Abstract
Intravenous immunoglobulin (IVIG) decreases neutrophil adhesion to endothelium and red blood cell-neutrophil interactions in sickle cell mice undergoing vaso-occlusion. In this Phase I clinical trial of sickle cell anemia (SCA) patients admitted with pain crisis, we evaluated the status of adhesion molecules on neutrophils in control and IVIG-treated subjects pre- and post-infusion up to 800 mg/kg, the same dose used in murine studies. Mac-1 function significantly decreased from baseline in the low-dose IVIG (200–400 mg/kg) cohorts. IVIG-related adverse events may have occurred in the high-dose (600–800 mg/kg) cohorts. There were no significant increases in neutrophil and leukocyte counts, suggesting that IVIG may more selectively inhibit Mac-1 function as opposed to neutrophil adhesion. This study provides the first in-human validation of pre-clinical murine studies that IVIG can decrease Mac-1 function.
Keywords: sickle cell anemia, vaso-occlusion, pain crisis, Mac-1, intravenous gammaglobulin
Introduction
In murine models of sickle cell acute pain crisis, intravenous immunoglobulin (IVIG) reduces neutrophil adhesion to post capillary venular endothelium and adherent neutrophil interactions with circulating red blood cells (RBCs), thus increasing microcirculatory blood flow and survival.[1, 2] These effects were observed at IVIG doses between 200–800 mg/kg as early as 20 minutes after IVIG administration.[1, 2] While neutrophil rolling and adhesion are mediated via P and E-selectins on endothelium, E-selectin ligand-1 and Src family kinase activation mediate a secondary wave of signals polarizing activated Mac-1 (αMβ2) on the leading edge of neutrophils, with subsequent capture of sickle RBCs.[3] IVIG inhibits Mac-1 dependent RBC capture by binding to the activating Fcγ receptor FcγRIII on neutrophils, resulting in recruitment of the protein tyrosine phosphatase SHP-1 to FcγRIII and subsequent inhibition of activated Mac-1.[4]
The potentially important role of neutrophil adhesion and sickle RBC capture in sickle cell mouse models of acute pain crisis has recently been supported by the success of the Phase II trial of the pan-selectin inhibitor GMI-1070 in reducing time to resolution of pain crisis and opioid use[5–7].
We conducted a Phase I study of the safety and effect on neutrophil activation status of IVIG administered, owing to its long half-life, as a single-dose infusion upon hospital admission for acute pain crisis.
Methods
Study design and conduct
A Phase I randomized, double-blind, dose-finding study of IVIG (Gamunex-C, Grifols, Clayton, NC) was conducted in children and adults with Hb SS or Sβ0-thalassemia requiring hospital admission for uncomplicated (unaccompanied by infection or other acute processes) acute pain crisis between January 2009 and December 2013. The study took place at 2 collaborating hospitals, The Mount Sinai and Montefiore Medical Centers. Gamunex-C is hypo-osmolar (258 mOsm/kg), sucrose-free, and contains only trace amounts of sodium, and thus has an excellent risk profile with regard to volume overload/renal toxicity.[8, 9] 15 subjects were randomized by pharmacy staff using a computer-generated randomization algorithm to a total of 20 acute pain crises at a ratio of 3 IVIG: 1 equivalent-volume normal saline control at each dosing cohort of 100, 200, 400, 600, and 800 mg/kg IVIG (a modified Fibonacci dose escalation design). Subject re-enrollment at subsequent dosing levels occurred in 3 subjects after a 3-month washout period.
Study drug was administered as soon as logistically feasible after inpatient admission. Actual patient weight was used for dose calculation. Standard-dose oral acetaminophen and diphenhydramine pre-medication, as well as bolus opioids as needed with single line availability, was administered and then study drug/placebo was infused undiluted (100 mg/mL) at the recommended manufacturer rate (starting at 1 mg/kg/min up to 8 mg/kg/min for dosing cohorts 100, 200 and 400mg/kg, up to 4mg/kg/min for 600mg/kg, and over 6 hours for 800mg/kg). Clinicians (including investigators) and patients were blinded to treatment by masking of the infusion bag and tubing by pharmacy staff. Outpatient hydroxyurea (HU) dosing was continued during the hospitalization. Pain was managed with morphine or hydromorphone patient controlled analgesia adjusted per usage and the FACES or numeric rating scales at least every 8 hours throughout the hospital stay by anesthesiologist co-investigators. The non-steroidal anti-inflammatory drugs ibuprofen or ketorolac were also allowed, but there was no significant difference in their usage rate between groups. Subcutaneous heparin prophylaxis was administered to adult patients per routine standard at Mount Sinai, but was not administered to pediatric patients at Montefiore.
