevance for translational studies, there is a great need for further characterization of the TIC injury model to increase our understanding of the behavioral characteristics of the model. For example, one important aspect of the clinical presentation of trigeminal neuropathic pain is the common comorbidity of psychological disorders and emotional distress (Wall and Melzack, 1999). In clinical populations, symptoms of anxiety and depression in particular have been consistently observed in patients with chronic trigeminal-mediated pain (Averill et al., 1996, Fishbain, 1999a, b, McWilliams et al., 2003, Nicholson and Verma, 2004, Robinson et al., 2009, Burris et al., 2010).
The measurement of constructs such as anxiety and depression in animal models, however, has proven more difficult than in clinical populations. Fortunately, the use of cognitive dependent tests offers a more thorough examination of psychological constructs such as anxiety, and are increasingly used by researchers seeking to understand chronic neuropathic pain conditions (Mao et al., 2008, Mogil, 2009). Measures of anxiety-like behaviors in animals have been extensively studied, and numerous validated protocols have been developed (Belzung and Griebel, 2001). Three assays that are particularly well understood in measuring animal behavior associated with psychological constructs such as anxiety are: the light-dark preference test, the open field exploratory test, and the elevated plus maze task. Furthermore, the activity and rearing behavior in each of these tasks has been previously shown to be affected by pain (Crawley and Goodwin, 1980, Belzung and Griebel, 2001, Bouwknecht and Paylor, 2002, Roeska et al., 2008, Parent et al., 2012).
The aim of the current study was to further characterize the novel TIC injury model by examining mechanical allodynia and heat hypersensitivity, as well as by measuring anxiety-like behaviors with cognitive dependent operant tests. The hypothesis was that mice with TIC injury would display greater mechanical allodynia, cold hypersensitivity, and more anxiety-like behaviors than naïve mice or animals undergoing sham surgical procedures.
2. Experimental Procedures
2.1. Animals
All experiments were performed with C57Bl/6 male, wild-type mice that weighed between 25 and 35 grams purchased from Harlan Laboratories (Indianapolis, IN). All mice were age matched. Animals were randomly assigned to receive either experimental (TIC injury model) surgical procedures, sham surgical procedures, or to remain naïve. Mice were housed in a well-ventilated room (maintained at 27°C) with a reversed 10/14 h dark/light cycle so that testing could be performed during the active period. All mice had access to food and water ad libitum throughout the duration of the experiment. Regular rodent chow diet with low soy bean content was provided (Teklab 8626, Harlan, Indiana). All experimental procedures were completed according to the guidelines provided by the National Institute of Health (NIH) regarding the care and use of animals for experimental procedures. Animal protocols were approved by the University of Kentucky’s Institutional Animal Care and Use Committee (IACUC). All animals were housed in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) and the United States Department of Agriculture (USDA).
2.2. Trigeminal Inflammatory Compression (TIC) Injury Surgery
Mice were anesthetized with sodium pentobarbital (70 mg/kg, i.p.). Under standard sterile surgery conditions, the hair on the top of their head was shaved and the area disinfected. Ophthalmic cream was applied over their eyes to protect them from over-dryness. Mice were then fully constrained in a stereotaxic frame. A small 15 mm incision was made along the midline of the head and the orbicularis oculi muscle was gently dissected and retracted away from the bone. Small cotton balls were packed into the orbital cavity to control bleeding, and the infraorbital nerve was located (approximately 5mm deep within cavity). Animals randomly assigned to receive the TIC injury surgery underwent surgical placement of a 2 mm length of chromic gut suture (6-0). Chromic gut suture was inserted parallel to the edge and among the infraorbital nerve fibers adjacent to the maxillary bone infraorbital fissure where it can be observed adhered to the nerve when dissected at the end of the experiment. This is done in order to prevent the chromic gut suture from being lost in the orbital cavity, but not to pierce the entire infraorbital nerve. Mechanical allodynia is induced in the mouse whisker pad due to the physical stimulation of the nerve by the suture as well as due to the irritative chromate salt released from the suture. Animals assigned to receive sham surgical procedures did not have the chromic gut suture placement, but only received the skin incision and muscle retraction. Naive animals did not receive any surgery.
2.3. Behavioral Tests
All behavioral tests were conducted during the animals’ active cycle (i.e. dark phase of the dark/light cycle) during the hours of 8:00 am to 6:00 pm. During testing, either a red-light or a dim lamp was illuminated to allow light for the experimenters. The light-dark preference and open field tests were repeated 4 weeks apart, and the elevated plus maze was given only once to avoid over-testing the mice (Walf and Frye, 2007).
2.3.1. Assessment of Mechanical Allodynia
Mechanical threshold of the whisker pad was measured before and after surgery with a modified up/down method using a graded series of von Frey fiber filaments (force:0.008 g (size:1.65); 0.02 g (2.36); 0.07 g (2.83); 0.16 g (3.22); 0.4 g (3.61); 1.0 g (4.08); 2.0 g (4.31); 6.0 g (4.74); Stoelting, Wood Dale, IL). One experimenter gently restrained the mouse in their palm (2–5 minutes) with a cotton glove until the mouse was acclimated and calm. A second experimenter applied the von Frey filaments to the mouse’s whisker pad. The 0.16 g (3.22) fiber was applied first. If the mouse responded three or more times out of five trials to the fiber, this was considered a positive response and the next lower gram force filament was applied. However, if the mouse responded two or fewer times out of five to the fiber applied, this was recorded as a negative response and then the filament with the next higher gram force was applied. Head withdrawal, front paw sweeping, and biting were considered positive responses. Time between applications of each filament was 2–10 seconds. After one fiber successfully caused positive responses, application of the subsequent fibers continued until four fibers were applied or until the animal responded to the lowest gram force fiber. Data were analyzed with a curve-fitting algorithm that allowed for estimation of the 50% mechanical withdrawal threshold (measured in gram force). In this test, decreased mechanical threshold value as compared to baseline is considered to be indicative of mechanical allodynia. Responses to the von Frey fiber stimulations were recorded on day 7 post surgery (TIC and sham) and testing continued once a week post-surgery for both the ipsilateral and contralateral whisker pads. A cohort of naïve mice were tested intermittently (weeks 2, 4, 8, 10, 11) only for mechanical threshold alongside the sham animals and mice with TIC injury.
2.3.2. Assessment of Thermal Hypersensitivity
The protocol used to measure thermal hypersensitivity was adapted from previous experimental protocols (Neubert et al., 2005). Neubert and colleagues used a metal probe connected to a water bath placed in an operant licking box. The present study utilized a looped copper coil probe (0.065″ I.D.; 1/8″ O.D.; 0.030″ Wall Thickness, Restek Corporation, Bellefonte, PA) with a 5 × 3 × 3 mm tip connected to an insulated rubber tubing and attached directly to an Isotemp bath circulator (39.5 × 24.5 × 39 cm, Isotemp 3016S; Fisher Scientific). The water bath was filled with antifreeze liquid, and digitally set to a specified temperature for testing. The temperature of the copper-wire probe was measured with a Physiotemp temperature monitor (Thermalert Model TH-8; PHYSIOTEMP INSTRUMENTS INC. Clifton, NJ, USA). The temperatures reported in this study were measured from the tip of the copper-wire probe. The 10–11 °C was chosen to activate cold nociceptors; likewise 45–46.5 °C was chosen as this temperature was proved to discriminate sensitization in inflammatory conditions (not in normal conditions) (Neubert et al., 2005, Rossi and Neubert, 2009). Room temperature (23–24.3°C) and mouse body skin temperature (32–32.5°C) were chosen to act as controls for this experiment to ensure that contact with the probe was not causing an adverse mechanical stimulation to the animals. Mouse skin was shaved on the ipsilateral V2 (trigeminal nerve second branch) region just behind the whisker pad 24 hours before testing (Cha et al., 2012). One experimenter gently restrained the mouse in their palm with a cotton glove until the mouse was acclimated and calm (5 minutes). The other experimenter then applied the looped copper-wire probe to the shaved V2 region (Figure 1.A). Head withdrawal latency, the time in seconds from which the stimulus was applied to the time the mouse reacted with head withdrawal, front paw sweeping, and/or biting, was recorded. Three trials for each temperature were conducted with 1 minute intervals between each trial. Mice were tested with only one temperature per testing day and thermal testing for all animal groups occurred after post-operative week 8.
