Abstract
Objective:
To determine whether a structured and quantitative assessment of differential olfactory performance—recognized between a blast-injured traumatic brain injury (TBI) group and a demographically comparable blast-injured control group—can serve as a reliable antecedent marker for preclinical detection of intracranial neurotrauma.
Methods:
We prospectively and consecutively enrolled 231 polytrauma inpatients, acutely injured from explosions during combat operations in either Afghanistan or Iraq and requiring immediate stateside evacuation and sequential admission to our tertiary care medical center over a 2½-year period. This study correlates olfactometric scores with both contemporaneous neuroimaging findings as well as the clinical diagnosis of TBI, tabulates population-specific incidence data, and investigates return of olfactory function.
Results:
Olfactometric score predicted abnormal neuroimaging significantly better than chance alone (area under the curve = 0.78, 95% confidence interval [CI] 0.70–0.87). Normosmia was present in all troops with mild TBI (i.e., concussion) and all control subjects. Troops with radiographic evidence of frontal lobe injuries were 3 times more likely to have olfactory impairment than troops with injuries to other brain regions (relative risk 3.0, 95% CI 0.98–9.14). Normalization of scores occurred in all anosmic troops available for follow-up testing.
Conclusion:
Quantitative identification olfactometry has limited sensitivity but high specificity as a marker for detecting acute structural neuropathology from trauma. When considering whether to order advanced neuroimaging, a functional disturbance with central olfactory impairment should be regarded as an important tool to inform the decision process.
Classification of evidence:
This study provides Class III evidence that central olfactory dysfunction identifies patients with TBI who have intracranial radiographic abnormalities with a sensitivity of 35% (95% CI 20.6%–51.7%) and specificity of 100% (95% CI 97.7%–100.0%).
An absent or diminished ability to smell is an infrequent sequela of blunt trauma to the head region and is a poorly understood neuropathologic phenomenon. Currently, a standardized clinical assessment of cranial nerve I, as well as the intracranial olfactory processing structures and higher-order neuronal networks, is not commonplace in the trauma arena, especially during the acute phase of injury. The result of this inattention is that the incidence and prognostic significance have remained unknown.
A noninvasive, physiologically innocuous, objective diagnostic test that can quantitatively profile acute injury using organs of special sense (e.g., vestibular balance posturography, eye-tracking oculography, etc.), to aid in the early detection of structural intracranial injury, would have great value to a deployed military as well as civilian neurotraumatology.1–3 To date, no single clinical factor or combination of such factors has been found that can reliably predict a priori which acute trauma patients presenting emergently to a medical treatment facility will have a subclinical traumatic brain injury (TBI). As a result, neuroimaging is routinely used as a screening instrument. However, the indications remain ill-defined, discretionary, and highly subjective.4,5 This study investigates whether a formal examination of olfactory function, involving an assessment directed specifically toward odorant identification, can be used as a suitable detection tool for subclinical stages of structural neurologic injury, thereby expanding the selection criteria and clinical decision rules for ordering neuroimaging studies in patients with suspected closed (blunt) head trauma.
METHODS
Participants.
The study cohort was restricted to US service members acutely injured during combat operations either in Afghanistan or Iraq and having complex blast-related injuries sufficient to require immediate, contiguous, interhospital inpatient transfer back to the United States. In accord with official policy for intercontinental Air Force casualty evacuation movements, all troops with this elevated severity of injury were required to be triaged exclusively to the National Capital Region of Washington, DC.6 This unique wartime triaging protocol afforded the opportunity to carefully study olfactory function and features of brain trauma on a distinct, confined, and well-delineated cohort of the most critically injured combat survivors. On admission, all troops were prospectively subdivided into 4 mechanistic trauma domains, as outlined in figure 1. Polytrauma patients (injury severity score [ISS] of ≥15 and/or injury to multiple areas of the body) injured by blast explosive-related brisance formed both the study and control groups.
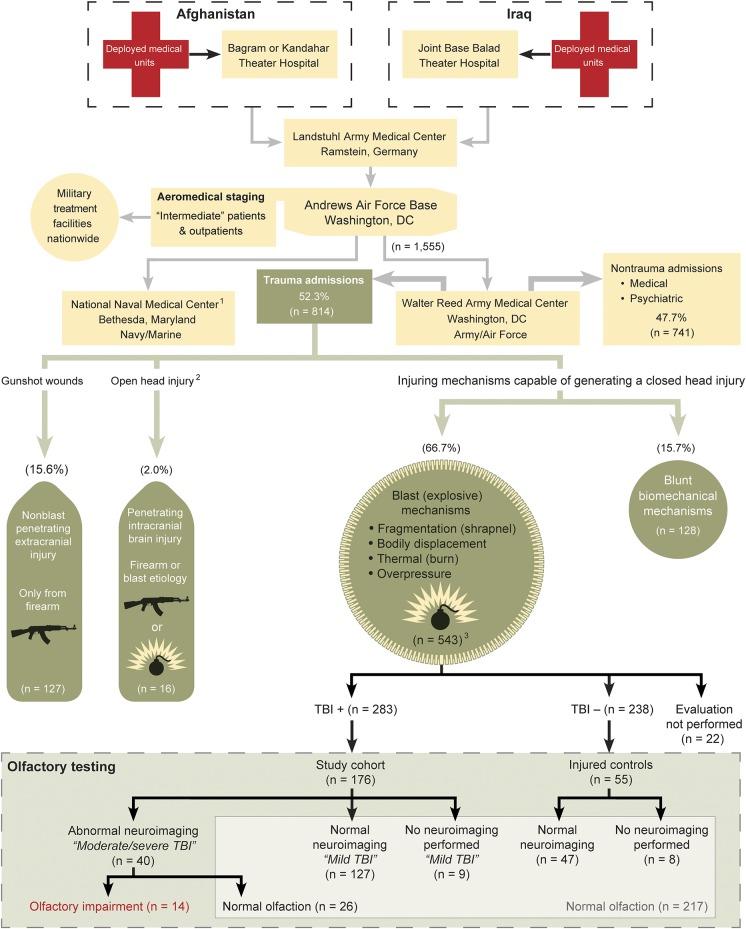
Figure 1. Combat Casualty Care Pathway: Interhospital transfer of inpatients from Afghanistan and Iraq to Walter Reed Army Medical Center.
