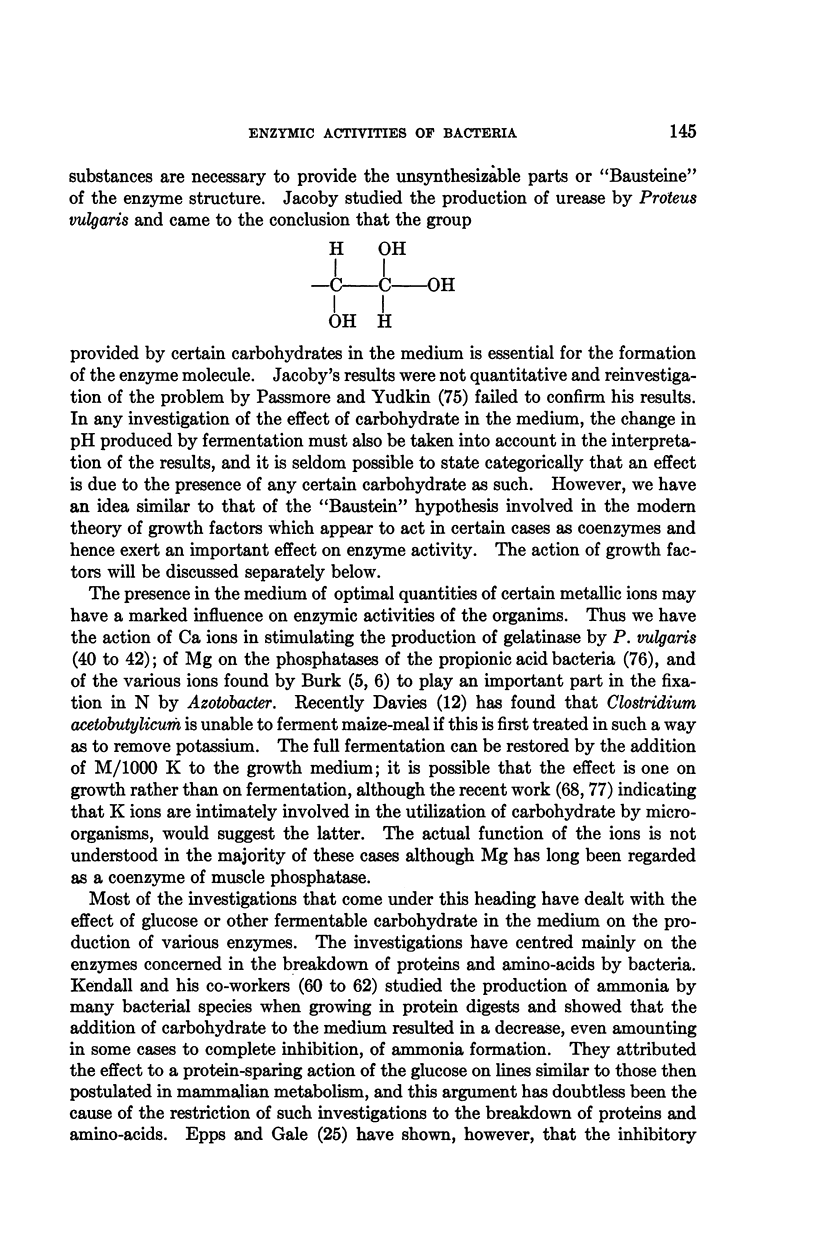
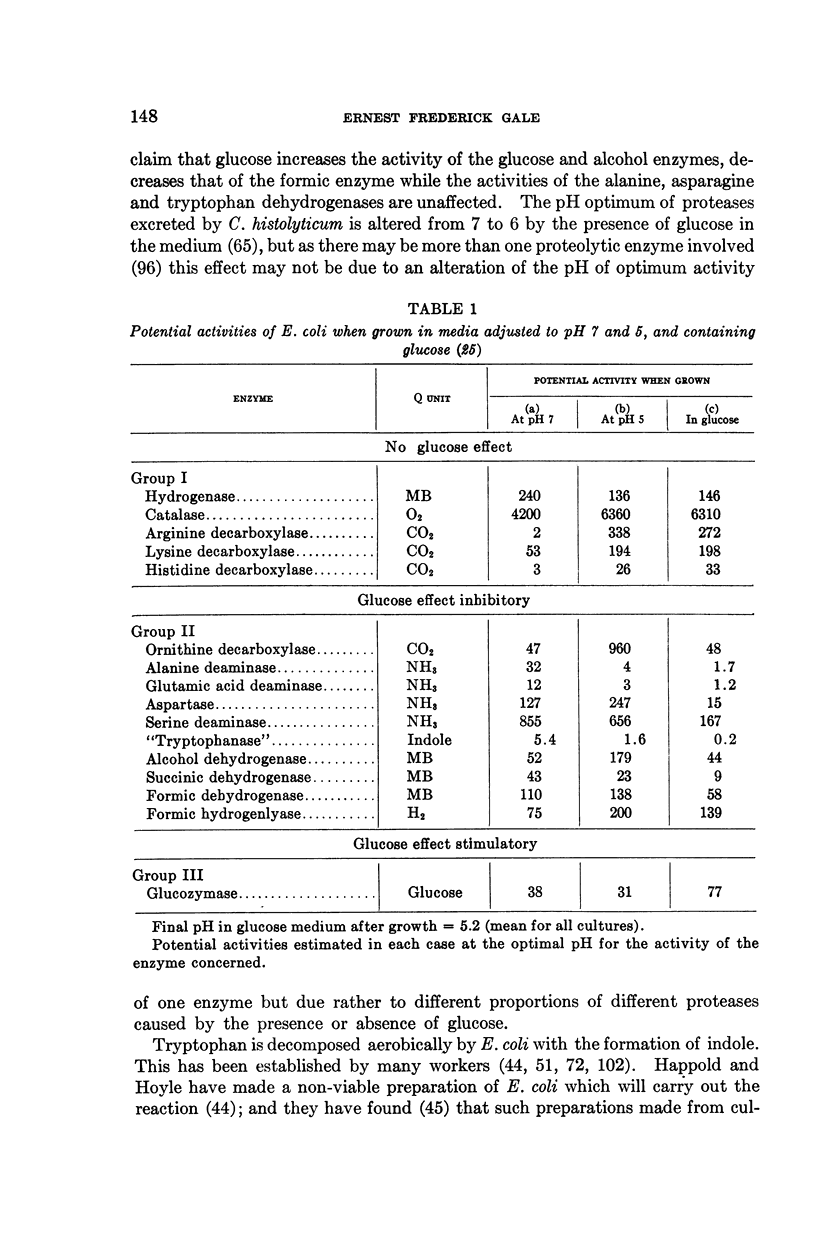
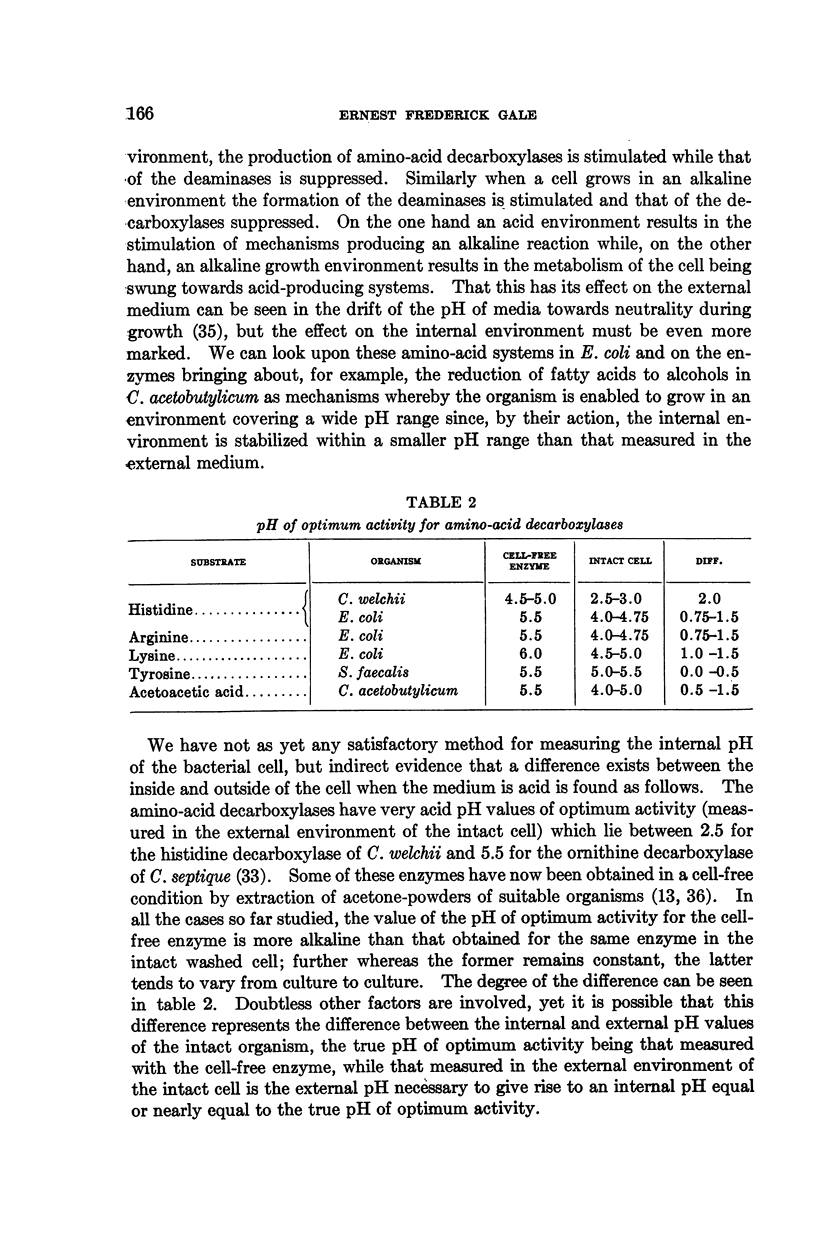
Full text
PDF































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman N., Rettger L. F. The Influence of Carbohydrate on the Nitrogen Metabolism of Bacteria. J Bacteriol. 1918 Jul;3(4):389–402. doi: 10.1128/jb.3.4.389-402.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton C. E. A Comparison of the Metabolic Activities of Aerobacter aerogenes, Eberthella typhi and Escherichia coli. J Bacteriol. 1937 Feb;33(2):145–162. doi: 10.1128/jb.33.2.145-162.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Clark W. M. The Growth of Certain Bacteria in Media of Different Hydrogen Ion Concentrations. J Bacteriol. 1919 Jul;4(4):409–427. doi: 10.1128/jb.4.4.409-427.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R., Stephenson M. Studies on the acetone-butyl alcohol fermentation: Nutritional and other factors involved in the preparation of active suspensions of Cl. acetobutylicum (Weizmann). Biochem J. 1941 Dec;35(12):1320–1331. doi: 10.1042/bj0351320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. Studies on the acetone-butyl alcohol fermentation: Intermediates in the fermentation of glucose by Cl. acetobutylicum. 3. Potassium as an essential factor in the fermentation of maize meal by Cl. acetobutylicum (BY). Biochem J. 1942 Sep;36(7-9):582–599. doi: 10.1042/bj0360582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deere C. J., Dulaney A. D., Michelson I. D. The Lactase Activity of Escherichia coli-mutabile. J Bacteriol. 1939 Apr;37(4):355–363. doi: 10.1128/jb.37.4.355-363.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deere C. J. On the "Activation" of the Lactase of Escherichia coli-mutabile. J Bacteriol. 1939 May;37(5):473–483. doi: 10.1128/jb.37.5.473-483.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux Woods D. Indole formation by Bacterium coli: The breakdown of tryptophan by washed suspensions of Bacterium coli. Biochem J. 1935 Mar;29(3):640–648. doi: 10.1042/bj0290640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos R. J. THE ADAPTIVE PRODUCTION OF ENZYMES BY BACTERIA. Bacteriol Rev. 1940 Mar;4(1):1–16. doi: 10.1128/br.4.1.1-16.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerth A. H. The Production of Histamine in Bacterial Cultures. J Bacteriol. 