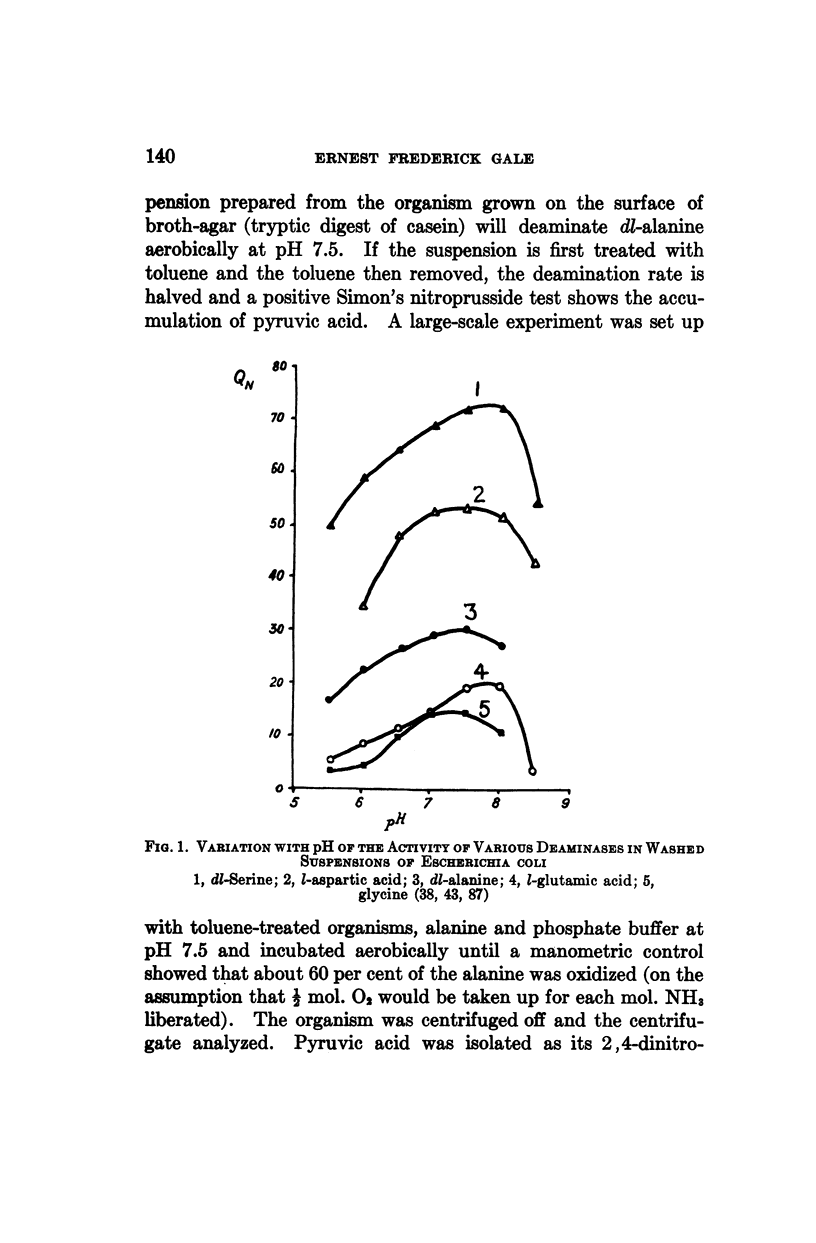
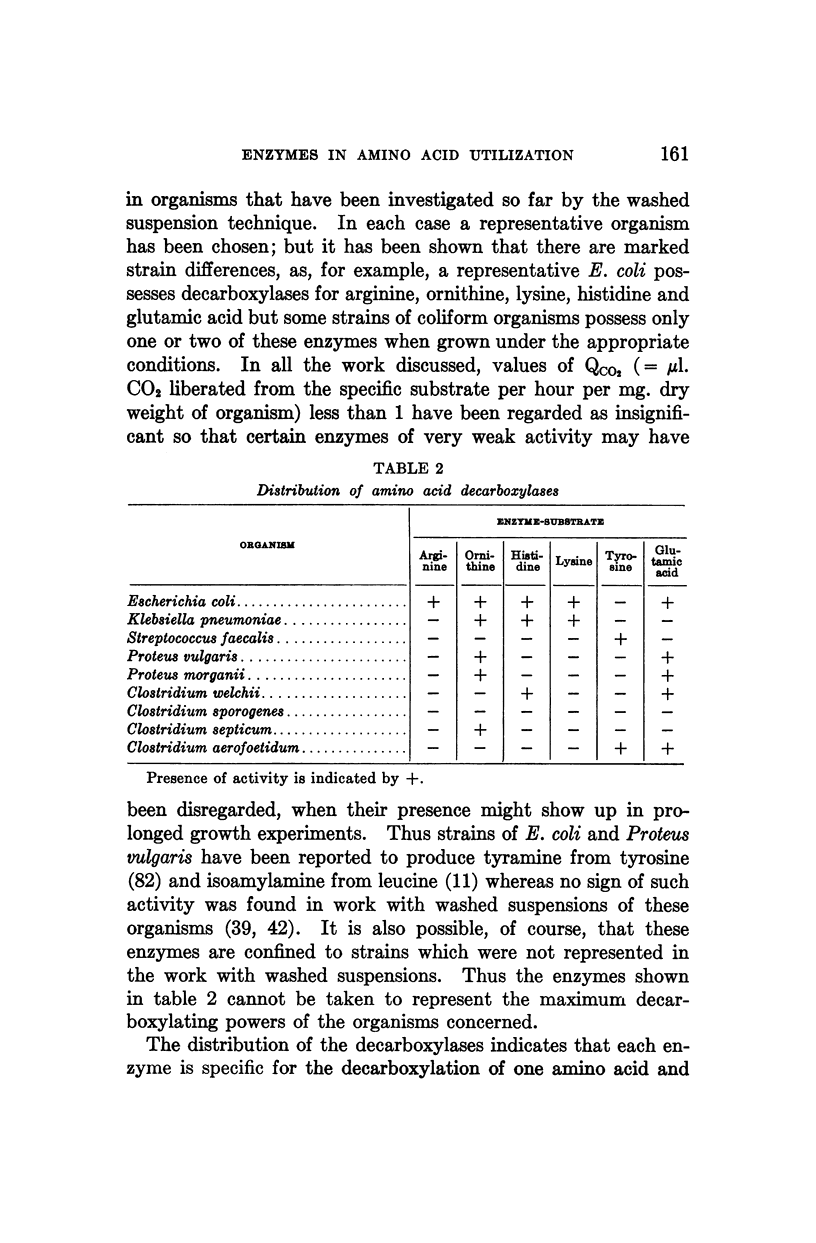
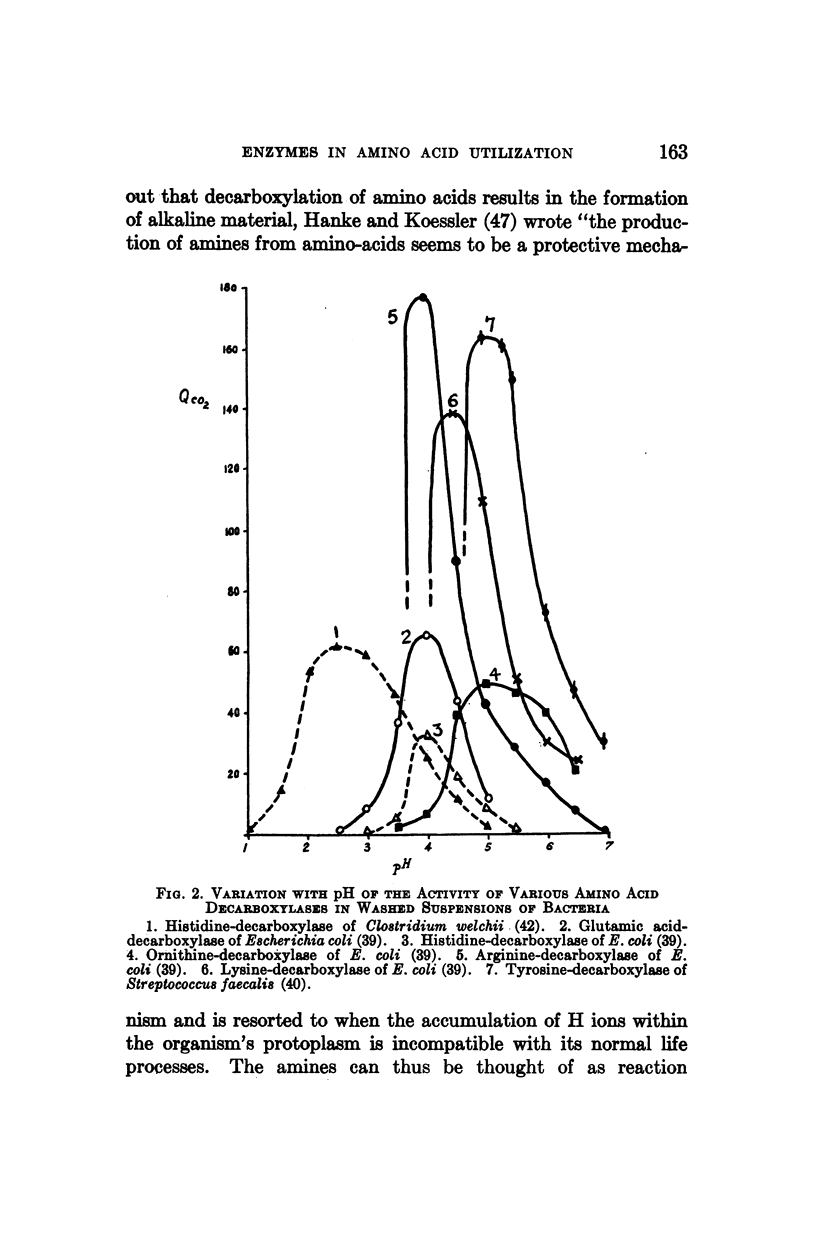
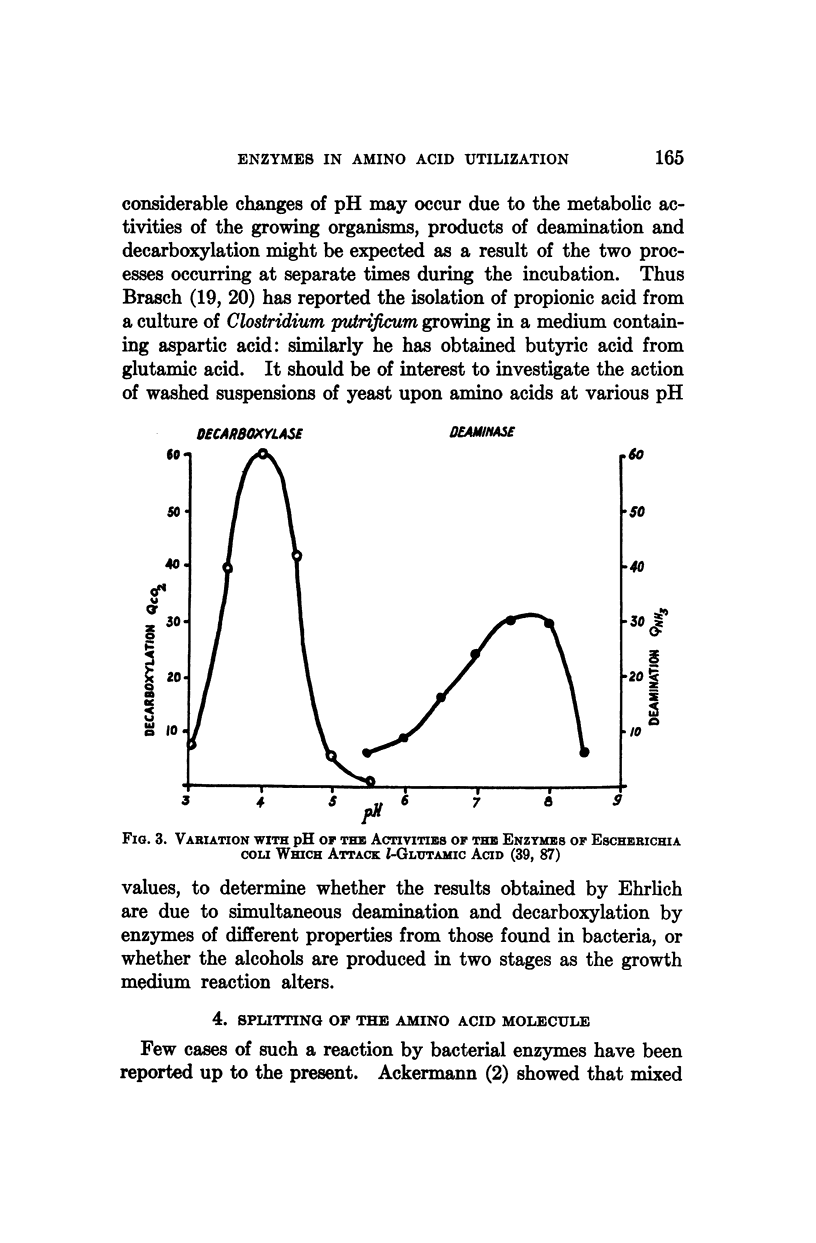
Full text
PDF





































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. W., Happold F. C. The coli-tryptophan-indole reaction: Essential structural conditions for the enzymic degradation of tryptophan to indole. Biochem J. 1940 May;34(5):657–663. doi: 10.1042/bj0340657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger G., Walpole G. S. Isolation of the pressor principles of putrid meat. J Physiol. 1909 Mar 22;38(4):343–352. doi: 10.1113/jphysiol.1909.sp001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth V. H., Green D. E. A wet-crushing mill for micro-organisms. Biochem J. 1938 May;32(5):855–861. doi: 10.1042/bj0320855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton C. E. The Utilization of Amino Acids and of Glucose by Clostridium botulinum. J Bacteriol. 1940 May;39(5):485–497. doi: 10.1128/jb.39.5.485-497.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. P., Woolf B. The deamination and synthesis of l-aspartic acid in the presence of bacteria. Biochem J. 1928;22(2):474–481. doi: 10.1042/bj0220474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux Woods D. Indole formation by Bacterium coli: The action of washed suspensions of Bacterium coli on indole derivatives. Biochem J. 1935 Mar;29(3):649–655. doi: 10.1042/bj0290649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux Woods D. Indole formation by Bacterium coli: The breakdown of tryptophan by washed suspensions of Bacterium coli. Biochem J. 1935 Mar;29(3):640–648. doi: 10.1042/bj0290640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerth A. H., Littwin R. J., Deutsch J. V. The Determination of Histamine in Bacterial Cultures. J Bacteriol. 