Abstract
Some of the most common symptoms of the inflammatory bowel diseases (IBD, which include ulcerative colitis and Crohn’s disease) are abdominal pain, diarrhea, and weight loss. It is therefore not surprising that clinicians and patients have wondered whether dietary patterns influence the onset or course of IBD. The question of what to eat is among the most commonly asked by patients and among the most difficult to answer by clinicians. There are therefore substantial variations in dietary behaviors of patients and recommendations for them, although clinicians do not routinely endorse specific diets for patients with IBD. Dietary clinical trials have been limited by their inability to include a placebo control, contamination of study groups, and inclusion of patients receiving medical therapies. Further challenges include accuracy of information on dietary intake, complex interactions between foods consumed, and differences in food metabolism among individuals. We review the roles of diet in the etiology and management of IBD, based on plausible mechanisms and clinical evidence. Researchers have learned much about the effects of diet on the mucosal immune system, epithelial function, and the intestinal microbiome; these findings could have significant practical implications. Controlled studies of patients receiving enteral nutrition and observations made from patients on exclusion diets have shown that components of whole foods can have deleterious effects for patients with IBD. Additionally, studies in animal models suggested that certain nutrients can reduce intestinal inflammation. In the future, engineered diets that restrict deleterious components but supplement beneficial nutrients could be used to modify the luminal intestinal environment of patients with IBD—these might be used alone or in combination with immunosuppressive agents, or as salvage therapy for patients who do not respond or lose responsiveness to medical therapies. Stricter diets might be required to induce remission, whereas more sustainable exclusion diets could be used to maintain long-term remission.
Keywords: IBD, diet, pathogenesis, therapy
The inflammatory bowel diseases (IBD) comprise chronic immune-mediated inflammatory diseases of the intestinal tract, typified by Crohn’s disease (CD) and ulcerative colitis (UC). Their pathogenesis is complex and involves genetic and environmental factors. Advances in DNA sequencing technology led to the association of 163 genetic polymorphisms with risk for IBD1, and efforts are underway to identify additional risk loci. However, in total, these loci only account for about 13% of CD and 7% of UC disease variance. Furthermore, studies of the level of concordance of CD or UC between identical twins estimated the maximum contribution of genetic factors to IBD to be approximately 10% for UC and 30%–40% for CD. Therefore, it appears that environmental factors make largest contributions to IBD risk.
IBD is believed to arise in individuals with a genetic predisposition to immune dysregulation upon exposure to specific environmental factors (reviewed in2). Among the environmental factors associated with IBD, diet and the intestinal microbiota are the most likely to be modifiable, making them targets for prevention and treatment of IBD. However, the diet can modify the composition of the gut microbiota and their production of absorbable metabolites, so studying the effects of these factors on IBD pathogenesis is complicated. We review the relationship between diet and IBD with a focus on current and future strategies for prevention and treatment of patients with IBD (Figure 1).
Figure 1. Effects of Diet on the Intestinal Microbiota and Immune System During Development of IBD.
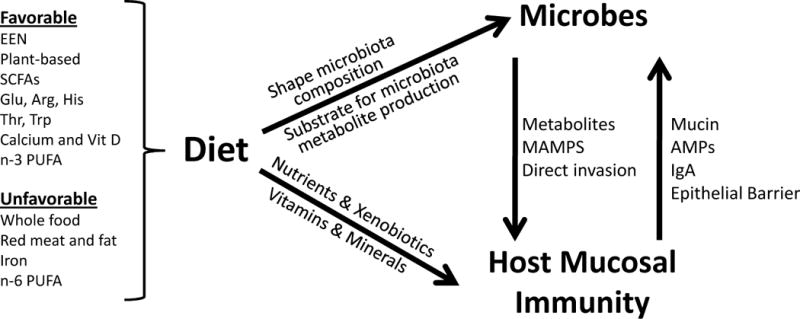
There is evidence from epidemiologic, animal, and clinical studies that certain components of diet can promote or prevent against intestinal inflammation. Diet not only affects the composition of the gut microbiota but also serves as substrate for microbial synthesis of metabolites, which affect the mucosal immune system. Microbes also act the immune system of the intestinal mucosa, through the engagement of innate immune receptors with specific microbial products (mucosally-associated molecular patterns, MAMPS). In turn, the host mucosal immune system fortifies mucosal barrier function and interacts with the gut microbiota through the production of mucin, anti-microbial peptides (AMPs), and IgA.
Epidemiology
IBD is most prevalent in northern Europe and North America,3 and less common in the Asia-Pacific region, with the exception of Australia.4 The incidence of IBD has been rapidly increasing in many parts of the world, including Asia, typically occurring first in more-industrialized countries.5 This contributed to the hypothesis that Westernization of our lifestyle is linked to the rising incidence of IBD, as well as other immune-mediated diseases.6–9 Diet is one of the more obvious environmental factors linked to industrialization.
Immigrants often change their diet and have been studied to assess environmental risk factors. Several studies found that immigrants to Israel had a higher prevalence of IBD than Israeli born populations.10–12 However, later studies found the incidence and prevalence of CD to be comparable among Jews in southern Israel, but a lower incidence in Arab Israelis; this difference could be attributed to genetic and/or environmental factors.13
Early-life exposures are important determinants of risk for IBD, and could affect results of immigrant studies. In Sweden, a region of high IBD incidence, second-generation but not firstgeneration immigrants developed IBD at rates comparable to the native Swedish population, suggesting the importance of early-life exposures.14 However, later environmental changes can also be important. For example, Acosta et al. observed that people newly diagnosed with IBD in the Galicia region of Spain were more likely than those without IBD to have immigrated to an industrialized European country and then returned.15
Humans’ first dietary exposure is invariably breast milk or formula, which is typically derived from cow’s milk. Breastfeeding was associated with lower incidence rates of IBD in a recent meta-analysis (pooled odds ratio, 0.69).16 This is likely to result from the protective effects of breast milk,17 rather than a harmful effect of cow’s milk-based formula, since IBD is rare before weaning.18 Researchers have shown that breast milk alters the composition of the gut microbiota in neonates19. However, there are dramatic changes in the gut microbiota after weaning regardless of whether the initial diet is breast milk or formula.20 So, early-life solid food exposures might also be important in determinants of risk for IBD.
Numerous observational studies have attempted to identify dietary patterns that contribute to the risk for IBD. These studies point to an increased risk of IBD among people who consume greater amounts of meat and fats—particularly polyunsaturated fatty acids and omega-6 fatty acids—and lower risk among people with diets high in fiber, fruits, and vegetables.21 Findings from the European Investigation into Cancer and Nutrition (EPIC) Study and the Nurses’ Health Study are particularly noteworthy because of their prospective design.22–24 The EPIC study associated greater consumption of linoleic acid (an n-6 polyunsaturated fatty acids [PUFA] present in high concentrations in red meat, cooking oils, and margarine) with a higher incidence of UC.22 In contrast, people who consumed higher levels of n-3 PUFA docosahexanoic acid were less likely to be diagnosed with UC.
In the Nurses’ Health Study, greater consumption of long-chain n-3 PUFAs and a higher ratio of n-3:n-6 PUFAs again appeared to protect against development of UC.23 The Nurses’ Health Study has also examined the association between fiber intake and incident IBD. Nurses consuming large amounts of fiber, particularly fruits, were approximately 40% less likely to be subsequently diagnosed with CD, although no association was observed for UC.25 Of course diet alone is inadequate to cause IBD. There is evidence for a gene–diet interaction, in which variants in genes for fatty acid metabolism affect the relationship between IBD risk and PUFA consumption.26 Together, these findings support the hypothesis that consumption of fruits and possibly vegetables, rather than meats and fats, can lower the risk of IBD.
Nutrient-Dependent Regulation of Intestinal Inflammation in Animal Models
Many dietary nutrients have been shown to help regulate mucosal immune function, such as vitamins, amino acids, and short-chain fatty acids—many of which are influenced by the gut microbiota (reviewed in27) (Figure 1). We focus on studies describing the effects of diet on animal models of intestinal inflammation (Table 1). Given the epidemiologic associations between diet and IBD pathogenesis, various nutrients have been studied in rodent models of IBD. The nutrients studied include macronutrients, which can be roughly classified into fats, proteins, carbohydrates, and minerals or vitamins.
Table 1.
Dietary Interventions in Animal Models of IBD.
| Food Category | Study, Year | Methods/animal model | Results |
|---|---|---|---|
| Immunologically-mediated colitis: | |||
| Dietary milk fat | Devkota et al, 20121 | Wild type and Il10 −/− animals were fed one of 3 diets – low fat, high fat with 37% PUFA, high fat with 37% milk fat. | Bloom of Bilophila wadsworthia in the stool from animals fed the milk fat (MF) diet. The MF Il10 −/− group had an increase in colitis incidence and severity. There was also an increase in proinflammatory cytokines in this group. |
| High cholesterol | Gao et al, 20102 | 2-glutathione peroxidase-deficient mice were fed one of two atherogenic diets (high cholesterol or high cholic acid) | The high cholesterol diet aggravated colitis by weakening the colon unfolded protein response. |
| High Fat | Gruber et al, 20133 | TNF-producing CD8 T cell-dependent ileitis; High fat diet versus control. | High fat diet aggravated ileal inflammation, reduced tight junction proteins, increased translocation of endotoxin, tissue pathology more severe. |
| Green tea polyphenols | Oz et al, 20134 | DSS and Il10 −/−; Once colitis developed, animals treated with sulfasalazine or 1 of 2 green tea polyphenols. | Green tea polyphenols improved antioxidant levels and attenuated severity of colitis analagous to sulfasalazine. |
| Semi-synthetic diet | Wagner et al, 20135 | TNF delta ARE/WT mice; Normal chow or semisynthetic diet with and without added gluten. | Transfer to semisynthetic diet at 7 weeks of age protected against ileitis. Glutenfortified semisynthetic diet induced ileal inflammation. |
| Omega-3 | Bosco et al, 20136 | Rag-2 | Increased dietary intake of fish oil did not prevent experimental colitis. |
| Omega-3 | Whiting et al, 20057 | SCID; ω-3-enriched or control diet for 3 weeks before colitis induction by transplantation of CD45RB T cells. | Transplanted ω -3-fed animals had significantly reduced pathology scores, colonic tumor necrosis factor-alpha, interleukin-12, and interleukin-1beta compared with animals fed standard diet. |
| Curcumin | Nones et al, 20098 | mdr1a −/−mice; Control (AIN-76A), control +0.2% curcumin or control +0.1% rutin | Curcumin, but not rutin, significantly reduced histological signs of colonic inflammation in mdr1a−/− mice. |
| Curcumin | Larmonier et al, 20089 | Investigated the effects of dietary curcumin (0.1–1%) on the development of colitis, immune activation, and in vivo NF-kappaB activity in germ-free IL-10−/− or IL-10−/−;NF-kappaB(EGFP) mice colonized with specific pathogen-free microflora. | Limited effectiveness on Th-1 mediated colitis in IL-10−/− mice, with moderately improved colonic morphology, but with no significant effect on pathogenic T cell responses and in situ NF-kappaB activity. |
| Curcumin | Ung et al, 201010 | IL-10 gene deficient mice gavaged daily for 2 weeks with 200 mg/kg per day curcumin emulsified in carboxymethyl cellulose | Both oral curcumin and carboxymethyl cellulose, appeared to have modifying effects on colitis. However, curcumin had additional anti-inflammatory effects mediated through a reduced production of pro-inflammatory mucosal cytokines. |
| Calcium | Schepens et al, 200911 | HLA-B27 transgenic rats were fed a purified high-fat diet containing either a low or high calcium concentration (30 and 120 mmol CaHPO4/kg diet, respectively) for 7 wk. | The calcium diet significantly inhibited the increase in intestinal permeability and diarrhea with time in HLA-B27 rats developing colitis compared with the control transgenic rats. |
