Abstract
We previously reported that murine norovirus (MNV), a virus prevalent in United States research institutions, increased atherosclerotic lesion size in Ldlr−/− mice when the mice were infected 8 wk after feeding an atherogenic diet. To determine whether the timing of MNV infection relative to atherosclerosis development altered the disease phenotype and to examine potential mechanisms by which MNV influences the disease process, we fed Ldlr−/− mice an atherogenic diet for 16 wk. Three days after initiating the atherogenic diet, half of the mice received MNV4 and the other half vehicle only (clarified cell-culture lysate; controls). Both groups of mice developed large aortic sinus lesions (control compared with MNV4: 133 ± 8 × 103 µm2 compared with 140 ± 7 × 103 µm2) that were not significantly different in size. Because the timing of MNV infection relative to atherosclerosis development and hypercholesterolemia differed between our previous and the current studies, we examined whether hypercholesterolemia altered MNV4-induced changes in bone-marrow–derived macrophages. MNV4 infection increased the potential of macrophages to take up and store cholesterol by increasing CD36 expression while suppressing the ABCA1 transporter. Thus, the effects of MNV4 infection on atherosclerotic lesion size appear to be dependent on the timing of the infection: MNV4 infection promotes only established lesions. This effect may be due to MNV4’s ability to increase cholesterol uptake and decrease efflux by regulating CD36 and ABCA1 protein expression.
Abbreviations: ABCA1, ATP-binding cassette A1; BMDM, bone-marrow–derived macrophage; iNOS, inducible nitric oxide synthase; MNV, murine norovirus
Chronic viral infection, such as occurs with HIV and hepatitis C virus, has been associated with an increased risk for atherosclerosis,2,19,46,48 although the mechanisms by which this occurs are not clearly defined. Our laboratory has been studying the effects of murine norovirus (MNV), which chronically infects immunocompetent mice, on murine models of inflammatory diseases, including atherosclerosis. MNV is a single-stranded RNA intestinal virus that belongs to the family Caliciviridae and has shown tropism toward antigen-presenting cells such as dendritic cells and macrophages.54 Whereas human norovirus is a major cause of nonbacterial acute gastroenteritis,52 MNV does not cause clinical disease in immunocompetent mice.55 However, the high prevalence of MNV in biomedical research facilities throughout the world,42,55 combined with its tropism for antigen-presenting cells, has prompted concern regarding potential effects on disease phenotypes in murine models of human diseases. Therefore, we previously examined 2 diseases, obesity and atherosclerosis, where macrophages have critical roles.41,42 We found that MNV infection did not influence glucose metabolism and weight gain,41 but it significantly increased the size and macrophage content of aortic sinus lesions in Ldlr−/− mice fed an atherogenic diet.42 These findings suggest that MNV might be a potential tool to determine how viral infection alters the risk of atherosclerosis.
Many factors influence the progression of atherosclerosis. Accordingly, we examined whether the timing of MNV infection relative to the stage of atherosclerosis progression influenced disease phenotype and evaluated potential mechanisms by which MNV could affect the disease process. To this end, we modeled the infection of macrophages by using in vitro cultures of bone-marrow–derived macrophages (BMDM).
Materials and Methods
Animals and diet.
Male B6.129S7-Ldlrtm1Her/J (Ldlr−/−; age, 4 wk) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and acclimated for 1 wk in an SPF facility42 with a 12:12-h light:dark cycle in a temperature-controlled room (20 to 23 °C). This mouse strain lacks the LDL receptor and thus is susceptible to developing atherosclerosis secondary to an elevated serum cholesterol level induced by the consumption of a high-fat diet. Mice were housed in groups of 5 in autoclaved ventilated microisolator cages, provided autoclaved and acidified water, and given an irradiated regular rodent chow (catalog no. 5053, PicoLab Rodent Diet 20, LabDiet, St Louis, MO) ad libitum during the 1-wk acclimation period. Mice were tested and determined to be free of MNV by the supplier. Our group has established standard operating protocols for our mouse housing facility to restrict MNV infection to intended groups only.41 All experimental protocols were reviewed and approved by the IACUC at the University of Washington.
After the acclimation period, all mice were fed an atherogenic (high fat, high cholesterol) diet (catalog no. TD88137, Harlan Laboratories, Dublin, VA) ad libitum for 16 wk to induce atherosclerosis. At 3 d after diet initiation, mice were infected with MNV4 (1 × 106 pfu, unknown passage) or vehicle (clarified RAW264.7 cell lysate) by oral gavage, as previously reported.42 Infection status was determined by fecal RT-PCR analysis 2 wk after MNV4 infection and at the end of the study.24 All MNV4-infected mice shed virus in the feces 2 wk after infection and remained positive for virus at the end of the study period (16 wk), whereas all mice treated with vehicle remained MNV-free throughout the study.
Atherosclerotic lesion analyses.
At the termination of the study, mice were euthanized by CO2 asphyxiation, followed by cardiocentesis to obtain blood. After perfusion with PBS, hearts and aortas were dissected and fixed in formalin for morphologic analyses. Aortic sinus sections were stained with Movat pentachrome stain, and lesion area was determined as previously described.42 The extent of macrophage infiltration in the lesion area was determined after Mac2 staining.42
Analysis of the Ly6Chi monocyte population in spleen.
