Abstract
Background: Measurement of fractional nitric oxide (NO) concentration in exhaled breath (FeNO) is a quantitative, noninvasive, simple, and safe method of measuring airway inflammation that provides a complementary tool to other ways of assessing airways disease, including asthma. While FeNO measurement has been standardized, there is currently no reference guideline for practicing health care providers to guide them in the appropriate use and interpretation of FeNO in clinical practice.
Purpose: To develop evidence-based guidelines for the interpretation of FeNO measurements that incorporate evidence that has accumulated over the past decade.
Methods: We created a multidisciplinary committee with expertise in the clinical care, clinical science, or basic science of airway disease and/or NO. The committee identified important clinical questions, synthesized the evidence, and formulated recommendations. Recommendations were developed using pragmatic systematic reviews of the literature and the GRADE approach.
Results: The evidence related to the use of FeNO measurements is reviewed and clinical practice recommendations are provided.
Conclusions: In the setting of chronic inflammatory airway disease including asthma, conventional tests such as FEV1 reversibility or provocation tests are only indirectly associated with airway inflammation. FeNO offers added advantages for patient care including, but not limited to (1) detecting of eosinophilic airway inflammation, (2) determining the likelihood of corticosteroid responsiveness, (3) monitoring of airway inflammation to determine the potential need for corticosteroid, and (4) unmasking of otherwise unsuspected nonadherence to corticosteroid therapy.
Keywords: nitric oxide, asthma, inflammation, airway disease, exhaled breath, clinical application
Contents
Executive Summary
Introduction
Methods
Committee Composition, Meetings, and Document Preparation
Document Structure
Quality of Evidence and Strength of Recommendations
Why Should a FeNO Test be Obtained?
Can FeNO Be Used to Diagnose Asthma?
FeNO Is Associated with Eosinophilic Airway Inflammation
FeNO Predicts Likelihood of Corticosteroid Responsiveness
FeNO Can Support a Diagnosis of Asthma
FeNO May Predict AHR
Is There a Normal FeNO Value?
Normal Values versus Relevant Cut Points for FeNO
Confounding Factors that May Affect FeNO
What Are the Clinically Significant Cut Points for FeNO?
Low FeNO (< 25 ppb in Adults; 20 ppb in Children)
High FeNO (> 50 ppb in Adults, 35 ppb in Children)
Intermediate FeNO (between 25 ppb and 50 ppb in Adults; 20–35 ppb in Children)
Persistently High FeNO (> 50 ppb in adults, 35 ppb in Children)
Can FeNO Be Used to Monitor Airway Inflammation?
Monitoring Airway Inflammation in Asthma
Minimally Important Differences, and Prognostic Significance of FeNO
How Should a FeNO Measurement Be Interpreted and Reported?
Other Situations in which FeNO May Be Useful
COPD
Pulmonary Hypertension
Cystic Fibrosis and Nasal NO Measurements
Conclusions and Future Directions
Online Supplement
Appendix E1: Methods Checklist
Appendix E2: Technical Considerations and Sources of Variation in FeNO
Appendix E3: Causes of High and Low FeNO Levels
Appendix E4: Case Studies
Executive Summary
Nitric oxide (NO) is now recognized as a biological mediator in animals and humans. NO is produced by the human lung and is present in the exhaled breath. It has been implicated in the pathophysiology of lung diseases, including asthma. The measurement of exhaled NO has been standardized for clinical use. Numerous studies have provided evidence regarding the applications of NO measurements in clinical practice, together with the performance characteristics and the strengths and the weaknesses of the test. Based on this evidence, this Clinical Practice Guideline is designed to guide clinicians as to how exhaled NO measurements should be used and interpreted.
Evidence Quality and Recommendations
These recommendations may vary with respect to the particular target population. Where this is the case, this has been included in the recommendation. If not stated, then the recommendation applies to patients with asthma.
We recommend the use of FeNO in the diagnosis of eosinophilic airway inflammation (strong recommendation, moderate quality of evidence).
We recommend the use of FeNO in determining the likelihood of steroid responsiveness in individuals with chronic respiratory symptoms possibly due to airway inflammation (strong recommendation, low quality of evidence).
We suggest that FeNO may be used to support the diagnosis of asthma in situations in which objective evidence is needed (weak recommendation, moderate quality of evidence).
We suggest the use of cut points rather than reference values when interpreting FeNO levels (weak recommendation, low quality of evidence).
We recommend accounting for age as a factor affecting FeNO in children younger than 12 years of age (strong recommendation, high quality of evidence).
We recommend that low FeNO less than 25 ppb (< 20 ppb in children) be used to indicate that eosinophilic inflammation and responsiveness to corticosteroids are less likely (strong recommendation, moderate quality of evidence).
We recommend that FeNO greater than 50 ppb (> 35 ppb in children) be used to indicate that eosinophilic inflammation and, in symptomatic patients, responsiveness to corticosteroids are likely (strong recommendation, moderate quality of evidence).
We recommend that FeNO values between 25 ppb and 50 ppb (20–35 ppb in children) should be interpreted cautiously and with reference to the clinical context. (strong recommendation, low quality of evidence).
We recommend accounting for persistent and/or high allergen exposure as a factor associated with higher levels of FeNO (strong recommendation, moderate quality of evidence).
We recommend the use of FeNO in monitoring airway inflammation in patients with asthma (strong recommendation, low quality of evidence).
We suggest using the following values to determine a significant increase in FeNO: greater than 20% for values over 50 ppb or more than 10 ppb for values lower than 50 ppb from one visit to the next (weak recommendation, low quality of evidence).
We suggest using a reduction of at least 20% in FeNO for values over 50 ppb or more than 10 ppb for values lower than 50 ppb as the cut point to indicate a significant response to antiinflammatory therapy (weak recommendation, low quality of evidence).
Conclusion: Advances in technology and standardization have made FeNO measurement simple, permitting its use as a biomarker that adds a new dimension to the traditional clinical tools in the assessment and management of airways diseases. These guidelines for interpretation of FeNO measurements are meant to enhance their clinical utility, but more work is still needed to better define the use of FeNO in different clinical settings.
Introduction
NO has long been known as an atmospheric pollutant present in vehicle exhaust emissions and cigarette smoke, and more recently its clinical importance as a biological mediator in animals and humans has been recognized (1, 2). NO is present in virtually all mammalian organ systems and is produced by the human lung. It is present in the exhaled breath of all humans (3). NO is recognized to play key roles in virtually all aspects of lung biology and has been implicated in the pathophysiology of lung diseases, including asthma (4). The functions and effects of NO in the lung/airways reflect its key roles as a vasodilator, bronchodilator, neurotransmitter, and inflammatory mediator (3). Patients with asthma have high levels of NO in their exhaled breath and high levels of inducible nitric oxide synthase (NOS2) enzyme expression in the epithelial cells of their airways, suggesting a role for NO in asthma pathogenesis (5). NO is a highly reactive molecule/free radical and may have oxidant properties directly or in the form of the more noxious peroxynitrite. These properties give NO its bactericidal and cytotoxic effects and may participate in host defense by mediating antimicrobial activity and cytotoxicity for tumor cells (4). The exact pathophysiological role of NO in the airways and lungs is complex (4, 6–8). On the one hand, it may act as a proinflammatory mediator predisposing to the development of airway hyperresponsiveness (AHR) (4, 9). On the other, under physiological conditions NO acts as a weak mediator of smooth muscle relaxation, and protects against AHR (4, 10). In exhaled air, NO appears to originate in the airway epithelium (5, 11–15), as a result of NOS2 up-regulation which occurs with inflammation (5, 12, 13, 16). Thus, exhaled NO may be regarded as an indirect marker for up-regulation of airway inflammation.
The field of exhaled NO measurement has developed remarkably over the last 15 years. The use of chemiluminescence analyzers allowed for the detection of NO in exhaled breath in the early 1990s (17). Patients with asthma were found to have high FeNO in their exhaled breath (18–20) that decreased in response to treatment with corticosteroids (21). This quickly prompted the evaluation of FeNO as a potential noninvasive method to diagnose asthma and monitor the response to antiinflammatory therapy.
Advantages for FeNO include the noninvasive nature of the test, ease of repeat measurements, and the relatively easy use in patients with severe airflow obstruction where other techniques are difficult to perform (22). By providing information about airway inflammation (23, 24), FeNO adds a new dimension to the traditional clinical tools (history, physical exam, and lung function tests).
Before FeNO could become useful as a clinical tool, several issues needed to be addressed (25). In particular, the methods and equipment for measuring FeNO needed to be standardized (26, 27). Large population studies were needed to determine effect of confounding factors and provide the normal range or useful cutoff points of FeNO levels (22, 25). Most of these issues have either already been addressed or are currently under investigation, allowing FeNO measurement to make the transition from research into the clinical arena. Last, but not least, interpretative strategies need to be devised and put in place for the different potential uses and applications (28). The purpose of this document is to address this last requirement.
Wherever possible, the recommendations are based on published material, including abstracts, as referenced, but they are supplemented by nonsystematic observations of experts in the field. The guidelines are provided with the clear understanding that this will be a rapidly evolving area and that periodic updating will be required.
Methods
Committee Composition, Meetings, and Document Preparation
The project Chair (R.A.D.) assembled a group of international experts in exhaled nitric oxide. Their expertise was in clinical care, clinical research, or basic science in the area of asthma and/or nitric oxide (five pulmonologists [R.A.D., S.C.E., A.C.O., A.L.P., D.R.T.], an allergist [P.B.B.], two physiologists [C.G.I., J.O.L.], and one pediatric pulmonologist [M.W.L.]). The outline of the Report was proposed by the Chair and modified and agreed upon following input from all Committee members. The Committee was divided into subgroups, each was assigned a specific section, and preliminary drafts were developed. Three face-to-face meetings and nine teleconferences were held. The outline and the drafts were reviewed, and evidence-based recommendations were discussed and finalized by consensus. Committee members disclosed all potential conflicts of interest. All disclosed conflicts of interests were reported to the Chair of the Ethics and Conflict of Interest Committee of the ATS. These were reviewed in detail, and members with perceived conflicts abstained from the discussion of specific questions related to their conflicts of interest. Furthermore, members were reminded to consider their own and other members’ potential conflicts of interest during the meetings. The Chair (R.A.D.) integrated the draft sections and composed the entire document into a preliminary document that was circulated among the committee members for further input. The revised document incorporated the comments and input from all Committee members.