A Data Safety and Monitoring Board approved progression to the next cohort after completion of each dosing cohort. Stopping rules for study hold pending IRB review were 2 unexpected possibly-related Grade 3 severe adverse events (SAE), 1 unexpected possibly-related thrombo-embolic event, or 1 unexpected possibly-related Grade 4–5 SAE.
The study was institutional review board approved at all sites, and written informed consent was obtained from all subjects. All investigators involved in data collection and analysis had access to primary clinical trial data. The trial is registered as “Intravenous Gamma globulin for Sickle Cell Pain Crisis” under the ClinicalTrials.gov identifier NCT01757418.
Study subjects
Inclusion criteria were as follows: SS or Sβ0-thalassemia sickle cell disease genotype; age 8–65 years for dosing cohorts up to 400mg/kg and 12–65 years for subsequent dosing cohorts; normal stroke risk as assessed by transcranial Doppler or MRA; and acute pain crisis requiring hospital admission and parenteral opioids.
Exclusion criteria were as follows: concomitant acute process, fever >38.5° C and clinical suspicion of infection, serum alanine aminotransferase >2X ULN; serum creatinine > 1.3 mg/dL or spot urine protein >300 mg/dL ; Hb > 10 g/dL or Hct > 30%; concomitant condition associated with renal dysfunction; prior stroke or thrombosis; current estrogen use; pregnancy or breastfeeding; current participation in another investigational drug study or a chronic transfusion program; known IgA deficiency or allergy to gamma globulin; vaccination with a live attenuated virus in the preceding 6 weeks; documented history of illicit drug abuse.
Clinical outcomes
Safety outcome variables were treatment-emergent adverse events (AE) followed through 30 days after hospital discharge. IVIG complications that were pro-actively assessed included thrombosis, immune hemolysis (as detected by a positive direct antiglobulin test), high serum viscosity, high serum troponin, and high serum creatinine. Acute pain crisis-associated complications such as acute chest syndrome, RBC transfusion, and hospital re-admission were also monitored. The following efficacy markers were analyzed: time to pain crisis resolution from the end of study drug infusion (resolution defined as pain scores consistently ≤ 5 out of 10 and off IV opioids); cumulative opioid use from the end of study drug infusion (in morphine equivalent units (MEU)/kg), change in hemoglobin (Hb) post-infusion, and 24 hour (hr) post-infusion lactate dehydrogenase (LDH) and high-sensitivity C-reactive protein (CRP).
Neutrophil activation assessment
Venous blood into EDTA or ACD drawn immediately pre- and 24±4 hours post- infusion was evaluated by flow cytometry for adhesion markers on CD16b+ (ID3, Beckman Coulter) neutrophils: activated Mac-1 (CBRM1/5, eBioscience) or activated β2 integrin (ab13219, abcam), Mac-1 (ICRF44, BD Pharmingen), CD44 (G44-26, BD Pharmingen), E-selectin-Fc chimera (R&D Systems), L-selectin (DREG-56, eBioscience), LFA-1 (HI111 BD Pharmingen), and PSGL-1 (KPL-1, BD Pharmingen). Activated Mac-1 Ab detects the functionally active form of Mac-1(was performed on 200–800 mg/kg cohorts) and activated β2 integrin recognizes the high affinity conformation of β2 integrin, the β subunit of LFA-1 and Mac-1 (was performed on 100 mg/kg cohort).
With late afternoon or evening study drug administration, samples were stored overnight as needed (range 8–24 hours) at 4°C prior to analysis. When stored overnight, all paired pre-IVIG and 24 hour post-IVIG samples per patient were stored for uniform lengths of time. No adhesion markers except activated Mac-1 show expression increase at 24 hr compared to immediate analysis (Figure 1B). Since the time interval to analysis was constant for each subject’s two samples, the ratio between the two samples (Figure 1A) was unaffected by storage.
Figure 1. Neutrophil Mac-1 expression and influence of 24 hour storage.