| Germinated barley (GBF; prebiotic) | Kanauchi et al, 200812 | CD45RB(high) T cell chronic colitis model | Body-weight loss and occult blood were reduced in the mice that had been fed with GBF. In these mice, there were also reductions in IFN-gamma mRNA expressions and IL-6 in the colonic mucosa, as compared with the control group. GBF also attenuated mucosal damage and mucin positive goblet cell depletion. Conversely, TGF-beta expression significantly increased in the GBF group, compared with the control group. |
| Elemental diet | Kajiura et al, 200913 | IL-10 −/− | Elemental diet suppressed inflammation in IL-10 −/− mice. There were changes in the microbiome associated with the elemental diet by T-RLFP. |
| Elemental diet | Andou et al, 200914 | IL-10 −/−; Single amino acid diet versus mixture. | In the IL-10−/−transfer model, dietary histidine, but not alanine, reduced histologic damage and colon weight and TNF-alpha mRNA expression. Histidine inhibited LPS-induced TNF-alpha and IL-6 production by mouse macrophages in a concentration-dependent manner, whereas alanine or histidine-related metabolites had no such effect. Histidine inhibited LPS-induced NF-KB in macrophages. |
| TGF-beta | Schiffrin et al, 200515 | HLA B-27 rats; Casein-based rat-adapted diet containing TGF-beta or a control casein-based diet without TGF-beta. | The test diet improved diarrhea and the colonic inflammation as shown by a lower inflammatory score (2.43 +/− 1.13 vs 4.42 +/− 0.53, p < .05), lower mucosal thickness (431.25 +/− 72.29 vs 508.57 +/− 81.32 microm, p = .08) and decreased IFN-gamma mRNA expression. |
| TGF-beta | Oz et al, 200416 | Five-week-old IL-10−/− mice (in BALB/c background) were fed either an enteral diet (Diet-A) containing TGF-beta 2 or a control enteral diet (Diet-B) not rich in TGF-beta 2. | IL-10−/− mice fed a TGF-beta 2 containing diet gained more weight, did not develop diarrhea or prolapse, had lower pathological scores. |
| Fiber | Koleva et al, 201217 | 4-week-old HLA B-27 transgenic rats were fed 8 g/kg body weight inulin or fructooligosaccharides (FOS) for 12 weeks, or not. | Colitis was significantly reduced in all FOS-fed rats compared to the control diet, whereas inulin decreased chronic intestinal inflammation in only half the number of animals. |
| Chemical colitis: | |||
| Monotonous diet | Nagy-Szakal et al, 201318 | DSS colitis- mice; Monotonous diet versus cycling 2 different diets | Monotonous diet improved colitis. |
| Olive oil | Takashima et al, 201419 | DSS colitis – rats; 5% extra virgin olive oil added to AIN diet | Olive oil improved disease activity score, body weight, histological score, gene expression, cell proliferation and apoptosis. |
| Olive oil | Camuesco et al, 200520 | DSS colitis – rats; Olive oil based diet with or without fish oil | Colitic rats fed the olive oil-based diet had a lower colonic inflammatory response than those fed the soybean oil diet, and this effect was increased by the dietary incorporation of (ω-3) PUFA. |
| MCT-rich formula | Papada et al, 201421 | TNBS colitis – rats; 5 groups – control, TNBS-colitis, TNBS colitis + LCT rich diet, TNBS colitis + MCT rich diet, TNBS colitis + infliximab | The MCT-rich diet decreased IL-6, IL-8 and intercellular adhesion molecule-1 (ICAM-1) levels and glutathione S-transferase (GST) activity, while the LCT-rich diet reduced only ICAM-1 levels and GST activity. Neither elemental formula affected IL-10 levels. Diet did not affect colitis score. |
| Red meat | Le Leu et al, 201322 | DSS colitis – mice; 4 groups – control diet, high amylose maize starch diet, cooked red meat diet, amylose maize starch + cooked red meat diet | Intake of red meat aggravated DSSinduced colitis whereas co-consumption of resistant starch reduced the severity of colitis. |
| Red meat | Schepens et al, 201123 | TNBS colitis – rats; High-fat control diet vs. similar diet supplemented with heme | Heme rats: Higher fecal wet weight, increased luminal cytotoxicity by fecal water cytotoxicity assay, decreased food intake. No change in histologic score. |
| High fat | Vieira de Barros et al, 201124 | DSS colitis – rats; Five groups: control normal fat non-colitic or control colitis, high soybean fat group colitis, high fish fat group colitis, or high-fat soybean plus fish oil colitis. | High-fat diets did not exacerbate colitis. |
| High fat | Van der Logt et al, 201325 | DSS colitis – mice; Normal chow versus high-fat diet with or without heme | High fat diet mice lost more weight. Heme supplementation further aggravated weight loss. No difference in histological score. |
| Green tea and other polyphenols | Bruckner et al, 201226 | DSS colitis – mice; green tea polyphenol | Improved body weight, less histological damage, increased antioxidant activity with green tea polyphenol supplementation. |
| Green tea and other polyphenols | Oz et al, 200527 | DSS colitis – mice; Treatment with 3 antioxidants including green tea polyphenols | All 3 antioxidants provided protection against DSS-induced colitis. |
| Green tea and other polyphenols | Mazzon et al, 200528 | DNBS colitis – rats; Green tea extract vs. no green tea extract | Treatment with green tea extract attenuated diarrhea and weight loss. There was also improvement in histological score in green tea group. |
| Green tea and other polyphenols | Youn et al, 200929 | DSS colitis – mice; Oral administration of resveratrol or piceattanol | Oral administration of resveratrol or piceattanol for 7 days attenuated DSSinduced inflammatory injury. |
| Omega-3 | Mbodji et al, 201330 | TNBS colitis – rats; 5-ASA or 5-ASA plus PUFA | Decreased inflammatory score and reduced NF-kB activation in PUFA group. |
| Omega-3 | Ibrahim et al, 201231 | TNBS colitis – rats; ALA-rich formula vs. isocaloric corn oil formula. | Decreased expression of adhesion molecules in ALA group. |
| Omega-3 | Tyagi et al, 201232 | DSS colitis – rats; Varying rations of dietary LA to ALA | Substitution of 1/3 LA with ALA mitigated experimental colitis by clinical, biochemical, and histological parameters. |
| Omega-3 | Matsunaga et al, 200833 | DSS colitis – mice; Mice were fed a control diet, omega-3 fat-rich diet, omega-6 fat-rich diet, or saturated fat-rich diet. | The omega-3 fat diet group, but not the other fat diet groups, showed exacerbated colitis with a further decrease of adiponectin expression. |
| Omega-3 | Nieto et al, 200234 | TNBS colitis – rats; Rats were treated with TNBS and fed diets enriched in olive oil (OO), fish oil (FO), or purified pig brain phospholipids (BPL), as sources of monounsaturated and PUFA of the (ω-3) and (ω-3) + (ω-6) series. | Rats with colitis fed FO at 1 wk showed significantly less macroscopic and microscopic colonic damage. |
| Curcumin | Sugimoto et al, 200235 | TNBS colitis – mice; 0.5%, 2.0%, or 5.0% curcumin was added to chow | Treatment prevented and improved wasting and histologic scores. |
| Curcumin | Jian et al, 200536 | TNBS colitis – rats; 2.0% curcumin in the diet after colitis induced. | Treatment prevented and treated wasting and histopathologic signs of colitis. |
| Curcumin | Zeng et al, 201337 | TNBS colitis – rats; Intragastric administration of curcumin or sulfasalazine daily for one week. | Disease activity index decreased more rapidly in the curcumin-treated group than in the sulfasalazine-treated group (p< 0.05). |
| Resistant starch | Moreau et al, 200338 | DSS colitis – rats; DSS or no DSS. The rats were fed a basal diet (BD), or a FOS or resistant starch (RS) diet creating six groups: BD-control, BD-DSS, FOS-control, FOS-DSS, RS-control and RS-DSS. | At days 7 and 14, caecal and distal macroscopic and histological observations were improved in RS-DSS compared with BD-DSS and also with FOS-DSS rats. Caeco-colonic SCFA were reduced in FOSDSS and RS-DSS groups compared with controls. |
| Amino acids | Xue et al, 201139 | DSS colitis – rats; glutamine (0.75 g/kg/d) or sham was administered to rats by oral gavage during 7-day DSS treatment. | Decreased bleeding and diarrhea in glutamine group. |
| Amino acids | Liu et al, 201340 | DSS colitis – rats; Mixture of threonine (0.50 g/d), methionine (0.31 g/d), and monosodium glutamate (0.57 g/d) or an isonitrogenous amount of alanine (control group). | Increased regeneration/reepithelialization after 10-day supplementation. The spontaneous resolution of inflammation was not affected by the supplementation. |
| Amino acids | Coburn et al, 201241 | DSS colitis – mice; L-Arg supplementation | L-Arg supplementation improved the clinical parameters of survival, body weight loss, and colon weight. |
| Amino acids | Ren et al, 201442 | DSS colitis – mice; Effects of graded dose of arginine (0.4%, 0.8%, and 1.5%) or glutamine (0.5%, 1.0% and 2.0%) supplementation | Dietary arginine or glutamine supplementation had significant (P<0.05) influence on the clinical and biochemical parameters (T-SOD, IL-17 and TNF-α). |
| Amino acids | Giris et al, 200743 | TNBS colitis – rats; The rats were divided into four groups. Group 1 had TNBS colitis alone, group 2 had TNBS-induced colitis and glutamine 1 g/kg/day intragastric gavage for 3 days before TNBS solution administration and 15 days following TNBS solution administration, group 3 had glutamine alone 1 g/kg/day intragastric gavage for 18 days before being euthanized, and group 4 had gavage for 18 days before being euthanized | Glutamine reduced colonic damage (histology) in TNBS-induced colitis. |
| Fermented brown rice | Kataoka et al, 200844 | DSS colitis – rats; The inhibitory effects of brown rice fermented by Aspergillus oryzae (FBRA), a fiber-rich food, on the induction of acute colitis by dextran sulfate sodium (DSS) were examined. | Feeding a 5% and 10% FBRA-containing diet significantly decreased the ulcer and erosion area in the rat colon stained with Alcian blue. In another experiment, 10% FBRA feeding decreased the ulcer index (percentage of the total length of ulcers in the full length of the colon) and colitis score, which were determined by macroscopic observation. |
| Combination | Joo et al, 201345 | DSS colitis – mice; C57BL/6 mice received 2.5% DSS in drinking water for 5 d to induce colitis. Then, they were given 0.25 mL of glutamine, fiber, and oligosaccharide (GFO) mixture or a 20% glucose solution twice daily for 10 d. Another set of mice receiving unaltered drinking water was used as the control group. | The body weight loss and disease activity index were significantly lower in the GFOtreated mice compared with the glucosetreated mice (P < 0.05). The decrease in colon length induced by DSS was significantly alleviated in GFO-treated mice compared with glucose-treated mice (P < 0.01). In addition, the histologic findings showed that intestinal inflammation was significantly attenuated in mice treated with GFO. Furthermore, treatment with GFO significantly inhibited the DSS-induced increase in the mRNA expression of IL-1β. |
Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012;487:104–8.
Gao Q, Esworthy RS, Kim BW, Synold TW, Smith DD, Chu FF. Atherogenic diets exacerbate colitis in mice deficient in glutathione peroxidase. Inflamm Bowel Dis 2010;16:2043−54.
Gruber L, Kisling S, Lichti P, Martin FP, May S, Klingenspor M, Lichtenegger M, Rychlik M, Haller D. High fat diet accelerates pathogenesis of murine Crohn’s disease-like ileitis independently of obesity. PLoS One 2013;8:e71661.
Oz HS, Chen T, de Villiers WJ. Green Tea Polyphenols and Sulfasalazine have Parallel Anti-Inflammatory Properties in Colitis Models. Front Immunol 2013;4:132.
Wagner SJ, Schmidt A, Effenberger MJ, Gruber L, Danier J, Haller D. Semisynthetic diet ameliorates Crohn’s diseaselike ileitis in TNFDeltaARE/WT mice through antigen-independent mechanisms of gluten. Inflamm Bowel Dis 2013;19:1285–94.
Bosco N, Brahmbhatt V, Oliveira M, Martin FP, Lichti P, Raymond F, Mansourian R, Metairon S, Pace-Asciak C, Bastic Schmid V, Rezzi S, Haller D, Benyacoub J. Effects of increase in fish oil intake on intestinal eicosanoids and inflammation in a mouse model of colitis. Lipids Health Dis 2013;12:81.
Whiting CV, Bland PW, Tarlton JF. Dietary n-3 polyunsaturated fatty acids reduce disease and colonic proinflammatory cytokines in a mouse model of colitis. Inflamm Bowel Dis 2005;11:340–9.
Nones K, Dommels YE, Martell S, Butts C, McNabb WC, Park ZA, Zhu S, Hedderley D, Barnett MP, Roy NC. The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (mdr1a−/−) mice, a model of inflammatory bowel diseases. Br J Nutr 2009;101:169–81.
Larmonier CB, Uno JK, Lee KM, Karrasch T, Laubitz D, Thurston R, Midura-Kiela MT, Ghishan FK, Sartor RB, Jobin C, Kiela PR. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am J Physiol Gastrointest Liver Physiol 2008;295:G1079–91.
