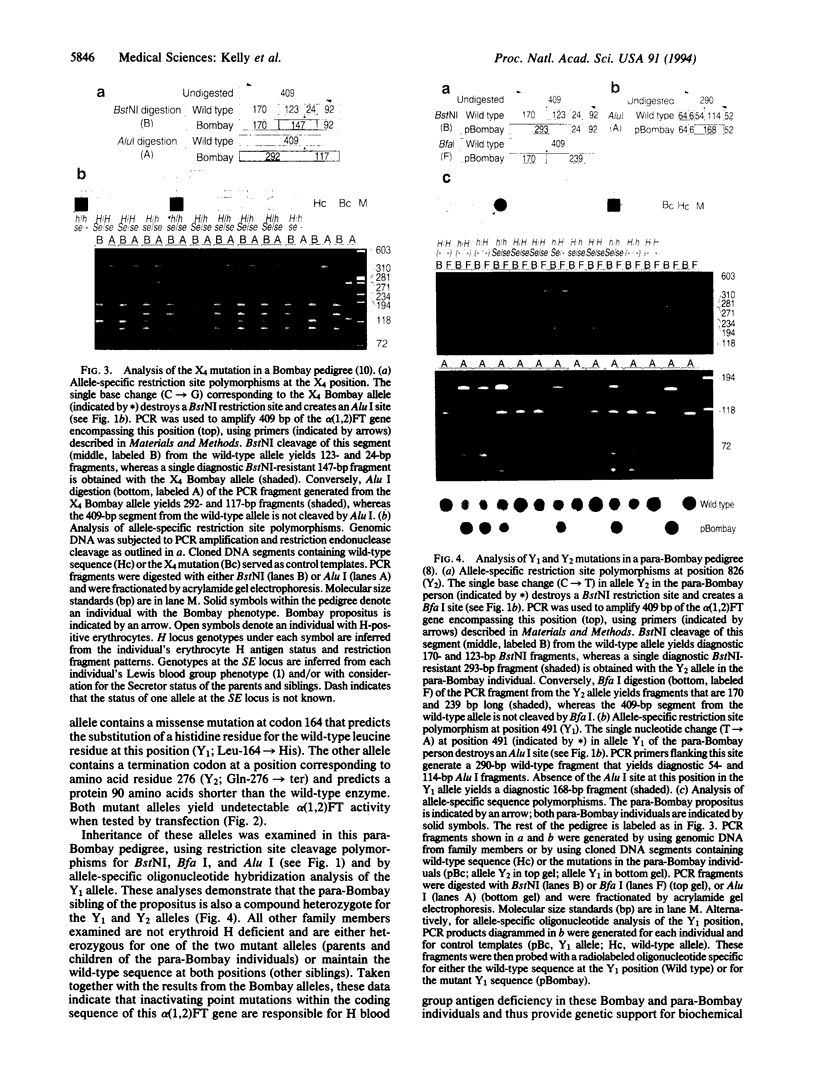
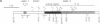Abstract
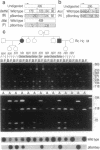
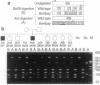
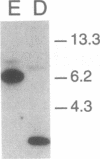
The penultimate step in the biosynthesis of the human ABO blood group oligosaccharide antigens is catalyzed by alpha-(1,2)-fucosyltransferase(s) (GDP-L-fucose: beta-D-galactoside 2-alpha-L-fucosyltransferase, EC 2.4.1.69), whose expression is determined by the H and Secretor (SE) blood group loci (also known as FUT1 and FUT2, respectively). These enzymes construct Fuc alpha 1-->2Gal beta-linkages, known as H determinants, which are essential precursors to the A and B antigens. Erythrocytes from individuals with the rare Bombay and para-Bombay blood group phenotypes are deficient in H determinants, and thus A and B determinants, as a consequence of apparent homozygosity for null alleles at the H locus. We report a molecular analysis of a human alpha-(1,2)-fucosyltransferase gene, thought to correspond to the H blood group locus, in a Bombay pedigree and a para-Bombay pedigree. We find inactivating point mutations in the coding regions of both alleles of this gene in each H-deficient individual. These results define the molecular basis for H blood group antigen deficiency in Bombay and para-Bombay phenotypes, provide compelling evidence that this gene represents the human H blood group locus, and strongly support a hypothesis that the H and SE loci represent distinct alpha-(1,2)-fucosyltransferase genes. Candidate sequences for the human SE locus are identified by low-stringency Southern blot hybridization analyses, using a probe derived from the H alpha-(1,2)-fucosyltransferase gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BHENDE Y. M., DESHPANDE C. K., BHATIA H. M., SANGER R., RACE R. R., MORGAN W. T. J., WATKINS W. M. A "new" blood group character related to the ABO system. Lancet. 1952 May 3;1(6714):903–904. [PubMed] [Google Scholar]
- Bhatia H. M., Sathe M. S. Incidence of "Bombay" (Oh) phenotype and weaker variants of A and B antigen in Bombay (India). Vox Sang. 1974;27(6):524–532. doi: 10.1111/j.1423-0410.1974.tb02450.x. [DOI] [PubMed] [Google Scholar]
- Blaszczyk-Thurin M., Sarnesto A., Thurin J., Hindsgaul O., Koprowski H. Biosynthetic pathways for the Leb and Y glycolipids in the gastric carcinoma cell line KATO III as analyzed by a novel assay. Biochem Biophys Res Commun. 1988 Feb 29;151(1):100–108. doi: 10.1016/0006-291x(88)90564-5. [DOI] [PubMed] [Google Scholar]
- Chan L. RNA editing: exploring one mode with apolipoprotein B mRNA. Bioessays. 1993 Jan;15(1):33–41. doi: 10.1002/bies.950150106. [DOI] [PubMed] [Google Scholar]
- Higuchi R., von Beroldingen C. H., Sensabaugh G. F., Erlich H. A. DNA typing from single hairs. Nature. 1988 Apr 7;332(6164):543–546. doi: 10.1038/332543a0. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Smithies O. Altering genes in animals by gene targeting. Annu Rev Immunol. 1992;10:705–730. doi: 10.1146/annurev.iy.10.040192.003421. [DOI] [PubMed] [Google Scholar]
- Kukowska-Latallo J. F., Larsen R. D., Nair R. P., Lowe J. B. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4)fucosyltransferase. Genes Dev. 1990 Aug;4(8):1288–1303. doi: 10.1101/gad.4.8.1288. [DOI] [PubMed] [Google Scholar]