Document Structure
This document is structured to provide an evidence-based review of the current state of knowledge regarding the application and interpretation of FeNO measurements in clinical practice. The recommendations regarding interpretive strategy were organized around specific questions according to the GRADE approach to assessing the quality of the evidence (Summary Table E1 in Appendix in online supplement) (29, 30). Relevant section topics and questions were identified by the Committee. Committee members were asked to review the current evidence by independently completing a pragmatic systematic review of the literature using PubMed and OVID. Each Committee member was asked to assess the identified literature relevant to his/her section, and decide about inclusion of individual articles. MEDLINE searches from 1993 to December 2008 were performed by Committee members, with periodic updates during document development and finalization. Searching the literature before 1993 was not done systematically since the discovery of nitric oxide in asthma was first reported in 1993. The search was augmented by searches of Committee member files. The literature search was limited to all relevant studies including randomized controlled trials, cohort studies, case-control studies, and cross-sectional studies published in the English language. Sections that did not yield specific recommendations were written after a thorough review of the available literature in a narrative review format.
Quality of Evidence and Strength of Recommendations
The quality of evidence was determined according to the ATS GRADE criteria (30). For each question, the Committee graded the quality of the evidence available (high, moderate, low, or very low), and made a recommendation for or against. Recommendations were decided by consensus. Recommendations were either “strong” or “weak.” The strength of a recommendation reflects the extent to which one can, across the range of patients for whom the recommendation is intended, be confident that desirable effects outweigh undesirable effects (30). Consensus on the recommendations was reached among all the members of the Committee. The strength of a recommendation has important implications for patients, clinicians, and policy makers (30).
Strong recommendation.
Patients: Most people in this situation would want the recommended course of action and only a small proportion would not
Clinicians: Most patients should receive the recommended course of action
Policy makers: The recommendation can be adopted as a policy in most situations
Weak recommendation.
Patients: The majority of people in this situation would want the recommended course of action, but many would not
Clinicians: Be more prepared to help patients to make a decision that is consistent with the patient's own values
Policy makers: There is a need for substantial debate and involvement of stakeholders
Why Should a FeNO Test Be Obtained?
Common reasons for measuring FeNO.
To assist in assessing the etiology of respiratory symptoms
To help identify the eosinophilic asthma phenotype
To assess potential response or failure to respond to antiinflammatory agents, notably inhaled corticosteroids (ICS)
To establish a baseline FeNO during clinical stability for subsequent monitoring of chronic persistent asthma
To guide changes in doses of antiinflammatory medications: step-down dosing, step-up dosing, or discontinuation of antiinflammatory medications
To assist in the evaluation of adherence to antiinflammatory medications
To assess whether airway inflammation is contributing to poor asthma control particularly in the presence of other contributors (e.g., rhinosinusitis, anxiety, gastro-esophageal reflux, obesity, or continued allergen exposure).
Can FeNO Be Used to Diagnose Asthma?
Asthma is a clinical diagnosis and there is no single diagnostic test for the disease. The background pathology of asthma is often but not always due to eosinophilic airway inflammation. The two are not synonymous. This is extremely important in the interpretation of FeNO measurements. It is often claimed that FeNO is a diagnostic test for asthma, but in cases of asthma not due to airway eosinophilia, FeNO may be low. Similarly, the value of exhaled FeNO as a predictor of steroid responsiveness is high even in the absence of induced sputum eosinophils (31).
■ Recommendations:
We recommend the use of FeNO in the diagnosis of eosinophilic airway inflammation (strong recommendation, moderate quality of evidence).
We recommend the use of FeNO in determining the likelihood of steroid responsiveness in individuals with chronic respiratory symptoms possibly due to airway inflammation (strong recommendation, low quality of evidence).
We suggest that FeNO may be used to support the diagnosis of asthma in situations in which objective evidence is needed (weak recommendation, moderate quality of evidence).
FeNO is associated with eosinophilic airway inflammation.
There are several inflammatory phenotypes in asthma most commonly described as eosinophilic, neutrophilic, mixed, and paucigranulocytic (32). Determination of the subtype may help a physician decide which therapies to select or stop (33–35). Given the long-established relationship between eosinophilic inflammation and steroid responsiveness in airways disease, the finding that FeNO correlates with eosinophilic inflammation suggests its use as indirect indicator not only of eosinophilic inflammation, but more importantly of the potential for steroid responsiveness (36–42).
There is little evidence directly demonstrating that eosinophilic airway inflammation increases FeNO by increasing NOS2 expression or activity (43). However, eosinophilic airway inflammation may affect FeNO indirectly through NOS2 or via other enzyme pathways. Numerous studies describe the relationship between FeNO and eosinophilic airway inflammation. Eosinophils can be measured in sputum, bronchoalveolar lavage, and biopsies. There are also reports of correlation between FeNO and blood eosinophils (44–46). Warke and coworkers reported that in bronchoalveolar lavage fluid the correlation between eosinophils and FeNO was 0.78 (P < 0.001) (40). Payne and colleagues reported that the correlation between FeNO and eosinophils in bronchial biopsies was 0.54 (P = 0.03) (47), but in contrast Lim and coworkers were unable to find a significant correlation in the biopsies (48). In induced sputum, the correlation between FeNO levels and eosinophils ranges from 0.35 (n = 25, P = 0.09) (36) to 0.48 (n = 35, P = 0.003) (49) to 0.62 (n = 78, P < 0.001) (50). In the largest study to date (n = 566), the correlation was of a similar order (0.59, P < 0.001) (39). In this last study, FeNO of 36 ppb (at a flow rate of 50 ml/s) had a sensitivity and specificity for sputum eosinophilia of more than 3% (the cut point deemed by the authors to be clinically significant) of 78% and 72%, respectively. In the study by Shaw and colleagues, a FeNO of less than 26 ppb had a negative predictive value of 85% for sputum eosinophils less than 3% (51). Similarly, Porsbjerg and coworkers have reported that with FeNO less than 27ppb, it is unlikely that sputum eosinophils will be greater than 1% (52). Thus a low FeNO is of value in determining the absence of eosinophilic, and, by inference, the likely absence of steroid-responsive airway inflammation.
These limited correlations reflect the fact that whereas sputum eosinophilia is always abnormal, exhaled nitric oxide is present even in health with its distribution skewed to the right. It is also necessary to bear in mind that negative and positive predictive values are limited in their generalizablity, given that they depend on the prevalence of the condition in the tested population. Importantly, two studies have shown that the relationship between FeNO levels and airway eosinophilia is independent of the diagnosis of asthma as reported in patients with chronic obstructive pulmonary disease (COPD) (53), and with eosinophilic bronchitis (54). Furthermore, NO and NO metabolites in the airway (e.g., peroxynitrite) alter the REDOX balance in the airways, may cause inflammation, and are in some part steroid sensitive. Thus NO production is to some extent independent of eosinophilic inflammation (4).
FeNO predicts likelihood of corticosteroid responsiveness.
Treatment response in asthma is heterogeneous (55). Not all patients respond to corticosteroids and an important reason to use FeNO is to help decide who might benefit from steroid treatment, and who should try other medications (e.g., leukotriene modifiers). FeNO may also be used to determine patients in whom steroid therapy may be safely withdrawn. FeNO has been shown to predict the likelihood of steroid responsiveness more consistently than spirometry, bronchodilator response, peak flow variation, or AHR to methacholine (56–58). The optimum cut point in the study by Smith and coworkers (56), was 47 ppb, with a negative predictive value of 89% for the change in FEV1 with inhaled steroids. The predictive values were similar for alternative end-points. Even when patients do not demonstrate sputum eosinophilia, FeNO is highly predictive of steroid response (at a cut point of 33 ppb) (31). These data are consistent with studies in which high FeNO (> 47 ppb) predicts the likelihood of loss of control when inhaled steroids are reduced or withdrawn in children with a confirmed diagnosis of asthma (59). Conversely, low FeNO (< 22 ppb) predicts the likelihood of successful reduction or withdrawal of inhaled steroids (positive predictive value, 92%) (60). Again, these outcomes may differ somewhat depending on the target population: for the most part these data are derived from patients with mild to moderate asthma. In summary, depending on the prevalence of eosinophilic airway inflammation in the target population, FeNO measurements may provide a signal that is helpful in identifying patients with asthma-like symptoms who are likely to benefit (or not) from corticosteroid treatment.
FeNO can support a diagnosis of asthma.
The diagnosis of asthma is well defined, and the background pathology is often but not always due to eosinophilic airway inflammation. Early studies in populations comprising mainly patients with eosinophilic asthma explored the performance characteristics of FeNO as a diagnostic test. The predictive values for FeNO (usually at cut points of > 25 ppb) were shown to be sufficiently robust for it to be used in this context (23, 61, 62). Further, the predictive values for FeNO are higher than for conventional measurements such as peak flows and spirometry (23), and similar to those associated with bronchial challenge tests (62). However, in general, in patients presenting with variable cough, wheeze, and shortness of breath, an increased FeNO provides supportive rather than conclusive evidence for an asthma diagnosis. As stated, the limitations to the diagnostic role of FeNO arise principally because airway inflammation in asthma is heterogeneous and is not always associated with increased FeNO (e.g., neutrophilic airway inflammation). Similarly, in patients who have already been treated with inhaled steroids, the test may be falsely negative. Thus, the importance of FeNO lies in its potential to identify steroid responsiveness, rather than the exact clinical diagnosis. This information is much more clinically relevant because it enables the clinician to bypass an empiric “trial of steroids” or unnecessary long-term corticosteroid treatment.
FeNO may predict AHR.
Irrespective of the specific underlying inflammatory signal which FeNO represents, measurements appear to reflect the dynamic interrelationships between the response to allergen or other triggers and evolving eosinophilic airway inflammation/AHR (4, 7, 8, 63). Serial FeNO levels increase progressively in response to allergen exposure and the advent of airway symptoms (63). Because of the practical difficulties involved in measuring AHR, especially in children, it was initially thought that FeNO might be used as a surrogate marker for AHR. The relationship between NO metabolism and AHR in asthma is complex (64). When FeNO was used to predict the presence of AHR, the studies reveal inconsistent relationships and correlations are generally low. The clinical interpretation of FeNO in relation to AHR is even more problematic in subjects who are taking ICS (9, 65) and with long-standing as opposed to recently developed asthma (66). This is demonstrated in studies designed to evaluate pathophysiological relationships in clinical asthma using factor analysis: AHR, airway inflammation, and FeNO belong to different domains (66–68). However, in one study FeNO has been used as a surrogate for AHR testing to support the diagnosis of asthma in children, and the data appear to support its use in this limited context (62).
Is There a Normal FeNO Value?
This section will discuss the normal ranges of FeNO. We will also discuss the important clinical cut points and the rationale for selecting these cut points to be used in the interpretation of an elevated or reduced FeNO value. It is important to choose the appropriate cut point in relation to the clinical setting and question. While this section and the accompanying tables (see Tables 3–5) focus on asthma and airway diseases/inflammation, other causes of high and low FeNO levels are listed in the Appendix in the online supplement.
TABLE 3.