Single-cell suspensions were generated from the spleen at necropsy,8 and cellularity was determined by using a hemocytometer. Cell subsets were analyzed as previously described.50 Cells were blocked with antiCD16/CD32 (BD Biosciences, San Jose, CA) and then stained with antigen-specific antibodies for linage markers (Lin: NK1.1, CD90, CD45R, Ly6G), CD11b, CD11c, F4/80, MHC class II, and Ly6C (all obtained from BD Biosciences). Data were collected by flow cytometry (model LSRII, BD Biosciences) and analyzed by using FlowJo software (Tree Star, Ashland, OR). Single cells were gated by using forward-scatter parameters A and W, and leukocytes were gated for size via forward-scatter A and side-scatter A parameters. Cells were identified on the basis of surface staining as neutrophils (Lin+CD11b+F480–class II–), dendritic cells (CD11chi), macrophages (Lin–CD11c–class II+ and CD11b+ or F4/80+ or both), or monocytes (Lin–CD11b+class II–F4/80–). Monocytes were further characterized according to Ly6C staining.
BMDM.
Bone marrow cells were isolated from Ldlr−/− mice (age, 10 to 12 wk) and differentiated to macrophages (7 to 10 d of culture) by using a previously reported method.32 Differentiated macrophages were plated at 1 to 1.4 × 106 cells per well in 6-well plates and infected for 24 h with MNV4 (multiplicity of infection, 0.2) with various amounts of oxLDL. For qRT–PCR analysis, RNA was extracted by using the RNeasy kit (Qiagen, Venlo, Limburg, The Netherlands), and for western blot analysis, protein was extracted with the MPER protein extraction kit (Thermo Scientific, Waltham, MA). For flow cytometric analysis, BMDM were dissociated by cold shock in PBS (Ca++- and Mg++-free).
qRT-PCR.
Total RNA (0.5 to 1 µg) was converted to cDNA by using the Superscript III first-strand synthesis system for RT-PCR (Invitrogen) and used for qRT-PCR with specific primers for inducible nitric oxide synthase (iNOS),35 IFNβ,53 IL10,1 CD36,43 ATP-binding cassette A1 (ABCA1),43 and IL6 (forward, 5′ AGA GTT GTG CAA TGG CAA TTC TGA 3′; reverse, 5′ TGG TAC TCC AGA AGA CCA GAG GAA 3′). The housekeeping gene HPRT14 was used to normalize the expression levels, and data are presented relative to a single replicate of the control (untreated) sample.
Western blot analysis.
Protein concentrations were determined by using BCA protein assay reagent (Thermo Scientific). Samples (15 to 20 µg total protein) were separated by denaturing gel electrophoresis (4% to 15% gradient gel; BioRad, Hercules, CA) and transferred onto polyvinylidene difluoride membranes (BioRad) with 1× CAPS buffer (10 mM CAPS in 10% methanol, pH 11). The membrane was incubated with ABCA1 antibody (polyclonal; Novus Biologic, Littleton, CO) in 5% bovine serum albumin in TBST (1× Tris-buffered saline containing 0.1% Tween 20) overnight, washed with TBST, and incubated with antirabbit–horseradish peroxidase secondary antibody (Thermo Scientific). Signal was detected by using SuperSignal West Femto chemiluminescent substrate (Thermo Scientific). For a loading control, the housekeeping protein GAPDH was detected (antiGAPDH, catalog no. G9545, Sigma, St Louis, MO) on the same blot.
Flow cytometric analysis.
The expression of CD36 in BMDM was detected by using flow cytometry. Cells were stained with antiCD36 (dilution, 1:50; clone number 72-1, allophycocyanin conjugate, eBiosciences, San Diego, CA) and analyzed by using flow cytometry (FACSCanto II, BD Biosciences) and FlowJo software (version 10). Specificity of the antiCD36 antibody was confirmed by using an isotype-matched control antibody (rat IgG2a K isotype, eBiosciences).
Statistics.
All data was analyzed by using Prism statistical software (GraphPad, La Jolla, CA). A 2-tailed t test was performed for comparison between the 2 treatment groups, and statistical significance was defined as a P value of less than 0.05.
Results
Effects of MNV4 infection during early-stage atherosclerosis on Ly6Chi monocytes and lesion size in Ldlr−/− mice.
We previously determined that hyperlipidemic Ldlr−/− mice infected with MNV4 during lesion development (8 wk after initiation of an atherogenic diet) had significantly larger lesions in the aortic sinus than did uninfected controls.42 To determine whether MNV4 infection alters atherosclerosis development and progression when mice are infected before they develop lesions due to sustained hypercholesterolemia, we infected Ldlr−/− mice with MNV4 3 d after the atherogenic diet was initiated.