- Kumazaki T., Yoshida A. Biochemical evidence that secretor gene, Se, is a structural gene encoding a specific fucosyltransferase. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4193–4197. doi: 10.1073/pnas.81.13.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M., Burnashev N., Sakmann B., Seeburg P. H. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993 Mar;10(3):491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- Larsen R. D., Ernst L. K., Nair R. P., Lowe J. B. Molecular cloning, sequence, and expression of a human GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6674–6678. doi: 10.1073/pnas.87.17.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pendu J., Cartron J. P., Lemieux R. U., Oriol R. The presence of at least two different H-blood-group-related beta-D-gal alpha-2-L-fucosyltransferases in human serum and the genetics of blood group H substances. Am J Hum Genet. 1985 Jul;37(4):749–760. [PMC free article] [PubMed] [Google Scholar]
- Lindenberg S., Sundberg K., Kimber S. J., Lundblad A. The milk oligosaccharide, lacto-N-fucopentaose I, inhibits attachment of mouse blastocysts on endometrial monolayers. J Reprod Fertil. 1988 May;83(1):149–158. doi: 10.1530/jrf.0.0830149. [DOI] [PubMed] [Google Scholar]
- Oriol R., Danilovs J., Hawkins B. R. A new genetic model proposing that the Se gene is a structural gene closely linked to the H gene. Am J Hum Genet. 1981 May;33(3):421–431. [PMC free article] [PubMed] [Google Scholar]
- REGE W. P., PAINTER T. J., WATKINS W. M., MORGAN W. T. ISOLATION OF SEROLOGICALLY ACTIVE FUCOSE-CONTAINING OLIGOSACCHARIDES FROM HUMAN BLOOD-GROUP H SUBSTANCE. Nature. 1964 Jul 25;203:360–363. doi: 10.1038/203360a0. [DOI] [PubMed] [Google Scholar]
- Rajan V. P., Larsen R. D., Ajmera S., Ernst L. K., Lowe J. B. A cloned human DNA restriction fragment determines expression of a GDP-L-fucose: beta-D-galactoside 2-alpha-L-fucosyltransferase in transfected cells. Evidence for isolation and transfer of the human H blood group locus. J Biol Chem. 1989 Jul 5;264(19):11158–11167. [PubMed] [Google Scholar]
- Roth M. S., Antin J. H., Bingham E. L., Ginsburg D. Use of polymerase chain reaction-detected sequence polymorphisms to document engraftment following allogeneic bone marrow transplantation. Transplantation. 1990 Apr;49(4):714–720. doi: 10.1097/00007890-199004000-00012. [DOI] [PubMed] [Google Scholar]
- SOLOMON J. M., WAGGONER R., LEYSHON W. C. A QUANTITATIVE IMMUNOGENETIC STUDY OF GENE SUPPRESSION INVOLVING A1 AND H ANTIGENS OF THE ERYTHROCYTE WITHOUT AFFECTING SECRETED BLOOD GROUP SUBSTANCES. THE ABH PHENOTYPES AHM AND OHM. Blood. 1965 Apr;25:470–485. [PubMed] [Google Scholar]
- Sarnesto A., Köhlin T., Thurin J., Blaszczyk-Thurin M. Purification of H gene-encoded beta-galactoside alpha 1----2 fucosyltransferase from human serum. J Biol Chem. 1990 Sep 5;265(25):15067–15075. [PubMed] [Google Scholar]
- Watkins W. M. Biochemistry and Genetics of the ABO, Lewis, and P blood group systems. Adv Hum Genet. 1980;10:1-136, 379-85. doi: 10.1007/978-1-4615-8288-5_1. [DOI] [PubMed] [Google Scholar]
- Weston B. W., Smith P. L., Kelly R. J., Lowe J. B. Molecular cloning of a fourth member of a human alpha (1,3)fucosyltransferase gene family. Multiple homologous sequences that determine expression of the Lewis x, sialyl Lewis x, and difucosyl sialyl Lewis x epitopes. J Biol Chem. 1992 Dec 5;267(34):24575–24584. [PubMed] [Google Scholar]
- Yamamoto F., Clausen H., White T., Marken J., Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990 May 17;345(6272):229–233. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]