Abstract
Introduction
Obesity in men is associated with infertility in numerous studies. The current trend for decline in semen parameters parallels the increasing prevalence of obesity worldwide. In addition to impaired semen quality, fertility among obese men may be affected by sexual dysfunction, endocrinopathy, aromatization activity, psychological and thermal effects, sleep apnea, leptin and minor toxins, and possibly the inflammatory and obstructive elements of epididymitis pathology. The variable degrees of certainty associated with these causes parallel the levels of supporting evidence. This search aims to shed lights on different conditions that obese men suffer from; as that makes the treatment of infertility more categorized.
Material and methods
A PubMed search was conducted to identify clinical and pathological mechanisms linking obesity to male infertility.
Results
Among the myriad of publications reviewed in this paper, impaired spermatogenesis and sexual dysfunction have been shown to drive other variables towards poor fertility potentials. The paper presented a new, detailed flow chart showing more factors and further interactions among conditions leading to infertility.
Conclusions
The prime hormonal defect in obese men is hypotestosteronaemia, which results in impaired spermatogenesis leading to poor fecundability. Studies have shown that most mechanisms accounting for reduced fertility potentials in overweight men are reversible.
Keywords: obesity, body mass index, erectile dysfunction, male infertility, spermatogenesis, hypogonadism
INTRODUCTION
Infertility, defined as the absence of conception after one year of unprotected intercourse, affects 1 in every 13 couples in the United States [1]. Infertility in men constitutes 25 to 30% of all the cases and contributes, in combination with female factors, to another 30%. Reduced semen quality has been found to be a universal trend in the last few decades due to dramatic changes in the lifestyle of civilized communities around the world. Obesity is one of the alarming health problems in modernized societies that leads to infertility.
According to the chemical calorie theory, endocrine disruptors are, at least in part, responsible for the pandemic of obesity in the last few decades [2]. Subfertile men have a significantly higher body mass index (BMI) than the general population. Both low and high BMIs (less than 19 kg/m2 or more than 30 kg/m2 respectively) have been associated with reduced testicular volume (WHO 1987) and reduced semen quality suggesting impairment of spermatogenesis [3]. A bulging belly once considered a sign of prosperity is now a sign of disease. Severe health conditions, including type 2 diabetes, high blood pressure, high cholesterol, bone problems, obstructive sleep apnea, cancer and infertility have been linked to BMI. It has become a serious health issue due to the various associated medical complications. It may also have a negative impact on quality of life, physical fitness, self esteem, and emotional and social well-being.
Considerable research has gone into obesity in recent years, and efforts have been made to control this growing health problem. Increased weight in men has been associated with a lower testosterone level, poorer sperm quality, and reduced fertility as compared to men of normal weight. The odds of infertility increase by 10% for every 9 kg (20 pounds) a man is overweight [4].
Multiple explanations have been proposed for the decline in male fertility. These include increased prevalence of obesity and exposure to environmental pollution during fetal or adult life. Obesity leap reveals that in the period from 1991 to 1998, the prevalence of obesity increased from 12 to 17.9% in the general population and from 11.7 to 17.9% in men [5]. Hedley et al., estimated the current prevalence of obesity to be as high as 30.6% [6]. The effect of obesity on male fertility is thought to be multifactorial and may be modulated by genetic, endocrinal and environmental influences. The surge in obesity has reached pandemic proportions with 1.6 billion adults classified as overweight and a further 400 million adults classified as obese [7]. It accounts for 7.5% of the total burden of disease [8]. Alarmingly, there is now evidence in animal models that paternal obesity increases the susceptibility to obesity and diabetes in offspring, suggesting a possible mechanism for the amplification of these chronic diseases. This article reviews the studies linking the pathology of obesity to male subfertility, and discusses the proposed pathophysiological mechanisms underlying these intricate relationships.
The proposed mechanisms for infertility in obese men: male obesity and semen parameters
There are several studies that have investigated the impact of male obesity on the traditional sperm parameters mandated by the World Health Organization (WHO), namely sperm concentration, sperm motility and sperm morphology [9, 10]. The effects of obesity on male reproduction are less well documented than in the female. However, several studies indicate that sperm quality and fertility are reduced in overweight and obese men. Male obesity is suspected to cause alterations in semen parameters, especially sperm concentration [11], total sperm count, total motile sperm count, [12] total progressively motile sperm count, [11] sperm morphology, and DNA fragmentation [12]. Other studies, however, show conflicting results. Therefore, it is possible that in each overweight individual, different mechanisms are involved leading to one or more of the alterations in semen parameters and not in others.