Low FeNO (< 25 ppb [< 20 ppb IN CHILDREN]): IMPLIES NONEOSINOPHILIC OR NO AIRWAY INFLAMMATION*
| Diagnosis |
| In a symptomatic patient (chronic cough and/or wheeze and/or shortness of breath for > 6 wk) presenting for the first time, the patient is unlikely to benefit from a trial of inhaled corticosteroid treatment, possible etiologies: |
| Other pulmonary/airway causes: |
| Rhinosinusitis |
| Noneosinophilic asthma |
| Reactive airways dysfunction syndrome |
| COPD |
| Bronchiectasis |
| Cystic fibrosis, primary ciliary dyskinesia |
| Extended post-viral bronchial hyperresponsiveness syndrome |
| Vocal cord dysfunction |
| Nonpulmonary/airway causes: |
| Anxiety-hyperventilation |
| Gastroesophageal reflux disease |
| Cardiac disease/pulmonary hypertension/pulmonary embolism |
| Confounding factors: |
| Smoking |
| Obesity |
| Monitoring |
| In a symptomatic patient with an established diagnosis of asthma, possible etiologies: |
| Asthma: |
| Noneosinophilic asthma (probably steroid unresponsive) |
| Additional or alternative diagnosis? |
| Vocal cord dysfunction |
| Anxiety-hyperventilation |
| Bronchiectasis, |
| Cardiac disease |
| Rhinosinusitis, |
| Gastroesophageal reflux disease |
| In an asymptomatic patient with an established diagnosis of asthma: |
| Implies adequate dosing and good adherence to antiinflammatory therapy |
| Inhaled corticosteroid dose may possibly be reduced (repeat FeNO 4 wk later to confirm this judgment; if it remains low then relapse is unlikely). |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; FeNO = fraction of exhaled nitric oxide.
The interpretation of FeNO is an adjunct measure to history, physical exam, and lung function assessment.
For intermediate FeNO levels (in the range 25–50 ppb [20–35 ppb in children]), refer to Table 5.
TABLE 4.
HIGH FeNO (> 50 ppb [> 35 ppb IN CHILDREN]) OR RISING FeNO (> 40% CHANGE FROM PREVIOUSLY STABLE LEVELS): Implies uncontrolled or deteriorating eosinophilic airway inflammation*
| Diagnosis |
| In a symptomatic patient (chronic cough and/or wheeze and/or shortness of breath during past > 6 wk) presenting for the first time, possible etiologies: |
| Atopic asthma |
| Eosinophilic bronchitis |
| COPD with mixed inflammatory phenotype |
| That the patient is likely to benefit from a trial of inhaled corticosteroid treatment |
| Monitoring |
| In a symptomatic patient with an established diagnosis of asthma, possible etiologies: |
| High persistent allergen exposure |
| Inhaled corticosteroid delivery problems: |
| Poor adherence |
| Poor inhaler technique |
| Proximal drug deposition, with untreated distal airway/alveolar inflammation |
| Inadequate inhaled corticosteroid dose: |
| Likely to respond to increased inhaled corticosteroid dose OR prednisone |
| Rarely: truly steroid resistant asthma (a trial of systemic steroid will confirm this: FeNO will remain high |
| Rarely: Churg Strauss syndrome, pulmonary eosinophilia |
| In an asymptomatic patient: |
| No change in inhaled corticosteroid dosing, but refer to FeNO trend over time in individual patient |
| Withdrawing inhaled corticosteroid is likely to be followed by relapse |
| An increase in therapy is indicated as some patients are asymptomatic, but the high FeNO could be a risk factor for an upcoming exacerbation. |
| “High” FENO may be normal in a certain percent of the population (Figure 1). |
Definition of abbreviation: FeNO = fraction of exhaled nitric oxide.
The interpretation of FeNO is an adjunct measure to history, physical exam, and lung function assessment.
For intermediate FeNO (levels in the range 25–50 ppb [20–35 ppb in children]), refer to Table 5.
TABLE 5.
GENERAL OUTLINE FOR FeNO INTERPRETATION: SYMPTOMS REFER TO COUGH AND/OR WHEEZE AND/OR SHORTNESS OF BREATH*
| FeNO < 25ppb | FeNO 25–50 ppb | FeNO > 50 ppb | |
| (<20 ppb in children) | (20–35 ppb in children) | (>35 ppb in children) | |
| Diagnosis | |||
| Symptoms present during past 6+ wk | Eosinophilic airway inflammation unlikely | Be cautiousEvaluate clinical context | Eosinophilic airway inflammation present |
| Alternative diagnoses | Monitor change in FeNO over time | Likely to benefit from ICS | |
| Unlikely to benefit from ICS | |||
| Monitoring (in Patients with Diagnosed Asthma) | |||
| Symptoms present | Possible alternative diagnoses | Persistent allergen exposure | Persistent allergen exposure |
| Unlikely to benefit from increase in ICS | Inadequate ICS dosePoor adherence | Poor adherence or inhaler techniqueInadequate ICS dose | |
| Steroid resistance | Risk for exacerbation | ||
| Steroid resistance | |||
| Symptoms absent | Adequate ICS doseGood adherenceICS taper | Adequate ICS dosingGood adherenceMonitor change in FeNO | ICS withdrawal or dose reduction may result in relapsePoor adherence or inhaler technique |
■ Recommendations:
We suggest the use of cut points rather than reference values when interpreting FeNO levels (weak recommendation, low quality of evidence).
We recommend accounting for age as a factor affecting FeNO in children younger than 12 years of age (strong recommendation, high quality of evidence).
Normal Values versus Relevant Cut Points for FeNO
This section will discuss the normal ranges of FeNO and what are the important clinical cut points to be used in the interpretation of an elevated or reduced FeNO value. It is unlikely that reference values derived from a “normal” population will be as helpful as cut points in patients with airways disease or respiratory symptoms. The distribution of FeNO in an unselected population is skewed to the right (see Figure 1). Even when individuals with atopy or diagnosed asthma are excluded, the upper limit of “normal” ranges from 27 to 57 ppb depending on sex (69). This overlaps with the range of values obtained in populations with asthma in relation to sputum eosinophilia (see Figure 1). In a clinical study, Shaw and colleagues reported that the optimum cut point for a clinically significant FeNO (corresponding to a sputum eosinophil count of ≥ 2%) was 26 ppb (51). Similarly, studies designed to determine the optimum cut point to diagnose asthma using FeNO have usually pointed to a diagnostic cut point ranging from 20 to 25 ppb (23, 70–72). However, in patients with stable, well-controlled asthma, FeNO values range from 22 to 44 ppb (73). Clearly, there is considerable overlap between mean FeNO levels in healthy and populations with stable asthma. This is illustrated in Figure 2.
Figure 1.
Schematic representation of the distribution of FeNO levels in an unselected population of 2,200 male and female subjects. The median value was 16.0 ppb with a range of 2.4 to 199 ppb. The cut point of 26 ppb is the optimum cut point for significant sputum eosinophilia, indicating that up to 20% of individuals with an FeNO greater than 25 ppb may not necessarily have sputum eosinophilia, and that the clinical context requires to be taken into account. The data used to prepare this composite figure were obtained from Shaw and colleagues (51) and Olin and colleagues (73) after consultation with the authors.
Figure 2.
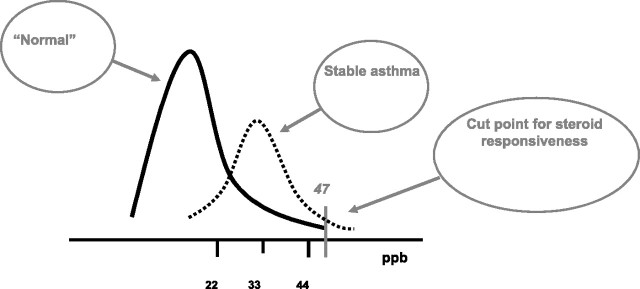
An amplification of Figure 1 in which the distribution of FeNO in stable asthma is depicted as a dotted line. Taken from Olin and colleagues (73). In that study, the 95% confidence intervals for FeNO in stable asthma was reported to be 22 to 44 ppb. The cut point of 47 ppb is the optimum cut point for steroid responsiveness in patients with nonspecific respiratory symptoms. The other data used to prepare this composite figure were obtained from Smith and colleagues (56) after consultation with the authors.
Confounding factors that may affect FeNO.
As discussed in the Appendix in the online supplement, FeNO values can be affected by several factors, including measurement technique, exhalation flow rate, nasal NO contamination, the NO analyzer used (74), age, height, smoking, and antiinflammatory medications. A number of recent publications have reported reference values for FeNO in adults (69, 75–79) (Table 1) and children (76, 80–83). There are important differences between these studies with regard to the size of the examined population, as well as the range of statistical variables that have been included or excluded, limiting their value (76, 77, 80–83). Factors affecting population FeNO levels may be due to one or more variables including genetics, age, sex, atopy, weight and height, current smoking, and diet. The importance of current smoking and atopic status is generally agreed upon (28), but there are inconsistencies between the studies regarding which other factors ought to be accounted for when deriving and applying reference values (Table 1). More detailed information on these biological sources of variability is provided in the Appendix in the online supplement.
TABLE 1.
STUDIES OF ONLINE FRACTION OF EXHALED NITRIC OXIDE VALUES AT EXHALATION FLOW RATE OF 50 ml/s IN HEALTHY SUBJECTS
| Author and Reference | N | Groups for which Reference Values Are Given | “Normal Values” (ppb) | Analyzer |
| Kharitonov 2003 (75) | 59 | Mixed population of adults and children | Mean 16.3 ppb, ULN 33. | NIOX (Aerocrine AB, Stockholm, Sweden) |
| Buchvald 2005 (76) | 405 | Children aged 4–17 yr | Mean 9.7 ppb, | NIOX (Aerocrine AB, Stockholm, Sweden) |
| Data also available by age stratification | Upper 95% CI: 25.2 | |||
| Olivieri 2006 (77) | 204 | Male, nonsmoker, nonasthmatic | 4.5–20.6 | (CLD88, Ecomedics, Switzerland) |
| Female, nonsmoker, nonasthmatic | 3.6–18.2 | |||
| (note: atopy not considered) | (note: values quoted are 5th and 95th centiles) | |||
| Olin 2007 (69) | 3,376 | Random population | See Table 2 | NIOX (Aerocrine AB, Stockholm, Sweden) |
| 1,131 never-smoking subjects not reporting any asthma symptom, dry cough or the use of inhaled corticosteroids | ||||
| Travers 2007 (78) | 3,500 | Male, nonsmoker, nonatopic | 9.5–47.4 | NIOX (Aerocrine AB, Stockholm, Sweden) |
| Male, nonsmoker, atopic | 11.2–56.5 | |||
| Male, smoker, nonatopic | 7.5–38.4 | |||
| Male, smoker, atopic | 8.8–45.9 | |||
| Female, nonsmoker, nonatopic | 7.5–37.4 | |||
| Female, nonsmoker, atopic | 8.8–44.6 | |||
| Female, smoker, nonatopic | 5.9–30.5 | |||
| Female, smoker, atopic | 6.9–36.4 | |||
| (note: values quoted are 90% confidence interval) | ||||
| Dressel 2008 (79) | 897 | Male, nonsmoker, nonatopic, 165 cm | 19.5 | (NOA 280, Sievers, Boulder, CO) |
| Male, nonsmoker, atopic, 165 cm | 29.1 | |||
| Male, smoker, nonatopic, 165 cm | 12.2 | |||
| Male, smoker, atopic, 165 cm | 18.3 | |||
| Female, nonsmoker, nonatopic, 160 cm | 15.7 | |||
| Female, nonsmoker, atopic, 160 cm | 23.5 | |||
| Female, smoker, nonatopic, 160 cm | 9.9 | |||
| Female, smoker, atopic, 160 cm | 14.7 |
Age seems to be important in children (81), but there is less agreement across the studies regarding age in adults, sex, and height. In the largest study to date, Olin and coworkers identified the importance of age and height as factors affecting FeNO, but did not find any differences between males and females (69). In contrast, Travers and colleagues (78) and Taylor and coworkers (84) reported consistently higher levels in males. The magnitude of the effect of the patient-related factors alone or in combination is potentially clinically significant. This is demonstrated in Table 2 (data from Reference 69).