Figure 1. TIC injury induced mechanical and cold allodynia.
Mice with TIC injury were tested for mechanical allodynia on the whisker pad and thermal hypersensitivity in the V2 area behind the whisker pad (A). The ipsilateral whisker pad mechanical threshold of the mice with TIC injury was significantly decrease compared to that of the contralateral side and the sham and naïve mice within the first week post injury. The decreased threshold persisted until euthanasia at 21weeks post injury (B). The receptive field pattern variations are shown in (C). It should be noted that 100% of the mice receiving the TIC surgery develop allodynia. Mice with TIC injury are hypersensitive to cold but not warm temperatures (D). n= 7–10; Two way ANOVA, Fisher post hoc test ****p<0.0001; One way ANOVA, Tukey post hoc ***p<0.001.
2.3.3. Acoustic Startle Disturbance
The acoustic startle disturbance test is a well-established measure of anxiety-like behaviors in response to a stressful stimulus (Geyer et al., 1982, Blaszczyk et al., 2000, Blaszczyk et al., 2010). Mice were placed in a vinyl cylinder container (radius: 21.5 cm; depth: 29.9 cm) with room for the animal to move. To produce an acoustic startle disturbance above the animal for a period of 2 min, one experimenter irregularly pressed a © Top Paw Dog Training Clicker (2″ Length “blue bone” clicker; Item # 39330 purchased from ©Pet Smart, Lexington, KY). The clicker was pressed with force eliciting an average frequency of 430.89 Hz (recorded with a KAYPENTAX COMPUTERIZED SPEECH LAB, Real-Time Pitch; Model #5121, version 3.4.1) and ranging from 70–110 dB (Decibel Meter App; Version 1.6; Device Type: iPhone4 iOS Version: 6.1.3). Mice not currently being tested were housed in a separate sound-proof room during the acoustic startle test. Immediately after exposure to the mild acoustic startle, mice were either placed in the dark side of the light-dark box or the central area of the elevated plus maze for some groups to begin the test designated for that day.
2.3.4. Two Compartment Light-Dark Box Preference Test
The light-dark box preference task has a long history of use in the measurement of anxiety-like behavior. In this task, anxiety-like behaviors are believed to manifest as a decrease in: 1) total time spent in the light area, 2) number of entries into the light area, and 3) number of rearing events and exploratory behavior (Crawley and Goodwin, 1980, Hascoet, 1998, Bouwknecht and Paylor, 2002). The light-dark box consists of two equally-sized chambers (one illuminated and one darkened; 11 × 19 × 12 cm/each) connected with a 5 × 5 cm doorway in which mice are allowed to move freely between chambers. Immediately after exposure to the mild acoustic startle disturbance (described above), mice were placed in the dark side of the box facing away from the light chamber. Animals remained in the light-dark preference box for a total of 10 minutes. Behaviors measured in this test included: (1) time spent in the light area, (2) number of transitions into the light and dark chambers, defined as at least partial passage between chambers with extension of at least one of the animal’s back legs from one chamber to the next; (3) number of rearing events, a measurement for exploratory behavior, (4) latency of the first transition into the light chamber, and (5) latency of first re-entry (transition) back into the dark chamber. Behaviors were measured at postoperative weeks 1, 4, and 8.
2.3.5. Open Field Exploratory Activities
Exploratory behaviors were measured using a Flexfield Animal Activity System (San Diego Instruments, San Diego, CA). This apparatus consists of two Plexiglas chambers (40 × 40 × 36 cm) equipped with Photobeam Activity System (PAS) software coupled to a Compaq 486 computer (Hewlett Packard, Palo Alto, CA). Each chamber contained infrared photobeam sensors with 16 beams on each axis (total of 32 beams) that are arranged 1.25 cm above the chamber floor. Obstruction of these photo beams constitutes movements in the x- and y- plane. The x- and y- plane were further divided into a central and peripheral area. Another set of 16 beams is located 8 cm above the chamber floor to record movements along the z-axis measured i.e. rearing events and rearing duration (Zhang et al., 2004). Data were collected in 5-min intervals for a total of 45 minutes to record: (1) number and duration of rearing events (2) active time vs. rest time, (3) overall distance travelled, (4) total beam breaks, and (5) time spent in the central verses peripheral areas of the chamber. Behaviors were measured at post-operative weeks 1, 4, and 8.
2.3.6. Elevated Plus Maze Task
The elevated plus maze task is a widely used test for measuring anxiety-like behavior, and behaviors in this test have been previously shown to be affected by rodent pain models (Kontinen et al., 1999, Belzung and Griebel, 2001, Roeska et al., 2008, Parent et al., 2012). The elevated plus maze (Bioseb, Vitrolles, France) consists of four arms arranged in a cross-shaped design (length: 35 cm, width: 5 cm/each, height from floor: 51 cm); two arms are enclosed on three sides by 15 cm high walls and the other two are not. All arms meet in a central area (5 cm × 5 cm) which allows animals to move freely throughout each zone of the maze. A computer equipped with automated program software (BIOEPM 1.1.14; BIOSEB, France) and linked with a camera head (DFK22AUC03) recorded each animal’s movement throughout the maze. Mice were placed in the central area of the maze and allowed to explore the maze for a period of 5 minutes. Separate groups of mice received acoustic startle disturbance (described above) and then immediately placed into the plus maze. Mouse behaviors were videotaped, coded, and analyzed off line by an observer blinded to experimental group for: (1) time spent in open arms, (2) number of transitions into the open and closed arms, and (3) number of head dips into the open arms, defined as the movement of the animals head from the closed arm to the open arm of the maze. This test was only given once to separate groups of mice with and without acoustic startle.
In this test, the open arms represent a potentially threatening environment. Thus anxiety-like response to this perceived threat results in decreased time spent in the open arms and decreased number of entries into the open arms of the maze (Belzung and Griebel, 2001). Acoustic startle disturbance just prior to the test induces panic anxiety-like behavior with increased time and entries in the open arms compared to controls (de Paula and Hoshino, 2003, 2004).
2.4. Statistical Analysis
GraphPad Prism 6 statistical program was used for all data analysis (Graph Pad Software, Inc. La Jolla, CA). Results are shown as the mean ± standard error. Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey post hoc test and two-way ANOVA followed by Fisher post hoc test where appropriate. A p<0.05 was considered significant for all tests.