Ung VY, Foshaug RR, MacFarlane SM, Churchill TA, Doyle JS, Sydora BC, Fedorak RN. Oral administration of curcumin emulsified in carboxymethyl cellulose has a potent anti-inflammatory effect in the IL-10 gene-deficient mouse model of IBD. Dig Dis Sci 2010;55:1272−7.
Schepens MA, Schonewille AJ, Vink C, van Schothorst EM, Kramer E, Hendriks T, Brummer RJ, Keijer J, van der Meer R, Bovee-Oudenhoven IM. Supplemental calcium attenuates the colitis-related increase in diarrhea, intestinal permeability, and extracellular matrix breakdown in HLA-B27 transgenic rats. J Nutr 2009;139:1525–33.
Kanauchi O, Oshima T, Andoh A, Shioya M, Mitsuyama K. Germinated barley foodstuff ameliorates inflammation in mice with colitis through modulation of mucosal immune system. Scand J Gastroenterol 2008;43:1346–52.
Kajiura T, Takeda T, Sakata S, Sakamoto M, Hashimoto M, Suzuki H, Suzuki M, Benno Y. Change of intestinal microbiota with elemental diet and its impact on therapeutic effects in a murine model of chronic colitis. Dig Dis Sci 2009;54:1892–900.
Andou A, Hisamatsu T, Okamoto S, Chinen H, Kamada N, Kobayashi T, Hashimoto M, Okutsu T, Shimbo K, Takeda T, Matsumoto H, Sato A, Ohtsu H, Suzuki M, Hibi T. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology 2009;136:564–74 e2.
Schiffrin EJ, El Yousfi M, Faure M, Combaret L, Donnet A, Blum S, Obled C, Breuille D. Milk casein-based diet containing TGF-beta controls the inflammatory reaction in the HLA-B27 transgenic rat model. JPEN J Parenter Enteral Nutr 2005;29:S141–8; discussion S149–50, S184–8.
Oz HS, Ray M, Chen TS, McClain CJ. Efficacy of a transforming growth factor beta 2 containing nutritional support formula in a murine model of inflammatory bowel disease. J Am Coll Nutr 2004;23:220–6.
Koleva PT, Valcheva RS, Sun X, Ganzle MG, Dieleman LA. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br J Nutr 2012;108:1633–43.
Nagy-Szakal D, Mir SA, Ross MC, Tatevian N, Petrosino JF, Kellermayer R. Monotonous diets protect against acute colitis in mice: epidemiologic and therapeutic implications. J Pediatr Gastroenterol Nutr 2013;56:544–50.
Takashima T, Sakata Y, Iwakiri R, Shiraishi R, Oda Y, Inoue N, Nakayama A, Toda S, Fujimoto K. Feeding with olive oil attenuates inflammation in dextran sulfate sodium-induced colitis in rat. J Nutr Biochem 2014;25:186–92.
Camuesco D, Galvez J, Nieto A, Comalada M, Rodriguez-Cabezas ME, Concha A, Xaus J, Zarzuelo A. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. J Nutr 2005;135:687–94.
Papada E, Kaliora AC, Gioxari A, Papalois A, Forbes A. Anti-inflammatory effect of elemental diets with different fat composition in experimental colitis. Br J Nutr 2014;111:1213–20.
Le Leu RK, Young GP, Hu Y, Winter J, Conlon MA. Dietary red meat aggravates dextran sulfate sodium-induced colitis in mice whereas resistant starch attenuates inflammation. Dig Dis Sci 2013;58:3475–82.
Schepens MA, Vink C, Schonewille AJ, Dijkstra G, van der Meer R, Bovee-Oudenhoven IM. Dietary heme adversely affects experimental colitis in rats, despite heat-shock protein induction. Nutrition 2011;27:590–7.
Vieira de Barros K, Gomes de Abreu G, Xavier RA, Real Martinez CA, Ribeiro ML, Gambero A, de Oliveira Carvalho P, Silveira VL. Effects of a high fat or a balanced omega 3/omega 6 diet on cytokines levels and DNA damage in experimental colitis. Nutrition 2011;27:221–6.
van der Logt EM, Blokzijl T, van der Meer R, Faber KN, Dijkstra G. Westernized high-fat diet accelerates weight loss in dextran sulfate sodium-induced colitis in mice, which is further aggravated by supplementation of heme. J Nutr Biochem 2013;24:1159–65.
Bruckner M, Westphal S, Domschke W, Kucharzik T, Lugering A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J Crohns Colitis 2012;6:226–35.
Oz HS, Chen TS, McClain CJ, de Villiers WJ. Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem 2005;16:297–304.
Mazzon E, Muia C, Paola RD, Genovese T, Menegazzi M, De Sarro A, Suzuki H, Cuzzocrea S. Green tea polyphenol extract attenuates colon injury induced by experimental colitis. Free Radic Res 2005;39:1017–25.
Youn J, Lee JS, Na HK, Kundu JK, Surh YJ. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr Cancer 2009;61:847–54.
Mbodji K, Charpentier C, Guerin C, Querec C, Bole-Feysot C, Aziz M, Savoye G, Dechelotte P, Marion-Letellier R. Adjunct therapy of n-3 fatty acids to 5-ASA ameliorates inflammatory score and decreases NF-kappaB in rats with TNBS-induced colitis. J Nutr Biochem 2013;24:700–5.
Ibrahim A, Aziz M, Hassan A, Mbodji K, Collasse E, Coeffier M, Bounoure F, Savoye G, Dechelotte P, Marion-Letellier R. Dietary alpha-linolenic acid-rich formula reduces adhesion molecules in rats with experimental colitis. Nutrition 2012;28:799–802.
Tyagi A, Kumar U, Reddy S, Santosh VS, Mohammed SB, Ehtesham NZ, Ibrahim A. Attenuation of colonic inflammation by partial replacement of dietary linoleic acid with alpha-linolenic acid in a rat model of inflammatory bowel disease. Br J Nutr 2012;108:1612–22.
Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y, Kawaguchi A, Nagao S, Itoh K, Miura S. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflamm Bowel Dis 2008;14:1348–57.
Nieto N, Torres MI, Rios A, Gil A. Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. J Nutr 2002;132:11–9.
Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, Koide Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 2002;123:1912–22.
Jian YT, Mai GF, Wang JD, Zhang YL, Luo RC, Fang YX. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol 2005;11:1747–52.
Zeng Z, Zhan L, Liao H, Chen L, Lv X. Curcumin improves TNBS-induced colitis in rats by inhibiting IL-27 expression via the TLR4/NF-kappaB signaling pathway. Planta Med 2013;79:102–9.
Moreau NM, Martin LJ, Toquet CS, Laboisse CL, Nguyen PG, Siliart BS, Dumon HJ, Champ MM. Restoration of the integrity of rat caeco-colonic mucosa by resistant starch, but not by fructo-oligosaccharides, in dextran sulfate sodium-induced experimental colitis. Br J Nutr 2003;90:75–85.
Xue H, Sufit AJ, Wischmeyer PE. Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. JPEN J Parenter Enteral Nutr 2011;35:188–97.
Liu X, Beaumont M, Walker F, Chaumontet C, Andriamihaja M, Matsumoto H, Khodorova N, Lan A, Gaudichon C, Benamouzig R, Tome D, Davila AM, Marie JC, Blachier F. Beneficial effects of an amino acid mixture on colonic mucosal healing in rats. Inflamm Bowel Dis 2013;19:2895–905.
Coburn LA, Gong X, Singh K, Asim M, Scull BP, Allaman MM, Williams CS, Rosen MJ, Washington MK, Barry DP, Piazuelo MB, Casero RA, Jr., Chaturvedi R, Zhao Z, Wilson KT. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS One 2012;7:e33546.
Ren W, Yin J, Wu M, Liu G, Yang G, Xion Y, Su D, Wu L, Li T, Chen S, Duan J, Yin Y, Wu G. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS One 2014;9:e88335.
Giris M, Erbil Y, Dogru-Abbasoglu S, Yanik BT, Alis H, Olgac V, Toker GA. The effect of heme oxygenase-1 induction by glutamine on TNBS-induced colitis. The effect of glutamine on TNBS colitis. Int J Colorectal Dis 2007;22:591–9.
Kataoka K, Ogasa S, Kuwahara T, Bando Y, Hagiwara M, Arimochi H, Nakanishi S, Iwasaki T, Ohnishi Y. Inhibitory effects of fermented brown rice on induction of acute colitis by dextran sulfate sodium in rats. Dig Dis Sci 2008;53:1601–8.
Joo E, Yamane S, Hamasaki A, Harada N, Matsunaga T, Muraoka A, Suzuki K, Nasteska D, Fukushima T, Hayashi T, Tsuji H, Shide K, Tsuda K, Inagaki N. Enteral supplement enriched with glutamine, fiber, and oligosaccharide attenuates experimental colitis in mice. Nutrition 2013;29:549–55.
High-fat diets have been found to increase the severity of colitis that develops in mice28, 29. The essential fatty acids have been studied extensively in animal models of IBD. Addition of PUFAs to the diets of mice had variable effects in preventing or treating colitis30–34. Specific amino acids are thought to be immunomodulatory, such as glutamine and arginine, and might be involved in mediating responses to metabolic stress, such as when the intestine in inflamed35. Glutamine and arginine have been shown to improve clinical and biochemical parameters of chemical-induced colitis when added to diets of mice36–39. Biogenic amines such as histamine, an important regulator of physiological functions of the gut, might also affect the immune response in patients with IBD. Histamine is derived from the amino acid histidine, and dietary histidine can reduce symptoms of immune-mediated colitis in mice40. Tryptophan is a precursor to immunoregulatory biogenic amines, such as kynurenine, via indoleamine 2,3-dioxygenase activity, promotes development of T-regulatory cells and immune tolerance41. Additionally, threonine could enhance barrier function by augmenting intestinal mucus production. Dietary supplementation with tryptophan and threonine has been shown to reduce features of colitis in piglets and mice42, 43.
Plant polysaccharides and poorly digestible fibrous plant components have, overall, been shown to reduce features of colitis in mice (reviewed in44). This is believed to occur via increased production of short-chain fatty acids (SCFAs), which might increase barrier function by serving as a source of energy for colonocytes45. SCFAs also promote immune tolerance by increasing development of T-regulatory cells, via G-protein coupled receptor-dependent and epigenetic effects through histone acetylation (recently reviewed in46). The anti-colitic effects of plant-based compounds have also been studied, including curcurmin47, green tea,48 and other polyphenols such as resveratrol49, and fermented grains50.
There are several dietary vitamins and minerals that are thought to be involved in the pathogenesis of IBD, based on studies of rodents and observational studies of humans. Dietary calcium and vitamin D are important for patients with IBD, not only for bone health, but also because vitamin D is involved in anti-inflammatory pathways51. Studies of animal models of IBD have supported the importance of calcium and vitamins. In HLA-B27 transgenic rats, a highcalcium diet inhibited diarrhea and intestinal permeability52. Mice with dextran sulfate sodiuminduced colitis had firmer stools, less blood loss, and weight recovery following administration of the active form of vitamin D53. Vitamin D was shown to promote epithelial cell resistance to injury and suppress the inflammatory response to luminal antigens.
Iron also appears to have a complex role in development of intestinal inflammation54, 55. Iron catalyzes the formation of oxygen radicals, which can cause cellular injury and increase activation of the transcription factor NFκB, which perpetuates inflammation56–58. Excess iron can increase the ratio of CD8+ T cells to CD4+ T cells. Iron is also used by bacteria and has been linked with invasive strains that promote inflammation54. Ingested iron, particularly iron sulfate, has been directly implicated in intestinal inflammation and alteration of gut microbiota in mice59, 60. For example, TNF∆ARE/WT mice develop ileitis with when fed an iron sulfate containing diet, but are protected from ileitis when placed on iron sulfate-free diets, even with parenteral iron replacement59. In this model, iron induces endoplasmic reticulum stress in intestinal epithelial cells and sensitizes the epithelium to apoptosis, induced by cytotoxic T-cells59. Whether heme iron, the predominant form in meat, has the same effects is unknown. However, dietary heme has been shown to increase the severity of colitis in rodents61, 62.
The purpose of animal studies is to identify mechanisms of pathogenesis that can be translated to human disease. For example, defined-formula diets are powerful tools for treatment of IBD and have been evaluated in rodent models of IBD63. Devkota et al demonstrated how diet can alter the intestinal microbiota to contribute to the development of colitis in mice; a diet high in milk fat promoted the expansion of the low-abundance sulphitereducing bacteria Bilophila wadsworthia and the increased incidence of colitis64. These bacteria caused induction of an inflammatory immune response, and also promoted development of colitis in interleukin-10 knockout, but not wild-type, mice. These findings reveal a possible mechanism by which the Western diet alters the composition of the gut microbiota to promote inflammation and other immune disorders.