TABLE 2.
FRACTION OF EXHALED NITRIC OXIDE 95% UPPER LIMITS, STRATIFIED FOR SEX AND ATOPY, ACCORDING TO HEIGHT AND AGE AMONG 1,131 HEALTHY LIFELONG NEVER-SMOKING SUBJECTS
| Height (cm) | Age 25–49 yr |
Age 50–75 yr |
||
| Women | Men | Women | Men | |
| Subjects without Atopy (n = 845) | ||||
| 150–159 | 25 | 27 | 34 | 32 |
| 160–169 | 26 | 30 | 36 | 35 |
| 170–179 | 28 | 33 | 39 | 39 |
| 180–189 | 30 | 37 | 41 | 44 |
| 190–199 | — | 42 | — | 49 |
| Subjects with Atopy (n = 286) | ||||
| 150–159 | 30 | 58 | 37 | 65 |
| 160–169 | 36 | 63 | 45 | 63 |
| 170–179 | 43 | 54 | 53 | 62 |
| 180–189 | 51 | 50 | 64 | 57 |
| 190–199 | — | 50 | — | 56 |
Data taken from Reference 69.
Thus, in our present state of knowledge the problems of multiple confounding factors and overlap between normal populations and populations with asthma preclude the routine application of reference values in the clinical setting. The Committee felt that it is more relevant to identify clinically meaningful cut points rather than reference values to interpret FeNO levels as outlined below, keeping in mind that very few of these cut points are well validated. At any one time, however, the most important consideration is whether or not the patient has current respiratory symptoms or a prior diagnosis of airways disease; that is, the interpretation of FeNO levels should be determined in individual patients with reference to the context in which the measurement is being obtained.
What Are the Clinically Significant Cut Points for FeNO?
It is important to choose the appropriate cut point in relation to the clinical setting and question. In this section, we discuss the rationale for selecting these cut points (see Tables 3–5). While this section and the accompanying tables focus on asthma and airway diseases/inflammation, other causes of high and low FeNO levels are listed in the Appendix in the online supplement.
■ Recommendations:
We recommend that low FeNO (< 25 ppb [< 20 ppb in children]) be used to indicate that eosinophilic inflammation and responsiveness to corticosteroids are less likely (strong recommendation, moderate quality of evidence).
We recommend that FeNO > 50ppb (> 35 ppb in children) be used to indicate that eosinophilic inflammation and, in symptomatic patients, responsiveness to corticosteroids are likely (strong recommendation, moderate quality of evidence).
We recommend that FeNO values between 25 ppb and 50 ppb (20–35 ppb in children) should be interpreted cautiously with reference to the clinical context (strong recommendation, low quality of evidence).
Low FeNO (< 25 ppb in adults; 20 ppb in children).
In a symptomatic adult patient with a FeNO of less than 25 ppb (20 ppb in children), eosinophilic airway inflammation is unlikely. This cut point is based on evidence from a number of sources including the study by Shaw and colleagues (51) and Porsbjerg and coworkers (52), studies investigating the role of FeNO measurements to diagnose asthma (23, 70–72), and studies designed to optimize ICS use (56, 60). The differential diagnosis for symptomatic patients with a low FeNO is given in Table 3. In patients presenting with nonspecific respiratory symptoms, low FeNO suggests alternative diagnoses which are not amenable to an increase in inhaled or oral steroid therapy.
High FeNO (> 50 ppb in adults, 35 ppb in children).
High FeNO is likely to indicate significant airway eosinophilia. It is also likely to indicate that a symptomatic patient has steroid-responsive airways inflammation (56, 57, 85, 86). The clinically significant cut point of 50 ppb is based on the results of pragmatic studies. However, this is a general guide and may vary slightly in individual patients. Symptomatic steroid-naïve patients with high FeNO are more likely to exhibit responsiveness to inhaled steroid therapy, irrespective of the diagnostic label (e.g., asthma or nonasthma), with an optimum cut point of 47 ppb (56). In asymptomatic patients with stable asthma, the likelihood of relapse following withdrawal of ICS therapy is greatest in patients whose FeNO increases to above 49 ppb during the 4 weeks after steroid withdrawal (59). The differential diagnosis for high FeNO is shown in Table 4.
Intermediate FeNO (between 25 ppb and 50 ppb in adults; 20–35 ppb in children).
The above data indicate that for FeNO values between 25 and 50 ppb, cautious interpretation is required. The weight placed on an FeNO result within this range will depend on whether the test is being used diagnostically in a symptomatic steroid-naïve subject, or whether the patient's FeNO has increased or decreased from a previous value by what is deemed to be a clinically significant amount in a patient who is being monitored over time.
Recommendation
We recommend accounting for persistent and/or high allergen exposure as a factor associated with higher levels of FeNO (strong recommendation, moderate quality of evidence).
Persistently high FeNO (> 50 ppb in adults, 35 ppb in children).
In a patient with ongoing asthma, symptoms may occur despite apparently adequate antiinflammatory treatment (87). In the collective experience of the Committee, a common cause of persistently high FeNO is poor adherence to ICS therapy. Other explanations could be poor inhaled drug delivery or continued exposure to allergen (7, 8).
Continuing or increasing exposure to aeroallergens to which a patient is sensitized may result in a rise in FeNO, or the persistence of an elevated FeNO. The magnitude of the effect may be sufficient for FeNO levels to increase beyond the cut point of 50 ppb, and in some patients may occur even in the absence of respiratory symptoms (88–91). More recent evidence suggests that persistent high FeNO in corticosteroid-treated individuals with asthma may also reflect a highly reactive asthma phenotype, and such patients need to be managed with caution (35). However, if the patient is asymptomatic and has a high FeNO, then no change in treatment is required. There is a small group of patients whose FeNO remains high despite good asthma control. This probably results from the fact that more than one factor (i.e., not just eosinophilic airway inflammation) is responsible for the elevated FeNO. Another explanation may be that the high exhaled NO is derived from constitutive NOS sources which are steroid insensitive. Thus, levels greater than 50 ppb in a well-treated asymptomatic patient may be “normal” for that specific patient.
Can FeNO Be Used to Monitor Airway Inflammation?
The change in FeNO value following corticosteroid intervention may be more valid than the absolute FeNO value. The definition of a clinically significant change in FeNO, however, remains to be established.
■ Recommendations
We recommend the use of FeNO in monitoring airway inflammation in patients with asthma (strong recommendation, low quality of evidence).
We suggest using the following values to determine a significant increase in FeNO: greater than 20% for values over 50 ppb or more than 10 ppb for values lower than 50 ppb from one visit to the next (weak recommendation, low quality of evidence).
We suggest using a reduction of at least 20% in FeNO for values over 50 ppb or more than 10 ppb for values lower than 50 ppb as the cut point to indicate a significant response to antiinflammatory therapy (weak recommendation, low quality of evidence).
Monitoring Airway Inflammation in Asthma
Serial measurements obtained when patients’ asthma is both stable and unstable allows each patient to act as his/her own control when assessing subsequent measurements and as a result “personal best” can be used (92). The same cut points used in detecting airway inflammation apply when monitoring patients with asthma. In asymptomatic individuals, including patients with well-controlled asthma, low FeNO suggests that ICS dose could be reduced or even that ICS treatment may be withdrawn altogether. In a study of children with stable asthma, withdrawal of ICS did not result in symptom relapse when FeNO remained consistently low (optimum cut point 22 ppb) when measured 2 to 4 weeks after treatment withdrawal (60). In symptomatic patients with low FeNO, strategies other than increasing the ICS dose should be pursued. Thus, FeNO values which are either high or low are informative as to the etiology of current symptoms particularly in patients with difficult asthma. Sequential measurements may be important in determining trends. The relatively rapid change in FeNO in response to ICS is thought to add to its utility in monitoring adherence to and response to such therapy (93). However, as a predictor of asthma control, FeNO is no better than more conventional lung function tests (51, 87, 94, 95). The predictive values of a single measurement of FeNO for loss of asthma control are insufficiently sensitive or specific to justify its use for this specific purpose (51, 94, 95).
Minimally Important Differences, and Prognostic Significance of FeNO
The within-subject coefficient of variation for FeNO in healthy subjects is approximately 10%, or up to 4 ppb (75, 96). The variation increases to approximately 20% in patients with asthma (75, 96, 97). Since a change of 20% could be due to the variation in the FeNO measurement, the Committee recommends a change of at least 20% to indicate a significant rise or fall in FeNO over time or following an intervention. However, there are very few data that clarify what constitutes a clinically important change in individual patients. In one study, FeNO levels were 50% higher during acute asthma compared with when stability was restored (98). Data obtained from steroid withdrawal studies show that the mean increase in FeNO associated with the advent of loss of control ranges from 16 ppb (99) to 25 ppb (50), the latter representing a 60% increase from baseline. However, the range of the increase in FeNO between stability and loss of control is high (up to 141 ppb) (50). More recently, Michils and colleagues have reported that the transition from good control to poorly controlled asthma is likely to be associated with a rise in FeNO of 40% or greater (100). An acute rise (over 12–24 h) in FeNO may occur after infection or exposure to an allergen to which the patient is sensitized. The magnitude of the rise may be as high as 150 ppb. Ideally, one would wish that a minimally important change in FeNO to a level that is above or below a particular cut point would provide justification for a specific interpretation. Unfortunately, there are insufficient data to recommend this approach. Rather, the current FeNO level, the direction and magnitude of any recent change, and where the measured level sits in relation to the cut points for “high” or “low” values need to be taken into account.