US Air Force Transportation Regulating Command and Control Evacuation System aircrew mission manifests provided longitudinal tracking of all patient movements from the combat zones to our hospital. Twenty Army/Air Force casualties were diverted to NNMC for treatment of penetrating and complex blunt head injuries; 13 penetrating are included in the totals. However, 7 Army/Air Force closed head injuries at NNMC (5 blast mechanisms and 2 blunt mechanisms) are excluded from our dataset. When penetrating intracranial and blast trauma were in conflict, penetrating was always taken in preference to all other mechanisms. Neuroimaging in Afghanistan or Iraq was often performed as a routine component of whole-body trauma scans: head, chest, abdomen, and pelvis. NNMC = National Naval Medical Center; TBI = traumatic brain injury.
Assessment of closed head trauma.
All troops were considered to have met the inclusion criteria for a closed neurologic head trauma event due to elevated ISS resulting from close proximity to the epicenter of the explosion and were required to undergo compulsory de novo evaluations for potential TBI. Assessments were independently performed by civilian psychologists and accompanying subordinate caregivers using identification and stratification criteria initially established by the American Congress of Rehabilitation Medicine and adopted by both the Departments of Defense and Veterans Affairs6–10 (figure e-1 on the Neurology® Web site at Neurology.org).
Diagnostic categories.
Broad-based and progressive use of neuroimaging in deployed combat casualty care, typically performed as a routine component of in-theater, whole-body trauma scans, allowed our cohorts to be further stratified into 5 discrete categories: moderate/severe TBI (i.e., neuroimage-abnormal), mild TBI with normal neuroimaging, mild TBI with no neuroimaging, controls with normal neuroimaging, and controls with no neuroimaging. Neuroradiology results were used as a definitive demarcation between mild TBI (also known as concussion) and moderate/severe TBI and were required to be normal for the clinical diagnosis of a mild TBI, which was assigned at the time of provider interview (figure 2). Troops with abnormal neuroimaging were automatically upstaged to moderate/severe TBI. The comparison control group consisted of blast-injured troops who were comparable in demographic features and severity of polytrauma to the TBI-positive group. In all cases, initial neuroimaging was performed before the date of the TBI diagnosis and by radiologists who were unaware of the olfactometric results.
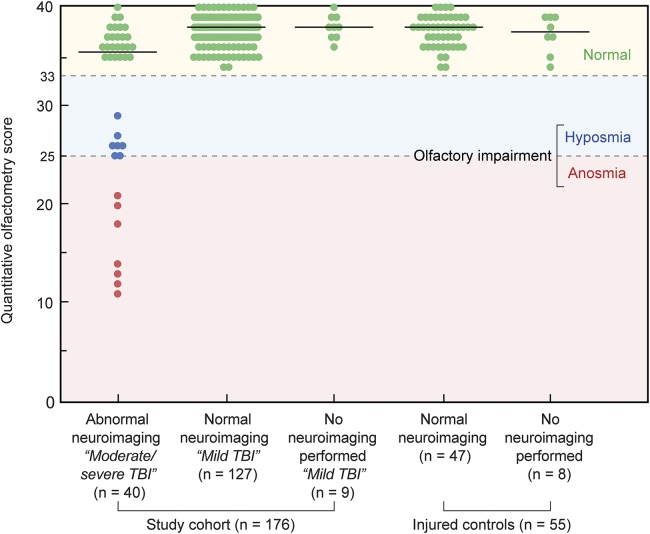
Figure 2. Associations among olfactory performance, neuroimaging status, and TBI status (n = 231).
No attempt was made to further differentiate gradations of hyposmia because the delimiters that define such alterations have not been uniformly standardized. Olfactometry scores differed significantly among groups (p < 0.001, Kruskal–Wallis test) with significant differences noted between the group with abnormal neuroimaging (i.e., moderate/severe TBI) and each of the 4 groups, based on post hoc comparisons using a stepwise step-down procedure. No significant differences were found between any of the other groups. TBI = traumatic brain injury.
Otorhinolaryngology evaluation.