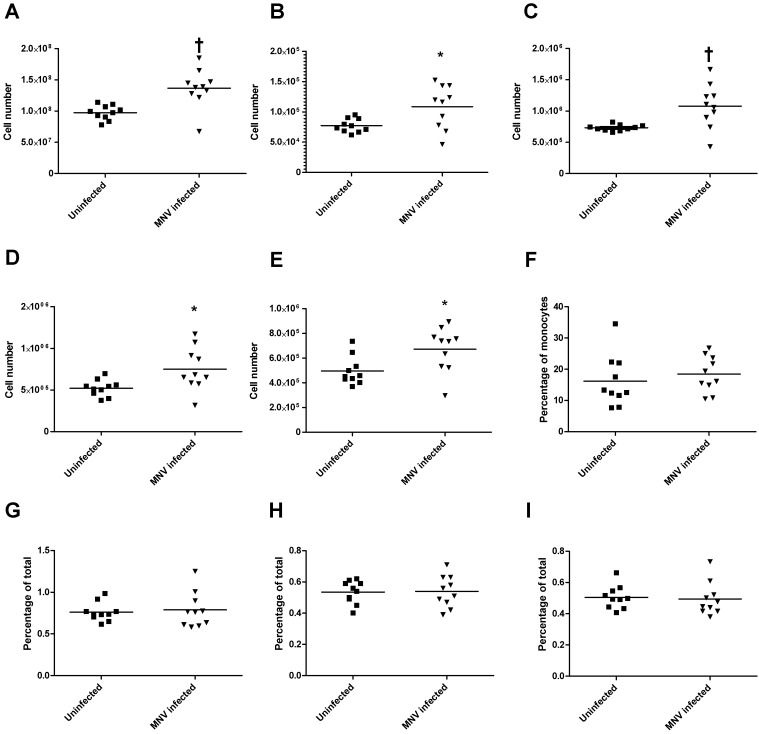
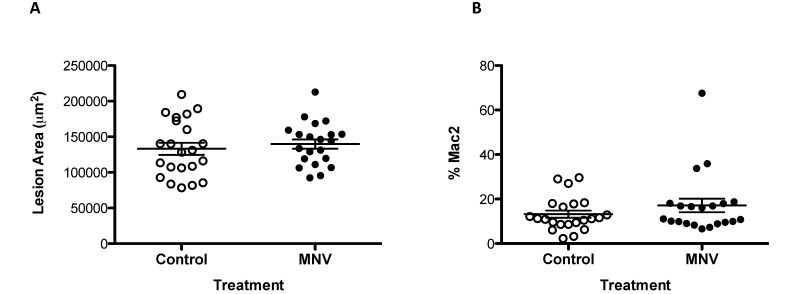
Development of atherosclerotic lesions is associated with increased numbers of circulating Ly6Chi monocytes,50 therefore, we characterized splenic monocytes and other cell populations and analyzed aortic sinus lesions at the end of the study period. The splenic cellularity (Figure 1 A) and absolute cell numbers of Ly6Chi monocytes (Figure 1 B), MHC class II-positive macrophages (Figure 1 C), dendritic cells (Figure 1 D), and neutrophils (Figure 1 E) were significantly (P < 0.05) increased at the study endpoint. However, the cell percentages of these populations (Figure 1 F through I) did not differ between treatment groups, indicating that MNV infection was associated with a general increase in cell numbers and not with a specific increase in any particular cell type in the context of atherosclerosis development. In agreement with the lack of specific difference in monocyte populations in response to MNV4 infection, the size of atherosclerotic lesions in the aortic sinus at the end of the study did not differ between MNV4-infected mice compared with uninfected controls (Figure 2 A); the percentage of the lesion area showing macrophage infiltration was also similar between the 2 groups (Figure 2 B). This result is in contrast to our previous report,42 where MNV4 infection significantly increased lesion size. However, in the previous study, MNV4 infection occurred 8 wk after initiation of the atherogenic diet and thus, the mice would have sustained hyperlipidemia and developed lesions42 by the time of infection. Another infectious agent, Chlamydia pneumoniae, also alters lesion size only in hyperlipidemic mice and not in mice prior to the induction of hyperlipidemia.6 To explain potential differences between our previous study and the results we present here, we next examined the effect of hyperlipidemia on the responses of macrophages to MNV4 infection in vitro by using BMDM.
Figure 1.
MNV infection does not alter the frequency of Ly6Chi monocytes. Spleens of Ldlr−/− mice were processed at the end of the study to determine whether MNV influences composition of immune cell subtypes by staining for antigen-specific antibodies for linage markers (Lin: NK1.1, CD90, CD45R, Ly6G), CD11b, CD11c, F4/80, MHC class II, and Ly6C. Total cell numbers of (A) the spleen, (B) Ly6Chi monocytes (Lin–CD11b+F4/80–class II–CD11c–Ly6Chi), (C) macrophages (Lin–CD11c–class II+ and CD11b+ or F4/80+ or both), (D) dendritic cells (CD11chi), and (E) neutrophils (Lin+CD11b+class II–F4/80–) are shown. (F) The percentage of Ly6Chi monocytes (of total monocytes) as well as percentages of total splenocytes of (G) macrophages, (H) dendritic cells, and (I) neutrophils are shown. Bars represent means; values significantly (*, P < 0.05; †, P < 0.01) different from those of uninfected animals are indicated.
Figure 2.
MNV infection at early stages of atherosclerosis development does not alter lesion size. (A) The size of the aortic sinus lesion was determined from Movat-pentachrome–stained serial sections of hearts of Ldlr−/− mice fed an atherogenic diet and treated with either MNV or vehicle only (clarified RAW264.7 cell lysate). (B) Macrophage infiltration was assessed by using antiMac2 antibody and is expressed as percentage of lesion area. Bars represent means ± SEM.
Effect of MNV4 infection on cytokine expression in BMDM.
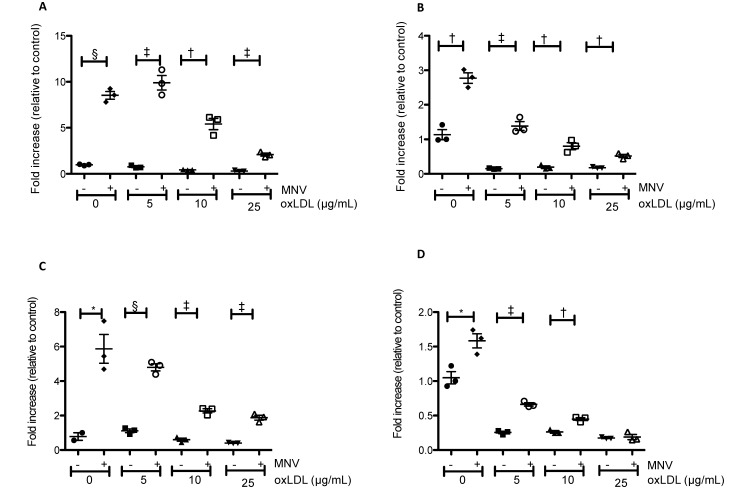
Modified LDL, such as oxLDL, are a risk factor for atherosclerosis because they are more readily taken up by macrophages than are native LDL.49 We therefore treated BMDM with various amounts of oxLDL with or without MNV4 infection and assessed the expression of inflammation-associated genes. MNV4 infection increased expression levels of the inflammatory cytokines IL6 and IFNβ and of a classic macrophage activation marker, iNOS, in all treatment groups (comparison between no infection and MNV infection at different levels of oxLDL, Figure 3). In addition, the expression of the antiinflammatory cytokine IL10 was increased with MNV4 infection except at high levels of oxLDL (25 µg/mL; Figure 3).