Endocrinopathy and impaired spermatogenesis
Several studies document that increased male BMI is associated with reduced plasma concentrations of sex hormone binding globulin (SHBG) and testosterone with a concomitant rise in plasma concentration of estrogen [13]. Decreased testosterone and increased estrogen have long been associated with subfertility and reduced sperm counts by disrupting the negative feedback loop of the hypothalamic pituitary gonadal (HPG) axis. Other hormones involved in the regulation of Sertoli cell function and spermatogenesis, such as FSH/LH ratios, inhibin B and SHBG levels have all been observed to be decreased in males with increased BMI [14]. Targeted disruption of FSH signals and receptors leads to aberrant gametogenesis and hormonal imbalance [15]. Therefore, it remains plausible that decreased sperm counts observed in male obesity are, at least in part, a result of changes to the HPG axis through testosterone and estrogen, and likely reduced Sertoli cell function. Male obesity is associated with lower total and free testosterone levels. This decrease in androgen levels is proportional to the degree of obesity. Various mechanisms account for reduced total testosterone levels and are defined within a reversible hypogonadotropic hypogonadism pathway [16]. Reduced pituitary function or hypogonadotropic hypogonadism in obese men is most likely multifactorial. It is known that in obese men both estrone and estradiol are increased due to increased peripheral aromatization of androgens. Estrogens have a negative effect on the hypothalamus by altering the gonadotropin releasing hormone (GnRH) pulses, resulting in suppression of gonadotropin FSH and LH secretions. Apart from hyperestrogenemia, different factors have been proposed to explain the hypogonadotropism seen in obesity. Endogenous opioids have been suggested to have a role in the pathophysiology of hypogonadotropic hypoandrogenism in extremely obese males. The effect of type 2 diabetes, frequently associated with obesity, on the HPG axis is increasingly being appreciated [17].
Insulin resistance
The Endocrine Society Clinical Practice Guidelines (2010), recommend that men with type 2 diabetes have to be screened for low testosterone levels [18]. Along with the mechanisms mentioned previously, obese men with type 2 diabetes can have secondary hypogonadism because of peripheral and central insulin resistance and the effect of proinflammatory cytokines (TNFα and IL-6) on the HPG axis [19]. SHBG levels are reduced in obese men as a result of increased circulating insulin levels, associated with the insulin resistance of obesity. However, after adjusting for SHBG levels, low testosterone levels are shown to be correlated with insulin resistance and obesity, denoting an independent effect of insulin resistance on testosterone production [20].
Sleep apnea
Sleep apnea (SA) is a type of sleep disorder characterized by pauses in breathing or instances of shallow or infrequent breathing during sleep. Each pause in breathing causes hypoxemia. It is more common among obese individuals. Although its role in male infertility is not well elucidated, it is commonly associated with decreased pituitary gonadal function and a decline in morning testosterone concentrations [21]. Sleep fragmentation has been proposed as the mechanism by which sleep apnea disrupts nocturnal testosterone rhythm. Moreover, the adjusted mean (corrected for age and BMI) total testosterone is reduced proportionally to the severity of the SA. It can affect both testosterone levels as well as, independently, erectile function. Furthermore, it can negatively affect testosterone levels independent of BMI. SA has also been associated with reduced sexual quality of life. The combination of the aforementioned factors may result in a compounding effect on male fertility [22].
The thermal effect on spermatogenesis
One of the demerits of obesity that may potentially contribute to altered sperm production/parameters is raised gonadal heat resulting from increased scrotal adiposity. The process of spermatogenesis is highly heat sensitive, with optimal temperature in humans ranging between 34–35°C [23]. Elevated temperatures within the scrotum, due to fat tissue, could harm sperm cells. The deleterious effect of heat is associated with reduced sperm motility, increased sperm DNA fragmentation and increased sperm oxidative stress. Changes in testicular temperature can occur via a number of mechanisms such as varicoceles, using a laptop computer positioned on the lap [24], and immersion in a sauna bath [25]. Increased scrotal adiposity, along with the scrotum being surrounded completely by suprapubic and thigh fat may contribute to reduced sperm function and subfertility. It is noteworthy that increased sperm DNA damage and oxidative stress are identified in obese patients. The surgical removal of scrotal fat has been reported to improve sperm parameters [26].