All troops who met the inclusion criteria were evaluated by the military otorhinolaryngologist from the research team. In view of the possibility that concomitant neuropsychological symptoms and disorders might detract from the olfactory test performance, a brief pretest neuropsychological assessment was administered. Subjects with any evidence of global cognitive impairment were excluded. No antecedent history of smell deficiency predating the current trauma was present in any of the participants. Anterior rhinoscopy was performed by the otorhinolaryngologist at the bedside immediately before the olfactory testing session. Inspection of the nasal cavities assessed patency for nasal airflow and excluded potential infectious, inflammatory, and other obstructive causes of smell deficiency. In addition, visualized portions of the paranasal sinuses, noted on CT and MRI scans, were examined to rule out any additional potential subclinical causes of impairment. Troops with objective radiographic evidence of bone fractures involving the anterior skull base cribriform plate were identified and were excluded (n = 3/543 or 0.55%). As an aside, these 3 blast-injured troops were tested apart from the study and were completely anosmic, with no return of olfactory function by follow-up testing by 6 months.
Quantitative identification olfactometry index test.
The 40-item University of Pennsylvania Smell Identification Test (UPSIT or SIT; Sensonics, Haddon Heights, NJ) was then directly administered birhinically to the patient by the otorhinolaryngologist. When activated, the UPSIT dispenses microencapsulated vapors and is designed specifically for assessing odor identification. The UPSIT has been shown to exhibit a 6-month interval test-retest reliability of 92% (r = 0.918, p < 0.001).11 The complete 40-item version was selected as the index test, as opposed to abbreviated screening instruments (e.g., Brief Smell Identification Test), to obtain a more precise evaluation because of the higher test-retest reliability.11 Olfactometric scores were assigned to 3 categories: normosmic (normal), hyposmic (decreased), and anosmic (absent), with the latter 2 classified as having olfactory impairment. Use of an objective, quantitative assessment tool was necessary because self-report interview methodology has been shown to be an inadequate screening method and an insensitive measure of olfactory dysfunction.12,13 Moreover, self-awareness of impairment is typically only present in the most severe cases and only after a prolonged latency period.14–16
Standard protocol approvals, patient consents, and registrations.
The protocol was approved by the department of clinical investigations and was conducted in compliance with all applicable federal regulations governing the protection of human subjects. Written informed consent was obtained from all participants. This study provides Class III evidence that central olfactory impairment can serve as a marker for detection of intracranial neurotrauma.
Statistical analyses.
Olfactometric scores were summarized as median (interquartile range) and compared between groups using Kruskal–Wallis and Mann–Whitney U tests. Categorical data, including presence/absence of olfactory impairment and location, were summarized as n (%) and compared using Fisher exact test (figure 3). Diagnostic accuracy regarding identifying troops with abnormal neuroimaging was assessed using the area under the receiver operating characteristic curve.
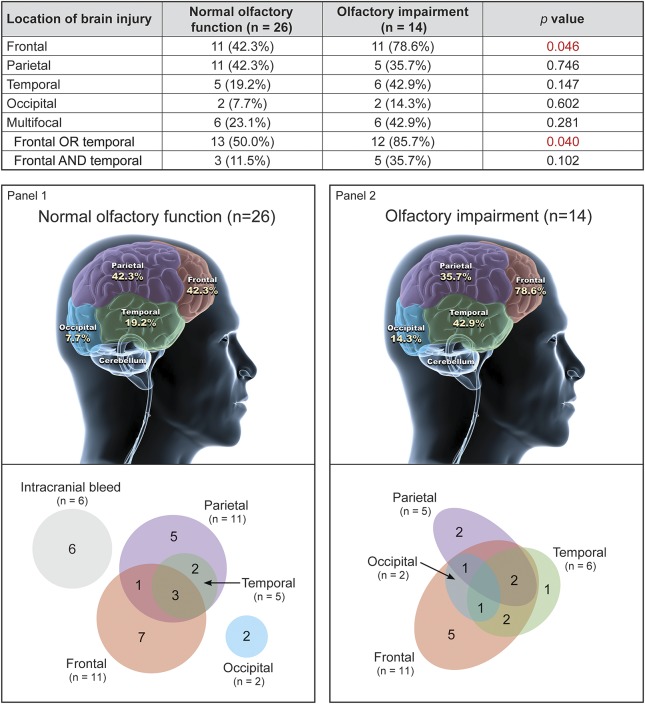
Figure 3. Troops with abnormal neuroimaging: Location of injury and its association with olfactory performance and multifocality (n = 40).
For specific brain regions, data do not sum to 100% because more than one region could be affected. A clinically relevant neuropathologic finding was defined as any acute intracranial finding due to trauma. Findings were substratified based only on location and multifocality and not by size or nature of the lesion (e.g., edema vs hemorrhage vs contusion). Multifocal was defined as 2 or more regions of the brain affected. Neuroimaging results involved a review of the most proximal, in-theater scans and follow-up stateside radiographic examinations. All studies were read by neuroradiology. Fisher exact test. The silhouette image of the brain was modified from an image generated by dreamstime.com, with permission.
RESULTS
Baseline characteristics.