Dietary Interventions in Humans with IBD
Findings from epidemiology studies, animal studies, and analyses of clinical anecdotes have provided the basis for prospective trials that modified diets and evaluated disease progression in patients with IBD. Enteral nutritional therapy can induce disease remission; this observation and results of epidemiology studies provide compelling evidence for the role of food in IBD pathogenesis and treatment. Human studies of food and IBD can generally be categorized into groups of elimination diets, exclusion of specific inflammatory mediators, inclusion of anti-inflammatory mediators, and inclusion of prebiotics (Table 2).
Table 2.
Human studies on diet and IBD
| Food category | Study, year | Methods | Results |
|---|---|---|---|
| Elimination | |||
|
| |||
| Crohn’s disease exclusion diet | Sigall-Boneh et al, 20141 | Retrospective review: 47 subjects treated with a “Crohn’s disease exclusion diet” and up to 50% calories from polymeric formula over 6 weeks. | Clinical remission achieved in 70% subjects. |
| Specific carbohydrate diet (SCD) | Cohen et al, 20142 | Prospective design: 10 subjects with CD on SCD followed over 12 weeks. | Improvement in PCDAI and intestinal appearance as assessed by capsule endoscopy. |
| Specific carbohydrate diet | Suskind et al, 20143 | Case series: 7 subjects with CD on SCD. | Duration on SCD: 5–30 months (mean 14.6 months). Improvements in PCDAI and CRP. |
| Anti-inflammatory diet | Olendzki et al, 20144 | Case series: 11 subjects (8 CD, 3 ulcerative colitis) followed diet, which targeted dysbiosis, for at least 4 weeks. | Improvement in clinical symptoms and ability to reduce medications. |
| Allergen Elimination Diet (IgG) | Rajendran et al, 20115 | Prospective study: 40 Pts with symptomatic CD tested for IgG4 antibodies to 14 specific food antigens. Top 4 foods removed for 4 week period. | 29/40 completed study. 26/29 had significant decrease in modified CDAI, ESR, and IgG4 titers for excluded foods. |
| Semi-Vegetarian | Chiba et al, 20106 | Prospective 2 year study evaluating efficacy of semi-vegetarian diet (SVD) on maintaining clinical remission. | 16/22 subjects maintained diet. 15/16 on SVD maintained remission. |
| FODMAP | Croagh et al, 20077 | Combined retrospective and prospective study assessing 13 subjects with ileal pouch, and 2 subjects with ileal rectal anastamosis on FODMAP exclusion diet | No improvement in pouchitis. |
| Low residue | Levenstein et al, 19858 | Randomized diet trial of 70 subjects with CD on low residue vs. normal Italian for mean 29 months. | Diet history showed normal diet had 3 times greater fiber intake. No differences in steroids, surgeries, or clinical disease activity. |
|
| |||
| Pro-inflammatory mediators | |||
|
| |||
| Low Microparticle diet | Butler et al, 20079* | Macrophages (from CD and healthy controls) incubated with microparticles (aluminosilicates and titanium dioxide) before assay for cytokine production and phagocytic activity | Microparticles along did not stimulate activity. In presence of lipopolysaccharide, microparticles could induce more profound cytokine response (IL-8, TNFa, IL-10). |
| Lomer et al, 200510 | RCT over 16 weeks with 83 subjects enrolled in 2×2 design: low or normal micro-particles and/or calcium. | No differences between groups in clinical disease activity or inflammatory markers. | |
| Emulsifiers | Roberts et al, 201311 | Evaluation of data on emulsifier consumption by country and the correlation with CD incidence. | Positive correlation between emulsifier exposure and CD incidence. |
| Roberts et al, 201012* | plant fibers vs. emulsifiers on translocation of mucosa-associated e.coli using M-cell monolayers and human Peyer’s patches. | reduced E coli translocation across M-cells; apple and leek fiber had no significant effect. Polysorbate-80 increased E coli translocation 59 fold. | |
|
| |||
| Anti-inflammatory | |||
|
| |||
| Omega 3 | Cabre et al, 201213 | Systematic review: included 19 RCTs | No clear benefit of omega-3 fatty acids. |
| Feagan et al, 200814 | Two randomized, placebo-controlled multi-center studies (EPIC-1 and EPIC-2). Subjects with quiescent Crohn’s disease were randomly assigned to receive either 4 grams/day of omega-3 free fatty acids or placebo for up to 58 weeks. | No difference in rates of clinical relapse at 1 year in either trial. | |
| Curcumin | Holt et al, 200515 | Pilot study of 5 subjects with ulcerative proctitis and 5 with CD treated with curcumin for 3 months | All 5 subjects with ulcerative proctitis had improvement in global score. CDAI improved in all CD subjects. |
| Vitamin D | Ananthakrishnan et al, 201316 | Retrospective cohort study: 3217 subjects; evaluating 25-OH vitamin D status and clinical outcomes. | Low 25-OH vitamin D levels associated with increased risk for surgery and hospitalization. Correction of 25-OH vitamin D associated with decreased risk for surgery |
| Jorgensen et al, 201017 | RCT with 94 subjects with CD in remission randomized to 1200 IU vitamin D3 daily vs. placebo for one year. | Rate of disease relapse lower in subjects treated with D3 (13%) vs. placebo (29%), p=0.06 | |
|
| |||
| Prebiotic | |||
|
| |||
| Inulin | Joossens et al, 201218 | RCT with 67 subjects randomized to oligofructose-enriched inulin (OF-IN) or placebo over 4 weeks. | In placebo group, no significant changes in fecal microbiota. In OFIN group, decrease in Ruminococcus gnavus and increase in B longum after 4 weeks. |
| Fructose oligosaccharides (FOS) | Benjamin et al, 201119 | Placebo-controlled RCT with 103 subjects randomized to 15 grams/day of FOS or placebo for 4 weeks | No difference in clinical response. No difference in fecal concentration of of bifidobacteria or F prausnitzii at week 0 or 4. |
| Fiber | Ritchie et al, 198720 | Multicenter prospective randomized trial over 2 years assessing a diet with unrestricted sugar intake and low in fiber vs. a diet with little sugar intake and high in unrefined carbohydrates; 352 subjects enrolled. | Difference in sugar and fiber intake between groups as expected, but no differences in clinical endpoints. |
| Berghouse et al, 198421 | Case-crossover study with 5 CD and 5 ulcerative colitis subjects with ileostomies. Evaluated on 2 weeks of a Western diet, then 2 weeks on a diet rich in unrefined cereals. | Unrefined cereal group had greater weight of ostomy output as well as higher bacterial flora per gram. | |
| Heaton et al, 197922 | Retrospective comparison between 32 subjects with CD instructed on fibrerich, unrefined carbohydrate diet vs. 32 subjects with no dietary instruction. | Mean follow-up of 52 months. Dietinstructed group had less refined sugar, greater fiber intake, as well as fewer admissions and less surgery. | |
| Wheat grass | Ben-Arye et al, 200223 | Placebo-controlled RCT of23 subjects with active UC randomized to 100 mL/day wheat grass juice vs. placebo for 1 month. | Wheat grass group with greater improvement in disease activity and physician global assessment. |
| Germinated barley | Kanauchi et al, 200224 | RCT with 18 subjects with mildmoderately active ulcerative colitis randomized to greminated barley 20–30 gram/day for 4 weeks vs. baseline anti-inflammatory therapy. | Germinated barley group with lower disease activity at 4 weeks. |
| Dietary yeast | Barclay et al, 199225 | Study of 19 subjects with dietary yeast inclusion for 1 month and yeast exclusion for 1 month. | Clinical disease activity higher during time of yeast exposure. |
Denotes study was conducted ex vivo
References:
Sigall-Boneh R, Pfeffer-Gik T, Segal I, Zangen T, Boaz M, Levine A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm Bowel Dis 2014;20:1353–60.
Cohen SA, Gold BD, Oliva S, Lewis J, Stallworth A, Koch B, Eshee L, Mason D. Clinical and Mucosal Improvement with the Specific Carbohydrate Diet in Pediatric Crohn’s Disease: A Prospective Pilot Study. J Pediatr Gastroenterol Nutr 2014.
Suskind DL, Wahbeh G, Gregory N, Vendettuoli H, Christie D. Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. J Pediatr Gastroenterol Nutr 2014;58:87–91.
Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr J 2014;13:5.
Rajendran N, Kumar D. Food-specific IgG4-guided exclusion diets improve symptoms in Crohn’s disease: a pilot study. Colorectal Dis 2011;13:1009–13.
Chiba M, Abe T, Tsuda H, Sugawara T, Tsuda S, Tozawa H, Fujiwara K, Imai H. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol 2010;16:2484–95.
Croagh C, Shepherd SJ, Berryman M, Muir JG, Gibson PR. Pilot study on the effect of reducing dietary FODMAP intake on bowel function in patients without a colon. Inflamm Bowel Dis 2007;13:1522–8.
Levenstein S, Prantera C, Luzi C, D’Ubaldi A. Low residue or normal diet in Crohn’s disease: a prospective controlled study in Italian patients. Gut 1985;26:989–93.
Butler M, Boyle JJ, Powell JJ, Playford RJ, Ghosh S. Dietary microparticles implicated in Crohn’s disease can impair macrophage phagocytic activity and act as adjuvants in the presence of bacterial stimuli. Inflamm Res 2007;56:353–61.
Lomer MC, Grainger SL, Ede R, Catterall AP, Greenfield SM, Cowan RE, Vicary FR, Jenkins AP, Fidler H, Harvey RS, Ellis R, McNair A, Ainley CC, Thompson RP, Powell JJ. Lack of efficacy of a reduced microparticle diet in a multi-centred trial of patients with active Crohn’s disease. Eur J Gastroenterol Hepatol 2005;17:377–84.
Roberts CL, Rushworth SL, Richman E, Rhodes JM. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J Crohns Colitis 2013;7:338–41.
Roberts CL, Keita AV, Duncan SH, O’Kennedy N, Soderholm JD, Rhodes JM, Campbell BJ. Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut 2010;59:1331–9.
Cabre E, Manosa M, Gassull MA. Omega-3 fatty acids and inflammatory bowel diseases – a systematic review. Br J Nutr 2012;107 Suppl 2:S240–52.
Feagan BG, Sandborn WJ, Mittmann U, Bar-Meir S, D’Haens G, Bradette M, Cohen A, Dallaire C, Ponich TP, McDonald JW, Hebuterne X, Pare P, Klvana P, Niv Y, Ardizzone S, Alexeeva O, Rostom A, Kiudelis G, Spleiss J, Gilgen D, Vandervoort MK, Wong CJ, Zou GY, Donner A, Rutgeerts P. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. JAMA 2008;299:1690–7.
Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci 2005;50:2191–3.
Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng SC, Savova G, Chen P, Szolovits P, Xia Z, De Jager PL, Shaw SY, Churchill S, Karlson EW, Kohane I, Plenge RM, Murphy SN, Liao KP. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm Bowel Dis 2013;19:1921–7.
Jorgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn’s disease – a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther 2010;32:377–83.
Joossens M, De Preter V, Ballet V, Verbeke K, Rutgeerts P, Vermeire S. Effect of oligofructose-enriched inulin (OF-IN) on bacterial composition and disease activity of patients with Crohn’s disease: results from a double-blinded randomised controlled trial. Gut 2012;61:958.
Benjamin JL, Hedin CR, Koutsoumpas A, Ng SC, McCarthy NE, Hart AL, Kamm MA, Sanderson JD, Knight SC, Forbes A, Stagg AJ, Whelan K, Lindsay JO. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 2011;60:923–9.
Ritchie JK, Wadsworth J, Lennard-Jones JE, Rogers E. Controlled multicentre therapeutic trial of an unrefined carbohydrate, fibre rich diet in Crohn’s disease. Br Med J (Clin Res Ed) 1987;295:517–20.
Berghouse L, Hori S, Hill M, Hudson M, Lennard-Jones JE, Rogers E. Comparison between the bacterial and oligosaccharide content of ileostomy effluent in subjects taking diets rich in refined or unrefined carbohydrate. Gut 1984;25:1071–7.
Heaton KW, Thornton JR, Emmett PM. Treatment of Crohn’s disease with an unrefined-carbohydrate, fibrerich diet. Br Med J 1979;2:764–6.