Metabolic syndrome
Metabolic syndrome (MetS) is a disorder of energy utilization and storage, diagnosed by cooccurrence of three out of five of the following conditions: abdominal (central) obesity, elevated blood pressure, elevated fasting plasma glucose, high serum triglycerides, and low high density cholesterol (HDL) levels. Metabolic syndrome increases the risk of developing cardiovascular disease, particularly heart failure, and diabetes. MetS has been associated with hypogonadism and erectile dysfunction (ED) [27]. Some studies have shown an estimated prevalence of 34% in the US adult population [28]. The prevalence increases with age. The elements of this condition have been examined with regard to its associated detrimental effects on male fertility [29]. Hyperinsulinemia and hyperglycemia are common occurrences in obese individuals, and are constant confounding factors in many studies of male obesity. Hyperinsulinemia and hyperglycemia have been shown to have an inhibitory effect on sperm quantity and quality and therefore, could be attributing factors to the reduced fertility seen in obese men.
Hyperestrogenemia and aromatization
Elevated estrogens in obese men may, in part, result from the increased mass of white adipose tissue. White adipose tissue is responsible for aromatase activity and adipose-derived hormones and adipokines, which are elevated in obese men [30]. The aromatase cytochrome P450 enzyme, is produced by many tissues, including adipose tissue and testicular Leydig cells. In men, aromatization activity converts testosterone to estrogens. It is suggested that elevated estrogen concentrations in obese men may result from an increased conversion of androgens to estrogens by white adipose tissue [31]. This contributes to the increased plasma estrogen levels. Along with aromatization, the expression of estrogen receptors’ genes have been speculated to play a role in obese individuals [32]. Another key hormone produced by white adipose tissue is leptin, which plays a pivotal role in the regulation of energy intake and expenditure.
Leptin and trace toxins
Leptin is a hormone that has demonstrated a relationship between body fat and the neuroendocrine axis, since it influences appetite and the reproductive axis. Leptin is produced primarily by fat cells and might damage sperm cells or the cells that produce them. Adipose tissue is no longer considered as a simple reservoir to store fat. Rather, it plays a dynamic role in whole-body energy homeostasis by acting as an endocrine organ. Collective evidence indicates a strong link between neural influences and adipocyte expression and secretion of leptin. Leptin augments secretion of gonadotropin hormones, which are essential for initiation and maintenance of normal reproductive function, by acting centrally at the hypothalamus to regulate GnRH, neuronal activity and secretion. Thus, leptin serves as a surreptitious signal that links metabolic status to the reproductive axis [33]. An increase in leptin levels significantly decreases the production of testosterone from Leydig cells [34]. This explains why elevated leptin levels, commonly found in obese males [35], could alter the HPG axis and contribute to the decreased testosterone production. Infertility in overweight/obese males may be explained by leptin insensitivity. Obesity was also shown to be associated with high homocysteine and low vitamin D levels. Both are suspected to affect semen parameters.
Sperm DNA damage and oxidative stress
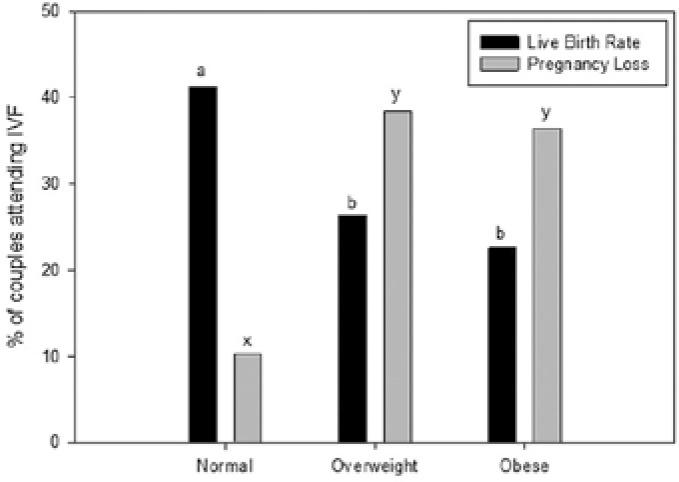
Numerous human and animal studies have determined that a relationship between obesity and reduced sperm DNA integrity exists. Male obesity has been linked with a reduction in sperm concentration and motility, an increase in sperm DNA damage and changes in reproductive hormones. One of the potential underlying pathological mechanisms behind diminished reproductive performance in obese men is sperm oxidative stress. Studies have shown that oxidative stress increases with an increase in BMI, primarily due to an increase in seminal macrophage activation. This leads to decreased sperm motility, increased sperm DNA damage, decreased acrosome reaction and lower embryo implantation rates following (Figure 1) IVF [9, 36] However, the magnitude of this increase was small and only of minor clinical significance, as there was no associated decline in sperm DNA integrity or sperm motility with increasing reacting oxygen species (ROS) production. Increased BMI was also found to be significantly linked to a fall in sperm concentration, serum testosterone, and an increase in serum estradiol [37].