1939 Feb;37(2):205–222. doi: 10.1128/jb.37.2.205-222.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Handley W. C., Happold F. C. The 'tryptophanase-tryptophan reaction: Possible mechanisms for the inhibition of indole production by glucose in cultures of B. coli. Biochem J. 1942 Apr;36(3-4):311–318. doi: 10.1042/bj0360311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Richard W., Handley C., Happold F. C. The tryptophanase-indole reaction: Some observations on the production of tryptophanase by Esch. coli; in particular the effect of the presence of glucose and amino acids on the formation of tryptophanase. Biochem J. 1941 Jan;35(1-2):207–212. doi: 10.1042/bj0350207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fildes P. The production of indole by suspensions of Bact. coli. Biochem J. 1938 Sep;32(9):1600–1606. doi: 10.1042/bj0321600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Epps H. M. The effect of the pH of the medium during growth on the enzymic activities of bacteria (Escherichia coli and Micrococcus lysodeikticus) and the biological significance of the changes produced. Biochem J. 1942 Sep;36(7-9):600–618. doi: 10.1042/bj0360600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. Factors influencing bacterial deamination: Aspartase II: its occurrence in and extraction from Bacterium coli and its activation by adenosine and related compounds. Biochem J. 1938 Sep;32(9):1583–1599. doi: 10.1042/bj0321583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. Formic dehydrogenase of Bacterium coli: its inactivation by oxygen and its protection in the bacterial cell. Biochem J. 1939 Jun;33(6):1012–1027. doi: 10.1042/bj0331012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. Production of amines by bacteria: The decarboxylation of amino-acids by organisms of the groups Clostridium and Proteus With an addendum by G. L. Brown, F. C. MacIntosh and P. Bruce White. Biochem J. 1941 Jan;35(1-2):66–80. doi: 10.1042/bj0350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Stephenson M. Factors influencing bacterial deamination: Factors influencing the activity of dl-serine deaminase in Bacterium coli. Biochem J. 1938 Feb;32(2):392–404. doi: 10.1042/bj0320392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Stephenson M. l-Malic dehydrogenase and codehydrogenase of Bacterium coli. Biochem J. 1939 Aug;33(8):1245–1256. doi: 10.1042/bj0331245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. The