Ben-Arye E, Goldin E, Wengrower D, Stamper A, Kohn R, Berry E. Wheat grass juice in the treatment of active distal ulcerative colitis: a randomized double-blind placebo-controlled trial. Scand J Gastroenterol 2002;37:444–9.
Kanauchi O, Suga T, Tochihara M, Hibi T, Naganuma M, Homma T, Asakura H, Nakano H, Takahama K, Fujiyama Y, Andoh A, Shimoyama T, Hida N, Haruma K, Koga H, Mitsuyama K, Sata M, Fukuda M, Kojima A, Bamba T. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: first report of a multicenter open control trial. J Gastroenterol 2002;37 Suppl 14:67–72.
Barclay GR, McKenzie H, Pennington J, Parratt D, Pennington CR. The effect of dietary yeast on the activity of stable chronic Crohn’s disease. Scand J Gastroenterol 1992;27:196–200.
Epidemiologic studies associated red meat and n-6 PUFA intake with incidence of IBD22, 24. Chiba et al prospectively studied the role of a semi-vegetarian diet in 22 subjects in Japan over the course of 2 years65. The semi-vegetarian diet (allowed milk and eggs; fish once per week; other meat once every 2 weeks) was associated with a high rate of maintenance of disease remission. Fiber has an important role in intestinal transit, and the soluble form is fermented to SCFAs—an important source of nutrients for colonocytes66. Although increased stool output and greater stool bacterial content have been associated with increases in fiber intake, high fiber intake has not been associated with clinical endpoints67–69. Omega-3-PUFAs have anti-inflammatory properties, but 2 randomized controlled trials found no effect on relapse, at 1 year, in patients with quiescent CD70.
Vitamin D can be obtained from food, supplements, or sun exposure. Vitamin D deficiency is common in the United States and among patients with IBD. Researchers have tested whether vitamin D can be used to treat IBD. In an observational study, patients with documented correction of vitamin D deficiency were less likely to require surgery for IBD, during a specific follow-up period, than those who remained vitamin-D deficient71. A small randomized trial of patients with CD in clinical remission demonstrated numerically and nearly statistically significant lower rates of clinical relapse among patients given 1200 IU daily of vitamin D3, compared with placebo72.
The specific-carbohydrate diet, which involves strict restriction of grains, most dairy, and refined sugars is gaining interest in the medical community, but has not been extensively studied. Aside from 1 randomized study, clinical studies of elimination diets have been limited by their non-randomized design. Researchers have performed a variety of smaller hypothesisgenerating studies on prebiotics, but 2 large, randomized, controlled studies of the effects of fructo-oligosaccharides and inulin showed no clear benefit for subjects with active CD73, 74. Studies of the effects of food on IBD are limited by the difficulty in accurately capturing dietary intake as well as the potential for complex interactions between foods. Further, proportions of food intake, compared with other dietary components, are a challenge to determine75.
Bowel Rest, Parenteral Nutrition, and Fecal Diversion
Parenteral nutrition (PN) has been shown to benefit malnourished, ill patients with CD, but little is known about the exact effects of eliminating oral intake of food, which promotes bowel rest76, 77. Observational studies have demonstrated short-term avoidance of surgery with PN and bowel rest, but little effect on eventual need for surgery76, 78, 79. The benefit of complete bowel rest was not demonstrated in a study that compared the effects of a combination of PN and bowel rest with those of PN and an oral diet—disease activity was similar between groups80.
Whereas bowel rest does not improve long-term outcomes of patients with CD, fecal diversion does81; this might provide insight into the pathogenesis of IBD. In subjects with active luminal CD who underwent ileocolonic resection and placement of a diverting ileostomy, the neo-terminal ileum had a normal appearance, based on endoscopy and histology evaluation, 3–6 months after surgery82, 83. In these patients, infusion of proximal ileum effluent into the distal ileum for 7 days resulted in histological changes consistent with infiltration of inflammatory cells82. Some component of the fecal stream therefore appears to contribute to recurrence of inflammation following surgery.
Fecal diversion is not always successful in inducing remission. However, response has been associated with serologic detection of an antigen from Pseudomonas fluorescens84, 85. Additionally, early use of antibiotics has been shown to delay recurrence of disease after surgery for CD86, 87. These findings suggest an interaction between diet and the gut microbiota that promote inflammation in patients with CD. Dietary components could alter the gut microbiota, directly stimulate the immune system, or be metabolized into compounds that induce inflammation. Further studies are needed to determine whether some or all of these are involved82, 88.
Exclusive Enteral Nutrition (EEN)
EEN therapy is the only dietary intervention that has been rigorously tested and shown to induce remission of CD89–91. EEN therapy with elemental, semi-elemental, or polymeric formula diets has been widely studied and shown to induce remission of CD; it is therefore the first-line therapy in many parts of the world92, 93. The most common protocol involves the administration of a defined formula at 100% of caloric needs for 4–12 weeks90. Although partial enteral nutritional therapy might also provide clinical benefit94, EEN appears superior for the induction of remission95. A smaller percentage of calories and nutrients, provided by the defined formula, may be required to maintain remission, allowing flexibility in the diet94.
In addition to reducing symptoms of CD, EEN has been associated with mucosal healing, which may be a superior predictor of long-term outcome89, 96–98. Perhaps the most promising findings came from a prospective trial of children with CD, randomized to groups given oral corticosteroids or EEN with a polymeric formula for 10 weeks89. In the short term, EEN was as effective as corticosteroids in producing clinical remission. However, EEN was significantly more effective than corticosteroids in healing the mucosa, based on endoscopic and histologic analyses. Overall, EEN has the ability to spare the use of steroids and other immunosuppressive therapies. In children, EEN can increase linear growth, bone health, and lean mass accrual99–102.
As for other therapies for IBD, patient selection appears to be important. EEN is most commonly prescribed for patients who have CD, rather than UC. Early studies demonstrated less satisfactory outcomes in patients with UC103 or CD that primarily involved the colon104. A prospective study that compared clinical and endoscopic remission by ileal, ileocolonic, or colonic disease location found that the rates of remission were the lowest among patients with only colonic disease104. However, 2 systematic reviews were unable to form a conclusion regarding disease phenotype and response to EEN105, 106.
A recent review of EEN in adults with CD demonstrated general poor compliance; the poor palatability of formula and low patient motivation were likely to be contributing factors107. Two studies of EEN in treatment-naïve adults demonstrated clinical remission, based on intention to treat analysis, in 80% and 82% of subjects, respectively108, 109. This suggests that prior exposure to immunosuppressive therapy is associated with poorer outcomes with EEN. However there are no data, as yet, that one factor causes the other. There might be a relationship between disease duration and reversibility of tissue damage.
Interestingly, it is widely recognized that the efficacy of EEN does not change with the composition of formula. A Cochrane review of EEN for induction of remission in CD evaluated the effects of formula composition and found no difference in efficacy105. A meta-analysis of adult study subjects demonstrated no significant differences in outcomes with protein content (elemental, semi-elemental, or polymeric). An analysis comparing low fat (<20 g fat/1000 kcal) vs high fat (>20 g fat/1000 kcal) formulas found no difference in outcomes, nor did a further analysis of very low-fat formula (<3 g fat/1000 kcal). Furthermore, an evaluation of long-chain triglyceride content at the thresholds 5%, 10%, and 15% of total energy similarly found no difference in outcomes105.
Digestion and Absorption of Whole Foods vs Defined-formula Diets
Although nutritional therapy, via EEN, is effective for patients with CD, little is known about its mechanisms. These could involve reductions in luminal antigens and food exclusion, direct anti-inflammatory effects of the formula, improved nutrition, or changes to the gut microbiota110–113. Several interesting clinical observations have been made. For example, the composition of the formula does not affect outcome, but increases in proportion of total caloric intake from formula are associated with greater effectiveness. Also, EEN is more effective in patients with ileocolonic, compared with colonic, disease alone or patients with UC. These observations will lead to better dietary recommendations for patients with IBD, but what insights do they provide into the mechanisms of EEN?
Mechanisms involving the physical properties of food
The mechanisms of digestion and absorption of food are well described (for a review, see114, 115), but affected by the food’s physical form. A diet of diverse, colorful, whole-foods may activate digestion differently than repetitive or bland diets such as EEN. The mechanoreceptor responses induced by the volume of ingested food at a meal might be reduced if formula is sipped slowly or infused at a slow rate.
Elemental formulas are prescribed for EEN in certain parts of the world. These formulas comprise L-amino acids or protein hydrolysates, simple carbohydrates (no fiber), fatty acids, vitamins, minerals, and trace elements. Elemental formulas are readily absorbed in the proximal small bowel, with minimal residue reaching the distal small bowel and colon.116 In fact, the proximal small bowel develops increased villous height but the distal small bowel and colon develop atrophy, similar to that observed in animals fed intravenously. Elemental formulas slow gastric emptying and acid secretion and release of pancreatic enzymes, and are thought to reduce overall bacterial mass.117 It may be that EEN is effective by completely eliminating the residue that reaches the distal small intestine and colon; diets that include table foods are unlikely to be able to reproduce this. Ultimately, if specific residues promote inflammation, restriction diets could be used in management of IBD.
Mechanisms involving the delivery of essential nutrients
Another reason that the EEN is effective could involve improved delivery of essential nutrients. Patients with active IBD have lower levels of several amino acids, such as tryptophan and histidine118, and other micronutrients. Some of these, such as tryptophan, can modulate immune function and affect the composition of the gut microbiota41, 119. Others, such as threonine, help maintain barrier function and an adequate mucous layer120. Patients with acute inflammation are likely to have higher nutrient demands in the restitution process, so there would be different nutritional requirements during disease exacerbation and remission. This would support the concept of EEN for induction of remission and PEN for maintenance.
Formulas enter the intestine in an emulsified liquid solution that readily exposes the nutrients to digestive enzymes. Formulas containing whole proteins have similar nitrogen and fat absorption to that of whole foods, but fecal wet weight, dry weight and transit time are somewhat reduced.121 The absorption of energy and lipids from pureed whole foods is similar to that from an elemental formula, but protein absorption is greater with the whole foods, likely due to increased pancreatic enzyme secretion.122 The lipid components of formula include a mixture of FA, with up to 60% medium chain triglycerides that are rapidly absorbed without emulsification and longer chain FA, all in an emulsified liquid form. The total fat content, however, is rarely greater than 30% of energy and thus unlikely to overwhelm bile acid emulsification capacity. By contrast, the fat content of whole foods meals varies considerably, and may be quite high. Studies that have compared high vs. low fat formula for EEN have not consistently demonstrated different efficacy,105 indicating that fat content of the formula is less important, although differing types of fats may be important. Maltodextrin usually provides the carbohydrate of non-elemental EEN formulas; it is readily digested and absorbed. Carbohydrates from whole foods may be presented as long-chain polysaccharides embedded inside a grain or vegetable matrix or simple sugars.
EEN also provides Dietary Reference Intake levels of 24 micronutrients (vitamins, minerals, trace elements) in a volume of 1200–1500 mL/day. By contrast, the micronutrient content of whole foods will vary considerably based on the specific foods ingested, whether they are raw or cooked, the micronutrient content of the soil where plants were grown, whether fish were exposed to sunlight, the diet of cattle, and many more variables. Bioavailability will also be affected, by the presence of other micronutrients from the meal that can increase it or compete for absorption. Phytic acid, oxalate, and polyphenols, typically found in the bran portion of grains,123, 124 are considered anti-nutrients because they interfere with mineral absorption. For example, phytates bind iron and zinc, polyphenols bind iron and oxalates bind calcium,125 thus reducing their absorption. Formulas do not contain phytates or oxalates. However, they provide only 24 micronutrients, whereas whole foods, especially those from plant origins, are a complex matrix of up to thousands of phytochemicals126 of variable physiological significance.
Mechanisms involving the composition and function of the gut microbiota
IBD is associated with alterations in the composition of the intestinal microbiota characterized by decreased diversity, reduced proportions of Firmicutes, and increased proportions of Proteobacteria and Actinobacteria127. EEN might benefit patients via its effects on the composition of the gut microbiota. Although modest dietary changes have a relatively limited effect on the composition of the gut microbiota128, extreme interventions, such as use of EEN, are likely to have more substantial effects129, in part due to altered carbohydrate composition.