3. Results
3.1. Animals with TIC Injury Displayed Unilateral Mechanical Allodynia of the Whisker Pad
The mean 50% mechanical threshold of the ipsilateral side for mice with TIC injury was 0.03 ± 0.28g indicating mechanical allodynia, while the mean 50% mechanical threshold of the contralateral side (3.72 ± 0.12g) was not significantly different from baseline (n=13; p>0.05, Figure 1.B). All mice (100%) that underwent TIC surgery developed mechanical allodynia on the ipsilateral but not the contralateral whisker pad as determined with von Frey fibers thus confirming the results of our previously published paper describing this model (Ma et al., 2012). The 50% mechanical thresholds for sham and naïve animals remained consistently similar to baseline values (sham: ipsilateral, 3.36 ± 0.07g; contralateral, 3.49 ± 0.05g; n=12; naïve: ipsilateral, 3.55 ± 0.019g; contralateral, 3.50 ± 0.21g, n=5). For animals with TIC injury, a two-way ANOVA demonstrated statistically significant effects for time (F (4,227) =13.26, p<0.001), group (F (5,227) =144.30, p<0.001), and the interaction of group x time (F (20,227) =11.07, p<0.001). Fisher post hoc analyses revealed significant differences between mechanical thresholds of the ipsilateral side for the mice with TIC injury and sham animals (t=9.53, p<0.001) and for the mice with TIC injury and naïve animals (t=12.67, p<0.001). The decreased mechanical threshold in the animals with TIC injury was present post injury only on the ipsilateral side and persisted until the animals were euthanized in week 21.
The previously published study mentioned the presence of a receptive field on the mouse whisker pad, which was not discussed in detail (Ma et al., 2012). Initially, in the present study, von Frey fibers <0.16 g (3.22) were applied to the whisker pad sporadically until a withdrawal response occurred in order to find the specific receptive field for each animal. It is important to note that all mice with TIC injury responded to the 0.4 g fiber (3.61) regardless of specific receptive field stimulation, whereas the sham and naïve animals did not respond to the 0.4 g (3.61) fiber. The location of the receptive field for each animal remained consistent across time points and rarely changed once established. This provides evidence that placement of the chromic gut suture not only produces an injury to a localized area of axonal fibers along the infraorbital nerve, but also suggests that injury to these fibers correlates with a specific receptive field on the whisker pad.
Three different patterns for receptive fields were identified on the whisker pads of injured mice (Figure 1. C). Mouse #1 is representative of the most typical receptive field observed in 50% of the mice with TIC injury. The second most common pattern is represented by Mouse #2 and was observed in 38% of mice with TIC injury. Mouse #3 represents a uniquely different receptive field seen in only 11% of mice with TIC injury in which the receptive field was scattered sporadically throughout the whisker pad. Interestingly, all mice (100%) subjected to TIC surgery showed a sensitive spot at the 6 o’clock position on the whisker pad (Figure 1.C, indicated by the red x with a circle). The slightly different patterns of receptive fields are likely dependent upon the specific ION fibers in contact with the chromic gut suture.
3.2. TIC Injury Induced a Unilateral Cold Allodynia of the V2 Area
Withdrawal latencies for four thermal stimuli applied to the skin were recorded (n=8; Figure 1D). For the 10–11°C (i.e., cold) temperature stimuli, the head withdrawal latency of animals with TIC injury (5.11 ± 0.62s) was significantly reduced compared to that of sham animals (9.21 ± 0.88s) and naïve animals (9.75 ± 0.71s; F (2, 14) =17.50, p<0.001). The head withdrawal latency of animals with TIC injury for the 45–46.5°C (i.e., heat) temperature stimuli was 10.21± 1.20s which was not significantly different compared to that of sham (9.42 ±1.11s) or naïve animals (8.63 ±0.90s; p>0.05). At room temperature (23.24.3°C), the head withdrawal latency of animals with TIC injury (15.54 ± 1.52s) was not significantly different from sham (16.79 ± 0.83s) and naïve animals (17.17 ± 1.13s; p>0.05). For stimulation at mouse skin temperature (32–32.5°C), the head withdrawal latency of animals with TIC injury was 20.42 ± 2.37s, which was not significantly different from the sham (17.25 ± 1.87s) and naïve animals (15.92 ± 1.75s; p>0.05; Figure 1.D). These results indicate that animals with TIC injury were sensitive to mild cold stimuli as compared to sham and naïve animals, but had normal reactions to heat, room temperature, and skin temperature stimuli.
3.3. Animals with TIC Injury Displayed Anxiety-Like Behaviors in Two Compartment Light-Dark Preference Testing
Immediately after the 2-min acoustic disturbance, mice were placed in the dark side of the light-dark box facing away from the light box to begin the 10 min experiment. Total time spent in the light and dark sides, light-dark transitions, rearing events and latencies to the first cross into the light side and re-entry into the dark side were measured over 10 min. There were no significant differences in time spent in the light/dark sides or numbers of rearing events in weeks 1 or 4 post-injury (all p values >0.05). There was no significant difference between mice with TIC injury and sham animals when comparing latency of the first cross into the light side, latency to re-enter dark side, or the number of light-dark transitions (all p values >0.05; Table 1). Both mice with TIC injury and sham animals spent almost equal amounts of time in the light and dark sides of the box at post-injury week 1 and week 4 (p>0.05). At post-injury week 8, mice with TIC injury spent less time in the light, 231.60 ± 25.55s, i.e. they remained in the dark chamber over 70% of the testing time. In contrast, the sham mice remained consistent (330.39 ± 43.72s; i.e., over 50%) in time spent in the light chamber (TIC vs. sham, t (74) =2.11 p<0.05, Figure 2.A). Mice with TIC injury also had significantly fewer rearing events (9.77 ± 1.89/10min) at week 8 post injury compared to sham animals at week 8 post surgery (18.82 ±1.95/10min, t (74) =3.01, p<0.005, Figure 2. B). This difference remained significant even when the number of rearing events was analyzed by the first and second five minute intervals of the light-dark box preference (Table 1). The results of the light-dark box preference tests indicate that mice with TIC injury develop responses consistent with anxiety-like behavior at eight weeks post-injury.
Table 1.
Light-Dark Box Data
| Week | Group | Latency to first cross (s) | Light/dark Transitions (#) | Latency to re-enter dark side (s) | Rearing Events |
|---|---|---|---|---|---|

| |||||
| 0 | Naïve | 41.53±12.45 | 9.88±1.37 | 21.06±8.70 |
|
| 1 | TIC | 56.17±12.53 | 10.38±1.29 | 21.09±3.77 |
|
| 1 | SHAM | 40.55±13.91 | 11.13±1.29 | 20.29±5.67 |
|
| 4 | TIC | 23.48±9.88 | 9.83±1.90 | 65.66±24.86 |
|
| 4 | SHAM | 23.85±10.26 | 11.17±1.36 | 48.64±35.44 |
|
| 8 | TIC | 35.52±11.70 | 9.38±1.47 | 31.61±16.18 |
|
| 8 | SHAM | 13.08±5.47 | 11.55±1.89 | 18.33±3.59 |
|
Figure 2. Mice with TIC injury showed anxiety-like behavior in the light-dark box.
Mice with TIC injury spent significantly less time in the light side of the light-dark box at post injury week 8 compared with that of the sham animals (A). Mice with TIC injury showed a decreased number of rearing events at post injury week 8 compared to the sham mice (B). Behavioral baselines for naïve animals are indicated by the dotted line; n= 8–21, Two way ANOVA Fisher post hoc test **p<0.01,*p<0.05.