oxidation of amines by bacteria. Biochem J. 1942 Feb;36(1-2):64–75. doi: 10.1042/bj0360064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. The production of amines by bacteria: The decarboxylation of amino-acids by strains of Bacterium coli. Biochem J. 1940 Mar;34(3):392–413. doi: 10.1042/bj0340392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. The production of amines by bacteria: The production of tyramine by Streptococcus faecalis. Biochem J. 1940 Jun;34(6):846–852. doi: 10.1042/bj0340846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Van Heyningen W. E. The effect of the pH and the presence of glucose during growth on the production of alpha and theta toxins and hyaluronidase by Clostridium welchii. Biochem J. 1942 Sep;36(7-9):624–630. doi: 10.1042/bj0360624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines R. B. Further studies of the effect of the medium on the production of bacterial gelatinase. Biochem J. 1933;27(2):466–474. [PMC free article] [PubMed] [Google Scholar]
- Haines R. B. The formation of bacterial proteases, especially in synthetic media. Biochem J. 1931;25(6):1851–1859. doi: 10.1042/bj0251851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines R. B. The influence of the medium on the production of bacterial gelatinase. Biochem J. 1932;26(2):323–336. doi: 10.1042/bj0260323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happold F. C., Hoyle L. The coli-tryptophan-indole reaction: Enzyme preparations and their action on tryptophan and some indole derivatives. Biochem J. 1935 Aug;29(8):1918–1926. doi: 10.1042/bj0291918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D., Todd E. W. Purification and properties of a haemolysin produced by group A haemolytic streptococci (streptolysin O). Biochem J. 1941 Nov;35(10-11):1124–1139. doi: 10.1042/bj0351124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey A. D. Factors Limiting Bacterial Growth: IV. The Age of the Parent Culture and the Rate of Growth of Transplants of Escherichia coli. J Bacteriol. 1939 Mar;37(3):285–299. doi: 10.1128/jb.37.3.285-299.