Prebiotic oligosaccharides, such as inulin and its fructo-oligosaccharide derivatives or galactooligosaccharide (GOS), have been proposed to improve gut microbial health130–132. Prebiotics are resistant to digestion in the stomach and small bowel, but ferment in the colon. Breast milk contains substantial quantities of GOS, which promotes growth of bifidobacteria in the infant colon, and vegetables are a source of inulin. Although ingestion of excess prebiotics can produce flatulence or diarrhea, due to gas produced by fermentation, the SCFAs produced aid in the absorption of sodium and hepatic control of lipid and carbohydrate metabolism, and supply energy to colon, heart, brain, and muscle cells131. In addition, SCFAs are important for T-regulatory cell development and maintenance of mucosal immune homeostasis133, 134. Resistant starch, a component of potatoes, bananas, pasta, and bread, is similar to prebiotics in that it is not digested in the upper gastrointestinal tract, but fermented in the colon to SCFAs132.
There is controversy over whether IBD can be effectively treated by altering the composition of the gut microbiota. We are only beginning to identify the specific bacteria involved in these processes. Faecalibacterium prauznitzii have anti-inflammatory properties,135 and inulin supplementation increases concentrations of F prauznitzii136. However, patients treated with EEN were reported to have reduced concentration of F prauznitzii and butyrate137. Furthermore, 2 large randomized controlled studies of fructo-oligosaccharides and inulin showed no clear benefit for patients with CD73, 74. It is possible that the effectiveness of EEN does not result from alterations in the composition of the gut microbiota, but rather alterations in the beneficial or harmful metabolites produced the gut microbiota. Examples of such associations are beginning to emerge in other fields138 and this is an important area of research of IBD.
Mechanisms involving alteration in bile acids
Diet can affect production of bile acids, which act on the gut microbiome. In a reciprocal manner, the gut microbiota regulates bile acid metabolism and synthesis139, 140. There are multiple potential mechanisms by which the effects of diet on bile acids could also affect development of IBD. One pathway could involve the farnesoid X receptor, a bile salt receptor proposed to preserve epithelial barrier function and downregulate inflammatory cytokines. Alternatively, diet-induced changes in bile acids that alter the composition of the gut microbiota, could affect IBD onset and progression by affecting production of potentially toxic compounds by sulfite-reducing bacteria, or by promoting T-helper 1 cell-mediated immune responses64, 141.
Mechanisms involving reduced exposure to deleterious compounds
EEN helps patients avoid many additives and other potentially detrimental ingredients found in prepared foods. In addition to the lack of phytates and oxalates, which might reduce absorption, formulas do not contain the chemical additives or preservatives found in many processed foods. Furthermore, formulas do not contain gluten, a normal component of wheat, barley, and rye that can reduce absorption in subjects with gluten sensitivity, celiac disease, or wheat allergy.142 The formulas prescribed for EEN are generally not immunogenic, although whey or casein can induce allergic reactions in individuals with a true milk allergy (rare).143 However, soy protein may be tolerated by these patients. Other potential protein-based allergens that have been identified in whole-food diets are generally not included in EEN formulas.
Other Approaches to Restriction Diets
Several diets popularized in recent years have focused on elimination of potential harmful substances144. The specific carbohydrate and Paleolithic diets aim to eliminate putative harmful food components. The specific carbohydrate diet excludes all sugars other than monosaccharides: glucose, fructose, and galactose. Processed meats are also avoided because they may contain other sugars. The Paleolithic diet emphasizes intake of lean meats from non-domesticated animals as well as fruits and vegetables. The diet aims to achieve a low ratio of n-6:n-3 PUFAs. However, there is little evidence of the efficacy of these diets from wellcontrolled studies. These restriction diets have been largely popularized through anecdotal evidence, shared without guidance from the medical community. Because they have little scientific basis, these diets require critical appraisal. Sigall-Boneh et al reported that the combination of a partial enteral nutritional and a restriction diet induced remission and mucosal healing in approximately 70% of patients with CD, thereby making partial enteral nutritional a more attractive option145. Unfortunately, the restriction diet used in the study may be difficult to maintain.
The superiority of EEN to partial enteral nutritional, and the rapid recurrence of inflammation following restoration of intestinal continuity, support the hypothesis that the exclusion of specific types of foods has therapeutic benefit. Ritchie et al found no difference in outcomes of CD between patients who consumed refined vs natural sugars69. Given the epidemiologic associations between red meat, n-6 PUFA, and incidence of IBD, researchers performed a prospective trial of a semi-vegetarian diet22, 24, 65. They showed that the diet was effective in maintaining remission over 2 years.65
Most studies of diet and IBD generally involved broad food avoidance or supplementation. However, individualized food avoidance, based on detection of IgG4 to specific food antigens, have been shown to reduceclinical symptoms but not C-reactive protein.146. Whereas formulas chosen for EEN are nutritionally complete, extreme exclusion diets may put patients at risk for caloric or specific-nutrient deficiencies.
Future Directions
Dietary interventions might be used to treat patients with active IBD, maintain their remission, or even prevent this disease. Dietary interventions for the treatment of UC are limited and generally have not been effective. In contrast, EEN has been consistently demonstrated to be effective for CD, although it is not effective for all patients. Based on these experiences, interest has turned to exclusion diets, which would allow patients to eat a limited diet of whole foods without exacerbating the disease. Patients’ own experimentation often leads them to identify a number of foods that they believe worsen their symptoms, in essence creating their own personal exclusion diet. For most, this approach is not sufficient to control inflammation. Given the evidence that EEN can reduce symptoms and mucosal inflammation, there is reason to believe that diets and/or dietary supplements could be derived that would provide therapeutic benefit. Improving our understanding of the mechanism of EEN could help to inform the creation of such diets.
An important first step in the development of therapeutic diets for IBD is to improve our methods of studying diet. Clinical studies on food and IBD are limited by the difficulty in accurately capturing dietary intake as well as the potential for complex interactions between foods. Further, proportions of food intake, relative to other dietary components, is challenging to capture75. Differences in food metabolism can vary among individuals. The same food products can even differ, based on preservation, processing, packaging, and preparation. Food science is an area open to further study.
Although data from animal models and in vitro experiments can help guide the development of dietary interventions, clinical trials are also needed. Dietary intervention trials face several challenges that traditional drug trials do not. Most obvious is the inability to use placebo as a control. The need for open-label designs could bias participants and investigators, so alternative strategies are needed. These could include having a separate blinded evaluator for subjective outcomes such as clinical remission or reduced mucosal ulcerations, or relying on objective measures such as biomarkers to assess efficacy. The gut microbiome might also be used as a biomarker of dietary intake in the context of subjects’ genotypes, disease phenotypes, and dietary intake exposures. Results from open-label studies can also be distorted if participants become aware of the study hypothesis; these studies may need to withhold information from participants on the study’s main question or the diets the participants are placed on.
It is extremely difficult to recruit newly diagnosed patients to clinical trials. As such, trials of dietary therapy will generally be conducted in subjects receiving other medical therapies. With availability of increasingly effective therapies, the ability to detect a difference in outcomes with dietary interventions may be limited. One approach to overcome this challenge could be studies of different diets in patients undergoing withdrawal from specific treatments.
Given that IBD encompasses likely far more than 2 diseases (CD and UC), it is unlikely that all patients with IBD can be managed with dietary interventions alone. However, the development of effective dietary interventions that could be used as sole therapy for subsets of patients and adjunctive therapy for other patients would be a major step forward. Adjunctive dietary therapy could reduce the frequency of disease relapse and/or the degree of immunosuppression necessary to control the disease—both of which are important and valued by patients.
Development of diets that prevent the onset of disease is a much greater challenge. It would require identification of people who are at high risk for disease, presumably based on genetic testing and/or use of other biomarkers. Diets aimed at preventing onset of disease would need to be highly palatable, since adherence to such a diet would need to be life long. Adherence to therapeutic diets is a major challenge even for patients with established diagnoses. However, if science is able to direct more specific and evidence-based dietary recommendations, it is likely that a market for palatable foods could be developed for patients with IBD.
Incorporating the advances in our understanding of diet and its effect on the gut microbiome and the mucosal immune system (Figure 1), we provide a vision for how dietary engineering might be used to modify the intestinal lumen and its microbes to treatIBD as either primary of adjunctive therapy (Figure 2). Several strategies are being developed to modify the gut microbiota, such as next-generation probiotics, whereas EEN has already been shown to be effective in inducing remission in patients with active CD. Although the magnitude of efficacy of defined formula diets to treat active CD appears to correlate with the extent of exclusion of whole foods, the specific foods that perpetuate active disease have not been identified. As further research identifies components of whole foods with deleterious effects, or nutrients in defined formula diets that benefit patients with IBD (Figure 1), it may be possible to develop sustainable long-term diets, composed of selected whole foods and a dietary supplement, for treatment of gastrointestinal diseases. The proportions of these would need to be determined empirically, based on the responses of individual patients. Upon relapse of disease, intensive environmental therapy could be reinitiated to induce remission—an approach currently used with defined formula diets in selected populations. Modifications to the intestinal environment might be effective as individual therapy, in combination with immunosuppressive agents to increase response and/or reduce drug dose, or as salvage therapy for patients who do not respond or lose responsiveness to immunosuppressive agents.
Figure 2. Altering the Luminal Environment of the Intestine as an Adjunct Therapy for IBD.