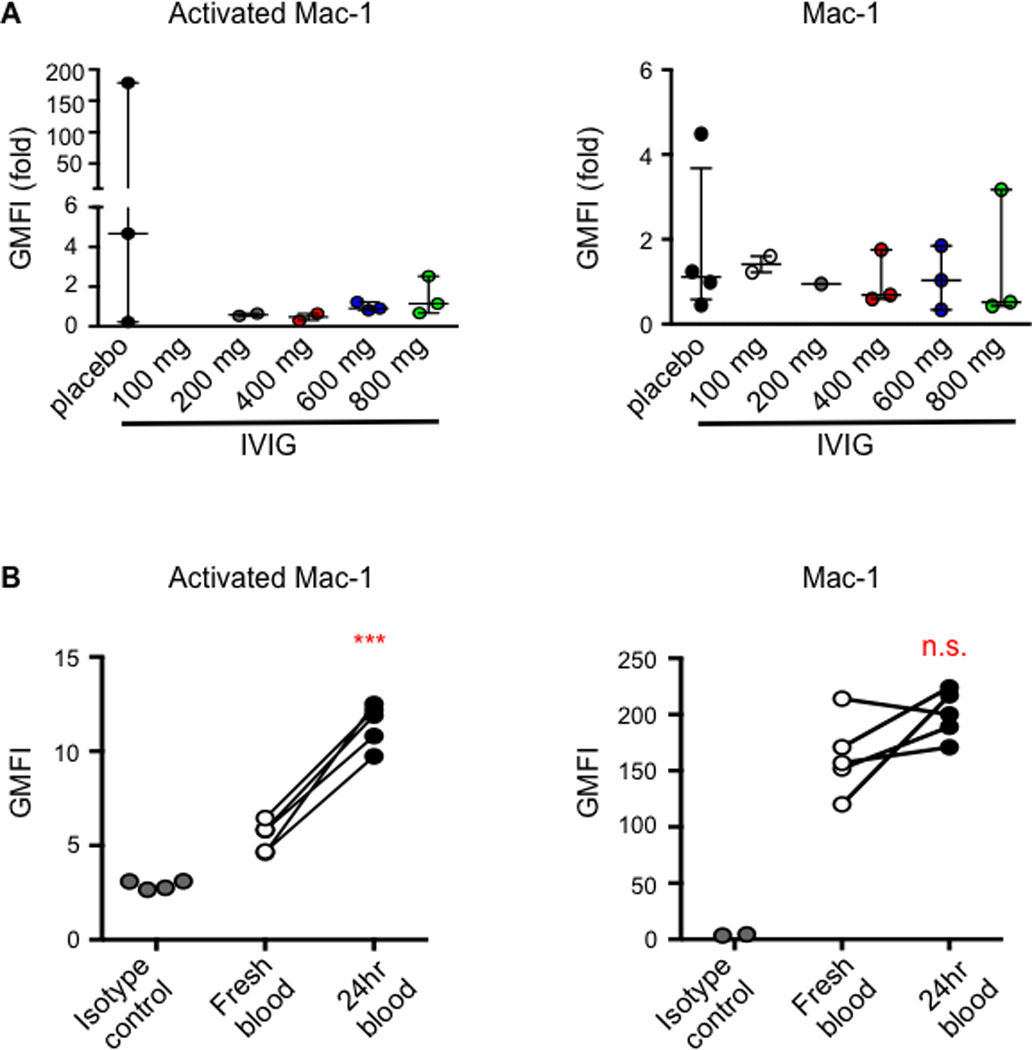
A) Ratio of the post-to-pre geometric mean fluorescent intensity (GMFI) for activated Mac-1 and total Mac-1 on CD16b-positive leukocytes by flow cytometry. B) 24 hour storage shows consistent increase in activated Mac-1 expression compared to fresh samples, but since the storage interval for the pre and post-sample was uniform in each subject, the ratio of post to pre GMFI is valid (paired t-test, ***p<0.001).
Statistical analysis
Analysis was of the intent-to-treat population, which was the same as the per-protocol population due to absence of deviations. For clinical outcomes, control and IVIG groups were compared using the Mann-Whitney U or Kruskal-Wallis tests for continuous variables and Pearson’s chi-squared testing for categorical variables (Excel 2010, Microsoft Corporation, Redmond, WA or SPSS Statistics 18, IBM Corp, Armonk, NY). Paired t-testing was also used to compare 24h to baseline values for neutrophil adhesion markers (GraphPad Prism 5, GraphPad Software, La Jolla, CA).
Results
Table I shows the baseline subject and pain crisis characteristics. There were no statistically significant differences between the IVIG and control groups as wholes except for a greater number of pain crisis admissions in the year preceding the study admission in the IVIG group. All subjects were on hydroxyurea except the control patient in the 100 mg/kg cohort.
Table I.