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills G. M. Ammonia production by pathogenic bacteria. Biochem J. 1940 Jul;34(7):1057–1069. doi: 10.1042/bj0341057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills G. M. Aneurin (vitamin B(1)) and pyruvate metabolism by Staphylococcus aureus. Biochem J. 1938 Feb;32(2):383–391. doi: 10.1042/bj0320383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins F. G., Morgan E. J. The influence of thiol-groups in the activity of dehydrogenases. Biochem J. 1938 Mar;32(3):611–620. doi: 10.1042/bj0320611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocholaty W., Hoogerheide J. C. Studies of the metabolism of the strict anaerobes (genus: Clostridium): Dehydrogenation reactions by suspensions of Cl. sporogenes. Biochem J. 1938 Mar;32(3):437–448. doi: 10.1042/bj0320437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocholaty W., Weil L. Enzymic adaptation in Clostridium histolyticum. Biochem J. 1938 Oct;32(10):1696–1701. doi: 10.1042/bj0321696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Hafez M. M., Eggleston L. V. Indole formation in Bacterium coli commune. Biochem J. 1942 Apr;36(3-4):306–310. doi: 10.1042/bj0360306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis I. M. Bacterial Variation with Special Reference to Behavior of Some Mutabile Strains of Colon Bacteria in Synthetic Media. J Bacteriol. 1934 Dec;28(6):619–639. doi: 10.1128/jb.28.6.619-639.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane M. G., Knight B. C. The biochemistry of bacterial toxins: The lecithinase activity of Cl. welchii toxins. Biochem J. 1941 Sep;35(8-9):884–902. doi: 10.1042/bj0350884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean D. Studies on diffusing factors: The hyaluronidase activity of testicular extracts, bacterial culture filtrates and other agents that increase tissue permeability. Biochem J. 1941 Jan;35(1-2):159–183. doi: 10.1042/bj0350159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCEEDINGS OF THE BIOCHEMICAL SOCIETY. Biochem J. 1942 Jun;36(5-6):v–x. doi: 10.1042/bj036000v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore R., Yudkin J. The effect of carbohydrates and allied substances on urease production by Proteus vulgaris. Biochem J. 1937 Feb;31(2):318–322. doi: 10.1042/bj0310318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pett L. B., Wynne A. M. Studies on bacterial phosphatases: The phosphatases of Clostridium acetobutylicum Weizmann and Propionibacterium jensenii Van Niel. Biochem J. 1933;27(5):1660–1671. doi: 10.1042/bj0271660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel J. H., Webley D. M. Vitamin B(1) and bacterial oxidation: The effects of magnesium, potassium and hexosediphosphate ions. Biochem J. 1942 Feb;36(1-2):8–33. doi: 10.1042/bj0360008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel J. H., Webley D. M. Vitamin B(1) and bacterial oxidations: Dependence of acetic acid oxidation on vitamin B(1). Biochem J. 1941 Jan;35(1-2):192–206. doi: 10.1042/bj0350192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel J. H., Woolf B. The Equilibrium between l-Aspartic Acid, Fumaric Acid and Ammonia in Presence of Resting Bacteria. Biochem J. 1926;20(3):545–555. doi: 10.1042/bj0200545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raistrick H., Clark A. B. Studies on the Cycloclastic Power of Bacteria: Part II. A Quantitative Study of the Aerobic Decomposition of Tryptophan and Tyrosine by Bacteria. Biochem J. 1921;15(1):76–82. doi: 10.1042/bj0150076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Werkman C. H. Adaptation of the Propionic-Acid Bacteria to Vitamin B(1) Synthesis Including a Method of Assay. J Bacteriol. 1939 Jul;38(1):25–32. doi: 10.1128/jb.38.1.25-32.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M., Gale E. F. Factors influencing bacterial deamination: The deamination of glycine, dl-alanine and l-glutamic acid by Bacterium coli. Biochem J. 1937 Aug;31(8):1316–1322.1. doi: 10.1042/bj0311316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M., Gale E. F. The adaptability of glucozymase and galactozymase in Bacterium coli. Biochem J. 1937 Aug;31(8):1311–1315. doi: 10.1042/bj0311311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M., Stickland L. H. Hydrogenlyases: Bacterial enzymes liberating molecular hydrogen. Biochem J. 1932;26(3):712–724. doi: 10.1042/bj0260712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M., Stickland L. H. Hydrogenlyases: Further experiments on the formation of formic hydrogenlyase by Bact. coli. Biochem J. 1933;27(5):1528–1532. doi: 10.1042/bj0271528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M., Yudkin J. Galactozymase considered as an adaptive enzyme. Biochem J. 1936 Mar;30(3):506–514. doi: 10.1042/bj0300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland L. H. The bacterial decomposition of formic acid. Biochem J. 1929;23(6):1187–1198. doi: 10.1042/bj0231187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still J. L. Alcohol enzyme of Bact. coli. Biochem J. 1940 Sep;34(8-9):1177–1182. doi: 