Figure 3.
MNV infection alters cytokine mRNA expression in BMDM in the presence and absence of oxLDL. (A) iNOS, (B) IFNβ, (C) IL6, and (D) IL10 expression were determined by qRT–PCR analysis. Samples were normalized to HPRT and plotted relative to a single replicate of the control sample (no oxLDL and no MNV). Bars represent means ± SEM; expression levels were compared between uninfected and infected samples of the same concentration of oxLDL by using the Student t test, and values that differ significantly (*, P < 0.05; †, P < 0.01; ‡, P < 0.001; §, P < 0.0001) are indicated.
Effects of MNV4 infection on CD36 and ABCA1 expression.
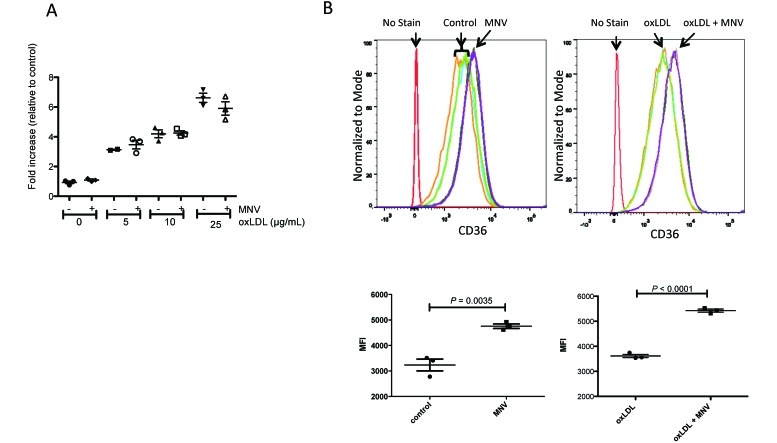
Cellular cholesterol levels are regulated by both uptake and efflux mechanisms, and an increase in uptake or decrease in efflux in macrophages could contribute to the development of atherosclerosis. We therefore determined whether MNV4 directly influences these processes by evaluating the expression of the scavenger receptor CD36 and efflux protein ABCA1. CD36 preferentially binds oxLDL and facilitates its uptake in macrophages,59 and its expression (RNA and protein) is induced as monocytes differentiate into macrophages in culture.25 We first determined whether MNV4 infection and oxLDL influence CD36 expression in BMDM by using qRT–PCR analysis and flow cytometry (Figure 4). In agreement with previous reports,47 oxLDL treatment increased CD36 RNA expression; specifically, CD36 RNA expression was significantly different among uninfected samples with varying amounts of oxLDL (P < 0.0001, one-way ANOVA). In addition, all samples treated with oxLDL differed from the control sample according to post hoc testing using Bonferroni correction for multiple comparisons (P < 0.01 for control compared with 5 µg/mL oxLDL; P < 0.001 for control compared with 10 or 25 μg/mL oxLDL). However, MNV4 infection did not further influence CD36 mRNA expression levels (no infection compared with MNV4 infection at all levels of oxLDL treatment). In contrast, cell-surface CD36 levels were significantly increased in MNV4-infected BMDM regardless of oxLDL treatment (P <0.05 for untreated groups ; P ≤ 0.0001 for oxLDL-treated groups, Figure 4 B), suggesting that MNV4 infection potentially increases oxLDL uptake in macrophages.
Figure 4.
CD36 expression is influenced by oxLDL treatment and MNV infection. (A) CD36 mRNA expression in BMDM in response to different levels of oxLDL and the presence or absence of MNV was determined by qRT-PCR analysis. Expression levels were normalized to HPRT and expressed relative to a single replicate of the control sample (no oxLDL, no MNV) (B) Cell-surface expression of CD36 protein was determined in response to MNV in the presence or absence of oxLDL from triplicate samples per treatment by using flow cytometry. The fluorescent intensity (bar, mean ± SEM) of triplicate samples is shown.
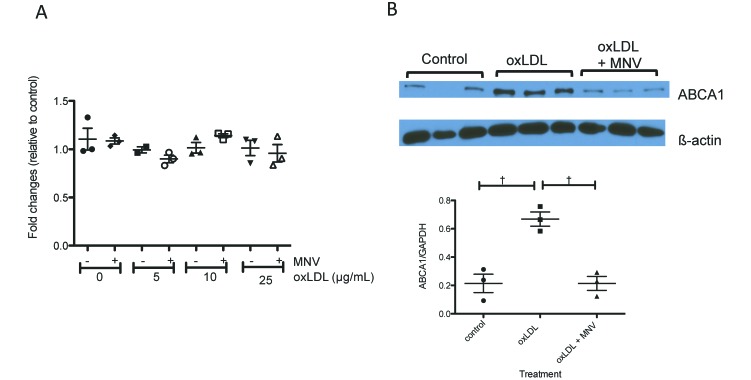
Increased cellular cholesterol levels trigger cholesterol efflux through the ABCA1 pathway.31 We therefore examined both the RNA and protein levels of Abca1 in response to oxLDL and MNV4 infection in BMDM. RNA expression levels of Abca1 did not change in response to either oxLDL treatment or MNV4 infection (Figure 5 A). However, ABCA1 protein levels were increased with oxLDL treatment (control compared with oxLDL, P < 0.01 for post hoc test after one-way ANOVA) and, notably, reduced with MNV4 infection in the presence of oxLDL (MNV4 + oxLDL compared with oxLDL, P < 0.01 for post hoc test after one-way ANOVA, Figure 5 B) indicating that MNV4 infection may decrease ABCA1-medated cholesterol efflux.