Clinical data
| 100 mg/kg (n=3) |
200 mg/kg (n=3) |
400 mg/kg (n=3) |
600 mg/kg (n=3) |
800 mg/kg (n=3) |
Saline control (n=5) |
p- value |
|
|---|---|---|---|---|---|---|---|
| Subject traits | |||||||
| Age (yr) | 24 (24–24) | 24 (10–35) | 25 (9–25) | 12 (12–13) | 14 (13–16) | 24 (13–28) | 0.31 |
| Female: male gender (%) | 100 | 67:33 | 33:67 | 67:33 | 100 | 40:60 | 0.29 |
| African-American: Hispanic race (%) | 33:67 | 67:33 | 33:67 | 67:33 | 67:33 | 60:40 | 1.0 |
| Hb genotype SS: SB0 (%) | 67:33 | 67:33 | 100:0 | 67:33 | 67:33 | 80:20 | 0.63 |
| G6PD carrier (%) | 0 | 0 | 0 | 0 | 0 | 20 | 0.25 |
| Hydroxyurea (%) | 100 | 100 | 100 | 100 | 100 | 80 | 0.25 |
| 1-year prior pain crisis admissions (n) | 7 (5–8) | 7 (1–7) | 3 (3–7) | 3 (3–6) | 3 (1–7) | 2 (0–4) | 0.045 |
| Pain crisis traits | |||||||
| Admission hemoglobin (mg/dL) | 8.8 (8.1–9.1) | 8.9 (7.9–9.4) | 8.4 (7.9–8.7) | 9.0 (8.7–9.3) | 9.4 (8.6–9.5) | 9.1 (6.1–9.8) | 0.61 |
| Admission white cells (K/µL) | 13.9 (11.3–24.1) | 12.6 (11.3–12.6) | 15.8 (4.8–15.6) | 15.1 (4.5–17) | 9.7 (8.6–13.9) | 18.6 (9.9–20.6) | 0.10 |
| Admission ANC (K/uL) | 9.7 (5.9–13.4) | 7 (3.1–9.4) | 5.7 (2.1–6.7) | 5.8 (2–9) | 7.8 (6.6–10.4) | 9.7 (5.7–13.1) | 0.23 |
| Admission platelets (K/uL) | 393 (371–446) | 263 (259–530) | 332 (237–434) | 477 (158–488) | 332 (218–446) | 320 (86–446) | 0.44 |
| Admission Hb S (%) Hb F (%) |
82 (77–84) 19 (5–24) |
87 (65–88) 8 (5–12) |
74 (68–75) 15 (16–21) |
72.5 (69.7–73.9) 19.8 (16.2–21.9) |
75.3 (69.7–77.4) 17.2 (13.7–21.9) |
82 (61–94) 11 (1–20) |
0.13 0.12 |
| Admission creatinine (mg/dL) | 0.5 (0.6–0.7) | 0.5 (0.4–1.1) | 0.6 (0.3–0.8) | 0.5 (0.5–0.6) | 0.5 (0.5–0.6) | 0.7 (0.4–0.8) | 0.50 |
| Admission pain score (0–10) Pre-infusion pain score (0–10) |
7 (7–7.5) 7 (7–7.5) |
8 (6–10) 8 (6–8) |
7 (6–8) 7 (6–8) |
6 (5–7) 5 (4–5) |
10 (9–10) 8 (8–9) |
9.5 (6–10) 7 (6–8) |
0.30 0.51 |
| Pre-infusion opioid use (ME/kilogram) | 1.55 (.54–3.05) | 0.41 (.06–1.67) | 1.27 (.31–1.35) | 0.6 (0.1–1.04) | 1.38 (0.6–1.66) | 0.81 (0.74–2.06) | 0.64 |
| Emergency admit to infusion start (h) | 14 (9–51) | 10 (8–22) | 24 (7–28) | 24 (19–33) | 30 (19–30) | 22 (10–78) | 0.40 |
Values are medians with ranges (continuous variables) or percentages (categorical variables). P-value assesses the entire IVIG group compared to the saline control group. G6PD = glucose-6-phophate dehydrogenase. ANC= absolute neutrophil count. ME = morphine equivalents.
Clinical outcomes
Safety outcomes are summarized in Table II. In both groups, the mean total number of adverse events per subject was 4, with no significant differences in total (p=0.9) or SAEs (p=0.8). IVIG was well tolerated, with possibly IVIG-related events being self-limited Grade 1–2 headache, fever, and hypertension observed in pediatric patients, mostly at the 600–800 mg/kg dosing levels. Pre- and 24h post- serum creatinine, serum viscosity, serum troponin, and direct antiglobulin tests remained within normal ranges in all subjects.
Table II.