Figure 1.
The effect of male obesity on conception success in couples going for IVF.
Psychological impact of obesity on sexuality
Erectile dysfunction (ED) has been correlated to fertility in men. In a survey of health professionals, obesity was associated with a 1.3 relative risk for ED [38]. In men reporting symptoms of ED, increased weight or obesity is found in 79% of subjects [39]. Data from the Agricultural Health Study in the United States indicate that, after adjusting for potential confounders, a 3-unit increase in male BMI was significantly associated with infertility (odds ratio 1.12). There was a dose–response relationship, and the association between BMI and infertility was similar in older and younger men, suggesting that erectile dysfunction in older men does not explain the association [40]. It has also been found that, in severely obese men, BMI was associated with increased avoidance of sexual encounters and increased difficulty with sexual performance, leading to lower satisfaction with sexual life. Sexual dysfunction in obese men is related to hypogonadism and elevated levels of proinflammatory cytokines. Clinical signs of these changes include reduced libido, reduced coital frequency, and ED. Obese individuals report higher incidences of sexual difficulties due to their weight and commonly experience some sexual dissatisfaction or sexual difficulties related to their weight. A higher BMI is associated with a greater impairment in sexual quality of life [41]. In males, for example, it has been proven that obesity can lower sexual satisfaction, lack of sexual enjoyment, lack of sexual desire, difficulty with sexual performance, and avoidance of sexual encounters and cause erectile dysfunction. Overweight people may consider themselves as sexual misfits, unattractive and undesirable, causing them to avoid potential or actual sexual relationships. Generally, those people who consider their weight to be a real problem and who seek treatment are those who have the highest rates of sexual problems. Obesity can also be related to sexuality in a reverse fashion. If an individual has had problems with sexuality or has been the victim of sexual abuse, he may turn to overeating as a means of dealing with his unpleasant experience [42]. The relation between obesity and depression is reciprocal. Overweight people are also frequently stigmatized and discriminated because of their body weight, which results in the development of a negative body image leading to low self-esteem, negative self-perception, lack of confidence, depression and anxiety disorders.
Epididymitis
There is no substantial evidence to relate obesity to epididymitis. Nevertheless, it is commonly observed that obese men have redundant lumps of fat at the suprapubic and inner thigh regions. These chunks of fat can cause mechanical inflammation to the scrotal contents, including epididymitis, by rubbing and sheering forces during various physical activities. Regardless of the cause, whether microbial, mechanical, or thermal, epididymitis can cause infertility.
Inflammation of the epididymis can affect epididymal function, by altering the environment within the epididymis, thus affecting sperm maturation, and fertilization ability. Inflammation can also cause blockage of epididymal ducts by scarring and cyst formation. Although epididymitis may occur on one or both sides, bilateral cases are more likely to impair male fertility. We concede that our envisage surrounding the link between epididymitis pathology and infertility requires additional high quality etiological research.