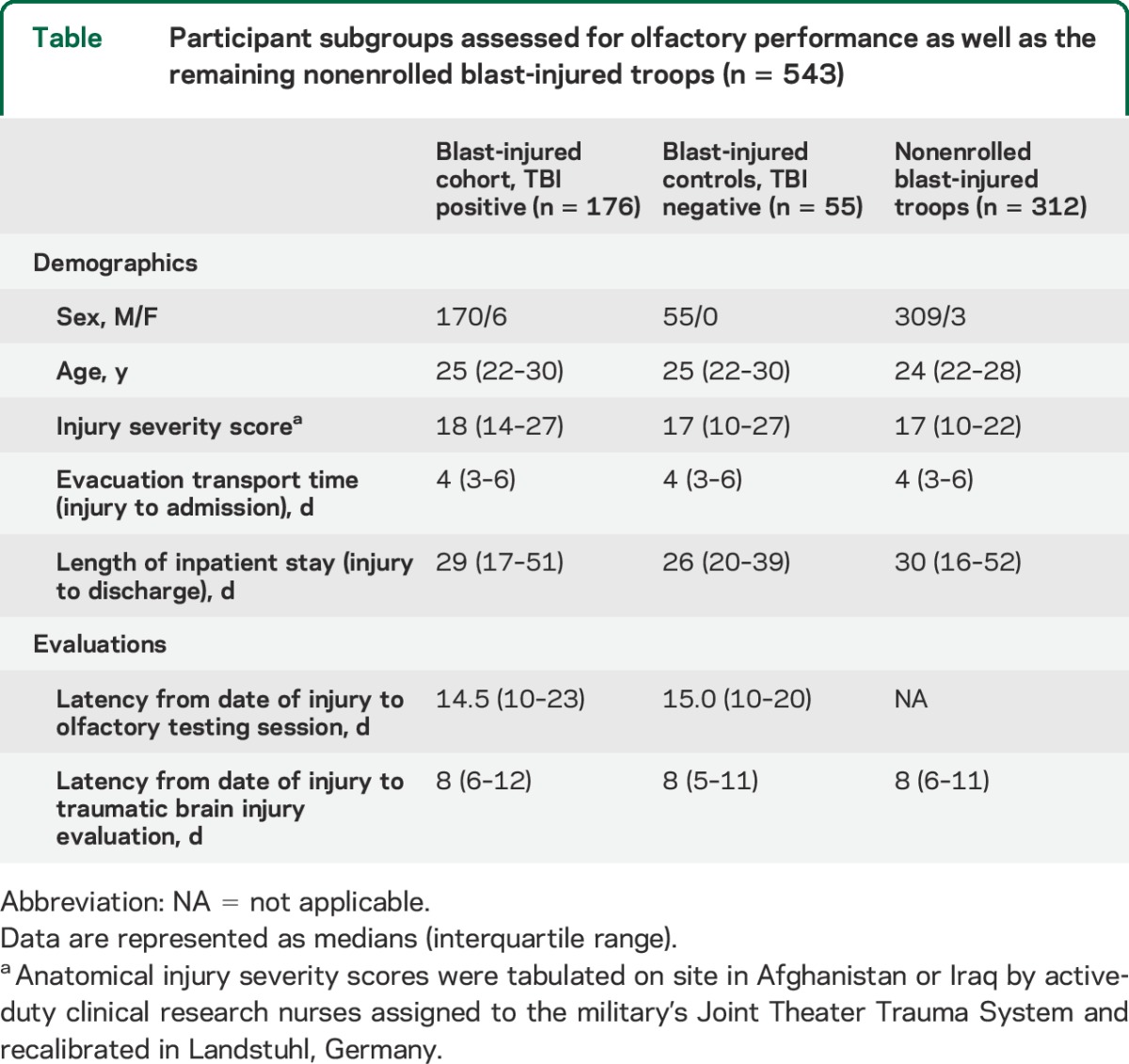
A total of 543 combat casualties sustained severe blast explosive injuries, which required immediate and contiguous interhospital inpatient transfer back to the United States. Our consecutive enrollment rate was 62.2% (176/283) for the TBI-positive study cohort and 23.1% (55/238) for the TBI-negative controls. Of note, we preferentially included blast-injured controls with available in-theater neuroimaging. No significant differences in demographics among the study cohort, the control group, and the overall nonenrolled blast group were observed (table).
Table.
Participant subgroups assessed for olfactory performance as well as the remaining nonenrolled blast-injured troops (n = 543)
Olfactory performance.
A total of 6.1% of the troops (14/231) exhibited impaired function. Normosmia was present in all subjects within both the mild TBI group (n = 136) and the blast-injured control group (n = 55). The median olfactometric scores did not differ significantly between these 2 groups (p = 0.918, Mann–Whitney U test). More important, normosmia was present in all subjects with normal neuroimaging, including 127 diagnosed with a mild TBI as well as 47 within the blast-injured controls (specificity = 100%, 95% [one-sided] confidence interval [CI] 97.7%–100.0%).