3.4. Acoustic Disturbance Affected Mice with TIC in the Elevated Plus Maze Task
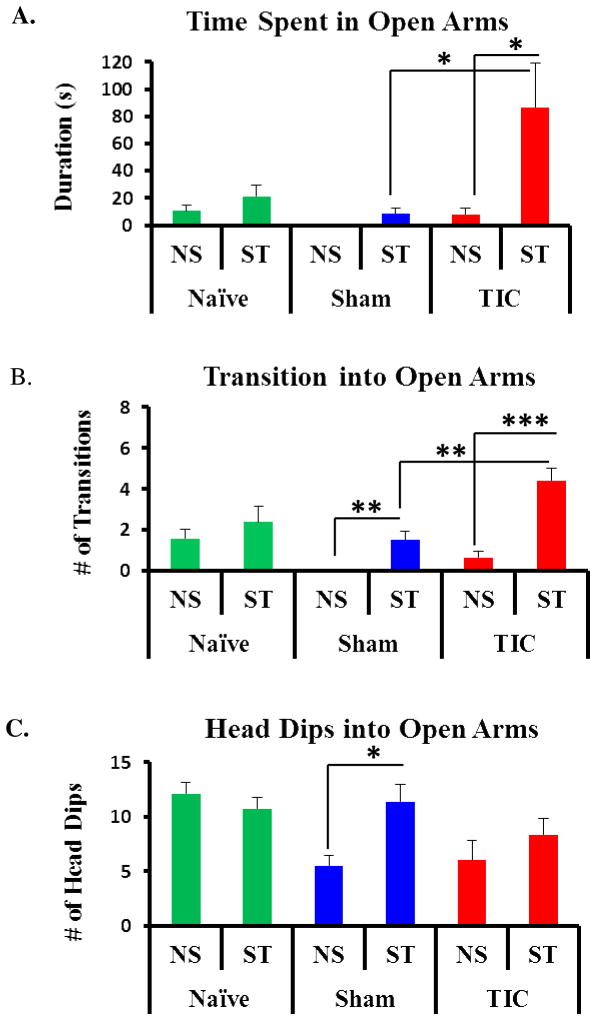
There was no evidence of anxiety-like behavior in any group in the elevated plus maze test administered in a quiet environment (Figure 3). However, separate groups of mice were subjected to plus maze testing immediately after a 2 min acoustic disturbance. In this case, mice with TIC spent significantly more time in the open arms (78.90 ± 30.45s) than did sham animals (8.89 ± 3.52s, t (51) =3.30, p<0.05, Figure 3. A). Mice with TIC injury also had more transitions into the open arms compared to sham animals at week 1 post-injury (TIC: 4.00 ± 0.67/5min; sham: 1.50 ± 0.42/5min; t (51) =3.52, p<0.01) Figure 3. B). The number of head dips into the open arms did not differ between mice with TIC injury (Figure 3. C).
Figure 3. Acoustic Disturbance Provides Susceptibility to Anxiety-like Behavior in Mice with TIC Injury in the Elevated Plus Maze.
No anxiety-like behaviors were observed in any of the groups tested in the plus maze in a quiet environment (NS). After acoustic startle (ST), testing separate groups determined mice with TIC injury spent significantly more time in the open arms (A) compared to sham or non-startled mice with TIC injury. Mice with TIC injury had significantly more transitions into the open arms as compared to the non-startled mice with TIC injury or sham group (B). Head dips into the open arms were significantly increased in the sham group after startle, but were not significantly different in other groups (C). n= 7–8, Comparison of non-startle (NS) vs startle (ST) group means for each conditions with t- test ***p<0.001, **p<0.01,*p<0.05.
3.5. Mice with TIC Injury Showed Less Exploratory Activity
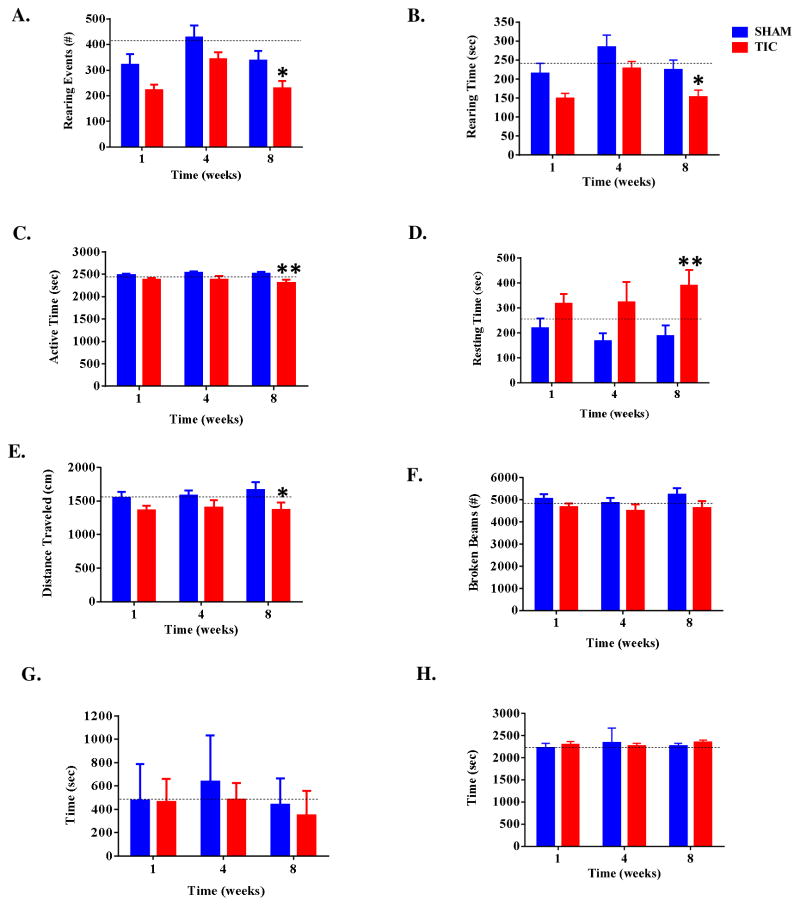
Exploratory behavior for animals with TIC injury and sham animals was compared via analyses of rearing duration and events, active time, resting time, total distance travelled, total beam breaks, and time spent in the center vs. periphery of the testing apparatus. Animals with TIC injury showed less rearing behavior than sham animals at week 8 post-injury as measured by both rearing duration (TIC: 153.11 ± 18.22s; sham: 224.75 ± 25.09s; t (64) =2.62, p<0.05; Figure 4A) and number of rearing events (TIC: 229.89 ± 27.36; sham: 337.46 ± 37.67; t (64) =2.62, p<0.05; Figure 4B). During week 8 post-injury, mice with TIC injury had lower active time (2311.50 ± 63.94s) and greater resting time (388.48 ± 63.94s) compared to sham animals (active, 2513.30 ± 43.15s; resting, 186.72 ± 43.15s; t (64) =2.73, p<0.05, Figure 4C & 4D). Mice with TIC injury also travelled less total distance than sham animals in week 8 post-injury (TIC: 1370.20 ± 110.60cm; sham: 1666.00 ± 114.38cm, t (64) =2.21, p<0.05; P<0.01; Figure 4E). There was no difference in the number of total beam breaks or center vs. peripheral time duration at any time point (all p values >0.05; Figure 4F, 4G, & 4H). Significant differences at weeks 1 or 4 post injury were not observed (all p values >0.05). Results in the open field test parallel the light-dark box data and support the development of anxiety-like behaviors in mice with TIC injury at week 8 post injury.
Figure 4. Mice Displayed Less Exploratory Activity in Open Field Testing.