Figure 5.
In the presence of oxLDL, MNV infection suppresses protein but not mRNA levels of ABCA1. (A) Abca1 mRNA levels in BMDM were determined by qRT-PCR assay and normalized to HPRT. Normalized values are expressed relative to a single replicate of the control sample (no oxLDL, no MNV). (B) ABCA1 protein levels (bar, mean ± SEM) were determined by western blot analysis followed by densitometry and normalized to GAPDH. Values that differ significantly (†, P < 0.01) are indicated.
Discussion
Since the first report of MNV4 in a research mouse colony,28 its high prevalence has been reported in mouse colonies in the United States, Canada, and Europe.21,23,29,38,44,57 In addition, many investigators have reported diverse strains of the virus and its varied influence on animal models.4,9,12,22,28,32,33,41,42 We have studied the effects of MNV4, the strain first reported by Hsu and colleagues,23 on various murine models of human disease.32,33,41,42 MNV4 infection persists for an extended period of time, and infected mice shed the virus in feces for as long as 16 wk after infection.41,42 However, the effects of MNV4 on disease phenotype vary depending on the disease and model. For example, MNV4 infection increased the size of atherosclerotic lesions but did not alter obesity or diabetes phenotypes in Ldlr−/− mice.41,42 MNV4 accelerated the development of inflammatory bowel disease in Mdr1a−/− mice33 but did not alter colitis-associated cancer in Smad3−/− mice.32 Therefore, it is difficult to predict whether MNV4 would be an intercurrent variable for studies using murine models.
Because of the tropism of MNV for macrophages, our laboratory examined the potential effects of MNV infection on atherosclerosis models.54 We previously reported that MNV4 infection during atherosclerosis development increases aortic sinus lesion area in Ldlr−/− mice.42 However, in research settings, MNV infection in mice can occur at different times. If breeding colonies are infected or newly purchased young mice are moved into a facility that does not exclude MNV, Ldlr−/− mice might be infected before significant atherosclerosis develops. Therefore, in these current studies, we examined whether MNV4 infection at an early stage of disease, that is, before hyperlipidemia is induced, alters disease outcome. We found that MNV infection during early atherosclerosis did not significantly alter lesion size or macrophage infiltration into the lesion area (Figure 2), suggesting that the timing of MNV4 infection may modulate its influence on atherosclerosis progression in this model.
Ldlr−/− mice were developed as a model to study familial hypercholesterolemia in humans due to defects in the LDLR pathway26 and were instrumental in understanding lipid uptake and metabolism. However, unlike humans with LDLR defects, Ldlr−/− mice do not develop severe hypercholesterolemia unless fed an atherogenic, high-cholesterol containing diet.26 This discrepancy is mainly due to the differential composition of apolipoprotein B associated with VLDL in humans compared with mice. In humans, VLDL is secreted with apoB100, a ligand for LDLR, whereas in mice, much VLDL is associated with apoB48, which is recognized by a remnant receptor10,26 rather than by LDLR. Therefore, LDLR defects in humans have more severe consequences in endogenous lipoprotein clearance and circulating cholesterol levels than do mice with LDLR defects, because these mice can clear LDL through remnant receptors in addition to LDLR. Consequently, a diet containing high cholesterol is needed to induce severe hypercholesterolemia and atherosclerosis in Ldlr−/− mice.27
Because of this characteristic of Ldlr−/− mice and because another infectious agent, Chlamydia pneumoniae, was found to increase lesion size only when the animals were hyperlipidemic,6 we postulated that the differential response to infection in our current and previous studies might be due to the timing of MNV4 infection in relation to disease stage or severity of hypercholesterolemia. To address this question, we examined whether MNV4 infection alters factors known to be associated with atherosclerosis in a manner dependent on oxLDL in vitro. MNV4 infection was associated with increased cytokine expression (IL6, IL1β, IFNβ) at all levels of macrophage exposure to oxLDL. Interestingly, oxLDL treatment decreased MNV4-induced cytokine expression in a dose-dependent manner, suggesting that MNV4’s effect on macrophages might differ depending on the severity of hyperlipidemia (Figure 3). In addition, MNV4 infection increased the expression of a marker of classically activated macrophages, iNOS, and oxLDL exposure suppressed iNOS expression in a dose-dependent manner. We also noted that expression of the antiinflammatory cytokine IL10 paralleled changes in proinflammatory cytokines, suggesting that IL10 expression likely mirrors proinflammatory cytokine signals in an attempt to control inflammation. These data suggest that the effects of MNV4 on macrophages are modulated by the presence of oxLDL. Our observation agrees with previous reports that oxLDL inhibits inflammatory cytokine (IL1β, IL6, and TNFα) and iNOS expression induced by lipopolysaccharide or IFNγ.17,20,40 How the oxLDL-associated suppression of immune responses in vitro relates to an increased risk of developing atherosclerosis in vivo is unclear. However, a decrease in normal inflammatory responses may allow macrophages to develop a chronic, smoldering inflammation, thus favoring progression to atherosclerosis.20 Therefore, increased oxLDL at the time of MNV4 infection may promote a chronic inflammatory state, resulting in the progression of lesions rather than in acute inflammation that can be controlled quickly.