Safety Outcomes
| 100 mg/kg (n=3) |
200 mg/kg (n=3) |
400 mg/kg (n=3) |
600 mg/kg (n=3) |
800 mg/kg (n=3) |
Saline control (n=5) |
p- value |
|
|---|---|---|---|---|---|---|---|
| Pre-specified adverse events | |||||||
| Acute chest syndrome (%) | 0 | 0 | 0 | 0 | 33 | 0 | 1.0 |
| Thrombosis (%) | 0 | 0 | 0 | 0 | 33 | 0 | 1.0 |
| Pain crisis re-admission (%) | 0 | 33 | 67 | 100 | 0 | 20 | 0.63 |
| Red cell transfusion (%) | 33 | 0 | 0 | 0 | 33 | 40 | 0.25 |
| 24h serum creatinine (mg/dL) | 0.4 (0.3–0.5) | 0.5 (0.5–0.6) | 0.5 (0.2–0.6) | 0.5 (0.5–0.5) | 0.6 (0.5–0.6) | 0.6 (0.4–0.8) | 0.28 |
| Possibly IVIG-related Grade 1–2 adverse events | |||||||
| Fever (%) | 0 | 0 | 0 | 0 | 100 | 40 | 0.56 |
| Hypertension (%) | 0 | 0 | 33 | 0 | 67 | 20 | 1.00 |
| Headache (%) | 0 | 0 | 0 | 100 | 33 | 0 | 0.53 |
There were 2 SAEs in the study, both in the 800 mg/kg IVIG cohort, that were deemed not likely to be IVIG related: progression to acute chest syndrome (ACS) 24 hours after IVIG infusion (2 days post-admission); and in another subject, a left brachial venous thrombosis associated with a peripherally inserted central catheter (PICC), diagnosed 17 days after IVIG infusion. Since these SAEs were expected, they did not meet criteria for stopping the trial.
All hospital re-admissions within the 30 day follow-up were for uncomplicated pain crisis. All re-admissions in the IVIG group occurred 20–30 days post-infusion (when the IVIG blood concentration is predicted to be ~40% of its peak) compared to the one control re-admission at 13 days post-infusion. There was a trend towards a higher re-admission rate with increasing doses of IVIG up to the 600 mg/kg cohort. This trend may be related to the IVIG group having a statistically greater number of pain crises in the preceding 12 months. There were no readmissions in the 800mg/kg cohort but several factors mitigated re-admission risk in this cohort: the patient with PICC-related venous thrombosis was started on heparin 17 days after IVIG infusion; the patient with ACS underwent red cell exchange 2 days after IVIG infusion, and the third patient initiated methadone therapy for recurrent acute pain post-discharge.
As expected for the small sample size, there were no statistically significant differences in time to crisis resolution, cumulative opioid use, and time to hospital discharge between groups (Table III). There was no significant difference between groups in the expected drop in Hb during hospitalization. There were smaller increases in LDH and CRP in the IVIG group, suggesting a potential salutary effect on hemolysis and inflammation. Notably, the IVIG group did not have an increase in WBC count or ANC, in contrast to expectation given IVIG’s purported effect on neutrophil adhesion.[1, 4] All subjects had decreases in WBC and ANC over time with no significant differences between groups.
Table III.
Efficacy Outcomes
| IVIG (n=15) | Control (n=5) | p-value | |
|---|---|---|---|
| Clinical endpoint outcomes | |||
| Time to crisis resolution* (hours) | 54 (1,215) | 63 (31,164) | 0.61 |
| Cumulative opioid use* (ME/kilogram) | 2.2 (0,8.8) | 1.6 (1.1,15.6) | 1.0 |
| Time to hospital discharge (days) | 4.4 (1,10) | 4.8 (3,5) | 0.67 |
| Clinical laboratory indicators | |||
| % Δ in hemoglobin# | −17 (−33,0) | −17 (−25, −3) | 0.76 |
| % Δ in white blood cell count | −15 (−48,13) | −22 (−41, 37) | 0.96 |
| % Δ in absolute neutrophil count | −18 (−65, 45) | −16 (−51,49) | 0.81 |
| % Δ in lactate dehydrogenase# | 4 (−48, 85) | 14 (−11, 679) | 0.46 |
| % Δ in high sensitivity C-reactive protein# | 38 (−16, 868) | 192 (1.5, 1427) | 0.43 |
Pre-specified primary outcome measure.
Pre-specified secondary outcome measure.
Values are medians with ranges. % change values are calculated as: Δ = (post value − pre value) / pre-value. The post-hemoglobin is the lowest value of all post-infusion measurements; all other post-values are from 24 hr post-infusion.
Neutrophil activation assessment
Consistent with murine data that IVIG decreases RBC-neutrophil interactions via Mac-1 inhibition[3, 4], IVIG treatment was associated with decreased surface expression of functional (activated) Mac-1 greater than the decreased surface expression of the total amount of Mac-1 (Figure 1A). In the combined low-dose IVIG cohort (200–400 mg/kg), the decrease in activated Mac-1 expression was significantly lower post- compared to pre-IVIG (p=.02). Increasing IVIG doses to 600 and 800 mg/kg did not further decrease activated Mac-1.