Reversibility
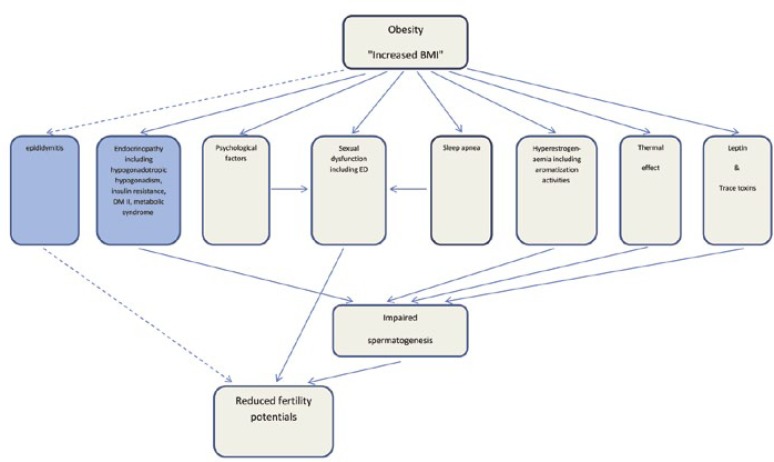
Turning the tide back is possible by treating obesity. While it is becoming clearer that male obesity has negative impacts on fertility, sperm function and, in the long-term, on the health of the offspring, it is equally clear that simple interventions, such as changes in diet and exercise, can reverse the disease state as well as offspring outcomes. Amending BMI status, correcting endocrinopathy and metabolic disturbances may affect obese patients at the molecular level by reducing oxidative stresses and its consequent DNA damages. To date, there is little information about the impact of diet and exercise interventions in obese men with regard to semen parameters. The mechanisms accounting for reduced total testosterone levels in obese men are diverse and are defined within a reversible hypogonadotropic hypogonadism pathway [16]. A flow chart showing how obesity factors lead to infertility is illustrated in Figure 2.
Figure 2.
Mechanisms linking being overweight to reduced fertility potential in men. The dashed arrows mean (indicate) the relation is yet to be confirmed.
CONCLUSIONS
There is substantial evidence that male obesity has negative impacts on fertility through changes at hormonal levels, as well as direct changes to sperm function and sperm molecular composition. Among the myriad of publications reviewed in this paper, impaired spermatogenesis and sexual dysfunction have been shown to drive other variables towards poor fertility potentials. Increased BMI was found to be significantly linked to a fall in sperm concentration, serum testosterone, and an increase in serum estradiol. The proposed mechanical theory of friction induced epididymitis and its sequelae demands further studies. Reversibility of infertility is a thrilling topic, as an enthusiastic overweight individual can undo the torsion and thus improve his condition.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Saima Salahuddin from London UK, for the English language review of the article.
References
- 1.Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982–2002. Fertil Steril. 2006;86:516–523. doi: 10.1016/j.fertnstert.2006.02.129. [DOI] [PubMed] [Google Scholar]
- 2.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8:185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- 3.Jensen TK, Andersson AM, Jørgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebaek NE. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82:863–870. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 4.Sallmén M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology. 2006;17:520–523. doi: 10.1097/01.ede.0000229953.76862.e5. [DOI] [PubMed] [Google Scholar]
- 5.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 6.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen DM, El-Serag HB. Review The epidemiology of obesity. Gastroenterol Clin North Am. 2010;39:1–7. doi: 10.1016/j.gtc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AIHW. Begg SVT, Barker B, Stevenson C, Stanley L, Lopez AD. Canberra: AIHW; 2007. The burden of disease and injury in Australia 2003; p. 337. ISBN 978 1 74024 648 4. [Google Scholar]
- 9.Palmer NO, Bakos HW, Fullston T, Lane M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis. 2012;2:253–263. doi: 10.4161/spmg.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90:2222–2225. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, Roudebush WE. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27:450–452. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16:293–311. doi: 10.1093/humupd/dmp047. [DOI] [PubMed] [Google Scholar]
- 13.Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76:1140–1146. doi: 10.1210/jcem.76.5.8496304. [DOI] [PubMed] [Google Scholar]
- 14.Kerr JB, Millar M, Maddocks S, Sharpe RM. Stage-dependent changes in spermatogenesis and Sertoli cells in relation to the onset of spermatogenic failure following withdrawal of testosterone. Anat Rec. 1993;235:547–559. doi: 10.1002/ar.1092350407. [DOI] [PubMed] [Google Scholar]