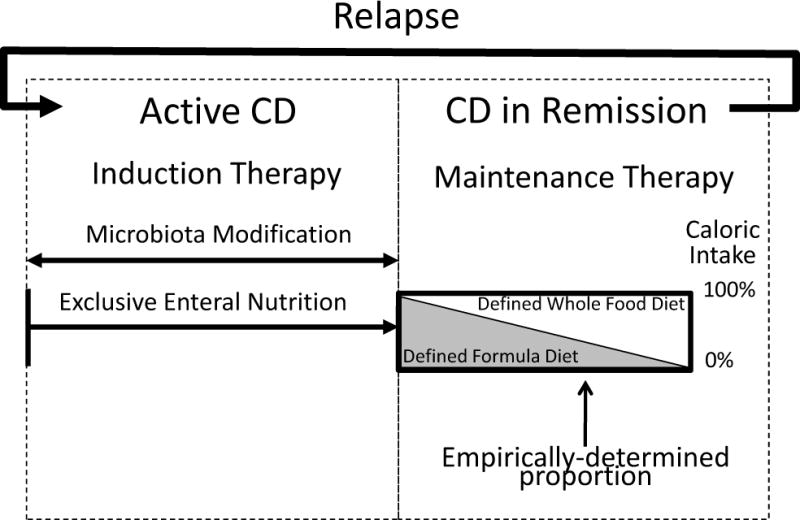
The luminal environment of the gut might be modified by alteration of the microbiome and/or diet to induce and/or maintain remission in patients with IBD. Such an approach might have value as an adjunct to currently used immunosuppressive agents, to increase response and/or reduce drug dose, or as salvage therapy for patients who do not respond or lose responsiveness to immunosuppressive agents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–94. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Han D, Walsh MC, Cejas PJ, et al. Dendritic cell expression of the signaling molecule TRAF6 is critical for gut microbiota-dependent immune tolerance. Immunity. 2013;38:1211–22. doi: 10.1016/j.immuni.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 6.Linneberg A, Nielsen NH, Madsen F, et al. Secular trends of allergic asthma in Danish adults. The Copenhagen Allergy Study Respir Med. 2001;95:258–64. doi: 10.1053/rmed.2001.1031. [DOI] [PubMed] [Google Scholar]
- 7.Magnus P, Jaakkola JJ. Secular trend in the occurrence of asthma among children and young adults: critical appraisal of repeated cross sectional surveys. BMJ. 1997;314:1795–9. doi: 10.1136/bmj.314.7097.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcalde-Cabero E, Almazan-Isla J, Garcia-Merino A, et al. Incidence of multiple sclerosis among European Economic Area populations, 1985–2009: the framework for monitoring. BMC Neurol. 2013;13:58. doi: 10.1186/1471-2377-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Icen M, Crowson CS, McEvoy MT, et al. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol. 2009;60:394–401. doi: 10.1016/j.jaad.2008.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niv Y, Abukasis G. Prevalence of ulcerative colitis in the Israeli kibbutz population. J Clin Gastroenterol. 1991;13:98–101. doi: 10.1097/00004836-199102000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Rozen P, Zonis J, Yekutiel P, et al. Crohn’s disease in the Jewish population of Tel-Aviv-Yafo. Epidemiologic and clinical aspects. Gastroenterology. 1979;76:25–30. [PubMed] [Google Scholar]
- 12.Krawiec J, Odes HS, Lasry Y, et al. Aspects of the epidemiology of Crohn’s disease in the Jewish population in Beer Sheva, Israel. Isr J Med Sci. 1984;20:16–21. [PubMed] [Google Scholar]
- 13.Odes HS, Locker C, Neumann L, et al. Epidemiology of Crohn’s disease in southern Israel. Am J Gastroenterol. 1994;89:1859–62. [PubMed] [Google Scholar]
- 14.Li X, Sundquist J, Hemminki K, et al. Risk of inflammatory bowel disease in first- and second-generation immigrants in Sweden: a nationwide follow-up study. Inflamm Bowel Dis. 2011;17:1784–91. doi: 10.1002/ibd.21535. [DOI] [PubMed] [Google Scholar]
- 15.Barreiro-de Acosta M, Alvarez Castro A, Souto R, et al. Emigration to western industrialized countries: A risk factor for developing inflammatory bowel disease. J Crohns Colitis. 2011;5:566–9. doi: 10.1016/j.crohns.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Barclay AR, Russell RK, Wilson ML, et al. Systematic review: the role of breastfeeding in the development of pediatric inflammatory bowel disease. J Pediatr. 2009;155:421–6. doi: 10.1016/j.jpeds.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Chatterton DE, Nguyen DN, Bering SB, et al. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol. 2013;45:1730–1747. doi: 10.1016/j.biocel.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Abramson O, Durant M, Mow W, et al. Incidence, prevalence, and time trends of pediatric inflammatory bowel disease in Northern California, 1996 to 2006. J Pediatr. 2010;157:233–239 e1. doi: 10.1016/j.jpeds.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Azad MB, Konya T, Maughan H, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185:385–94. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. American Journal of Gastroenterology. 2011;106:563–73. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 22.Investigators IBDiES. Tjonneland A, Overvad K, et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut. 2009;58:1606–11. doi: 10.1136/gut.2008.169078. [DOI] [PubMed] [Google Scholar]
- 23.Ananthakrishnan AN, Khalili H, Konijeti GG, et al. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut. 2013 doi: 10.1136/gutjnl-2013-305304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–73. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 25.Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145:970–7. doi: 10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costea I, Mack DR, Lemaitre RN, et al. Interactions between the dietary polyunsaturated Fatty Acid ratio and genetic factors determine susceptibility to pediatric Crohn’s disease. Gastroenterology. 2014;146:929–931 e3. doi: 10.1053/j.gastro.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676–84. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Logt EM, Blokzijl T, van der Meer R, et al. Westernized high-fat diet accelerates weight loss in dextran sulfate sodium-induced colitis in mice, which is further aggravated by supplementation of heme. J Nutr Biochem. 2013;24:1159–65. doi: 10.1016/j.jnutbio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Gruber L, Kisling S, Lichti P, et al. High fat diet accelerates pathogenesis of murine Crohn’s disease-like ileitis independently of obesity. PLoS One. 2013;8:e71661. doi: 10.1371/journal.pone.0071661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosco N, Brahmbhatt V, Oliveira M, et al. Effects of increase in fish oil intake on intestinal eicosanoids and inflammation in a mouse model of colitis. Lipids Health Dis. 2013;12:81. doi: 10.1186/1476-511X-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsunaga H, Hokari R, Kurihara C, et al. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflamm Bowel Dis. 2008;14:1348–57. doi: 10.1002/ibd.20491. [DOI] [PubMed] [Google Scholar]
- 32.Mbodji K, Charpentier C, Guerin C, et al. Adjunct therapy of n-3 fatty acids to 5-ASA ameliorates inflammatory score and decreases NF-kappaB in rats with TNBS-induced colitis. J Nutr Biochem. 2013;24:700–5. doi: 10.1016/j.jnutbio.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Nieto N, Torres MI, Rios A, et al. Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. J Nutr. 2002;132:11–9. doi: 10.1093/jn/132.1.11. [DOI] [PubMed] [Google Scholar]
- 34.Whiting CV, Bland PW, Tarlton JF. Dietary n-3 polyunsaturated fatty acids reduce disease and colonic proinflammatory cytokines in a mouse model of colitis. Inflamm Bowel Dis. 2005;11:340–9. doi: 10.1097/01.mib.0000164016.98913.7c. [DOI] [PubMed] [Google Scholar]
- 35.Coeffier M, Marion-Letellier R, Dechelotte P. Potential for amino acids supplementation during inflammatory bowel diseases. Inflamm Bowel Dis. 2010;16:518–24. doi: 10.1002/ibd.21017. [DOI] [PubMed] [Google Scholar]
- 36.Xue H, Sufit AJ, Wischmeyer PE. Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. JPEN J Parenter Enteral Nutr. 2011;35:188–97. doi: 10.1177/0148607110381407. [DOI] [PubMed] [Google Scholar]
- 37.Ren W, Yin J, Wu M, et al. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS One. 2014;9:e88335. doi: 10.1371/journal.pone.0088335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giris M, Erbil Y, Dogru-Abbasoglu S, et al. The effect of heme oxygenase-1 induction by glutamine on TNBS-induced colitis. The effect of glutamine on TNBS colitis. Int J Colorectal Dis. 2007;22:591–9. doi: 10.1007/s00384-006-0238-y. [DOI] [PubMed] [Google Scholar]
- 39.Coburn LA, Gong X, Singh K, et al. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS One. 2012;7:e33546. doi: 10.1371/journal.pone.0033546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andou A, Hisamatsu T, Okamoto S, et al. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology. 2009;136:564–74 e2. doi: 10.1053/j.gastro.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 41.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–43. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faure M, Mettraux C, Moennoz D, et al. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J Nutr. 2006;136:1558–64. doi: 10.1093/jn/136.6.1558. [DOI] [PubMed] [Google Scholar]
- 43.Kim CJ, Kovacs-Nolan JA, Yang C, et al. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutr Biochem. 2010;21:468–75. doi: 10.1016/j.jnutbio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Nanau RM, Neuman MG. Nutritional and probiotic supplementation in colitis models. Dig Dis Sci. 2012;57:2786–810. doi: 10.1007/s10620-012-2284-3. [DOI] [PubMed] [Google Scholar]
- 45.Roediger WE, Rae DA. Trophic effect of short chain fatty acids on mucosal handling of ions by the defunctioned colon. Br J Surg. 1982;69:23–5. doi: 10.1002/bjs.1800690108. [DOI] [PubMed] [Google Scholar]
- 46.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40:833–42. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Ung VY, Foshaug RR, MacFarlane SM, et al. Oral administration of curcumin emulsified in carboxymethyl cellulose has a potent anti-inflammatory effect in the IL-10 gene-deficient mouse model of IBD. Dig Dis Sci. 2010;55:1272–7. doi: 10.1007/s10620-009-0843-z. [DOI] [PubMed] [Google Scholar]
- 48.Bruckner M, Westphal S, Domschke W, et al. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J Crohns Colitis. 2012;6:226–35. doi: 10.1016/j.crohns.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Youn J, Lee JS, Na HK, et al. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr Cancer. 2009;61:847–54. doi: 10.1080/01635580903285072. [DOI] [PubMed] [Google Scholar]
- 50.Kataoka K, Ogasa S, Kuwahara T, et al. Inhibitory effects of fermented brown rice on induction of acute colitis by dextran sulfate sodium in rats. Dig Dis Sci. 2008;53:1601–8. doi: 10.1007/s10620-007-0063-3. [DOI] [PubMed] [Google Scholar]
- 51.Cantorna MT, Zhu Y, Froicu M, et al. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 52.Schepens MA, Schonewille AJ, Vink C, et al. Supplemental calcium attenuates the colitis-related increase in diarrhea, intestinal permeability, and extracellular matrix breakdown in HLA-B27 transgenic rats. J Nutr. 2009;139:1525–33. doi: 10.3945/jn.109.105205. [DOI] [PubMed] [Google Scholar]
- 53.Zhao H, Zhang H, Wu H, et al. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57. doi: 10.1186/1471-230X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nairz M, Schroll A, Sonnweber T, et al. The struggle for iron – a metal at the hostpathogen interface. Cell Microbiol. 2010;12:1691–702. doi: 10.1111/j.1462-5822.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 55.Porto G, De Sousa M. Iron overload and immunity. World J Gastroenterol. 2007;13:4707–15. doi: 10.3748/wjg.v13.i35.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Letters. 1978;92:321–6. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- 57.Hentze MW, Muckenthaler MU, Galy B, et al. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 58.Tsukamoto H, Lin M, Ohata M, et al. Iron primes hepatic macrophages for NF-kappaB activation in alcoholic liver injury. American Journal of Physiology. 1999;277:G1240–50. doi: 10.1152/ajpgi.1999.277.6.G1240. [DOI] [PubMed] [Google Scholar]
- 59.Werner T, Wagner SJ, Martinez I, et al. Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut. 2011;60:325–33. doi: 10.1136/gut.2010.216929. [DOI] [PubMed] [Google Scholar]
- 60.Weiss G. Iron in the inflammed gut: another pro-inflammatory hit? Gut. 2011;60:287–8. doi: 10.1136/gut.2010.229047. [DOI] [PubMed] [Google Scholar]
- 61.Le Leu RK, Young GP, Hu Y, et al. Dietary red meat aggravates dextran sulfate sodium-induced colitis in mice whereas resistant starch attenuates inflammation. Dig Dis Sci. 2013;58:3475–82. doi: 10.1007/s10620-013-2844-1. [DOI] [PubMed] [Google Scholar]
- 62.Schepens MA, Vink C, Schonewille AJ, et al. Dietary heme adversely affects experimental colitis in rats, despite heat-shock protein induction. Nutrition. 2011;27:590–7. doi: 10.1016/j.nut.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Kajiura T, Takeda T, Sakata S, et al. Change of intestinal microbiota with elemental diet and its impact on therapeutic effects in a murine model of chronic colitis. Dig Dis Sci. 2009;54:1892–900. doi: 10.1007/s10620-008-0574-6. [DOI] [PubMed] [Google Scholar]
- 64.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487:104–8. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010;16:2484–95. doi: 10.3748/wjg.v16.i20.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr Pharm Des. 2003;9:347–58. doi: 10.2174/1381612033391973. [DOI] [PubMed] [Google Scholar]
- 67.Berghouse L, Hori S, Hill M, et al. Comparison between the bacterial and oligosaccharide content of ileostomy effluent in subjects taking diets rich in refined or unrefined carbohydrate. Gut. 1984;25:1071–7. doi: 10.1136/gut.25.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levenstein S, Prantera C, Luzi C, et al. Low residue or normal diet in Crohn’s disease: a prospective controlled study in Italian patients. Gut. 1985;26:989–93. doi: 10.1136/gut.26.10.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ritchie JK, Wadsworth J, Lennard-Jones JE, et al. Controlled multicentre therapeutic trial of an unrefined carbohydrate, fibre rich diet in Crohn’s disease. Br Med J (Clin Res Ed) 1987;295:517–20. doi: 10.1136/bmj.295.6597.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feagan BG, Sandborn WJ, Mittmann U, et al. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. JAMA. 2008;299:1690–7. doi: 10.1001/jama.299.14.1690. [DOI] [PubMed] [Google Scholar]
- 71.Ananthakrishnan AN, Cagan A, Gainer VS, et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1921–7. doi: 10.1097/MIB.0b013e3182902ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn’s disease – a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–83. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 73.Benjamin JL, Hedin CR, Koutsoumpas A, et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut. 2011;60:923–9. doi: 10.1136/gut.2010.232025. [DOI] [PubMed] [Google Scholar]