Olfactory impairment was present in 35.0% of troops (14/40) with abnormal neuroimaging (i.e., moderate/severe TBI) (sensitivity 35.0%, 95% CI 20.6%–51.7%). The area under the receiver operating characteristic curve was 0.78 (95% CI 0.70–0.87), indicating that olfactometric score predicted abnormal neuroimaging significantly better than chance alone (p < 0.001). Of the 40 patients with abnormal neuroimaging, 18 were tested within 14 days postinjury, and the remaining 22 were tested ≥15 days postinjury. Nine of the 18 tested within 14 days had impaired function (sensitivity 50%, 95% CI 26%–74%) while only 5 of the 22 tested ≥14 days had impairment (sensitivity 23%, 95% CI 8%–45%). The difference in sensitivity between these small groups did not reach statistical significance (p = 0.101, Fisher exact test). However, these results suggest that it is worth testing the hypothesis that sensitivity of olfactory testing to identify patients with structural brain injury may be higher if testing is performed closer to the time of injury.
For those patients with olfactory impairment, the most common radiographic abnormalities involved injury to the frontal or temporal lobes. Specifically, 78.6% (11/14) had frontal lobe involvement, compared with 42.3% of normosmic patients (11/26) (p = 0.046, Fisher exact test). Moreover, 85.7% of troops (12/14) with olfactory impairment had either frontal or temporal involvement, compared with 50.0% of normosmic patients (13/26) (p = 0.040), and 35.7% of troops (5/14) with olfactory impairment had both frontal and temporal involvement, compared with only 11.5% of normosmic patients (3/26) (p = 0.10). Nearly half of troops (6/14) with olfactory impairment had involvement in 2 or more brain regions (multifocality), compared with less than one-quarter of normosmic troops (6/26) (relative risk 1.86, 95% CI 0.74–4.69, p = 0.28). While the association between olfactory impairment and involvement in 2 or more brain regions did not reach statistical significance, the magnitude of the association supports future investigation into whether olfactory impairment might occur more often in the context of multifocal intracranial injury.
Longitudinal changes were tested in a case series involving 7 troops with olfactory impairment who were available for follow-up. Normalization of scores was present in 6 of the 7 and within ≤160 days postinjury. The seventh showed improvement at the first follow-up but was not available for additional testing (figure e-2).
DISCUSSION
When normal transnasal airflow of odorant molecules is present, olfactory dysfunction is routinely subdivided into 2 basic groups: (1) peripheral disorders of sensation—involving direct injury to the intranasal olfactory receptor cells due to toxic inhalational chemical vapors, infectious agents, or fractures involving the anterior skull base; and (2) central disorders of perception—involving disruption of CNS neuronal network circuitry that makes odorant identification possible.
Across all study groups, we observed no neuroradiographic evidence of trauma to the cribriform plate, olfactory bulbs, olfactory tracts, or gyrus recti, as noted within the limits of scanner resolution and trauma protocol sequencing. The radiographic findings support a higher-order CNS etiology for the observed impairment. Furthermore, use of a demographically comparable blast-injured control group of patients, all of whom had normal olfactometric scores, served to discount the concern that any observed impairment was the result of peripheral trauma, from inhalational explosive blast vapors, at the intranasal receptor level.
Olfactory impairment was observed only in troops with concurrent acute traumatic radiographic abnormalities. This finding approximates to previous conclusions from other historical clinical research investigations of consecutive closed head injuries and supports the assertion that impaired olfactory identification is only present in the context of significant intracranial neurotrauma.16–21 However, our data stand in contrast with what is frequently noted or tacitly implied in the specialties of neurology, neurosurgery, or otorhinolaryngology, whereby olfactory impairment resulting from slight or minor blows to the head region—presumably due to partial stretching or sheering of fila olfactoria at the cribriform plate—has long-standing, albeit suppositious, clinical acceptance but no compelling advanced radiographic or histopathologic evidence for substantiation.12,13,22–27
Although 6.1% of the blast-injured troops (14/231) exhibited impaired olfactory performance, our data indicate that knowledge of the overall “headline” value for incidence distorts framing of the real risk and is not sufficient to be clinically informative. From our data, we conclude that ultimately it is the radiographic presence and the radiographic locations of the structural brain injuries that define the probability of subsequent olfactory performance degradation and not simply the abstract and unquantifiable risk factor of a “blow or hit to the head region.” In addition, despite the seemingly low sensitivity of olfactometry for detecting abnormal neuroimaging, the absolute specificity and the ability to presage troops with more serious frontal or temporal lobe injuries—those who may require emergent attention and potentially more invasive interventions—has salutary value in both an acute military and civilian trauma setting. This is because structural brain injuries involving the frontal or temporal lobes typically drive the behavioral and cognitive outcome for the patient.28
Our data are also consistent with the historical literature whereby the frontal and temporal regions have been shown to be selectively vulnerable to contusions from closed head trauma, independent of the site of impact. This is due to the kinematics of intrinsic pressure and strain responses within the brain tissue and rapid acceleration of cortical parenchymal surfaces along edges of the floor of the anterior cranial fossa.28,29 Moreover, damage to these regions, whether from brain trauma, stroke, or through neurodegenerative processes (e.g., premotor Parkinson disease, Alzheimer disease, multiple sclerosis, frontotemporal dementia), has been demonstrated to significantly impair memory and thereby the ability of the brain to correctly match up and link common inhalational odorant molecules to past learning and experience.30–34
Our study differs from previous olfactory investigations in ways that ultimately serve to enhance methodologic precision. First, the intrinsic demographic homogeneity and low intersubject variability, which are present within any military study whereby the cohort is restricted specifically to combat troops (young adult age range, sex, education, physical fitness standards), serves to discount multiple potential sources of bias and confounding, because of minimal individual differences among the participants at baseline.6 Second, anterior rhinoscopy and physician administration of the olfactory test by a dedicated otorhinolaryngologist rather than patient self-administration ensured compliance, motivation, consistency, and reliability of responses as well as normal intranasal dynamics and physiology. Specifically, direct physician monitoring of effort and task performance often prevented misattribution of seemingly incorrect responses to olfactory impairment resulting from sociocultural, geographic, and age-related maturational unfamiliarity with certain odorants (i.e., ascertainment bias), patient fatigue, as well as persistent intracohort difficulties with several indistinct and ambiguous odorants endemic to the UPSIT. Third, olfactometric scores were contemporaneously matched to posttraumatic anatomical changes on concurrent neuroimaging. As a practical matter, studies in which neuroimaging is performed with a lengthy latency from date of injury may no longer reveal structural pathology because of interval resolution of abnormal signals. This allows for the real possibility that impairment of the neuronal underpinnings essential to the olfactory processing network may be attributable to residual microstructure damage that exists below the threshold of detection for conventional radiographic resolution. Fourth, this is the only prospective olfactory study for which contemporaneous neuroimaging was available for a demographically comparable comparison control group.