Mac-1 activation may correlate with pain crisis severity. The control subject with the 178 times increase in surface expression of activated Mac-1 had a prolonged hospital course of 8 days marked by thrombocytopenia and the highest LDH increase (679%) and highest opioid usage of all study subjects. She was on HU but at a suboptimal dose of 12 mg/kg/day with HbF of only 4.4%. The control subject with the 4.5 times increase in surface expression of activated Mac-1, however, was on HU at 28 mg/kg/day with a HbF of 19.9%, yet had the highest LDH and CRP on hospital admission of all study subjects. The single control subject not on hydroxyurea had no higher β2-integrin activation (detects high-affinity conformation of β2 integrin) than the IVIG-treated subjects in his 100mg/kg cohort.
There were no significant differences in E-selectin binding, which mediates neutrophil recruitment/rolling[10], or neutrophil PSGL-1 and CD44 expression, which are ligands for E-selectin [11, 12], between control and IVIG groups nor within IVIG cohorts for 24 hr post- compared to pre- infusion values (data not shown). Other molecules involved in neutrophil recruitment/rolling[12], L-selectin and LFA-1, also showed no significant differences between or within groups (data not shown).
Discussion
This study supports the safety of IVIG in patients with sickle cell anemia screened by normal creatinine and lack of significant proteinuria. There was no evidence of post-IVIG renal dysfunction and the only probably-IVIG related adverse events were Grade 1–2 fever, hypertension, and headache, observed at the higher dosing levels. Furosemide has been used as prophylaxis for such IVIG-related symptoms and might have been helpful.
Although two subjects in the 800 mg/kg IVIG cohort suffered serious adverse events, these were deemed unlikely to be IVIG-related. The majority of IVIG related clots occur within 1–5 days of infusion [13, 14] in contrast to the PICC-line thrombosis occurring 17 days post-infusion. The venous thromboembolism rate is also higher with PICC lines than other central venous catheters [15]. The occurrence of ACS 2 days after admission is consistent with ACS epidemiology, where hospital admission, typically for pain crisis, precedes ACS in about 50% of patients, with ACS occurring at a mean 2.5 days after admission[16]. Analysis of the Montefiore pediatric patient population reveals that 20% of all pain crisis admissions progress to ACS (unpublished), which is consistent with 1 of 10 pediatric study subjects progressing to ACS. Finally, neutrophils lacking selectins and integrins may still adhere to blood vessels in the lungs [12], suggesting that IVIG might not mitigate progression to ACS.
Although not statistically significant, there was a higher rate of re-admission in the IVIG-treated group (40% versus 20%), which might be explained be its higher pain crisis admission frequency in the preceding 12 months as well as high average 30-day pediatric and adult readmission rates of 17 and 41%, respectively, in the SCD population.[17, 18] The lack of re-admission in the 800 mg/kg IVIG group may be due to initiation of mitigating treatment. Therefore, as neutrophil activation can be dose-related [19], the possibility of greater pain crisis re-admissions with high-dose IVIG cannot be excluded.
Because hydroxyurea treatment may decrease neutrophil-RBC interactions[20, 21], normalize L-selectin shedding[22] and decrease Mac-1 activation[21], that all IVIG-treated subjects were on chronic hydroxyurea (continued during hospitalization) may have decreased our ability to see an effect of IVIG. Nevertheless, our neutrophil adhesion studies provide support that IVIG treatment in human sickle cell pain crisis can decrease surface expression of activated Mac-1 as observed in the murine model. [3, 4] The E-selectin ligand PSGL-1 and the integrin LFA-1 were not decreased with IVIG in our study and were previously reported to be decreased with IVIG [23, 24], but on non-selected leukocytes or only on mononuclear cell populations, respectively. Our clinical data is consistent with our previous observations in the murine model, where no decrease of E-selectin binding, PSGL-1, or LFA-1 was observed 60 minutes after IVIG administration despite its effect on neutrophil adhesion and RBC interactions as early as 20 minutes after IVIG administration. [2] Therefore, although our data did not include evaluation of ESL-1, which is the third E-selectin ligand in addition to PSGL-1 and CD44[25] and can mediate Mac-1 activation in pain crisis[3], it is unlikely that ESL-1 expression would change after IVIG treatment. The murine sickle cell studies showing the importance of ESL-1 and E-selectin in Mac-1 activation were performed using E-selectin knockout mice transplanted with sickle cell bone marrow or with E-selectin Abs injected prior to surgery; therefore these studies demonstrate the necessity of ESL-1 for Mac-1 activation, but other mechanisms subsequently may impact Mac-1 activation. Indeed, IVIG inhibits Mac-1 activation through an independent antagonistic pathway involving Fc gammaRIII-mediated recruitment of Src-homology 2-containing tyrosine phosphatase-1.[4]
In summary, our study suggests that low-dose IVIG at 200–400 mg/kg has increased efficacy, as assessed by Mac-1 stabilization, and better tolerability compared to higher doses. Based on this encouraging data, we are working towards initiating a double blind, randomized, placebo controlled Phase II study at the 400 mg/kg dose. In order to rule out rebound neutrophil activation, neutrophil adhesion at 20 days (as well as 24 hours after infusion) may be added depending on complications observed.