Another potential reason for why MNV4’s influence on atherosclerosis differs between our current study and previous report is that the immune response to MNV4 infection may vary due to the age of the mice at the time of MNV 4 infection. In our previous experiment, we infected Ldlr−/− mice at 14 wk of age whereas in the current study, they were infected at approximately 6 wk of age. Because rodents’ immune system is considered mature by 1 mo of age and because they can effectively establish immunologic memory in the first 6 mo of age,30 we believe that the quality of the immune response to MNV (after what is a first-time exposure to MNV in our colony) is unlikely to differ according to the timing of infection during this time frame. However, this difference in infection timing introduced another variable, obesity. In our previous study, mice were fed a high-fat diet for 8 wk before the MNV4 infection and, thus, mice were obese prior to infection; in contrast, in the current study, infection occurred before significant obesity developed. Because obesity can influence macrophage response to infectious agents,3,58 obesity in the context of the atherosclerosis-promoting high-cholesterol diet may influence the influx of macrophages into lesion areas. Finally, another possible explanation for the differences between our current and previous studies could be related to differences in the biologic potential between the 2 viral isolates. RNA viruses such as MNV have a high mutation rate owing to the lack of a 3′-to-5′ exonuclease proofreading activity in their RNA-dependent RNA polymerase,13,56 allowing mutations to occur during viral propagation. Although the same viral stock was used to propagate MNV in Raw cells, viral propagation occurred at different times for the previous study compared with the study reported here, potentially resulting in the use of slightly different viral isolates between the 2 studies. Minor changes (even single amino-acid substitutions) in the MNV sequence can influence its biologic effects.5,7,39,51
The uptake and efflux of cholesterol regulates intracellular cholesterol content, which is a key factor in macrophage foam-cell formation.34 We therefore examined whether MNV4 infection alters one or both of these processes by analyzing the RNA and protein levels of CD36 (oxLDL uptake) and ABCA1 (cholesterol efflux) in Ldlr−/− BMDM. CD36 is a large glycoprotein that belongs to a scavenger receptor class B family and binds various ligands, including oxLDL.11 Studies to elucidate the role of CD36 in atherosclerosis in Apoe−/− Cd36−/− mice compared with Apoe−/− mice reported conflicting data: one study showed dramatic decreases in lesion size,15 whereas the other showed a slight or moderate increase in lesion size depending on the analysis site.36 However, in both studies, peritoneal macrophages showed impaired uptake of oxLDL and cholesterol in vitro15 and in vivo,36 demonstrating the involvement of CD36 in oxLDL uptake. In agreement with a previous study,11 we found that Ldlr−/− BMDM expressed elevated levels of CD36 in response to oxLDL treatment (Figure 4). Although mRNA levels of CD36 did not change after MNV4 infection, CD36 protein expression was increased in MNV4-infected BMDM regardless of oxLDL treatment (Figure 4 B), suggesting that MNV4 infection increases the oxLDL uptake capacity of macrophages.
Intracellular cholesterol levels also are regulated by efflux pathways, which involve ATP-binding cassette proteins. We found that Ldlr−/− BMDM treated with oxLDL showed significantly elevated levels of ABCA1, whereas ABCA1 expression was suppressed to control levels in BMDM infected with MNV4 despite concomitant treatment with oxLDL (Figure 5B). These data suggest that MNV4 infection induces macrophages to take up more oxLDL and not release it; consequently existing hypercholesterolemia and MNV4 infection would result in increased cholesterol levels in macrophages. This scenario may explain why MNV4 infection prior to induction of severe hypercholesterolemia (current study) did not alter lesion size, whereas MNV4 infection in animals with severe hypercholesterolemia increases lesion size. Virus-induced alterations in cholesterol transport mechanisms have been reported. For example, HIV is associated with an increased risk of developing atherosclerosis in part because of inhibition of cholesterol efflux via ABCA1.16,37 Monocytes and macrophages infected with HIV had a decreased levels of ABCA1 protein due to its increased degradation,37 and this accelerated degradation of ABCA1 is mediated by an HIV accessory protein, Nef, which secures sufficient cholesterol to improve the infectivity of HIV.37 Although MNV1 infection of macrophages are dependent on cholesterol,18,45 whether increased intracellular cholesterol improves its infectivity is unknown. Additional studies need to determine whether MNV alters cholesterol homeostasis to benefit its infectivity or ability to replicate.
In summary, we show that MNV4 infection influences cellular cholesterol levels in macrophages by regulating both uptake (CD36) and efflux (ABCA1) pathways in BMDM. Whether this modulation translates to a detectable in vivo effect is likely dependent on many factors, including the stage of atherosclerosis and the potency of the viral isolate. Our findings suggest that careful consideration should be given when performing atherosclerosis studies in MNV-infected mouse colonies, given that predicting the influence of MNV on the disease phenotype is difficult in murine models.
Acknowledgments
This research was supported by the NIH (R21 RR032289 to LMP) and the University of Washington Nutrition Obesity Research Center (P30 DK035816). We thank Drs Jennifer Finley, Karuna Patil, and Stacey Meeker and Mr Gary Knowels for their technical assistance.