1939 Feb;37(2):187–203. doi: 10.1128/jb.37.2.187-203.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerth A. H. The Production of Histamine in Bacterial Cultures. J Bacteriol. 1939 Feb;37(2):205–222. doi: 10.1128/jb.37.2.205-222.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fildes P. The production of indole by suspensions of Bact. coli. Biochem J. 1938 Sep;32(9):1600–1606. doi: 10.1042/bj0321600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. Factors influencing bacterial deamination: Aspartase II: its occurrence in and extraction from Bacterium coli and its activation by adenosine and related compounds. Biochem J. 1938 Sep;32(9):1583–1599. doi: 10.1042/bj0321583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Stephenson M. Factors influencing bacterial deamination: Factors influencing the activity of dl-serine deaminase in Bacterium coli. Biochem J. 1938 Feb;32(2):392–404. doi: 10.1042/bj0320392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Stephenson M. l-Malic dehydrogenase and codehydrogenase of Bacterium coli. Biochem J. 1939 Aug;33(8):1245–1256. doi: 10.1042/bj0331245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. The production of amines by bacteria: The decarboxylation of amino-acids by strains of Bacterium coli. Biochem J. 1940 Mar;34(3):392–413. doi: 10.1042/bj0340392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. The production of amines by bacteria: The production of putrescine from l(+)-arginine by Bacterium coli in symbiosis with Streptococcus faecalis. Biochem J. 1940 Jun;34(6):853–857. doi: 10.1042/bj0340853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. The production of amines by bacteria: The production of tyramine by Streptococcus faecalis. Biochem J. 1940 Jun;34(6):846–852. doi: 10.1042/bj0340846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happold