The recently completed Phase II GMI-1070 trial[7] suggests that inhibitors of neutrophil recruitment to vascular endothelium may be beneficial for acute pain crises. Possible advantages of IVIG over GMI-1070, assuming similar efficacy, are the longer half-life of ~30 days and known long-term safety profile. Since significant increases in WBC and neutrophil counts were observed with GMI-1070 but not with IVIG [26], IVIG may have a greater relative effect on RBC capture compared to neutrophil adhesion, which may be advantageous due to potential infectious risk with a direct neutrophil adhesion inhibitor. Possible disadvantages of IVIG include its cost and potential limited availability. Finally, our study also highlights the importance of clinical validation, as IVIG and GMI-1070 showed similar degrees of effect on WBC adhesion and RBC-WBC interactions in murine models.[2, 27]
Acknowledgements
The authors would like to thank Casimir Zygmunt of Talecris and Carol Dagney of Grifols who provided critical support for this study; the research nurses Crystal Miller and Caroline Canty; the research coordinators Karen Ireland and Erika Choi; Drs. Barry Coller, Mohandas Narla, and Janice Gabrilove for advice and expertise; and most importantly, the patients who participated in the study.
This study was supported by FDA grant R01FD003447 (P.A.S, D.M.) and NHLBI grant K23HL089217 (P.A.S.). This study was also supported in part by the CTSA Grant 1 UL1 TR001073-01, 1 TL1 TR001072-01, 1 KL2 TR001071-01 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and an Investigator Sponsored Research grant from Grifols (D.M). Talecris and Grifols provided Gamunex-C free of charge for this study.
Footnotes
Authorship Contributions and Disclosure of Conflicts of Interest
DM: Designed and conducted the study, analyzed data, enrolled patients, and wrote the manuscript.
GC: Performed laboratory measurements, analyzed data, and wrote the manuscript.
VC: Designed and conducted the study, analyzed data, and reviewed the manuscript.
SS: Designed and conducted the study, analyzed data, and reviewed the manuscript.
OO: Conducted the study and reviewed the manuscript.
JJ: Performed laboratory measurements and reviewed the manuscript.
MH: Performed laboratory measurements and reviewed the manuscript.
HC: Analyzed data and reviewed the manuscript.
HB: Designed the study and edited the manuscript.
GFA: Designed the study and edited the manuscript.
PSF: Designed the study and edited the manuscript.
PAS: Designed and conducted the study, analyzed data, enrolled patients, and wrote the manuscript.
The authors report no relevant conflict of interests.
References
- 1.Turhan A, Jenab P, Bruhns P, et al. Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytes. Blood. 2004;103:2397–2400. doi: 10.1182/blood-2003-07-2209. [DOI] [PubMed] [Google Scholar]
- 2.Chang J, Shi PA, Chiang EY, et al. Intravenous immunoglobulins reverse acute vaso-occlusive crises in sickle cell mice through rapid inhibition of neutrophil adhesion. Blood. 2008;111:915–923. doi: 10.1182/blood-2007-04-084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidalgo A, Chang J, Jang JE, et al. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nature medicine. 2009;15:384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang JE, Hidalgo A, Frenette PS. Intravenous immunoglobulins modulate neutrophil activation and vascular injury through FcgammaRIII and SHP-1. Circulation research. 2012;110:1057–1066. doi: 10.1161/CIRCRESAHA.112.266411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon SICS, 1, Thackray H, Magnani JL, Wun T. Inhibition of E-Selectin Inflammatory Function by the Glycomimetic GMI-1070. Blood: The American Society of Hematology. 2011 [Google Scholar]
- 6.Magnani JLKF, Patton JT, Larkin SK, Styles L, DeCastro LM, Telen MJ, Wun T, Thackray H. Pan-Selectin Antagonist GMI-1070 Affects Biomarkers of Adhesion, Activation and the Coagulation Cascade in Sickle Cell Adults At Steady State. Blood: The American Society of Hematology. 2012 [Google Scholar]