Mice with TIC injury had significantly fewer rearing events and rearing time compared to sham mice in post injury week 8 (A&B). Mice with TIC injury had significantly less active time and increased resting time in post-op week 8 compared to sham mice (C&D). Mice with TIC traveled less overall distance in post-op week 8 compared to sham mice (E). There were no significant differences for the number of beam breaks (F) or time in central (G) vs. peripheral (H) maze space. n=8–19, Two-way ANOVA, Fisher post hoc test, **p<0.01,*p<0.05.
4. Discussion
4.1. TIC Injury Model Mimics Clinical Neuropathic Pain
In the present study, we determined that the TIC injury model is efficacious for inducing hypersensitivity in 100% of the animals receiving the chromic gut suture placement. This is due to the adherence of the chromic gut suture to the infraorbital nerve edge. All mice experienced mechanical allodynia on the ipsilateral whisker pad with distinctive receptive fields. The receptive field pattern variations in the TIC injury model are believed to be due to the position of the chromic gut suture relative to specific infraorbital nerve fibers adjacent to the maxillary bone. The development of cold allodynia in the mice with TIC injury, but not heat hypersensitivity is another important feature of this model as clinic patients with neuropathic pain experience cold hypersensitivity but not heat sensitivity (Baron et al., 2010, Zakrzewska, 2013a, b).
The present study determined that the TIC injury model persists at least 21 weeks post-injury which is even longer than previously reported by our lab (Ma, et al., 2012). These are the first recorded data for a mouse facial pain model persisting for this extended length of time. The model does not cause nerve ischemia and only causes partial demyelination of the infraorbital nerve (Ma et al., 2012). Many acute and chronic orofacial models are in use that successful induce hypersensitivity in the mouse. However, many are acute models that do not persist longer than a week and fall short of reflecting the full clinical characteristics of chronic facial pain (Luccarini et al., 2006, Bornhof et al., 2011, Quintans et al., 2014). The chronic models of facial pain either differ significantly from the clinical presentation of patients with trigeminal pain or have low efficacy in mice (Vos et al., 1994, Saito et al., 2008, Xu et al., 2008, Zhang et al., 2012b, Siqueira-Lima et al., 2014).
The TIC injury model also mimics several other aspects consistent with the clinical presentation chronic facial pain in humans with this pain condition. For example, mice with TIC injury have distinct receptive fields that elicit withdrawal reflex responses just as human patients have specific receptive fields triggering pain. The pattern of the receptive field is not always the same in all animals. Likewise, the pattern of receptive fields varies from person to person (Simons and Travell, 1981, Travell, 1981, Siqueira et al., 2009). Moreover, mice with TIC injury display only cold but not heat hypersensitivity. Patients suffering with trigeminal neuropathic pain are not often bothered by heat stimuli, but instead more often complain that cold air, light touch, or blowing wind trigger their shooting pain (De Leeuw, 2008, Zakrzewska, 2013a, b).
4.2. Mice with TIC Injury Develop Anxiety-Like Behaviors
For the light-dark box preference and open field exploratory tests, the behavior of the mice with TIC injury at week 8 is significantly altered. The validity of repeating these tests every four weeks was indicated by the unaltered post injury behavior of the shams and the naïve mice which remained relatively consistent in weeks 1, 4, and 8 post surgery (Walf and Frye, 2007). In the light-dark box preference test, only the mice with TIC injury spend significantly less time in the light side of the box and have fewer numbers of rearing events at week 8 post-injury. Similarly for the open field testing, mice with TIC injury have fewer of the rearing events, less active time with increased resting time, and less total distance traveled only at post-injury week 8 compared to sham animals. The TIC injury induced anxiety-like behaviors persist well beyond 8 weeks and can be attenuated with pharmacological agents (manuscript in preparation). Although, operant behavioral testing beyond week 8 is not reported in this paper, these anxiety-like behaviors remained consistent throughout the 21 week experimental time course after the TIC injury, paralleling the whisker pad mechanical hypersensitivity reported here.
Previous research has shown that a decreased amount of time spent in the light side of the light-dark box, as well as a decreased number of rearing events and transitions into the light side, are anxiety-like behaviors (Costall and Naylor, 1997, Fedorova et al., 2003, Cryan and Holmes, 2005). Open field testing has been used to assess the exploratory and locomotor activity of animals in this case with automated counts for number of rearing events, measures of active vs. resting time, and time spent in the center vs. peripheral portions of the grid. Decreased number of rearing events, decreased active time, and decreased maze center occupancy time is considered a reliable index of anxiety-like behavior and can be used to measure response to anxiolytic agents (Katz and Roth, 1979, Costall and Naylor, 1997, Ramos et al., 1997).
4.3. Acoustic Disturbance Has Increased Effect on Mice with TIC in the Elevated Plus Maze Task
The plus maze can only be tested once without decrement due to acclimatization. Anxiety-like behavior was not evident in the shams or mice with TIC injury tested in the Plus Maze except when previously exposed to the 2 minute acoustic startle. Mice with TIC injury and acoustic startle disturbance spent significantly more time in the open arms and had a significantly greater number of transitions compared to non-startled mice with TIC, sham, and naïve mice. One could argue that these results represented panic-like anxiety behavior in the TIC mice (de Paula and Hoshino, 2003, 2004). Thus, mice with TIC injury with the acoustic disturbance had less evidence of open arm avoidance anxiety despite their reflexive pain related behaviors, similar to results in other studies utilizing acoustic startle (Davis et al., 1997, Bailey and Crawley, 2009).
Mice with TIC injury that were not exposed to the acoustic disturbance, however, did not have this increase in time spent in the open arms or number of transitions compared to sham animals without acoustic startle. Thus, the animals with both acoustic stress and injury displayed susceptibility to hyperarousal, revealing the panic-like anxiety behaviors of the mice with TIC injury already in a sensitized state. An acoustic startle disturbance has been shown to create “stress-induced analgesia” by inducing endogenous opioids release, thereby, creating the possibility that the animals with acoustic stress were also less aware of their painful condition (Lewis et al., 1980, Watkins and Mayer, 1982, Nencini et al., 1984, Terman et al., 1984, Vitale et al., 2005, Frew and Drummond, 2008). A human corollary of stress induced analgesia early after initiation of pain has been reported (Vachon-Presseau et al., 2013). Since the separate cohorts of animals without acoustic startle disturbance did not exhibit the same open arm behavior, the acoustic disturbance apparently induced a panic reaction resulting in increased time spent in open arms and an increased number of transitions. This is not unlike the wild running behavior mediated by the dorsal periaqueductal grey previously reported after acoustic startle (de Paulis and Hoshino, 2003, 2004). Chronic pain has been shown to be a predictor of panic attacks in patients (Smitherman et al., 2013; Kang et al., 2015).
Although the acoustic stimulation was an important stressor in the elevated plus maze test, it did not appear to have much effect on the behaviors of the mice in the light-dark box preference test. This suggests the plus maze was more stressful than the light-dark box and open field for triggering anxiety-like activities (Cruz et al., 1994, Rodgers, 1997, Rodgers and Dalvi, 1997, Salas et al., 2003, Walf and Frye, 2007).