F. C., Hoyle L. The coli-tryptophan-indole reaction: Enzyme preparations and their action on tryptophan and some indole derivatives. Biochem J. 1935 Aug;29(8):1918–1926. doi: 10.1042/bj0291918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills G. M. Ammonia production by pathogenic bacteria. Biochem J. 1940 Jul;34(7):1057–1069. doi: 10.1042/bj0341057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerheide J. C., Kocholaty W. Metabolism of the strict anaerobes (genus: Clostridium): Reduction of amino-acids with gaseous hydrogen by suspensions of Cl. sporogenes. Biochem J. 1938 Jun;32(6):949–957. doi: 10.1042/bj0320949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins F. G., Cole S. W. A contribution to the chemistry of proteids: Part II. The constitution of tryptophane, and the action of bacteria upon it. J Physiol. 1903 Jun 15;29(4-5):451–466. doi: 10.1113/jphysiol.1903.sp000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocholaty W., Hoogerheide J. C. Studies of the metabolism of the strict anaerobes (genus: Clostridium): Dehydrogenation reactions by suspensions of Cl. sporogenes. Biochem J. 1938 Mar;32(3):437–448. doi: 10.1042/bj0320437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Metabolism of amino-acids: Deamination of amino-acids. Biochem J. 1935 Jul;29(7):1620–1644. doi: 10.1042/bj0291620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Metabolism of amino-acids: The synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues. Biochem J. 1935 Aug;29(8):1951–1969. doi: 10.1042/bj0291951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby E., Twort F. W. On the presence of beta-imidazolethylamine in the intestinal wall; with a method of isolating a bacillus from the alimentary canal which converts histidine into this substance. J Physiol. 