- 15.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–2353. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 17.Dhindsa S, Furlanetto R, Vora M, Ghanim H, Chaudhuri A, Dandona P. Low estradiol concentrations in men with subnormal testosterone concentrations and type 2 diabetes. Diabetes Care. 2011;34:1854–1859. doi: 10.2337/dc11-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dandona P, Dhindsa S. Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96:2643–2651. doi: 10.1210/jc.2010-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guidelinase. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 20.Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27:861–868. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- 21.Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab. 2001;86:1134–1139. doi: 10.1210/jcem.86.3.7296. [DOI] [PubMed] [Google Scholar]
- 22.Luboshitzky R, Lavie L, Shen-Orr Z, Herer P. Altered luteinizing hormone and testosterone secretion in middle-aged obese men with obstructive sleep apnea. Obes Res. 2005;13:780–786. doi: 10.1038/oby.2005.88. [DOI] [PubMed] [Google Scholar]
- 23.Robinson D, Rock J, Menkin MF. Control of human spermatogenesis by induced changes of intrascrotal temperature. JAMA. 1968;204:290–297. [PubMed] [Google Scholar]
- 24.Sheynkin Y, Jung M, Yoo P, Schulsinger D, Komaroff E. Increase in scrotal temperature in laptop computer users. Hum Reprod. 2005;20:452–455. doi: 10.1093/humrep/deh616. [DOI] [PubMed] [Google Scholar]
- 25.Guo H, Zhang HG, Xue BG, Sha YW, Liu Y, Liu RZ. Effects of cigarette, alcohol consumption and sauna on sperm morphology. Zhonghua Nan Ke Xue. 2006;12:215–217. [PubMed] [Google Scholar]
- 26.Shafik A, Olfat S. Lipectomy in the treatment of scrotal lipomatosis. Br J Urol. 1981;53:55–61. doi: 10.1111/j.1464-410x.1981.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 27.Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009;30:10–22. doi: 10.2164/jandrol.108.005215. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Giles WH, Dietz WH. Prevalence of metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 29.Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl. 2008;29:251–259. doi: 10.2164/jandrol.107.003731. [DOI] [PubMed] [Google Scholar]
- 30.Wake DJ, Strand M, Rask E, Westerbacka J, Livingstone DE, Soderberg S, et al. Intra-adipose sex steroid metabolism and body fat distribution in idiopathic human obesity. Clin Endocrinol (Oxf) 2007;66:440–446. doi: 10.1111/j.1365-2265.2007.02755.x. [DOI] [PubMed] [Google Scholar]
- 31.Phillips KP, Tanphaichitr N. Mechanisms of obesity-induced male infertility. Expert Rev Endocrinol Metab. 2010;5:229–251. doi: 10.1586/eem.09.65. [DOI] [PubMed] [Google Scholar]
- 32.Esfahlan RJ, Zarghami N, Esfahlan AJ, Mollazadeh M, Nejati K, Nasiri M. The Possible Impact of Obesity on Androgen, Progesterone and Estrogen Receptors (ERα and ERβ) Gene Expression in Breast Cancer Patients. Breast Cancer (Auckl) 2011;5:227–237. doi: 10.4137/BCBCR.S7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hausman GJ, Barb CR, Lents CA. Leptin and reproductive function. Biochimie. 2012;94:2075–2081. doi: 10.1016/j.biochi.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Caprio M, Isidori AM, Carta AR, Moretti C, Dufau ML, Fabbri A. Expression of functional leptin receptors in rodent Leydig cells. Endocrinology. 1999;140:4939–4947. doi: 10.1210/endo.140.11.7088. [DOI] [PubMed] [Google Scholar]
- 35.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 36.Zorn B, Vidmar G, Meden-Vrtovec H. Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl. 2003;26:279–285. doi: 10.1046/j.1365-2605.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 37.Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43:121–128. doi: 10.1111/j.1439-0272.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 38.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161–168. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 39.Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, McKinlay JB. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000;30:328–338. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- 40.Sallmén M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology. 2006;17:520–523. doi: 10.1097/01.ede.0000229953.76862.e5. [DOI] [PubMed] [Google Scholar]
- 41.Kolotkin R, Binks M, Crosby R, Ostbye T, Gress R, Adams T. Obesity and sexual quality of life. Obesity. 2006;14:472–479. doi: 10.1038/oby.2006.62. [DOI] [PubMed] [Google Scholar]
- 42.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]