References
- 1.Abe K, Nguyen KP, Fine SD, Mo JH, Shen C, Shenouda S, Corr M, Jung S, Lee J, Eckmann L, Raz E. 2007. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc Natl Acad Sci USA 104:17022–17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adinolfi LE, Zampino R, Restivo L, Lonardo A, Guerrera B, Marrone A, Nascimbeni F, Florio A, Loria P. 2014. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol 20:3410–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. 2007. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci USA 104:20466–20471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammann CG, Messer RJ, Varvel K, Debuysscher BL, Lacasse RA, Pinto AK, Hasenkrug KJ. 2009. Effects of acute and chronic murine norovirus infections on immune responses and recovery from Friend retrovirus infection. J Virol 83:13037–13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey D, Thackray LB, Goodfellow IG. 2008. A single amino-acid substitution in the murine norovirus capsid protein is sufficient for attenuation in vivo. J Virol 82:7725–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blessing E, Campbell LA, Rosenfeld ME, Kuo CC. 2002. Chlamydia pneumoniae and hyperlipidemia are co-risk factors for atherosclerosis: infection prior to induction of hyperlipidemia does not accelerate development of atherosclerotic lesions in C57BL/6J mice. Infect Immun 70:5332–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borin BN, Tang W, Nice TJ, McCune BT, Virgin HW, Krezel AM. 2014. Murine norovirus protein NS1/2 aspartate to glutamate mutation, sufficient for persistence, reorients side chain of surface-exposed tryptophan within a novel structured domain. Proteins 82:1200–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brabb T, von Dassow P, Ordonez N, Schnabel B, Duke B, Goverman J. 2000. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med 192:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW. 2010. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell 141:1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan L. 1992. Apolipoprotein B, the major protein component of triglyceride-rich and low-density lipoproteins. J Biol Chem 267:25621–25624. [PubMed] [Google Scholar]
- 11.Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL. 2007. CD36 and macrophages in atherosclerosis. Cardiovasc Res 75:468–477. [DOI] [PubMed] [Google Scholar]
- 12.Doom CM, Turula HM, Hill AB. 2009. Investigation of the impact of the common animal facility contaminant murine norovirus on experimental murine cytomegalovirus infection. Virology 392:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elena SF, Sanjuán R. 2005. Adaptive value of high mutation rates of RNA viruses: separating causes from consequences. J Virol 79:11555–11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ericsson AC, Myles M, Davis W, Ma L, Lewis M, Maggio-Price L, Franklin C. 2010. Noninvasive detection of inflammation-associated colon cancer in a mouse model. Neoplasia 12:1054–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. 2000. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest 105:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feeney ER, McAuley N, O'Halloran JA, Rock C, Low J, Satchell CS, Lambert JS, Sheehan GJ, Mallon PW. 2013. The expression of cholesterol metabolism genes in monocytes from HIV-infected subjects suggests intracellular cholesterol accumulation. J Infect Dis 207:628–637. [DOI] [PubMed] [Google Scholar]
- 17.Fong LG, Fong TA, Cooper AD. 1991. Inhibition of lipopolysaccharide-induced interleukin 1β mRNA expression in mouse macrophages by oxidized low-density lipoprotein. J Lipid Res 32:1899–1910. [PubMed] [Google Scholar]
- 18.Gerondopoulos A, Jackson T, Monaghan P, Doyle N, Roberts LO. 2010. Murine norovirus 1 cell entry is mediated through a non-clathrin-, non-caveolae-, dynamin- and cholesterol-dependent pathway. J Gen Virol 91:1428–1438. [DOI] [PubMed] [Google Scholar]
- 19.Gibellini D, Borderi M, Clò A, Morini S, Miserocchi A, Bon I, Ponti C, Re MC. 2013. HIV-related mechanisms in atherosclerosis and cardiovascular diseases. J Cardiovasc Med (Hagerstown) 14:780–790. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton TA, Major JA, Chisolm GM. 1995. The effects of oxidized low-density lipoproteins on inducible mouse macrophage gene expression are gene- and stimulus-dependent. J Clin Invest 95:2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson KS. 2008. Murine norovirus, a recently discovered and highly prevalent viral agent of mice. Lab Anim (NY) 37:314–320. [DOI] [PubMed] [Google Scholar]
- 22.Hensley SE, Pinto AK, Hickman HD, Kastenmayer RJ, Bennink JR, Virgin HW, Yewdell JW. 2009. Murine norovirus infection has no significant effect on adaptive immunity to vaccinia virus or influenza A virus. J Virol 83:7357–7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu CC, Riley LK, Wills HM, Livingston RS. 2006. Persistent infection with and serologic cross-reactivity of 3 novel murine noroviruses. Comp Med 56:247–251. [PubMed] [Google Scholar]
- 24.Hsu CC, Wobus CE, Steffen EK, Riley LK, Livingston RS. 2005. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin Diagn Lab Immunol 12:1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. 1996. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood 87:2020–2028. [PubMed] [Google Scholar]
- 26.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. 1993. Hypercholesterolemia in low-density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 92:883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. 1994. Massive xanthomatosis and atherosclerosis in cholesterol-fed low-density lipoprotein receptor-negative mice. J Clin Invest 93:1885–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW. 2003 STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578 [DOI] [PubMed] [Google Scholar]
- 29.Kim M, Lee H, Chang KO, Ko G. 2010. Molecular characterization of murine norovirus isolates from South Korea. Virus Res 147:1–6. [DOI] [PubMed] [Google Scholar]
- 30.Landreth KS. 2002. Critical windows in development of the rodent immune system. Hum Exp Toxicol 21:493–498. [DOI] [PubMed] [Google Scholar]