Randomized trials designed to assess whether asthma outcomes are improved using regular FeNO measurements as the basis for adjusting the dose of ICS therapy have failed to show important benefits (51, 87, 95, 101, 102), although in one study ICS dose reduction was facilitated without compromising asthma control (103). Thus in general, FeNO measurements cannot be recommended for this purpose. A recent systematic assessment of published randomized trials of asthma therapy guided by FeNO concluded that the mixed results of these studies (the ASTRAL studies, an acronym for ASthma randomized TReatment ALgorighm studies) were due to specific design and methodological issues that may have led to incorrect conclusions (104). In his summary, Gibson highlights the following problems: (1) the dose–response relationship of the drugs used in relation to the outcomes measured; (2) the effects of adherence and nonadherence; (3) the algorithms used and their agreement with clinical decision making; (4) the selection of FeNO cut points/decision points. Gibson states that future studies would require the use of an additional metric to assess the likelihood that any two algorithms (conventional and biomarker-guided) will give different ICS dosing decisions (104). In a more recent study, investigators aimed to evaluate the accuracy of baseline FeNO to recognize individuals with difficult-to-treat asthma who have the potential to achieve control with a guideline-based stepwise strategy (105). One hundred two consecutive patients with suboptimal asthma control underwent stepwise increase in the treatment with maximal inhaled corticosteroids for 1 month. Then, those who remained uncontrolled received oral corticosteroids for an additional month. With this approach, 53 patients (52%) gained control. A FeNO cut point greater than or equal to 30 ppb demonstrated a sensitivity of 88% and a specificity of 91% for the identification of responsive individuals with asthma, and a value less than or equal to 30ppb had a negative predictive value for steroid response of 92% (105). Thus, incorporating optimal design features into future FeNO studies should help in obtaining a better estimate of the value of FeNO-guided asthma therapy (104). Otherwise, a study is unlikely to detect a positive result in favor of one decision-making algorithm versus the other, even if one truly exists.
How Should a FeNO Measurement Be Interpreted and Reported?
1. Assure proper methodology: follow ATS/ERS guidelines.
ATS/ERS guidelines for the measurement of FeNO have been published and are the current standard (26, 27). These guidelines should be followed carefully to obtain accurate and reproducible measurements. These guidelines should be used in conjunction with FDA-approved instructions for the use of specific nitric oxide analyzers. As additional instruments using different technologies to measure FeNO become available, these guidelines as well as the scope of FDA endorsements are likely to change.
2. Determine the reason for the test and the type of subject being tested: does the patient have asthma-like symptoms OR an already established diagnosis of asthma?
The interpretation of FeNO begins with whether a patient's symptoms are nonspecific and as yet undiagnosed, or whether they have a confirmed diagnosis of asthma. This upfront distinction between the diagnostic and monitoring uses of FeNO allows for a more appropriate interpretation of the results as outlined in Table 5. Other factors to take into account include whether the subject is a smoker or is on antiinflammatory medications, as well as his/her height and age.
3. Interpretation of FeNO measurement: clinically relevant cut points.
The purpose of measuring FeNO is to determine whether the value is within normal limits, high, or low. In addition, when monitoring over time, one must be able to determine when a significant change (increase) has taken place. After correct measurement, and with reference to factors which may be affecting the measurement (e.g., current smoking). Interpretation can be made as follows (see also Table 5):
< 25ppb (< 20ppb in children): eosinophilic inflammation and responsiveness to ICS (post-bronchodilator FEV1) are unlikely.
> 50ppb (> 35ppb in children): eosinophilic inflammation is likely; responsiveness to ICS (post-bronchodilator FEV1) is likely.
Values between 25ppb and 50ppb (20–35ppb in children) must be interpreted cautiously with reference to the clinical context.
An increase of > 20% and more than 25ppb (20ppb in children) may be significant but there are wide inter-individual differences.
A minimally important decrease of the FENO value is defined as a difference larger than 20% for values over 50ppb or more than 10ppb for values lower than 50ppb from one visit to the next. A reduction of an elevated FENO of more than 20% that often occurs 2–6 wk after initiation of anti-inflammatory therapy supports that the treatment was successful in reduction of inflammation.
4. Minimum reporting requirements for FeNO.
When reporting FeNO results, a minimum information set should be included. This should include but not be limited to: date, time of the day, age, sex, ethnicity, height, smoking status, reason for the test, and prior diagnosis (if known), and whether or not the patient was using inhaled or oral corticosteroids at the time of testing. The format of the reporting should include the device used to make the measurement, the number of measurements made, and the flow rate (currently approved FDA devices use 50 ml/s flow rate). One can choose to include all measurements performed or just the mean value. Results of previous testing (if available) should be included. A listing of the relevant cut point values is usually helpful.
Other Situations in which FeNO May Be Useful
These are emerging areas for the use of FeNO in the clinical setting, but there is not enough literature to provide specific guidelines for their application (106).
COPD.
The exact role of exhaled nitric oxide measurements in patients with established COPD remains to be defined. In a significant number of patients, an overlap syndrome comprising features of both asthma and COPD is found (53). The airway inflammatory cell infiltrate may be mixed, including eosinophilic inflammation. Studies show that, at least in the short term, the response to corticosteroids is likely to be greater in patients with COPD who also have sputum eosinophilia (107, 108) or elevated FeNO (109). This raises the possibility that FeNO measurements might be used in predicting steroid responsiveness in COPD. In a small group of 19 patients, Zietkowski and coworkers reported a significant correlation between baseline FeNO and ΔFEV1 after 2 months with inhaled budesonide 800μg/day (108). de Laurentiis and colleagues (110) reported greater FeNO variability in patients with COPD who subsequently develop exacerbations. More recently baseline FeNO was found to be a predictor for changes in airflow obstruction, but not improvements in functional exercise capacity or health-related quality of life, with corticosteroid therapy (56). There is also some early evidence that a raised FeNO predicts FEV1 response to ICS in COPD (111, 112).
Pulmonary hypertension.
NO is one of the important pathophysiological mediators of pulmonary hypertension (113, 114). It is important to point out, however, that while NO is the most recognized product of NOS, it is not the only one and an activity that is inhibited by NOS inhibition is not necessarily caused by NO (115–119). In the case of pulmonary hypertension for example, NO concentrations 1,000 times higher than those produced by NOS endogenously (normally present in the airways) are required for therapy, and pulmonary hypertension can be treated by nitrogen oxides such as ethyl nitrite that do not produce any nitric oxide at all (119). Thus in this sphere, we use NO to refer to NOS activity, recognizing that NO is a biomarker for NOS activity without always being the effector molecule. In addition to vasodilatation, NO regulates endothelial cell proliferation and angiogenesis, and maintains overall vascular health (121, 122). Interestingly, patients with pulmonary hypertension have low levels of FeNO (123). Although this is a far more complex issue than the simple lack of a vasodilator (124), giving NO therapeutically seems to work well (125). Therapies that target the NO pathway have revolutionized the treatment of this disease, including the widely used phosphodiesterase type 5 (PDE5) inhibitors, which prevent the breakdown of the NO effector molecule 3′,5′-cyclic guanosine monophosphase (cGMP), thus prolonging NO effects on tissues (122). The NO deficiency state in patients with pulmonary hypertension also improves with other therapies that do not directly target the NO pathway like prostacyclins and endothelin receptor antagonists (125, 126). This seems also to have a prognostic significance, with improved survival in patients who respond to therapy with higher FeNO levels compared with those who do not change their FeNO levels in response to therapy (127). The low FeNO levels in patients with pulmonary hypertension and the improvement with effective therapies suggest that monitoring NO levels over time may be a useful noninvasive marker to evaluate response to or failure of medical therapy in these patients (127).
Cystic fibrosis and nasal NO measurements.
Continuous and high production of NO takes place in the human nose and paranasal sinuses (128, 129), and this NO is readily measurable by noninvasive techniques (130). It has been shown that the nasal NO levels are altered in several respiratory disorders—including primary ciliary dyskinesia (PCD) (129), cystic fibrosis (CF) (131, 132), and allergic rhinitis (133, 134), and this has led to the proposal that nasal NO may be clinically useful in diagnosis and monitoring of these diseases. The levels of nasal NO are uniformly extremely low in patients with PCD, and the sensitivity and specificity of the test in this setting is excellent (135–139). The low levels of NO in CF are related to the absence of NOS2 expression in the airway epithelium, which supports the concept of NOS2 contribution to much of the NO detectable in exhaled breath (140–142). There is now abundant evidence that NO levels in CF are affected by a variety of other pathways as well. In addition to NOS2, determinants of exhaled NO in CF include arginase activity (143), superoxide levels (144), S-nitrosothiol metabolism (145), and denitrification pathways/prokaryotic nitrogen oxide metabolism (146, 147). Thus, these various determinants are all important when it comes to clinical interpretation FeNO in CF. As such, response to arginine, response to antioxidants, response to inhaled nitrosothiols, and response to antimicrobial therapy might potentially be monitored in CF, to some extent, by monitoring FeNO. Although FeNO is low in PCD, the diagnostic accuracy is considerably greater for a nasal NO test. Therefore this test is attractive for screening for PCD, prior to confirmatory testing (e.g., biopsies with analysis of ciliary structure). In contrast to FeNO, a single standardized procedure has not yet been defined for measuring nasal NO. Until this has been agreed upon, nasal NO levels are not yet recommended in routine clinical practice.
In summary, the use of FeNO in COPD and pulmonary hypertension and the use of nasal NO in diagnosis and monitoring of other respiratory disorders (e.g., allergic rhinitis, sinusitis, nasal polyposis, CF) are potentially of interest, but more research is needed before we know how clinically useful these tests can be for these disorders.
Conclusions and Future Directions
Advances in technology and standardization have made FeNO measurements simple, permitting their use as a biomarker in the assessment of inflammatory airways diseases. It is widely acknowledged that asthma is a heterogeneous disease with a variety of underlying pathophysiological abnormalities. FeNO plays a role in identifying these different phenotypes (4, 7, 8, 34, 35, 148). Measurements are easily performed in different settings and may be used in diagnosis and monitoring. Large population studies have identified various confounders that affect FeNO including age, sex, and height, among others. Consistent observations indicate that atopic individuals have higher FeNO levels while smokers tend to have lower FeNO levels (69, 76–78). Reference values have been derived from large population studies, but in practice they have limited application. Rather, evidence-based cut points that are shown to have diagnostic significance appear to be more relevant. When monitoring individual patients with asthma and assessing their treatment requirements, achieving “personal best” rather than “normal” values is more helpful. In many patients, changes in FeNO in relation to a baseline when clinically stable may be even more relevant. FeNO values of themselves do not justify a diagnosis or change in treatment. Rather, they need to be interpreted in relation to the clinical context as discussed in this Guideline. They may be particularly useful in understanding patients with asthma in whom more than one factor is contributing to respiratory symptoms (e.g., obesity, anxiety) and for whom clinical decision making is difficult. Another potential use of FeNO might be during inhalation challenge testing. That is, as with spirometry, giving an allergen inhalation challenge while measuring changes in FeNO before and after the challenge. This may be potentially useful in the assessment of occupational asthma (149, 150).