Another possible explanation for these data is that animals with TIC injury had more reactivity to the stressors and worse decision making capacity after the acoustic startle stimulation in the elevated plus maze task. This could be due to plastic changes in brain circuitry occurring long-term after injury. Neugebauer and colleagues (2004) described the altered physiological responses of the amygdala as a multi-functional pain integration site (Neugebauer et al., 2004). The pre-frontal cortex, amygdala, and ventral hippocampus are just a few areas of the brain crucial in anxiety responsive behaviors (Quirk et al., 2000, Neugebauer et al., 2004, Ji et al., 2010, Rea et al., 2013). Rodents with excitotoxic lesions in pre-frontal cortex, amygdala, and ventral hippocampus reportedly spend significantly more time in the open arms and have increased numbers of transitions compared to sham animals in the elevated plus maze test (Quinn et al., 2002, Shah and Treit, 2003, Ventura-Silva et al., 2013). This interpretation allows for the coexistence of anxiety/stress and chronic pain. Therefore, if pharmaceutical agents were available that not only alleviated chronic pain, but also anxiety, then perhaps our understanding of these mechanisms would be improved. A second report will present anxiety-like behaviors of mice with TIC injury at time points well beyond 8 weeks post-injury (weeks 8–21) as well as experiments with different pharmacological agents.
Together, the results of the light-dark preference, open field, and elevated plus maze testing have supported the TIC injury as an efficacious model for inducing anxiety-like behaviors as well as chronic facial pain. Other chronic pain models in animals have also observed similar anxiety-like behavior after migraine-induced pain and sciatic nerve injury (Robinson et al., 1988, Lipton et al., 2000, McWilliams et al., 2003, McWilliams et al., 2004, Dellarole et al., 2014). Of further note is that mice with TIC injury develop the anxiety-like behaviors by week 8 post-injury in each of the three tests. Yalcin and colleagues (2011) similarly reported that anxiety-like behaviors develop 6–8 weeks post injury (Yalcin et al., 2011). These data suggest that it is the chronicity of the TIC injury model that promotes the development of the “pain affect;” another facet similar to patients suffering from chronic pain.
Wall & Melzack (1999) described chronic pain as having two main features: “pain sensation” and the “pain affect” which incorporates the emotional distress that patients undergo due to pain (Wall and Melzack, 1999). The “pain affect” has also been described as the squeal of other physical and psychological disorders such as anxiety and depression (McWilliams et al., 2003, McWilliams et al., 2004, Maletic and Raison, 2009, Dellarole et al., 2014). These comorbidities have been so prevalent that approximately half of all patients suffering from chronic pain are also described as suffered from anxiety and depression (Asmundson and Katz, 2009, Asmundson and Taylor, 2009, Robinson et al., 2009, Macianskyte et al., 2011). The relationship between chronic pain, anxiety, and depression, is extremely complex with extensive overlap making it difficult to determine the cause and effect. This complex relationship is related to the numerous brain structures, such as the somatosensory cortex, prefrontal cortex, nucleus accumbens, insular cortex, anterior cingulate cortex, amygdala, hippocampus, thalamus, and cerebellum that become involved in pain perception which are also highlighted as major players in affective states, including depression, anxiety, and fear (Averill et al., 1996, Fishbain, 1999b, Apkarian, 2004, Apkarian et al., 2004a, Apkarian et al., 2004b, Becerra et al., 2006, Robinson et al., 2009, Dellarole et al., 2014). Thus, these studies validate the TIC injury as a representative model for future testing of chronic facial pain- and anxiety-related behaviors, as well as for testing pain and anxiolytic pharmaceutical agents, such as SSRIs, with potential to alleviate pain and comorbid psychological conditions. The model is particularly well suited for long-term, two week to three month treatments required for preclinical drug trials meeting Federal Food and Drug Administration standards (http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/ucm176522.htm 03/19/2015).
Several limitations existed for the present study. First, all of our animals were male C57Bl/6 mice. C57Bl/6 mice were chosen for their moderate levels of anxiety-like behavior ((Belzung, 2001, Belzung and Griebel, 2001, Kulesskaya et al., 2014, Kulesskaya and Voikar, 2014) In order to collect more consistent results, no females or other strains were tested in this study. However, future studies should be conducted to observe the behavior of different species or gender of mice with TIC injury since facial neuropathic pain in humans occurs preferentially in females (Rauhala et al., 2000, Macfarlane et al., 2001, Macfarlane et al., 2002). Secondly, the plus maze could only be administered once. The light/dark preference and open field tests were conducted four weeks apart in weeks 1, 4, and 8 post injury allowing determination of the development of anxiety-like behavior. This was done to prevent over-testing of the animals since testing minimally at 3 week intervals is recommended (Walf and Frye, 2007). Thirdly, although anxiety-like behavior was observed in mice with TIC injury, we did not conduct any traditional operant depressive tests such as forced swimming test, tail suspension test, sucrose consumption or sucrose splash tests (Wang et al., 2012, Zhang et al., 2012a). Future studies can be conducted that observe depressive-like behavior using forced swimming and tail suspension tests which subject the animals to a perceived life threatening, stressful environment ((Borsini and Meli, 1988, Sakakibara et al., 2005). Likewise, the sucrose consumption test would be another measure of depression in the mice with TIC injury as well as indication of their reward-seeking behavior (Wyvell and Berridge, 2000, Wang et al., 2012). Future studies could be conducted with these considerations in mind.
5. Conclusion
In summary, the TIC injury model is a robust, reliable, and reproducible translational relevant chronic trigeminal pain model mimicking the clinical injury due to its reliability, efficacy and persistence that can be used to study the course of chronic facial pain. This will help not only to identify certain molecular targets and signaling cascades that generate chronic pain, but also to define differences between trigeminal nerve related facial pain versus spinal nerve injury related pain. Since the TIC injury model has been developed in mice, gene therapies and specific gene knock-outs can be used to target particular cytokines, proteins, and receptors in hopes of revealing underlying mechanisms that influence trigeminal pain. Furthermore, since this model induces anxiety-like behaviors by week 8, it is a useful model to study pharmaceutical agents that perhaps can alleviate not only pain, but also the anxiety related to pain.
Highlights.
TIC injury in mice induces mechanical allodynia and cold hypersensitivity
TIC injury induces anxiety-like behaviors in mice 8 weeks after injury
When exposed to an acoustic startle stimulation, mice with TIC injury exhibit stress-induced analgesic behaviors.