- 31.Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE, Schmitz G. 1999. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun 257:29–33. [DOI] [PubMed] [Google Scholar]
- 32.Lencioni KC, Drivdahl R, Seamons A, Treuting PM, Brabb T, Maggio-Price L. 2011. Lack of effect of murine norovirus infection on a mouse model of bacteria-induced colon cancer. Comp Med 61:219–226. [PMC free article] [PubMed] [Google Scholar]
- 33.Lencioni KC, Seamons A, Treuting PM, Maggio-Price L, Brabb T. 2008. Murine norovirus: an intercurrent variable in a mouse model of bacteria-induced inflammatory bowel disease. Comp Med 58:522–533. [PMC free article] [PubMed] [Google Scholar]
- 34.Ley K, Miller YI, Hedrick CC. 2011. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol 31:1506–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lumeng CN, Bodzin JL, Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. 2005. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest 115:2192–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, Fu Y, Dart A, Orenstein JM, Bobryshev YV, Bukrinsky M, Sviridov D. 2006. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol 4:e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller B, Klemm U, Mas Marques A, Schreier E. 2007. Genetic diversity and recombination of murine noroviruses in immunocompromised mice. Arch Virol 152:1709–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nice TJ, Strong DW, McCune BT, Pohl CS, Virgin HW. 2013. A single-amino-acid change in murine norovirus NS1/2 is sufficient for colonic tropism and persistence. J Virol 87:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohlsson BG, Englund MC, Karlsson AL, Knutsen E, Erixon C, Skribeck H, Liu Y, Bondjers G, Wiklund O. 1996. Oxidized low-density lipoprotein inhibits lipopolysaccharide-induced binding of nuclear factor κB to DNA and the subsequent expression of tumor necrosis factor α and interleukin 1β in macrophages. J Clin Invest 98:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paik J, Fierce Y, Drivdahl R, Treuting PM, Seamons A, Brabb T, Maggio-Price L. 2010. Effects of murine norovirus infection on a mouse model of diet-induced obesity and insulin resistance. Comp Med 60:189–195. [PMC free article] [PubMed] [Google Scholar]
- 42.Paik J, Fierce Y, Mai PO, Phelps SR, McDonald T, Treuting P, Drivdahl R, Brabb T, LeBoeuf R, O'Brien KD, Maggio-Price L. 2011. Murine norovirus increases atherosclerotic lesion size and macrophages in Ldlr−/− mice. Comp Med 61:330–338. [PMC free article] [PubMed] [Google Scholar]
- 43.Park SH, Kim JL, Lee ES, Han SY, Gong JH, Kang MK, Kang YH. 2011. Dietary ellagic acid attenuates oxidized LDL uptake and stimulates cholesterol efflux in murine macrophages. J Nutr 141:1931–1937. [DOI] [PubMed] [Google Scholar]
- 44.Perdue KA, Green KY, Copeland M, Barron E, Mandel M, Faucette LJ, Williams EM, Sosnovtsev SV, Elkins WR, Ward JM. 2007. Naturally occurring murine norovirus infection in a large research institution. J Am Assoc Lab Anim Sci 46:39–45. [PubMed] [Google Scholar]
- 45.Perry JW, Wobus CE. 2010. Endocytosis of murine norovirus 1 into murine macrophages is dependent on dynamin II and cholesterol. J Virol 84:6163–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roed T, Kristoffersen US, Knudsen A, Wiinberg N, Lebech AM, Almdal T, Thomsen RW, Kjaer A, Weis N. 2014. Increased prevalence of coronary artery disease risk markers in patients with chronic hepatitis C—a cross-sectional study. Vasc Health Risk Manag 10:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiffman D, Mikita T, Tai JT, Wade DP, Porter JG, Seilhamer JJ, Somogyi R, Liang S, Lawn RM. 2000. Large-scale gene expression analysis of cholesterol-loaded macrophages. J Biol Chem 275:37324–37332. [DOI] [PubMed] [Google Scholar]
- 48.Shrestha S, Irvin MR, Grunfeld C, Arnett DK. 2014. HIV, inflammation, and calcium in atherosclerosis. Arterioscler Thromb Vasc Biol 34:244–250. [DOI] [PubMed] [Google Scholar]
- 49.Steinberg D, Parthasarathy S, Carew TE, Witztum JL. 1989. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 32:915–924. [DOI] [PubMed] [Google Scholar]
- 50.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. 2007. Ly6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thackray LB, Wobus CE, Chachu KA, Liu B, Alegre ER, Henderson KS, Kelley ST, Virgin HW., 4th 2007. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J Virol 81:10460–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thorne LG, Goodfellow IG. 2014. Norovirus gene expression and replication. J Gen Virol 95:278–291. [DOI] [PubMed] [Google Scholar]
- 53.Weiss G, Christensen HR, Zeuthen LH, Vogensen FK, Jakobsen M, Frøkiaer H. 2011. Lactobacilli and bifidobacteria induce differential interferon-β profiles in dendritic cells. Cytokine 56:520–530. [DOI] [PubMed] [Google Scholar]
- 54.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol 2:e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wobus CE, Thackray LB, Virgin HW., 4th 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol 80:5104–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Worobey M, Holmes EC. 1999. Evolutionary aspects of recombination in RNA viruses. J Gen Virol 80:2535–2543. [DOI] [PubMed] [Google Scholar]
- 57.Yeom SC, Yu SA, Choi EY, Lee BC, Lee WJ. 2009. Prevalence of Helicobacter hepaticus, murine norovirus, and Pneumocystis carinii and eradication efficacy of cross-fostering in genetically engineered mice. Exp Anim 58:497–504. [DOI] [PubMed] [Google Scholar]
- 58.Zhang AJ, To KK, Li C, Lau CC, Poon VK, Chan CC, Zheng BJ, Hung IF, Lam KS, Xu A, Yuen KY. 2013. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis 207:1270–1280. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Z, de Beer MC, Cai L, Asmis R, de Beer FC, de Villiers WJ, van der Westhuyzen DR. 2005. Low-density lipoprotein from apolipoprotein-E–deficient mice induces macrophage lipid accumulation in a CD36 and scavenger receptor class-A–dependent manner. Arterioscler Thromb Vasc Biol 25:168–173. [DOI] [PubMed] [Google Scholar]