- 74.Joossens M, De Preter V, Ballet V, et al. Effect of oligofructose-enriched inulin (OF-IN) on bacterial composition and disease activity of patients with Crohn’s disease: results from a double-blinded randomised controlled trial. Gut. 2012;61:958. doi: 10.1136/gutjnl-2011-300413. [DOI] [PubMed] [Google Scholar]
- 75.Willett W. Nutritional epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 76.Elson CO, Layden TJ, Nemchausky BA, et al. An evaluation of total parenteral nutrition in the management of inflammatory bowel disease. Dig Dis Sci. 1980;25:42–8. doi: 10.1007/BF01312731. [DOI] [PubMed] [Google Scholar]
- 77.Triantafillidis JK, Papalois AE. The role of total parenteral nutrition in inflammatory bowel disease: current aspects. Scand J Gastroenterol. 2014;49:3–14. doi: 10.3109/00365521.2013.860557. [DOI] [PubMed] [Google Scholar]
- 78.Reilly J, Ryan JA, Strole W, et al. Hyperalimentation in inflammatory bowel disease. Am J Surg. 1976;131:192–200. doi: 10.1016/0002-9610(76)90096-9. [DOI] [PubMed] [Google Scholar]
- 79.Vogel CM, Corwin TR, Baue AE. Proceedings: Intravenous hyperalimentation in the treatment of inflammatory diseases of the bowel. Arch Surg. 1974;108:460–7. doi: 10.1001/archsurg.1974.01350280066012. [DOI] [PubMed] [Google Scholar]
- 80.Lochs H, Meryn S, Marosi L, et al. Has total bowel rest a beneficial effect in the treatment of Crohn’s disease? Clin Nutr. 1983;2:61–4. doi: 10.1016/0261-5614(83)90033-x. [DOI] [PubMed] [Google Scholar]
- 81.Winslet MC, Andrews H, Allan RN, et al. Fecal diversion in the management of Crohn’s disease of the colon. Dis Colon Rectum. 1993;36:757–62. doi: 10.1007/BF02048367. [DOI] [PubMed] [Google Scholar]
- 82.D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–7. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 83.Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet. 1991;338:771–4. doi: 10.1016/0140-6736(91)90663-a. [DOI] [PubMed] [Google Scholar]
- 84.Spivak J, Landers CJ, Vasiliauskas EA, et al. Antibodies to I2 predict clinical response to fecal diversion in Crohn’s disease. Inflammatory Bowel Diseases. 2006;12:1122–30. doi: 10.1097/01.mib.0000235833.47423.d7. [DOI] [PubMed] [Google Scholar]
- 85.Yamamoto T, Allan RN, Keighley MR. Effect of fecal diversion alone on perianal Crohn’s disease. World J Surg. 2000;24:1258–62. doi: 10.1007/s002680010250. discussion 1262–3. [DOI] [PubMed] [Google Scholar]
- 86.Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology. 1995;108:1617–21. doi: 10.1016/0016-5085(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 87.Rutgeerts P, Van Assche G, Vermeire S, et al. Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2005;128:856–61. doi: 10.1053/j.gastro.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 88.Rhodes J, Rose J. Does food affect acute inflammatory bowel disease? The role of parenteral nutrition, elemental and exclusion diets. Gut. 1986;27:471–4. doi: 10.1136/gut.27.5.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006;4:744–53. doi: 10.1016/j.cgh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 90.Day AS, Whitten KE, Lemberg DA, et al. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: a feasible and effective approach. J Gastroenterol Hepatol. 2006;21:1609–14. doi: 10.1111/j.1440-1746.2006.04294.x. [DOI] [PubMed] [Google Scholar]
- 91.Heuschkel RB, Menache CC, Megerian JT, et al. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr. 2000;31:8–15. doi: 10.1097/00005176-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 92.Sandhu BK, Fell JM, Beattie RM, et al. Guidelines for the Management of Inflammatory Bowel Disease in Children in the United Kingdom. J Pediatr Gastroenterol Nutr. 2010 doi: 10.1097/MPG.0b013e3181c92c53. [DOI] [PubMed] [Google Scholar]
- 93.Caprilli R, Gassull MA, Escher JC, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: special situations. Gut. 2006;55(Suppl 1):i36–58. doi: 10.1136/gut.2005.081950c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takagi S, Utsunomiya K, Kuriyama S, et al. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: A randomized-controlled trial. Aliment Pharmacol Ther. 2006;24:1333–40. doi: 10.1111/j.1365-2036.2006.03120.x. [DOI] [PubMed] [Google Scholar]
- 95.Johnson T, Macdonald S, Hill SM, et al. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut. 2006;55:356–61. doi: 10.1136/gut.2004.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fell JM, Paintin M, Arnaud-Battandier F, et al. Mucosal healing and a fall in mucosal proinflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2000;14:281–9. doi: 10.1046/j.1365-2036.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- 97.Afzal NA, Van Der Zaag-Loonen HJ, Arnaud-Battandier F, et al. Improvement in quality of life of children with acute Crohn’s disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment Pharmacol Ther. 2004;20:167–72. doi: 10.1111/j.1365-2036.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- 98.Froslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–22. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 99.Thomas AG, Taylor F, Miller V. Dietary intake and nutritional treatment in childhood Crohn’s disease. J Pediatr Gastroenterol Nutr. 1993;17:75–81. doi: 10.1097/00005176-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 100.Gerasimidis K, Talwar D, Duncan A, et al. Impact of exclusive enteral nutrition on body composition and circulating micronutrients in plasma and erythrocytes of children with active Crohn’s disease. Inflamm Bowel Dis. 2012;18:1672–81. doi: 10.1002/ibd.21916. [DOI] [PubMed] [Google Scholar]
- 101.Newby EA, Sawczenko A, Thomas AG, et al. Interventions for growth failure in childhood Crohn’s disease. Cochrane Database Syst Rev. 2005:CD003873. doi: 10.1002/14651858.CD003873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Whitten KE, Leach ST, Bohane TD, et al. Effect of exclusive enteral nutrition on bone turnover in children with Crohn’s disease. J Gastroenterol. 2010;45:399–405. doi: 10.1007/s00535-009-0165-0. [DOI] [PubMed] [Google Scholar]
- 103.Seidman EG. Nutritional management of inflammatory bowel disease. Gastroenterol Clin North Am. 1989;18:129–55. [PubMed] [Google Scholar]
- 104.Afzal NA, Davies S, Paintin M, et al. Colonic Crohn’s disease in children does not respond well to treatment with enteral nutrition if the ileum is not involved. Dig Dis Sci. 2005;50:1471–5. doi: 10.1007/s10620-005-2864-6. [DOI] [PubMed] [Google Scholar]
- 105.Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2007:CD000542. doi: 10.1002/14651858.CD000542.pub2. [DOI] [PubMed] [Google Scholar]
- 106.Day AS, Whitten KE, Sidler M, et al. Systematic review: nutritional therapy in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2008;27:293–307. doi: 10.1111/j.1365-2036.2007.03578.x. [DOI] [PubMed] [Google Scholar]
- 107.Wall CL, Day AS, Gearry RB. Use of exclusive enteral nutrition in adults with Crohn’s disease: a review. World J Gastroenterol. 2013;19:7652–60. doi: 10.3748/wjg.v19.i43.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Okada M, Yao T, Yamamoto T, et al. Controlled trial comparing an elemental diet with prednisolone in the treatment of active Crohn’s disease. Hepatogastroenterology. 1990;37:72–80. [PubMed] [Google Scholar]
- 109.O’Morain C, Segal AW, Levi AJ. Elemental diet as primary treatment of acute Crohn’s disease: a controlled trial. Br Med J (Clin Res Ed) 1984;288:1859–62. doi: 10.1136/bmj.288.6434.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gassull MA. Review article: the role of nutrition in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):79–83. doi: 10.1111/j.1365-2036.2004.02050.x. [DOI] [PubMed] [Google Scholar]
- 111.Lionetti P, Callegari ML, Ferrari S, et al. Enteral nutrition and microflora in pediatric Crohn’s disease. JPEN J Parenter Enteral Nutr. 2005;29:S173–5. doi: 10.1177/01486071050290S4S173. discussion S175–8, S184–8. [DOI] [PubMed] [Google Scholar]
- 112.van den Bogaerde J, Kamm MA, Knight SC. Immune sensitization to food, yeast and bacteria in Crohn’s disease. Aliment Pharmacol Ther. 2001;15:1647–53. doi: 10.1046/j.1365-2036.2001.01032.x. [DOI] [PubMed] [Google Scholar]
- 113.Van Den Bogaerde J, Cahill J, Emmanuel AV, et al. Gut mucosal response to food antigens in Crohn’s disease. Aliment Pharmacol Ther. 2002;16:1903–15. doi: 10.1046/j.1365-2036.2002.01360.x. [DOI] [PubMed] [Google Scholar]
- 114.Farre R, Tack J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. Am J Gastroenterol. 2013;108:698–706. doi: 10.1038/ajg.2013.24. [DOI] [PubMed] [Google Scholar]
- 115.Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut. 2014;63:179–90. doi: 10.1136/gutjnl-2013-305112. [DOI] [PubMed] [Google Scholar]
- 116.Russell RI. Intestinal Adaptation to an Elemental Diet. Proceedings of the Nutrition Society. 1985;44:87–93. doi: 10.1079/pns19850014. [DOI] [PubMed] [Google Scholar]
- 117.Russell RI. Intestinal adaptation to an elemental diet. Proc Nutr Soc. 1985;44:87–93. doi: 10.1079/pns19850014. [DOI] [PubMed] [Google Scholar]
- 118.Hisamatsu T, Okamoto S, Hashimoto M, et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS One. 2012;7:e31131. doi: 10.1371/journal.pone.0031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–81. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Faure M, Moennoz D, Montigon F, et al. Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats. J Nutr. 2005;135:486–91. doi: 10.1093/jn/135.3.486. [DOI] [PubMed] [Google Scholar]
- 121.Kien CL, Cordano A, Cook DA, et al. Fecal characteristics in healthy young adults consuming defined liquid diets or a free-choice diet. Am J Clin Nutr. 1981;34:357–61. doi: 10.1093/ajcn/34.3.357. [DOI] [PubMed] [Google Scholar]
- 122.Hecketsweiler P, Vidon N, Emonts P, et al. Absorption of elemental and complex nutritional solutions during a continuous jejunal perfusion in man. Digestion. 1979;19:213–7. doi: 10.1159/000198347. [DOI] [PubMed] [Google Scholar]
- 123.Suma PF, Urooj A. Nutrients, antinutrients & bioaccessible mineral content (invitro) of pearl millet as influenced by milling. J Food Sci Technol. 2014;51:756–61. doi: 10.1007/s13197-011-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moretti D, Biebinger R, Bruins MJ, et al. Bioavailability of iron, zinc, folic acid, and vitamin A from fortified maize. Ann N Y Acad Sci. 2014;1312:54–65. doi: 10.1111/nyas.12297. [DOI] [PubMed] [Google Scholar]
- 125.Sandberg AS. Bioavailability of minerals in legumes. Br J Nutr. 2002;88(Suppl 3):S281–5. doi: 10.1079/BJN/2002718. [DOI] [PubMed] [Google Scholar]
- 126.Alarcon-Flores MI, Romero-Gonzalez R, Martinez Vidal JL, et al. Monitoring of phytochemicals in fresh and fresh-cut vegetables: a comparison. Food Chem. 2014;142:392–9. doi: 10.1016/j.foodchem.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 127.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–94. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 128.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Macfarlane GT, Macfarlane S. Models for intestinal fermentation: association between food components, delivery systems, bioavailability and functional interactions in the gut. Curr Opin Biotechnol. 2007;18:156–62. doi: 10.1016/j.copbio.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 131.Macfarlane GT, Steed H, Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol. 2008;104:305–44. doi: 10.1111/j.1365-2672.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- 132.Murphy O. Non-polyol low-digestible carbohydrates: food applications and functional benefits. Br J Nutr. 2001;85(Suppl 1):S47–53. doi: 10.1079/bjn2000261. [DOI] [PubMed] [Google Scholar]
- 133.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 134.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dewulf EM, Cani PD, Claus SP, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62:1112–21. doi: 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gerasimidis K, Bertz M, Hanske L, et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm Bowel Dis. 2014;20:861–71. doi: 10.1097/MIB.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 138.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gadaleta RM, van Erpecum KJ, Oldenburg B, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–72. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 140.Nolan JD, Johnston IM, Walters JR. Altered enterohepatic circulation of bile acids in Crohn’s disease and their clinical significance: a new perspective. Expert Rev Gastroenterol Hepatol. 2013;7:49–56. doi: 10.1586/egh.12.66. [DOI] [PubMed] [Google Scholar]
- 141.Sartor RB. Gut microbiota: Diet promotes dysbiosis and colitis in susceptible hosts. Nat Rev Gastroenterol Hepatol. 2012;9:561–2. doi: 10.1038/nrgastro.2012.157. [DOI] [PubMed] [Google Scholar]
- 142.Leonard MM, Vasagar B. US perspective on gluten-related diseases. Clin Exp Gastroenterol. 2014;7:25–37. doi: 10.2147/CEG.S54567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ludman S, Shah N, Fox AT. Managing cows’ milk allergy in children. BMJ. 2013;347:f5424. doi: 10.1136/bmj.f5424. [DOI] [PubMed] [Google Scholar]
- 144.Hou JK, Lee D, Lewis J. Diet and inflammatory bowel disease: review of patient-targeted recommendations. Clin Gastroenterol Hepatol. 2014;12:1592–600. doi: 10.1016/j.cgh.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sigall-Boneh R, Pfeffer-Gik T, Segal I, et al. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm Bowel Dis. 2014;20:1353–60. doi: 10.1097/MIB.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 146.Rajendran N, Kumar D. Food-specific IgG4-guided exclusion diets improve symptoms in Crohn’s disease: a pilot study. Colorectal Dis. 2011;13:1009–13. doi: 10.1111/j.1463-1318.2010.02373.x. [DOI] [PubMed] [Google Scholar]