Although these guidelines for interpretation of FeNO measurements will enhance their clinical utility, we need to continue to investigate how to interpret FeNO measurements in different clinical settings. Inclusion of FeNO as an endpoint in clinical trials would be very helpful in understanding the role of FeNO in monitoring response to therapy (151). Furthermore, FeNO measurement in large population-based studies like the National Health and Nutrition Examination Survey (NHANES) would provide more information on normative values (152). Thus, the guidelines provided here will need to be periodically updated with regard to new developments in this rapidly evolving field.
Acknowledgments
This official Clinical Practice Guideline was prepared by an ad hoc committee of the Assembly on Allergy, Immunology and Inflammation (AII).
Members of the Committee:
Raed A. Dweik (Chair), M.D.
Peter B. Boggs, M.D.
Serpil C. Erzurum, M.D.
Charles G. Irvin, M.D.
Margaret W. Leigh, M.D.
Jon O. Lundberg, Ph.D.
Anna-Carin Olin, Ph.D.
Alan L. Plummer, M.D.
D. Robin Taylor, M.D., D.Sc.
Footnotes
References
- 1.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 1987;84:9265–9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988;333:664–666. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell 1994;78:915–918. [DOI] [PubMed] [Google Scholar]
- 4.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA 2001;98:2622–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo FH, Comhair SA, Zheng S, Dweik RA, Eissa NT, Thomassen MJ, Calhoun W, Erzurum SC. Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol 2000;164:5970–5980. [DOI] [PubMed] [Google Scholar]
- 6.Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax 2003;58:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khatri SB, Hammel J, Kavuru MS, Erzurum SC, Dweik RA. Temporal association of nitric oxide levels and airflow in asthma after whole lung allergen challenge. J Appl Physiol 2003;95:436–440, discussion 435. [DOI] [PubMed] [Google Scholar]
- 8.Khatri SB, Ozkan M, McCarthy K, Laskowski D, Hammel J, Dweik RA, Erzurum SC. Alterations in exhaled gas profile during allergen-induced asthmatic response. Am J Respir Crit Care Med 2001;164:1844–1848. [DOI] [PubMed] [Google Scholar]
- 9.Reid DW, Johns DP, Feltis B, Ward C, Walters EH. Exhaled nitric oxide continues to reflect airway hyperresponsiveness and disease activity in inhaled corticosteroid-treated adult asthmatic patients. Respirology 2003;8:479–486. [DOI] [PubMed] [Google Scholar]
- 10.De Sanctis GT, MacLean JA, Hamada K, Mehta S, Scott JA, Jiao A, Yandava CN, Kobzik L, Wolyniec WW, Fabian AJ, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med 1999;189:1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane C, Knight D, Burgess S, Franklin P, Horak F, Legg J, Moeller A, Stick S. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax 2004;59:757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA 1995;92:7809–7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo FH, Erzurum SC. Characterization of inducible nitric oxide synthase expression in human airway epithelium. Environ Health Perspect 1998;106:1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dweik RA, Erzurum SC. Regulation of nitric oxide (NO) synthases and gas phase NO by oxygen. : , Marczin N, Kharitonov SA, Yacoub MH, Barnes PJ, Disease markers in exhaled breath (lung biology in health and disease). New York: Marcel Dekker, Inc; 2003. pp. 235–246. [Google Scholar]
- 15.Hansel TT, Kharitonov SA, Donnelly LE, Erin EM, Currie MG, Moore WM, Manning PT, Recker DP, Barnes PJ. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J 2003;17:1298–1300. [DOI] [PubMed] [Google Scholar]
- 16.Guo FH, Uetani K, Haque SJ, Williams BR, Dweik RA, Thunnissen FB, Calhoun W, Erzurum SC. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest 1997;100:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun 1991;181:852–857. [DOI] [PubMed] [Google Scholar]
- 18.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J 1993;6:1368–1370. [PubMed] [Google Scholar]
- 19.Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994;343:133–135. [DOI] [PubMed] [Google Scholar]
- 20.Gaston B, Drazen J, Chee CBE, Wohl MEB, Stamler JS. Expired nitric oxide concentrations are elevated in patients with reactive airways disease. Endothelium 1993;1:87–92. [Google Scholar]
- 21.Silkoff PE, McClean P, Spino M, Erlich L, Slutsky AS, Zamel N. Dose–response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest 2001;119:1322–1328. [DOI] [PubMed] [Google Scholar]
- 22.Ozkan M, Dweik RA. Nitric oxide and airway reactivity. Clin Pulm Med 2001;8:199–206. [Google Scholar]
- 23.Smith AD, Cowan JO, Filsell S, McLachlan C, Monti-Sheehan G, Jackson P, Taylor DR. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med 2004;169:473–478. [DOI] [PubMed] [Google Scholar]
- 24.Khalili B, Boggs PB, Shi R, Bahna SL. Discrepancy between clinical asthma control assessment tools and fractional exhaled nitric oxide. Ann Allergy Asthma Immunol 2008;101:124–129. [DOI] [PubMed] [Google Scholar]
- 25.Dweik RA. The promise and reality of nitric oxide in the diagnosis and treatment of lung disease. Cleve Clin J Med 2001;68:486, 488, 490, 493. [DOI] [PubMed] [Google Scholar]
- 26.American Thoracic Society. Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. Am J Respir Crit Care Med 1999;160:2104–2117. [DOI] [PubMed] [Google Scholar]
- 27.American Thoracic Society/European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912–930. [DOI] [PubMed] [Google Scholar]
- 28.Grob NM, Dweik RA. Exhaled nitric oxide in asthma. From diagnosis, to monitoring, to screening: are we there yet? Chest 2008;133:837–839. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt G, Vist G, Falck-Ytter Y, Kunz R, Magrini N, Schunemann H. An emerging consensus on grading recommendations? Evid Based Med 2006;11:2–4. [DOI] [PubMed] [Google Scholar]
- 30.Schunemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, Fahy BF, Gould MK, Horan KL, Krishnan JA, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 2006;174:605–614. [DOI] [PubMed] [Google Scholar]
- 31.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax 2010;65:384–390. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med 2004;170:579–580. [DOI] [PubMed] [Google Scholar]
- 33.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol 2007;119:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R, Castro M, Curran-Everett D, Fitzpatrick AM, et al. ; National Heart. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med 2010;181:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, Bleecker E, Busse W, Calhoun WJ, Castro M, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med 2010;181:1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattes J, Storm van's Gravesande K, Reining U, Alving K, Ihorst G, Henschen M, Kuehr K. NO in exhaled air is correlated with markers of eosinophilic airway inflammation in corticosteroid-dependent childhood asthma. Eur Respir J 1999;13:1391–1395. [PubMed] [Google Scholar]
- 37.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, et al. Eosinophilic inflammation in asthma. N Engl J Med 1990;323:1033–1039. [DOI] [PubMed] [Google Scholar]
- 38.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 2002;360:1715–1721. [DOI] [PubMed] [Google Scholar]
- 39.Berry MA, Shaw DE, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy 2005;35:1175–1179. [DOI] [PubMed] [Google Scholar]
- 40.Warke TJ, Fitch PS, Brown V, Taylor R, Lyons JD, Ennis M, Shields MD. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax 2002;57:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silkoff PE, Lent AM, Busacker AA, Katial RK, Balzar S, Strand M, Wenzel SE. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol 2005;116:1249–1255. [DOI] [PubMed] [Google Scholar]
- 42.Brown HM. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet 1958;2:1245–1247. [DOI] [PubMed] [Google Scholar]
- 43.Paoliello-Paschoalato AB, Oliveira SH, Cunha FQ. Interleukin 4 induces the expression of inducible nitric oxide synthase in eosinophils. Cytokine 2005;30:116–124. [DOI] [PubMed] [Google Scholar]
- 44.Barreto M, Villa MP, Monti F, Bohmerova Z, Martella S, Montesano M, Darder MT, Ronchetti R. Additive effect of eosinophilia and atopy on exhaled nitric oxide levels in children with or without a history of respiratory symptoms. Pediatr Allergy Immunol 2005;16:52–58. [DOI] [PubMed] [Google Scholar]
- 45.Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, Hodgdon K, Morgan W, Sorkness CA, Lemanske RF. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol 2003;112:883–892. [DOI] [PubMed] [Google Scholar]
- 46.van den Toorn LM, Overbeek SE, de Jongste JC, Leman K, Hoogsteden HC, Prins JB. Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med 2001;164:2107–2113. [DOI] [PubMed] [Google Scholar]
- 47.Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med 2001;164:1376–1381. [DOI] [PubMed] [Google Scholar]
- 48.Lim S, Jatakanon A, Meah S, Oates T, Chung KF, Barnes PJ. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in mild to moderately severe asthma. Thorax 2000;55:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 1998;53:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones SL, Kittelson J, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, Taylor DR. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med 2001;164:738–743. [DOI] [PubMed] [Google Scholar]
- 51.Shaw DE, Berry MA, Thomas M, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:231–237. [DOI] [PubMed] [Google Scholar]
- 52.Porsbjerg C, Lund TK, Pedersen L, Backer V. Inflammatory subtypes in asthma are related to airway hyperresponsiveness to mannitol and exhaled NO. J Asthma 2009;46:606–612. [DOI] [PubMed] [Google Scholar]
- 53.Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, Ligabue G, Ciaccia A, Saetta M, Papi A. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:418–424. [DOI] [PubMed] [Google Scholar]