Limitations of our study also warrant consideration. First, our testing strategies did not completely assay the olfactory system across its full spectrum of function. Additional features such as smell threshold and discrimination ability were not assessed. In patients with head trauma, intact sensation detection of an odorant is often present despite impaired perceptual capability (i.e., the ability to interpret, identify, and remember odors).20,35 Of note, these investigational tests are less widely used and employ methodology that currently lacks strict measures of both reliability and validity. Second, the level of generalizability of the criteria for assessing potential brain trauma occurring within the context of an explosion, in contrast to sports-related concussions or other civilian blunt force incidents, remains a topic of active investigation by the military. In explosion-related whole-body polytrauma, the unwitnessed event as well as the multimodal and “invisible” nature of component blast-injuring mechanisms (e.g., overpressure) has made it exceedingly difficult to identify the essential deterministic attributes necessary to construct discriminative inclusion criteria which can selectively identify—at the primary interface with patients—those troops within the spectrum of “sound-exposed” to “blast-exposed” to “blast-injured” that truly represent a potential neurologic head trauma case. The resultant usage of an overly broad and undifferentiated target population (i.e., inclusion of all troops involved in any manner with an explosive event) diminishes the predictive value of the follow-on, nonspecific, symptoms-based diagnostic rubric, with resultant ambiguity which favors correlation over causation. Current development of helmet-mounted quantitative dosimeters and accelerometers for both the military and the National Football League will facilitate movement toward causation and, ultimately, a more secure concussion diagnosis.
Third, once a potential closed head trauma case has been identified, lack of a reliable and valid methodology for incontrovertibly diagnosing a radiographically normal closed head injury also remains a critical gap in both military and civilian neurotraumatology. At this time, there is neither a universally applied case definition nor signature confirmatory tests for characterizing the phenomenon of “a transitory change in brain function” called mild TBI (concussion), particularly in patients with acute polytrauma. A lack of both a common standard of care and a defined scope of practice among the various types of caregivers who diagnose this condition have impeded meaningful comparisons and progress. Of note, this limitation permeates all clinical research studies dealing with concussions and forces all clinicians to continuously accept an immoderate level of uncertainty with the exactness of this amorphous and widely variegated diagnosis and, for the purposes of this study, its causal relationship with olfactory performance.6,36 The Glasgow Coma Scale has important disqualifying limitations and is not used to stratify the severity of a closed head injury in the military TBI program, or, for that matter, in athletic environments.37 Moreover, the most current recommendations state that the derived total Glasgow Coma Scale score should only be used to characterize groups rather than individual patients.38 In military polytrauma, the concussion diagnosis is ultimately based on a background level of awareness of a blast-related bodily injury, an autobiographical or observed report of immediate postincident changes from baseline neuro-status (loss of consciousness or an “apparent state of alteration in consciousness”), and depth of knowledge and breadth of experience of an advanced-level caregiver in managing neurotrauma.36 Despite advances in radiology and the arduous search for confirmatory neurodiagnostics, the diagnosis remains clinical and is reliant on each individual caregiver's retrospective assessment, interpretation, and judgment of both “scale” and concurrency of immediate postincident signs, symptoms, or deficits, believed to be pathognomonic and axiomatic for a clinically significant alteration in brain function.39 In lieu of these limitations, shortcomings in both the inclusion criteria and the case definition were partially offset by utilizing TBI evaluations that were performed by a dedicated team of psychologists with unrestricted access to intratheater medical records, superimposing the results of all available contemporaneous neuroimaging on 92.6% (214/231) of the troops, and use of an exceptionally homogeneous cohort consisting of the most critical blast-injured survivors with the highest ISS—whereby the presence of concurrent occult (i.e., radiographically normal) blast neurotrauma would be most likely.