1912 Aug 2;45(1-2):53–60. doi: 10.1113/jphysiol.1912.sp001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel J. H., Whetham M. D. Dehydrogenations produced by resting Bacteria. I. Biochem J. 1925;19(3):520–531. doi: 10.1042/bj0190520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel J. H., Whetham M. D. The Equilibria existing between Succinic, Fumaric, and Malic Acids in the presence of Resting Bacteria. Biochem J. 1924;18(3-4):519–534. doi: 10.1042/bj0180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel J. H., Woolf B. The Equilibrium between l-Aspartic Acid, Fumaric Acid and Ammonia in Presence of Resting Bacteria. Biochem J. 1926;20(3):545–555. doi: 10.1042/bj0200545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raistrick H., Clark A. B. Studies on the Cycloclastic Power of Bacteria: Part II. A Quantitative Study of the Aerobic Decomposition of Tryptophan and Tyrosine by Bacteria. Biochem J. 1921;15(1):76–82. doi: 10.1042/bj0150076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M., Gale E. F. Factors influencing bacterial deamination: The deamination of glycine, dl-alanine and l-glutamic acid by Bacterium coli. Biochem J. 1937 Aug;31(8):1316–1322.1. doi: 10.1042/bj0311316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. On lactic dehydrogenase: A cell-free enzyme preparation obtained from bacteria. Biochem J. 1928;22(2):605–614. doi: 10.1042/bj0220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland L. H. Studies in the metabolism of the strict anaerobes (genus Clostridium): The chemical reactions by which Cl. sporogenes obtains its energy. Biochem J. 1934;28(5):1746–1759. doi: 10.1042/bj0281746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland L. H. Studies in the metabolism of the strict anaerobes (genus Clostridium): The oxidation of alanine by Cl. sporogenes. IV. The reduction