- 54.Brightling CE, Symon FA, Birring SS, Bradding P, Wardlaw AJ, Pavord ID. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax 2003;58:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szefler SJ, Martin RJ. Lessons learned from variation in response to therapy in clinical trials. J Allergy Clin Immunol 2010;125:285–292, quiz 293–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, Herbison GP, Taylor DR. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med 2005;172:453–459. [DOI] [PubMed] [Google Scholar]
- 57.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, Craig TJ, Dolovich M, Drazen JM, Fagan JK, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol 2002;109:410–418. [DOI] [PubMed] [Google Scholar]
- 58.Knuffman JE, Sorkness CA, Lemanske RF, Mauger DT, Boehmer SJ, Martinez FD, Bacharier LB, Strunk RC, Szefler SJ, Zeiger RS, et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol 2009;123:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pijnenburg MW, Hofhuis W, Hop WC, De Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax 2005;60:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zacharasiewicz A, Wilson N, Lex C, Erin EM, Li AM, Hansel T, Khan M, Bush A. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med 2005;171:1077–1082. [DOI] [PubMed] [Google Scholar]
- 61.Deykin A, Massaro AF, Drazen JM, Israel E. Exhaled nitric oxide as a diagnostic test for asthma: online versus offline techniques and effect of flow rate. Am J Respir Crit Care Med 2002;165:1597–1601. [DOI] [PubMed] [Google Scholar]
- 62.Berkman N, Avital A, Breuer R, Bardach E, Springer C, Godfrey S. Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax 2005;60:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ihre E, Gustafsson LE, Kumlin M, Gyllfors P, Dahlen B. Early rise in exhaled no and mast cell activation in repeated low dose allergen challenge. Eur Respir J 2006;1:1. [DOI] [PubMed] [Google Scholar]
- 64.Meurs H, Maarsingh H, Zaagsma J. Arginase and asthma: novel insights into nitric oxide homeostasis and airway hyperresponsiveness. Trends Pharmacol Sci 2003;24:450–455. [DOI] [PubMed] [Google Scholar]
- 65.Dupont LJ, Rochette F, Demedts MG, Verleden GM. Exhaled nitric oxide correlates with airway hyperresponsiveness in steroid-naive patients with mild asthma. Am J Respir Crit Care Med 1998;157:894–898. [DOI] [PubMed] [Google Scholar]
- 66.Gronke L, Kanniess F, Holz O, Jorres RA, Magnussen H. The relationship between airway hyper-responsiveness, markers of inflammation and lung function depends on the duration of the asthmatic disease. Clin Exp Allergy 2002;32:57–63. [DOI] [PubMed] [Google Scholar]
- 67.Lapperre TS, Snoeck-Stroband JB, Gosman MM, Stolk J, Sont JK, Jansen DF, Kerstjens HA, Postma DS, Sterk PJ, The GSG. Dissociation of lung function and airway inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;1:1. [DOI] [PubMed] [Google Scholar]
- 68.Rosi E, Ronchi MC, Grazzini M, Duranti R, Scano G. Sputum analysis, bronchial hyperresponsiveness, and airway function in asthma: results of a factor analysis. J Allergy Clin Immunol 1999;103:232–237. [DOI] [PubMed] [Google Scholar]
- 69.Olin AC, Bake B, Toren K. Fraction of exhaled nitric oxide at 50 mL/s: reference values for adult lifelong never-smokers. Chest 2007;131:1852–1856. [DOI] [PubMed] [Google Scholar]
- 70.Arora R, Thornblade CE, Dauby PA, Flanagan JW, Bush AC, Hagan LL. Exhaled nitric oxide levels in military recruits with new onset asthma. Allergy Asthma Proc 2006;27:493–498. [DOI] [PubMed] [Google Scholar]
- 71.Deykin A, Massaro AF, Coulston E, Drazen JM, Israel E. Exhaled nitric oxide following repeated spirometry or repeated plethysmography in healthy individuals. Am J Respir Crit Care Med 2000;161:1237–1240. [DOI] [PubMed] [Google Scholar]
- 72.Dupont LJ, Demedts MG, Verleden GM. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest 2003;123:751–756. [DOI] [PubMed] [Google Scholar]
- 73.Olin AC, Rosengren A, Thelle DS, Lissner L, Bake B, Toren K. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest 2006;130:1319–1325. [DOI] [PubMed] [Google Scholar]
- 74.Borrill Z, Clough D, Truman N, Morris J, Langley S, Singh D. A comparison of exhaled nitric oxide measurements performed using three different analysers. Respir Med 2006;100:1392–1396. [DOI] [PubMed] [Google Scholar]
- 75.Kharitonov SA, Gonio F, Kelly C, Meah S, Barnes PJ. Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J 2003;21:433–438. [DOI] [PubMed] [Google Scholar]
- 76.Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol 2005;115:1130–1136. [DOI] [PubMed] [Google Scholar]
- 77.Olivieri M, Talamini G, Corradi M, Perbellini L, Mutti A, Tantucci C, Malerba M. Reference values for exhaled nitric oxide (reveno) study. Respir Res 2006;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Travers J, Marsh S, Aldington S, Williams M, Shirtcliffe P, Pritchard A, Weatherall M, Beasley R. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J Respir Crit Care Med 2007;176:238–242. [DOI] [PubMed] [Google Scholar]
- 79.Dressel H, de la Motte D, Reichert J, Ochmann U, Petru R, Angerer P, Holz O, Nowak D, Jorres RA. Exhaled nitric oxide: independent effects of atopy, smoking, respiratory tract infection, gender and height. Respir Med 2008;102:962–969. [DOI] [PubMed] [Google Scholar]
- 80.Baraldi E, Azzolin NM, Cracco A, Zacchello F. Reference values of exhaled nitric oxide for healthy children 6–15 years old. Pediatr Pulmonol 1999;27:54–58. [DOI] [PubMed] [Google Scholar]
- 81.Kovesi T, Kulka R, Dales R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest 2008;133:169–175. [DOI] [PubMed] [Google Scholar]
- 82.Wong GW, Liu EK, Leung TF, Yung E, Ko FW, Hui DS, Fok TF, Lai CK. High levels and gender difference of exhaled nitric oxide in Chinese schoolchildren. Clin Exp Allergy 2005;35:889–893. [DOI] [PubMed] [Google Scholar]
- 83.Malmberg LP, Petays T, Haahtela T, Laatikainen T, Jousilahti P, Vartiainen E, Makela MJ. Exhaled nitric oxide in healthy nonatopic school-age children: determinants and height-adjusted reference values. Pediatr Pulmonol 2006;41:635–642. [DOI] [PubMed] [Google Scholar]
- 84.Taylor DR, Mandhane P, Greene JM, Hancox RJ, Filsell S, McLachlan CR, Williamson AJ, Cowan JO, Smith AD, Sears MR. Factors affecting exhaled nitric oxide measurements: the effect of sex. Respir Res 2007;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meijer RJ, Postma DS, Kauffman HF, Arends LR, Koeter GH, Kerstjens HA. Accuracy of eosinophils and eosinophil cationic protein to predict steroid improvement in asthma. Clin Exp Allergy 2002;32:1096–1103. [DOI] [PubMed] [Google Scholar]
- 86.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, Zeiger RS, Larsen G, Spahn JD, Bacharier LB, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol 2005;115:233–242. [DOI] [PubMed] [Google Scholar]
- 87.Pijnenburg MW, Bakker EM, Lever S, Hop WC, De Jongste JC. High fractional concentration of nitric oxide in exhaled air despite steroid treatment in asthmatic children. Clin Exp Allergy 2005;35:920–925. [DOI] [PubMed] [Google Scholar]
- 88.Piacentini GL, Bodini A, Costella S, Vicentini L, Peroni D, Zanolla L, Boner AL. Allergen avoidance is associated with a fall in exhaled nitric oxide in asthmatic children. J Allergy Clin Immunol 1999;104:1323–1324. [DOI] [PubMed] [Google Scholar]
- 89.Vahlkvist S, Sinding M, Skamstrup K, Bisgaard H. Daily home measurements of exhaled nitric oxide in asthmatic children during natural birch pollen exposure. J Allergy Clin Immunol 2006;117:1272–1276. [DOI] [PubMed] [Google Scholar]
- 90.Pedroletti C, Millinger E, Dahlen B, Soderman P, Zetterstrom O. Clinical effects of purified air administered to the breathing zone in allergic asthma: a double-blind randomized cross-over trial. Respir Med 2009;103:1313–1319. [DOI] [PubMed] [Google Scholar]
- 91.Bodini A, Peroni D, Loiacono A, Costella S, Pigozzi R, Baraldi E, Boner AL, Piacentini GL. Exhaled nitric oxide daily evaluation is effective in monitoring exposure to relevant allergens in asthmatic children. Chest 2007;132:1520–1525. [DOI] [PubMed] [Google Scholar]
- 92.Smith AD, Cowan JO, Taylor DR. Exhaled nitric oxide levels in asthma: personal best versus reference values. J Allergy Clin Immunol 2009;124:714–718. [DOI] [PubMed] [Google Scholar]
- 93.Buchvald F, Eiberg H, Bisgaard H. Heterogeneity of FeNO response to inhaled steroid in asthmatic children. Clin Exp Allergy 2003;33:1735–1740. [DOI] [PubMed] [Google Scholar]
- 94.Gelb AF, Flynn Taylor C, Shinar CM, Gutierrez C, Zamel N. Role of spirometry and exhaled nitric oxide to predict exacerbations in treated asthmatics. Chest 2006;129:1492–1499. [DOI] [PubMed] [Google Scholar]
- 95.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O'Connor GT, Morgan WJ, Kattan M, Pongracic JA, Teach SJ, Bloomberg GR, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet 2008;372:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ekroos H, Karjalainen J, Sarna S, Laitinen LA, Sovijarvi AR. Short-term variability of exhaled nitric oxide in young male patients with mild asthma and in healthy subjects. Respir Med 2002;96:895–900. [DOI] [PubMed] [Google Scholar]
- 97.Pijnenburg MW, Floor SE, Hop WC, De Jongste JC. Daily ambulatory exhaled nitric oxide measurements in asthma. Pediatr Allergy Immunol 2006;17:189–193. [DOI] [PubMed] [Google Scholar]
- 98.Massaro AF, Gaston B, Kita D, Fanta C, Stamler JS, Drazen JM. Expired nitric oxide levels during treatment of acute asthma. Am J Respir Crit Care Med 1995;152:800–803. [DOI] [PubMed] [Google Scholar]
- 99.Beck-Ripp J, Griese M, Arenz S, Koring C, Pasqualoni B, Bufler P. Changes of exhaled nitric oxide during steroid treatment of childhood asthma. Eur Respir J 2002;19:1015–1019. [DOI] [PubMed] [Google Scholar]
- 100.Michils A, Baldassarre S, Van Muylem A. Exhaled nitric oxide and asthma control: a longitudinal study in unselected patients. Eur Respir J 2008;31:539–546. [DOI] [PubMed] [Google Scholar]
- 101.de Jongste JC, Carraro S, Hop WC, Baraldi E. Daily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthma. Am J Respir Crit Care Med 2009;179:93–97. [DOI] [PubMed] [Google Scholar]