The underlying olfactory impairment neuropathologic mechanisms remain unknown. Possibilities may include abrupt ionic shifts, altered metabolism, changes in neurotransmission due to cellular or interstitial edema, or impaired connectivity that ensue after objective trauma to the brain. However, at the functional level, the inability to specifically identify and verbally label odorants ultimately reflects posttraumatic impairment of memory-based cognitive networks in the CNS.40 Until more advanced structural and validated functional neuroimaging or postmortem pathologic evidence becomes available, the mechanism of injury will remain purely speculative. Future studies focusing on additional memory testing may also help to localize the affected circuitry.
The presence of measurable abnormalities with central olfactory dysfunction provides added value to the practicing physician for preclinical detection of intracranial injury and, accordingly, subsequent disease-modifying early interventions. While the level of sensitivity for screening purposes is insufficient to exclude all types of posttraumatic neuropathology, the absolute specificity and the association with frontal or temporal lobe injury enhance its value in clinical practice.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all of the injured troops for their inexorable sacrifices in support of our nation. The authors extend acknowledgment to Lieutenant Colonel Angela J. Masak, Joanna Chisar-Vivald, RN, CCRA, and Diane L. Garofolo for their logistical support and for their contributions on reviewing, commenting on, and editing the manuscript, as well as Randy J Thompson of biomedical graphics. The authors also thank CAPT Eric A. Elster, MD, Colonel Geoffrey S.F. Ling, MD, PhD, Colonel Dallas C. Hack, MD, Colonel George L. Coppit, MD, and the entire Otorhinolaryngology Clinic and Neurosurgery Clinic at Walter Reed National Military Medical Center for their sustained support.
GLOSSARY
- CI
confidence interval
- ISS
injury severity score
- TBI
traumatic brain injury
- UPSIT
University of Pennsylvania Smell Identification Test
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Michael Xydakis: principal investigator, design and conduct of the study, collection of data, management, analysis, and interpretation of the data, preparation of manuscript. Lisa Mulligan: design and conduct of study, collection and analysis of data. Alice Smith: design and conduct of study, collection and analysis of radiographic data. Cara Olsen: design of study, statistical data analysis, interpretation of data. Dina Lyon: collection of data, logistical management, preparation of manuscript. Leonardo Belluscio: design and conduct of the study, analysis and interpretation of the data, preparation of manuscript.
STUDY FUNDING
US Department of Defense Combat Casualty Care Medical Research and Development Program (DMRDP: ID-D10-I-AR-J6-626).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Xydakis MS, Bebarta VS, Harrison CD, Conner JC, Grant GA, Robbins AS. Tympanic-membrane perforation as a marker of concussive brain injury in Iraq. N Engl J Med 2007;357:830–831. [DOI] [PubMed] [Google Scholar]
- 2.Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train 2000;35:19–25. [PMC free article] [PubMed] [Google Scholar]
- 3.Maruta J, Heaton KJ, Maule AL, Ghajar J. Predictive visual tracking: specificity in mild traumatic brain injury and sleep deprivation. Mil Med 2014;179:619–625. [DOI] [PubMed] [Google Scholar]
- 4.Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT head rule for patients with minor head injury. Lancet 2001;357:1391–1396. [DOI] [PubMed] [Google Scholar]
- 5.Haydel MJ, Preston CA, Mills TJ, et al. Indications for computer tomography in patients with minor head injury. N Engl J Med 2000;343:100–105. [DOI] [PubMed] [Google Scholar]
- 6.Xydakis MS, Ling GS, Mulligan LP, Olsen CH, Dorlac WC. Epidemiologic aspects of traumatic brain injury in acute combat casualties at a major military medical center: a cohort study. Ann Neurol 2012;72:673–681. [DOI] [PubMed] [Google Scholar]
- 7.USAISR. Joint Trauma System Clinical Practice Guidelines. DoD policy guidance for management of mild traumatic brain injury/concussion in the deployed setting. Available at: http://www.usaisr.amedd.army.mil/cpgs.html. Accessed August 10, 2014.
- 8.Department of Defense Instruction 6490.11. DoD policy guidance for management of mild traumatic brain injury/concussion in the deployed setting. 2012. Available at: http://www.dtic.mil/whs/directives. Accessed August 10, 2014.
- 9.Defense and Veterans Brain Injury Center. VA/DOD clinical practice guidelines for management of mild TBI 2013. Available at: www.dvbic.org/concussionmtbi-information-and-tools-providers. Accessed December 10, 2013.