- 7.Telen MJWT, McCavit TL, DeCastro LM, Krishnamurti L, Lanzkron S, Hsu LL, Smith WR, Rhee S, Magnani JL, Thackray H. GMI 1070: Reduction In Time To Resolution Of Vaso-Occlusive Crisis and Decreased Opioid Use In a Prospective, Randomized, Multi-Center Double Blind, Adaptive Phase 2 Study In Sickle Cell Disease. Blood: The American Society of Hematology. 2013 [Google Scholar]
- 8.Lin RY, Rodriguez-Baez G, Bhargave GA, et al. Intravenous gammaglobulin-associated renal impairment reported to the FDA: 2004 – 2009. Clinical nephrology. 2011;76:365–372. doi: 10.5414/cn106824. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Ahmed I, Nissel-Horowitz S, et al. Intravenous gammglobulin-associated acute renal failure. American journal of hematology. 2001;66:151–152. doi: 10.1002/1096-8652(200102)66:2<151::AID-AJH1035>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Turhan A, Weiss LA, Mohandas N, et al. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama Y, Hidalgo A, Chang J, et al. CD44 is a physiological E-selectin ligand on neutrophils. The Journal of experimental medicine. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews Immunology. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 13.Go RS, Call TG. Deep venous thrombosis of the arm after intravenous immunoglobulin infusion: case report and literature review of intravenous immunoglobulin-related thrombotic complications. Mayo Clin Proc. 2000;75:83–85. doi: 10.4065/75.1.83. [DOI] [PubMed] [Google Scholar]
- 14.Funk MB, Gross N, Gross S, et al. Thromboembolic events associated with immunoglobulin treatment. Vox Sang. 2013;105:54–64. doi: 10.1111/vox.12025. [DOI] [PubMed] [Google Scholar]
- 15.Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382:311–325. doi: 10.1016/S0140-6736(13)60592-9. [DOI] [PubMed] [Google Scholar]
- 16.Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 17.Sobota A, Graham DA, Neufeld EJ, et al. Thirty-day readmission rates following hospitalization for pediatric sickle cell crisis at freestanding children's hospitals: risk factors and hospital variation. Pediatric blood & cancer. 2012;58:61–65. doi: 10.1002/pbc.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. Jama. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 19.Casulli S, Topcu S, Fattoum L, et al. A differential concentration-dependent effect of IVIg on neutrophil functions: relevance for anti-microbial and anti-inflammatory mechanisms. PloS one. 2011;6:e26469. doi: 10.1371/journal.pone.0026469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finnegan EM, Turhan A, Golan DE, et al. Adherent leukocytes capture sickle erythrocytes in an in vitro flow model of vaso-occlusion. American journal of hematology. 2007;82:266–275. doi: 10.1002/ajh.20819. [DOI] [PubMed] [Google Scholar]
- 21.Almeida CB, Scheiermann C, Jang JE, et al. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood. 2012;120:2879–2888. doi: 10.1182/blood-2012-02-409524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benkerrou M, Delarche C, Brahimi L, et al. Hydroxyurea corrects the dysregulated L-selectin expression and increased H(2)O(2) production of polymorphonuclear neutrophils from patients with sickle cell anemia. Blood. 2002;99:2297–2303. doi: 10.1182/blood.v99.7.2297. [DOI] [PubMed] [Google Scholar]
- 23.Gill V, Doig C, Knight D, et al. Targeting adhesion molecules as a potential mechanism of action for intravenous immunoglobulin. Circulation. 2005;112:2031–2039. doi: 10.1161/CIRCULATIONAHA.105.546150. [DOI] [PubMed] [Google Scholar]
- 24.Rigal D, Vermot-Desroches C, Heitz S, et al. Effects of intravenous immunoglobulins (IVIG) on peripheral blood B, NK, and T cell subpopulations in women with recurrent spontaneous abortions: specific effects on LFA-1 and CD56 molecules. Clinical immunology and immunopathology. 1994;71:309–314. doi: 10.1006/clin.1994.1091. [DOI] [PubMed] [Google Scholar]
- 25.Hidalgo A, Peired AJ, Wild MK, et al. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wun T, Styles L, DeCastro L, et al. Phase 1 Study of the E-Selectin Inhibitor GMI 1070 in Patients with Sickle Cell Anemia. PloS one. 2014;9:e101301. doi: 10.1371/journal.pone.0101301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang J, Patton JT, Sarkar A, et al. GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood. 2010;116:1779–1786. doi: 10.1182/blood-2009-12-260513. [DOI] [PMC free article] [PubMed] [Google Scholar]