of glycine by Cl. sporogenes. Biochem J. 1935 Apr;29(4):889–898. doi: 10.1042/bj0290889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr H. L. The anaerobic decomposition of l-cystine by washed cells of Proteus vulgaris. Biochem J. 1933;27(3):759–763. doi: 10.1042/bj0270759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen A. I., Laine T. Investigations on the root nodule bacteria of leguminous plants: The excretion products of root modules. The mechanism of N-fixation. Biochem J. 1939 Apr;33(4):412–427. doi: 10.1042/bj0330412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. D., Clifton C. E. Studies in the metabolism of the strict anaerobes (genus Clostridium): Hydrogen production and amino-acid utilization by Clostridium tetanomorphum. Biochem J. 1937 Oct;31(10):1774–1788. doi: 10.1042/bj0311774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. D., Clifton C. E. Studies in the metabolism of the strict anaerobes (genus Clostridium): The decomposition of pyruvate and l-(+)glutamate by Clostridium tetanomorphum. Biochem J. 1938 Feb;32(2):345–356. doi: 10.1042/bj0320345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. D. Studies in the metabolism of the strict anaerobes (genus Clostridium): Further experiments on the coupled reactions between pairs of amino-acids induced by Cl. sporogenes. Biochem J. 1936 Oct;30(10):1934–1946. doi: 10.1042/bj0301934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge W. R., Knox R., Glass V. Variability in the activity of bacterial enzymes: The effect of the age of the culture. Biochem J. 1936 May;30(5):926–931. doi: 10.1042/bj0300926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf B. Some enzymes in B. coli communis which act on fumaric acid. Biochem J. 1929;23(3):472–482. doi: 10.1042/bj0230472. [DOI] [PMC free article] [PubMed] [Google Scholar]