10.1042/bj0341177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heyningen W. E. The biochemistry of the gas gangrene toxins: Estimation of the alpha toxin of Cl. welchii, type A. Biochem J. 1941 Nov;35(10-11):1246–1256. doi: 10.1042/bj0351246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heyningen W. E. The biochemistry of the gas gangrene toxins: Partial purification of the toxins of Cl. welchii, type A. Separation of alpha and theta toxins. Biochem J. 1941 Nov;35(10-11):1257–1269. doi: 10.1042/bj0351257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heyningen W. E. The proteinases of Clostridium histolyticum. Biochem J. 1940 Dec;34(12):1540–1545. doi: 10.1042/bj0341540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C. E. WHAT DO WE MEAN BY A BACTERIAL LIFE CYCLE? Science. 1935 Mar 29;81(2100):314–315. doi: 10.1126/science.81.2100.314. [DOI] [PubMed] [Google Scholar]
- Winslow C. E., Walker H. H. THE EARLIER PHASES OF THE BACTERIAL CULTURE CYCLE. Bacteriol Rev. 1939 Dec;3(2):147–186. doi: 10.1128/br.3.2.147-186.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. D., Trim A. R. Studies in the metabolism of the strict anaerobes: The metabolism of amino-acids by Cl. welchii. Biochem J. 1942 Jun;36(5-6):501–512. doi: 10.1042/bj0360501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge W. R., Glass V. Variability in the activity of bacterial enzymes: Factors associated with viability and growth. Biochem J. 1937 Apr;31(4):526–531. doi: 10.1042/bj0310526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge W. R., Knox R., Glass V. Variability in the activity of bacterial enzymes: The effect of the age of the culture. Biochem J. 1936 May;30(5):926–931. doi: 10.1042/bj0300926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin J. Hydrogenlyases: Some factors concerned in the production of the enzymes. Biochem J. 1932;26(6):1859–1871. doi: 10.1042/bj0261859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin J. The dehydrogenases of Bacterium coli.: The coenzyme of glucose dehydrogenase. Biochem J. 1934;28(4):1463–1473. doi: 10.1042/bj0281463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin J. The dehydrogenases of Bacterium coli: The effect of dilution: with a note on the existence of a co-enzyme of glucose dehydrogenase. Biochem J. 1933;27(6):1849–1858. doi: 10.1042/bj0271849. [DOI] [PMC free article] [PubMed] [Google Scholar]