Acknowledgments
This study was supported by NIH COBRE 2P20RR020145-06 (RJD), NINDS R01-039041 (KNW), and a $20,000 donation to student salaries (KNW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apkarian AV. Cortical pathophysiology of chronic pain. Novartis Found Symp. 2004;261:239–245. [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004a;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004b;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26:888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Taylor S. PTSD diagnostic criteria: understanding etiology and treatment. Am J Psychiatry. 2009;166:726. doi: 10.1176/appi.ajp.2009.08121799. author reply 727. [DOI] [PubMed] [Google Scholar]
- Averill PM, Novy DM, Nelson DV, Berry LA. Correlates of depression in chronic pain patients: a comprehensive examination. Pain. 1996;65:93–100. doi: 10.1016/0304-3959(95)00163-8. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. Boca Raton (FL): 2009. [Google Scholar]
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. The Lancet Neurology. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behavioural brain research. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Bennett GaX, YK A peripheral mononeuropathy in rat that produces disorderso pain sensation like those seen in man. Pain physician. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Blaszczyk JW, Lapo IB, Werka T, Sadowski B. Differential startle magnitude in mice selected for high and low swim analgesia is not related to difference in nociception. Acta neurobiologiae experimentalis. 2010;70:398–405. doi: 10.55782/ane-2010-1812. [DOI] [PubMed] [Google Scholar]
- Blaszczyk JW, Tajchert K, Lapo I, Sadowski B. Acoustic startle and open-field behavior in mice bred for magnitude of swim analgesia. Physiology & behavior. 2000;70:471–476. doi: 10.1016/s0031-9384(00)00289-4. [DOI] [PubMed] [Google Scholar]
- Bornhof M, Ihmsen H, Schwilden H, Yeomans DC, Tzabazis A. The orofacial formalin test in mice revisited--effects of formalin concentration, age, morphine and analysis method. The journal of pain: official journal of the American Pain Society. 2011;12:633–639. doi: 10.1016/j.jpain.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology. 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behavioural brain research. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Burris JL, Evans DR, Carlson CR. Psychological correlates of medical comorbidities in patients with temporomandibular disorders. Journal of the American Dental Association. 2010;141:22–31. doi: 10.14219/jada.archive.2010.0017. [DOI] [PubMed] [Google Scholar]
- Cha M, Kohan KJ, Zuo X, Ling JX, Gu JG. Assessment of chronic trigeminal neuropathic pain by the orofacial operant test in rats. Behavioural brain research. 2012;234:82–90. doi: 10.1016/j.bbr.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. The influence of 5-HT2 and 5-HT4 receptor antagonists to modify drug induced disinhibitory effects in the mouse light/dark test. Br J Pharmacol. 1997;122:1105–1118. doi: 10.1038/sj.bjp.0701513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, GFK Preliminary Report of a Simple Animal Behavior Model for the Anxiolytic Effects of Benzodiazepines. Pharmacology Biochemistry and Behavior. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leeuw R, editor. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management. Quintessence Publishing Co, Inc; Hanover Park (IL): 2008. [Google Scholar]
- de Paula HM, Hoshino K. Antipanic procedures reduce the strychnine-facilitated wild running of rats. Behav Brain Res. 2003;147(1–2):157–62. doi: 10.1016/s0166-4328(03)00147-5. [DOI] [PubMed] [Google Scholar]
- de Paula HM, Hoshino K. Potentiation of panic-like behaviors of the rat by subconvulsive doses of strychnine. Physiol Behav. 2004;80(4):459–64. doi: 10.1016/j.physbeh.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Dellarole A, Morton P, Brambilla R, Walters W, Summers S, Bernardes D, Grilli M, Bethea JR. Neuropathic pain-induced depressive-like behavior and hippocampal neurogenesis and plasticity are dependent on TNFR1 signaling. Brain Behav Immun. 2014;41:65–81. doi: 10.1016/j.bbi.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova IM, Jacobson MA, Basile A, Jacobson KA. Behavioral characterization of mice lacking the A3 adenosine receptor: sensitivity to hypoxic neurodegeneration. Cell Mol Neurobiol. 2003;23:431–447. doi: 10.1023/A:1023601007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbain DA. Approaches to treatment decisions for psychiatric comorbidity in the management of the chronic pain patient. The Medical Clinics of North America. 1999a;83:737–760. vii. doi: 10.1016/s0025-7125(05)70132-2. [DOI] [PubMed] [Google Scholar]
- Fishbain DA. The association of chronic pain and suicide. Seminars in clinical neuropsychiatry. 1999b;4:221–227. doi: 10.153/SCNP00400221. [DOI] [PubMed] [Google Scholar]
- Frew AK, Drummond PD. Stress-evoked opioid release inhibits pain in major depressive disorder. Pain. 2008;139:284–292. doi: 10.1016/j.pain.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Flicker CE, Lee EH. Effects of tactile startle on serotonin content of midbrain raphe neurons in rats. Behavioural brain research. 1982;4:369–376. doi: 10.1016/0166-4328(82)90061-4. [DOI] [PubMed] [Google Scholar]
- Hascoet MBM. A new approach to the Light/Dark Test Procedure in Mice. Pharmacology Biochemistry and Behavior. 1998;60:645–653. doi: 10.1016/s0091-3057(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci. 2010;30:5451–5464. doi: 10.1523/JNEUROSCI.0225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EH, Kim B, Choe AY, Lee JY, Choi TK, Lee SH. Panic disorder and health-related quality of life: the predictive roles of anxiety sensitivity and trait anxiety. Psychiatry Res. 2015;225:157–63. doi: 10.1016/j.psychres.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth K. Open field behavior after chronic self stimulation. Int J Neurosci. 1979;9:17–19. [PubMed] [Google Scholar]
- Kawamura T, Akira T, Watanabe M, Kagitani Y. Prostaglandin E1 prevents apoptotic cell death in superficial dosal horn of rat spinal cord. Neuropharmacology. 1997;36:1023–1030. doi: 10.1016/s0028-3908(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Kim Sa, JC An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kontinen VK, Kauppila T, Paananen S, Pertovaara A, Kalso E. Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy. Pain. 1999;80:341–346. doi: 10.1016/s0304-3959(98)00230-9. [DOI] [PubMed] [Google Scholar]
- Kulesskaya N, Karpova NN, Ma L, Tian L, Voikar V. Mixed housing with DBA/2 mice induces stress in C57BL/6 mice: implications for interventions based on social enrichment. Front Behav Neurosci. 2014;8:257. doi: 10.3389/fnbeh.2014.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesskaya N, Voikar V. Assessment of mouse anxiety-like behavior in the light-dark box and open-field arena: role of equipment and procedure. Physiology & behavior. 2014;133:30–38. doi: 10.1016/j.physbeh.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–625. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Hamelsky SW, Kolodner KB, Steiner TJ, Stewart WF. Migraine, quality of life, and depression: a population-based case-control study. Neurology. 2000;55:629–635. doi: 10.1212/wnl.55.5.629. [DOI] [PubMed] [Google Scholar]
- Luccarini P, Childeric A, Gaydier AM, Voisin D, Dallel R. The orofacial formalin test in the mouse: a behavioral model for studying physiology and modulation of trigeminal nociception. The journal of pain: official journal of the American Pain Society. 2006;7:908–914. doi: 10.1016/j.jpain.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Ma F, Zhang L, Lyons D, Westlund KN. Orofacial neuropathic pain mouse model induced by Trigeminal Inflammatory Compression (TIC) of the infraorbital nerve. Molecular brain. 2012;5:44. doi: 10.1186/1756-6606-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane TV, Blinkhorn AS, Davies RM, Kincey J, Worthington HV. Association between female hormonal factors and oro-facial pain: study in the community. Pain. 2002;97:5–10. doi: 10.1016/s0304-3959(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Macfarlane TV, Glenny AM, Worthington HV. Systematic review of population-based epidemiological studies of oro-facial pain. Journal of dentistry. 2001;29:451–467. doi: 10.1016/s0300-5712(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Macianskyte D, Januzis G, Kubilius R, Adomaitiene V, Sciupokas A. Associations between chronic pain and depressive symptoms in patients with trigeminal neuralgia. Medicina (Kaunas) 2011;47:386–392. [PubMed] [Google Scholar]
- Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Frontiers in bioscience. 2009;14:5291–5338. doi: 10.2741/3598. [DOI] [PubMed] [Google Scholar]
- Mao SC, Lin HC, Gean PW. Augmentation of fear extinction by D-cycloserine is blocked by proteasome inhibitors. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:3085–3095. doi: 10.1038/npp.2008.30. [DOI] [PubMed] [Google Scholar]
- McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111:77–83. doi: 10.1016/j.pain.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nature reviews Neuroscience. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Nencini P, Ahmed AM, Anania MC, Moscucci M, Paroli E. Prolonged analgesia induced by cathinone. The role of stress and opioid and nonopioid mechanisms. Pharmacology. 1984;29:269–281. doi: 10.1159/000138023. [DOI] [PubMed] [Google Scholar]
- Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain medicine. 2004;5(Suppl 1):S9–S27. doi: 10.1111/j.1526-4637.2004.04019.x. [DOI] [PubMed] [Google Scholar]
- Parent AJ, Beaudet N, Beaudry H, Bergeron J, Berube P, Drolet G, Sarret P, Gendron L. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behavioural Brain Research. 2012;229:160–167. doi: 10.1016/j.bbr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto F, dL R, Evans DR, Carlson CR, Yepes JF, Branscum A, Okeson JP. Differences in psychosocial functioning and sleep quality between idiopathic continuous orofacial neuropathic pain patients and chronic masticatory muscle pain patients. J Orofac Pain. 2011;25:117–124. [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Quintans JS, Antoniolli AR, Almeida JR, Santana-Filho VJ, Quintans-Junior LJ. Natural products evaluated in neuropathic pain models - a systematic review. Basic Clin Pharmacol Toxicol. 2014;114:442–450. doi: 10.1111/bcpt.12178. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Berton O, Mormede P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behavioural brain research. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- Rea K, Olango WM, Harhen B, Kerr DM, Galligan R, Fitzgerald S, Moore M, Roche M, Finn DP. Evidence for a role of GABAergic and glutamatergic signalling in the basolateral amygdala in endocannabinoid-mediated fear-conditioned analgesia in rats. Pain. 2013;154:576–585. doi: 10.1016/j.pain.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Renton TYZ. Profiling of patients presenting with posttraumatic neuropathy of the trigeminal nerve. J Orofac Pain. 2011;25:333–344. [PubMed] [Google Scholar]
- Robinson MJ, Edwards SE, Iyengar S, Bymaster F, Clark M, Katon W. Depression and pain. Frontiers in bioscience. 2009;14:5031–5051. doi: 10.2741/3585. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Boston JD, Starkstein SE, Price TR. Comparison of mania and depression after brain injury: causal factors. Am J Psychiatry. 1988;145:172–178. doi: 10.1176/ajp.145.2.172. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ. Animal models of ‘anxiety’: where next? Behav Pharmacol. 1997;8:477–496. doi: 10.1097/00008877-199711000-00003. discussion 497–504. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2008;139:349–357. doi: 10.1016/j.pain.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Rossi HL, Neubert JK. Effects of hot and cold stimulus combinations on the thermal preference of rats. Behavioural brain research. 2009;203:240–246. doi: 10.1016/j.bbr.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Hitomi S, Suzuki I, Masuda Y, Kitagawa J, Tsuboi Y, Kondo M, Sessle BJ, Iwata K. Modulation of trigeminal spinal subnucleus caudalis neuronal activity following regeneration of transected inferior alveolar nerve in rats. J Neurophysiol. 2008;99:2251–2263. doi: 10.1152/jn.00794.2007. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Ishida K, Izawa Y, Minami Y, Saito S, Kawai Y, Butterweck V, Tamaki T, Nakaya Y, Terao J. Effects of forced swimming stress on rat brain function. The journal of medical investigation. JMI. 2005;52(Suppl):300–301. doi: 10.2152/jmi.52.300. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- Simons DG, Travell J. Myofascial trigger points, a possible explanation. Pain. 1981;10:106–109. doi: 10.1016/0304-3959(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Siqueira-Lima PS, Araujo AA, Lucchese AM, Quintans JS, Menezes PP, Alves PB, de Lucca W, Junior, Santos MR, Bonjardim LR, Quintans-Junior LJ. beta-cyclodextrin complex containing Lippia grata leaf essential oil reduces orofacial nociception in mice - evidence of possible involvement of descending inhibitory pain modulation pathway. Basic Clin Pharmacol Toxicol. 2014;114:188–196. doi: 10.1111/bcpt.12145. [DOI] [PubMed] [Google Scholar]
- Siqueira SR, Teixeira MJ, Siqueira JT. Clinical characteristics of patients with trigeminal neuralgia referred to neurosurgery. Eur J Dent. 2009;3:207–212. [PMC free article] [PubMed] [Google Scholar]
- Smitherman TA, Kolivas ED, Bailey JR. Panic disorder and migraine: comorbidity, mechanisms, and clinical implications. Headache. 2013;53(1):23–45. doi: 10.1111/head.12004. [DOI] [PubMed] [Google Scholar]
- Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: activation by stress. Science. 1984;226:1270–1277. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- Travell J. Identification of myofascial trigger point syndromes: a case of atypical facial neuralgia. Arch Phys Med Rehabil. 1981;62:100–106. [PubMed] [Google Scholar]
- Vachon-Presseau E, Martel MO, Roy M, Caron E, Albouy G, Marin MF, Plante I, Sullivan MJ, Lupien SJ, Rainville P. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci. 2013;33:6826–33. doi: 10.1523/JNEUROSCI.4584-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Silva AP, Melo A, Ferreira AC, Carvalho MM, Campos FL, Sousa N, Pego JM. Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front Behav Neurosci. 2013;7:32. doi: 10.3389/fnbeh.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Arletti R, Sandrini M. Acute noise stress analgesia in relation to 5-HT2 and mu-opioid receptor changes in the frontal cortex of young mice. Life Sci. 2005;77:2500–2513. doi: 10.1016/j.lfs.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Vos BP, Strassman AM. Behavioral Evidence Chronic Constriction of Trigeminal Neuropathic Pain Following Injury to the Rat’s Infraoribatl Nerve. Journal of Neuroscience. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Melzack R, editors. Wall & Melzack’s Textbook of Pain. 1999. [Google Scholar]
- Wang C, Wu HM, Jing XR, Meng Q, Liu B, Zhang H, Gao GD. Oxidative parameters in the rat brain of chronic mild stress model for depression: relation to anhedonia-like responses. The Journal of membrane biology. 2012;245:675–681. doi: 10.1007/s00232-012-9436-4. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Mayer DJ. Organization of endogenous opiate and nonopiate pain control systems. Science. 1982;216:1185–1192. doi: 10.1126/science.6281891. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Aita M, Chavkin C. Partial infraorbital nerve ligation as a model of trigeminal nerve injury in the mouse: behavioral, neural, and glial reactions. The journal of pain: official journal of the American Pain Society. 2008;9:1036–1048. doi: 10.1016/j.jpain.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier MJ, Barrot M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatry. 2011;70:946–953. doi: 10.1016/j.biopsych.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Zakrzewska JM. Differential diagnosis of facial pain and guidelines for management. British journal of anaesthesia. 2013a;111:95–104. doi: 10.1093/bja/aet125. [DOI] [PubMed] [Google Scholar]
- Zakrzewska JM. Multi-dimensionality of chronic pain of the oral cavity and face. The journal of headache and pain. 2013b;14:37. doi: 10.1186/1129-2377-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang X, Westlund KN. Restoration of spontaneous exploratory behaviors with an intrathecal NMDA receptor antagonist or a PKC inhibitor in rats with acute pancreatitis. Pharmacology Biochemistry and Behavior. 2004;77:145–153. doi: 10.1016/j.pbb.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Wu LN, Song JG, Li WQ. Correlations between cognitive impairment and brainderived neurotrophic factor expression in the hippocampus of post-stroke depression rats. Molecular medicine reports. 2012a;6:889–893. doi: 10.3892/mmr.2012.1009. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Dong YL, Lu Y, Cao S, Zhao ZQ, Gao YJ. Chemokine CCL2 and its receptor CCR2 in the medullary dorsal horn are involved in trigeminal neuropathic pain. J Neuroinflammation. 2012;9:136. doi: 10.1186/1742-2094-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]