- 102.Petsky HL, Cates CJ, Lasserson TJ, Li AM, Turner C, Kynaston JA, Chang AB. A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils). Thorax [DOI] [PubMed] [Google Scholar]
- 103.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 2005;352:2163–2173. [DOI] [PubMed] [Google Scholar]
- 104.Gibson PG. Using fractional exhaled nitric oxide to guide asthma therapy: design and methodological issues for ASthma TReatment ALgorithm studies. Clin Exp Allergy 2009;39:478–490. [DOI] [PubMed] [Google Scholar]
- 105.Perez-de-Llano LA, Carballada F, Castro Anon O, Pizarro M, Golpe R, Baloira A, Vazquez Caruncho M, Boquete M. Exhaled nitric oxide predicts control in patients with difficult-to-treat asthma. Eur Respir J 2010;35:1221–1227. [DOI] [PubMed] [Google Scholar]
- 106.Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, Pavord ID, Ratjen F, Silkoff PE, Taylor DR, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest 2010;138:682–692. [DOI] [PubMed] [Google Scholar]
- 107.Pizzichini E, Pizzichini MM, Gibson P, Parameswaran K, Gleich GJ, Berman L, Dolovich J, Hargreave FE. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med 1998;158:1511–1517. [DOI] [PubMed] [Google Scholar]
- 108.Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, Pavord ID. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2000;356:1480–1485. [DOI] [PubMed] [Google Scholar]
- 109.Zietkowski Z, Kucharewicz I, Bodzenta-Lukaszyk A. The influence of inhaled corticosteroids on exhaled nitric oxide in stable chronic obstructive pulmonary disease. Respir Med 2005;99:816–824. [DOI] [PubMed] [Google Scholar]
- 110.de Laurentiis G, Maniscalco M, Cianciulli F, Stanziola A, Marsico S, Lundberg JO, Weitzberg E, Sofia M. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm Pharmacol Ther 2008;21:689–693. [DOI] [PubMed] [Google Scholar]
- 111.Dummer JF, Epton MJ, Cowan JO, Cook JM, Condliffe R, Landhuis CE, Smith AD, Taylor DR. Predicting corticosteroid response in chronic obstructive pulmonary disease using exhaled nitric oxide. Am J Respir Crit Care Med 2009;180:846–852. [DOI] [PubMed] [Google Scholar]
- 112.Lehtimaki L, Kankaanranta H, Saarelainen S, Annila I, Aine T, Nieminen R, Moilanen E. Bronchial nitric oxide is related to symptom relief during fluticasone treatment in COPD. Eur Respir J 2010;35:72–78. [DOI] [PubMed] [Google Scholar]
- 113.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 1995;333:214–221. [DOI] [PubMed] [Google Scholar]
- 114.Ghamra ZW, Dweik RA. Primary pulmonary hypertension: an overview of epidemiology and pathogenesis. Cleve Clin J Med 2003;70:S2–S8. [DOI] [PubMed] [Google Scholar]
- 115.Mayer B, Pfeiffer S, Schrammel A, Koesling D, Schmidt K, Brunner F. A new pathway of nitric oxide/cyclic GMP signaling involving S-nitrosoglutathione. J Biol Chem 1998;273:3264–3270. [DOI] [PubMed] [Google Scholar]
- 116.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 2005;310:1966–1970. [DOI] [PubMed] [Google Scholar]
- 117.Schmidt HH, Hofmann H, Schindler U, Shutenko ZS, Cunningham DD, Feelisch M. No. NO from NO synthase. Proc Natl Acad Sci USA 1996;93:14492–14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature 2008;452:646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med 2006;173:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moya MP, Gow AJ, Califf RM, Goldberg RN, Stamler JS. Inhaled ethyl nitrite gas for persistent pulmonary hypertension of the newborn. Lancet 2002;360:141–143. [DOI] [PubMed] [Google Scholar]
- 121.Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research “Work in progress”. Circulation 2000;102:2781–2791. [DOI] [PubMed] [Google Scholar]
- 122.Dweik R. Pulmonary hypertension and the search for the selective pulmonary vasodilator. Lancet 2002;360:886. [DOI] [PubMed] [Google Scholar]
- 123.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med 1998;158:917–923. [DOI] [PubMed] [Google Scholar]
- 124.Dweik RA. The lung in the balance: arginine, methylated arginines, and nitric oxide. Am J Physiol Lung Cell Mol Physiol 2007;292:L15–L17. [DOI] [PubMed] [Google Scholar]
- 125.Ozkan M, Dweik RA, Laskowski D, Arroliga AC, Erzurum SC. High levels of nitric oxide in individuals with pulmonary hypertension receiving epoprostenol therapy. Lung 2001;179:233–243. [DOI] [PubMed] [Google Scholar]
- 126.Girgis RE, Champion HC, Diette GB, Johns RA, Permutt S, Sylvester JT. Decreased exhaled nitric oxide in pulmonary arterial hypertension: response to bosentan therapy. Am J Respir Crit Care Med 2005;172:352–357. [DOI] [PubMed] [Google Scholar]
- 127.Machado RF, Londhe Nerkar MV, Dweik RA, Hammel J, Janocha A, Pyle J, Laskowski D, Jennings C, Arroliga AC, Erzurum SC. Nitric oxide and pulmonary arterial pressures in pulmonary hypertension. Free Radic Biol Med 2004;37:1010–1017. [DOI] [PubMed] [Google Scholar]
- 128.Lundberg JON, Farkas-Szallasi T, Weitzberg E, Rinder J, Lidholm J, Änggård A, Hökfelt T, Lundberg JM, Alving K. High nitric oxide production in human paranasal sinuses. Nat Med 1995;1:370–373. [DOI] [PubMed] [Google Scholar]
- 129.Lundberg JO, Weitzberg E, Nordvall SL, Kuylenstierna R, Lundberg JM, Alving K. Primarily nasal origin of exhaled nitric oxide and absence in Kartagener's syndrome. Eur Respir J 1994;7:1501–1504. [DOI] [PubMed] [Google Scholar]
- 130.Palm JP, Graf P, Lundberg JO, Alving K. Characterization of exhaled nitric oxide: introducing a new reproducible method for nasal nitric oxide measurements. Eur Respir J 2000;16:236–241. [DOI] [PubMed] [Google Scholar]
- 131.Lundberg JON, Nordvall SL, Weitzberg E, Kollberg H, Alving K. Exhaled NO in pediatric asthma and cystic fibrosis. Arch Dis Child 1996;75:323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Balfour-Lynn IM, Laverty A, Dinwiddie R. Reduced upper airway nitric oxide in cystic fibrosis. Arch Dis Child 1996;75:319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arnal JF, Didier A, Rami J, M'Rini C, Charlet JP, Serrano E, Besombes JP. Nasal nitric oxide is increased in allergic rhinitis. Clin Exp Allergy 1997;27:358–362. [PubMed] [Google Scholar]
- 134.Kharitonov SA, Rajakulasingam K, O'Connor B, Durham SR, Barnes PJ. Nasal nitric oxide is increased in patients with asthma and allergic rhinitis and may be modulated by nasal glucocorticoids. J Allergy Clin Immunol 1997;99:58–64. [DOI] [PubMed] [Google Scholar]
- 135.Horvath I, Loukides S, Wodehouse T, Csiszer E, Cole PJ, Kharitonov SA, Barnes PJ. Comparison of exhaled and nasal nitric oxide and exhaled carbon monoxide levels in bronchiectatic patients with and without primary ciliary dyskinesia. Thorax 2003;58:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karadag B, James AJ, Gultekin E, Wilson NM, Bush A. Nasal and lower airway level of nitric oxide in children with primary ciliary dyskinesia. Eur Respir J 1999;13:1402–1405. [DOI] [PubMed] [Google Scholar]
- 137.Wodehouse T, Kharitonov SA, Mackay IS, Barnes PJ, Wilson R, Cole PJ. Nasal nitric oxide measurements for the screening of primary ciliary dyskinesia. Eur Respir J 2003;21:43–47. [DOI] [PubMed] [Google Scholar]
- 138.Bodini A, Rugolotto S, Pradal U, Zanotto G, Peroni D. Nasal nitric oxide for early diagnosis of familial primary ciliary dyskinesia. Arch Dis Child 2008;93:452–453. [DOI] [PubMed] [Google Scholar]
- 139.Stehling F, Roll C, Ratjen F, Grasemann H. Nasal nitric oxide to diagnose primary ciliary dyskinesia in newborns. Arch Dis Child Fetal Neonatal Ed 2006;91:F233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zheng S, Xu W, Bose S, Banerjee AK, Haque SJ, Erzurum SC. Impaired nitric oxide synthase-2 signaling pathway in cystic fibrosis airway epithelium. Am J Physiol Lung Cell Mol Physiol 2004;287:L374–L381. [DOI] [PubMed] [Google Scholar]
- 141.Kelley TJ, Drumm ML. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest 1998;102:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Meng QH, Springall DR, Bishop AE, Morgan K, Evans TJ, Habib S, Gruenert DC, Gyi KM, Hodson ME, Yacoub MH, et al. Lack of inducible nitric oxide synthase in bronchial epithelium: a possible mechanism of susceptibility to infection in cystic fibrosis. J Pathol 1998;184:323–331. [DOI] [PubMed] [Google Scholar]
- 143.Grasemann H, Kurtz F, Ratjen F. Inhaled L-arginine improves exhaled nitric oxide and pulmonary function in patients with cystic fibrosis. Am J Respir Crit Care Med 2006;174:208–212. [DOI] [PubMed] [Google Scholar]
- 144.Jones KL, Hegab AH, Hillman BC, Simpson KL, Jinkins PA, Grisham MB, Owens MW, Sato E, Robbins RA. Elevation of nitrotyrosine and nitrate concentrations in cystic fibrosis sputum. Pediatr Pulmonol 2000;30:79–85. [DOI] [PubMed] [Google Scholar]
- 145.Snyder AH, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am J Respir Crit Care Med 2002;165:922–926. [DOI] [PubMed] [Google Scholar]
- 146.Yoon SS, Coakley R, Lau GW, Lymar SV, Gaston B, Karabulut AC, Hennigan RF, Hwang SH, Buettner G, Schurr MJ, et al. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J Clin Invest 2006;116:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gaston B, Ratjen F, Vaughan JW, Malhotra NR, Canady RG, Snyder AH, Hunt JF, Gaertig S, Goldberg JB. Nitrogen redox balance in the cystic fibrosis airway: effects of antipseudomonal therapy. Am J Respir Crit Care Med 2002;165:387–390. [DOI] [PubMed] [Google Scholar]
- 148.Lara A, Khatri SB, Wang Z, Comhair SA, Xu W, Dweik RA, Bodine M, Levison BS, Hammel J, Bleecker E, et al. Alterations of the arginine metabolome in asthma. Am J Respir Crit Care Med 2008;178:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ferrazzoni S, Scarpa MC, Guarnieri G, Corradi M, Mutti A, Maestrelli P. Exhaled nitric oxide and breath condensate ph in asthmatic reactions induced by isocyanates. Chest 2009;136:155–162. [DOI] [PubMed] [Google Scholar]
- 150.Hewitt RS, Smith AD, Cowan JO, Schofield JC, Herbison GP, Taylor DR. Serial exhaled nitric oxide measurements in the assessment of laboratory animal allergy. J Asthma 2008;45:101–107. [DOI] [PubMed] [Google Scholar]
- 151.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180:59–99. [DOI] [PubMed] [Google Scholar]
- 152.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendices I-IV, Tables S1-S4