- 10.Kay T, Harrington DE, Adams RE, et al. Definition of mild traumatic brain injury: report from the Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. J Head Trauma Rehabil 1993;8:86–87. [Google Scholar]
- 11.Doty RL, McKeown DA, Lee WW, Shaman P. A study of the test-retest reliability of ten olfactory tests. Chem Senses 1995;20:645–656. [DOI] [PubMed] [Google Scholar]
- 12.Fortin A, Lefebvre MB, Ptito M. Traumatic brain injury and olfactory deficits: the tale of two smell tests. Brain Inj 2010;24:27–33. [DOI] [PubMed] [Google Scholar]
- 13.Ruff RL, Riechers RG, Wang XF, Piero T, Ruff SS. A case control study examining whether neurological deficits and PTSD in combat veterans are related to episodes of mild TBI. BMJ Open 2012;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA 2002;288:2307–2312. [DOI] [PubMed] [Google Scholar]
- 15.Callahan CD, Hinkebein JH. Assessment of anosmia after traumatic brain injury: performance characteristics of the University of Pennsylvania Smell Identification Test. J Head Trauma Rehabil 2002;17:251–256. [DOI] [PubMed] [Google Scholar]
- 16.Haxel BR, Grant L, Mackay-Sim A. Olfactory dysfunction after head injury. J Head Trauma Rehabil 2008;23:407–413. [DOI] [PubMed] [Google Scholar]
- 17.Sandford AA, Davidson TM, Herrera N, et al. Olfactory dysfunction: a sequela of pediatric blunt head trauma. Int J Pediatr Otorhinolaryngol 2006;70:1015–1025. [DOI] [PubMed] [Google Scholar]
- 18.De Kruijk JR, Leffers P, Menheere PP, Meerhoff S, Rutten J, Twijnstra A. Olfactory function after mild traumatic brain injury. Brain Inj 2003;17:73–78. [DOI] [PubMed] [Google Scholar]
- 19.Green P, Rohling ML, Iverson GL, Gervais RO. Relationships between olfactory discrimination and head injury severity. Brain Inj 2003;17:479–496. [DOI] [PubMed] [Google Scholar]
- 20.Levin HS, High WM, Eisenberg HM. Impairment of olfactory recognition after closed head injury. Brain 1985;108:579–591. [DOI] [PubMed] [Google Scholar]
- 21.Gudziol V, Hoenck I, Landis B, Podlesek D, Bayn M, Hummel T. The impact and prospect of traumatic brain injury on olfactory function: a cross-sectional and prospective study. Eur Arch Otorhinolaryngol 2014;271:1533–1540. [DOI] [PubMed] [Google Scholar]
- 22.Shofield PW, Moore TM, Gardner A. Traumatic brain injury and olfaction: a systematic review. Front Neurol 2014;5:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charland-Verville V, Lassonde M, Frasnelli J. Olfaction in athletes with concussion. Am J Rhinol Allergy 2012;26:222–226. [DOI] [PubMed] [Google Scholar]
- 24.Coello AF, Canals AG, Gonzalez JM, Martin JJ. Cranial nerve injury after minor head trauma. J Neurosurg 2010;113:547–555. [DOI] [PubMed] [Google Scholar]
- 25.Vent J, Koenig J, Hellmich M, Huettenbrink KB, Damm M. Impact of recurrent head trauma on olfactory function in boxers: a matched pairs analysis. Brain Res 2010;1320:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Kern RC, Quinn B, Rosseau G, Farbman AI. Post-traumatic olfactory dysfunction. Laryngoscope 2000;110:2106–2109. [DOI] [PubMed] [Google Scholar]
- 27.Jafek BW, Eller PM, Esses BA, Moran DT. Post-traumatic anosmia: ultra structural correlates. Arch Neurol 1989;46:300–304. [DOI] [PubMed] [Google Scholar]
- 28.McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci 2011;13:287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams JH, Graham DI, Scott G, Parker LS, Doyle D. Brain damage in fatal non-missile head injury. J Clin Pathol 1980;33:1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter H, Butters N. An assessment of olfactory deficits in patients with damage to prefrontal cortex. Neuropsychologia 1980;18:621–628. [DOI] [PubMed] [Google Scholar]
- 31.Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 2015;84:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolet A, Magnin E, Millot JL, et al. Olfactory dysfunction in multiple sclerosis: evidence of a decrease in different aspects of olfactory function. Eur Neurol 2013;69:166–170. [DOI] [PubMed] [Google Scholar]
- 33.Berendse HW, Booij J, Francot CM, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson's disease patients' relatives with a decreased sense of smell. Ann Neurol 2001;50:34–41. [DOI] [PubMed] [Google Scholar]
- 34.Heyanka DJ, Golden CJ, McCue RB, Scarisbrick DM, Linck JF, Zlatkin NI. Olfactory deficits in frontotemporal dementia as measured by the Alberta Smell Test. Appl Neuropsychol Adult 2014;21:176–182. [DOI] [PubMed] [Google Scholar]
- 35.Levin HS, Amparo E, Eisenberg HM, et al. Magnetic resonance imaging and computerized tomography in relation to the neurobehavioral sequela of mild and moderate head injuries. J Neurosurg 1987;66:706–713. [DOI] [PubMed] [Google Scholar]
- 36.Xydakis MS, Ling GS, Mulligan LP, Dorlac WC, Hack DC. Reply: loss of consciousness and concussion. Ann Neurol 2013;74:154–156. [DOI] [PubMed] [Google Scholar]
- 37.Riechers RG, Ramage A, Brown W, et al. Physician knowledge of the Glasgow Coma Scale. J Neurotrauma 2005;22:1327–1334. [DOI] [PubMed] [Google Scholar]
- 38.Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol 2014;13:844–854. [DOI] [PubMed] [Google Scholar]
- 39.Carney N, Ghajar J, Jagoda A, et al. Concussion guidelines step 1: systematic review of prevalent indicators. Neurosurgery 2014;75:3–15. [DOI] [PubMed] [Google Scholar]
- 40.Wilson DA, Stevenson RJ. The fundamental role of memory in olfactory perception. Trends Neurosci 2003;26:243–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.