Abstract
Background
Recent developments in neuroimaging have advanced understanding biological mechanisms underlying schizophrenia. However, neuroimaging correlates of treatment-resistant schizophrenia (TRS) and superior effects of clozapine on TRS remain unclear.
Methods
Systematic search was performed to identify neuroimaging characteristics unique to TRS and ultra-resistant schizophrenia (i.e. clozapine-resistant [URS]), and clozapine's efficacy in TRS using Embase, Medline, and PsychInfo. Search terms included (schizophreni*) and (resistan* OR refractory OR clozapine) and (ASL OR CT OR DTI OR FMRI OR MRI OR MRS OR NIRS OR PET OR SPECT).
Results
25 neuroimaging studies have investigated TRS and effects of clozapine. Only 5 studies have compared TRS and non-TRS, collectively providing no replicated neuroimaging finding specific to TRS. Studies comparing TRS and healthy controls suggest hypometabolism in the prefrontal cortex, hypermetabolism in the basal ganglia, and structural anomalies in the corpus callosum contribute to TRS. Clozapine may increase prefrontal hypoactivation in TRS although this was not related to clinical improvement; in contrast, evidence has suggested a link between clozapine efficacy and decreased metabolism in the basal ganglia and thalamus.
Conclusion
Existing literature does not elucidate neuroimaging correlates specific to TRS or URS, which, if present, might also shed light on clozapine's efficacy in TRS. This said, leads from other lines of investigation, including the glutamatergic system can prove useful in guiding future neuroimaging studies focused on, in particular, the frontocortical-basal ganglia-thalamic circuits. Critical to the success of this work will be precise subtyping of study subjects based on treatment response/nonresponse and the use of multimodal neuroimaging.
Keywords: schizophrenia, treatment-resistance, clozapine, neuroimaging, glutamate
1. Introduction
All currently available antipsychotics for schizophrenia are, to varying degrees, antagonists of the dopamine D2 receptor (D2R) (Kapur et al., 2000; Mamo et al., 2007; Seeman and Kapur, 2000). The efficacy of D2R antagonism is premised on the dopamine hypothesis of schizophrenia (Howes and Kapur, 2009), which proposes that aberrant dopaminergic functioning is critical in schizophrenia (Abi-Dargham et al., 1998; Hietala et al., 1999; Kapur, 2003; Laruelle et al., 1996; Sato et al., 1992). While the dopamine hypothesis remains central to our current understanding of schizophrenia, approximately 20% to 35% of patients show partial or no response to standard antipsychotic treatment (i.e. conventional or atypical antipsychotics, excepting clozapine [CLZ]) (Lindenmayer, 2000). Individuals in this sample, termed treatment-resistant schizophrenia (TRS), are candidates for CLZ, the one antipsychotic with established efficacy in TRS (Chung and Remington, 2005). However, response to CLZ is limited, in the range of 30-70% (Buchanan et al., 1998; Chakos et al., 2001; Conley and Kelly, 2001; Kane et al., 1988), and for those who are not responsive to CLZ, “ultra-resistant” schizophrenia (URS), there are no treatments to date that have proven consistently effective (Cipriani et al., 2009; Sommer et al., 2012).
Recent developments in neuroimaging techniques have substantially advanced our understanding of the biological mechanisms underlying schizophrenia, in particular from the standpoint of dopamine, with studies demonstrating that dopamine synthesis capacity, dopamine release and baseline dopamine levels are elevated in the striatum of patients with schizophrenia (Abi-Dargham et al., 2000; Fusar-Poli and Meyer-Lindenberg, 2013; Howes et al., 2012; Howes et al., 2007; Laruelle et al., 1996). With respect to TRS specifically, Demjaha et al. (2012) compared presynaptic dopaminergic dysfunction among patients with TRS, patients with non-TRS, and healthy controls (HC) using 18[F]-DOPA PET (Demjaha et al., 2012). Dopamine synthesis capacity was lower in patients with TRS than in patients with non-TRS, but not different between TRS and healthy controls (HC). Coppens et al. (1991) reported greater than 95% blockade of D2Rs in the striatum of patients with TRS, concluding that lack of therapeutic response cannot be attributed to insufficient blockade of D2Rs in this population (Coppens et al., 1991). Focusing presynaptically, it has also been reported that augmentation with tetrabenazine, a presynaptic vesicular monoamine transporter inhibitor, is not effective in patients with TRS (Remington et al., 2012). Taken together, these data indicate that patients meeting criteria for TRS have a form (or forms) of the illness that are mediated beyond dopamine neurotransmission.
To this last point, it has been proposed that TRS represents at least several distinct forms from the standpoint of pathophysiology (Farooq et al., 2013). One form is responsive to CLZ, an atypical antipsychotic with superior efficacy in 30% to 70% of patients with TRS (Agid et al., 2011; Chakos et al., 2001; Kane et al., 1988; Lieberman et al., 1994; Meltzer et al., 1990). Whether part of its efficacy in this group is related to D2R binding is not entirely clear; CLZ occupies less than 50% of D2Rs 2-hours following administration and has a very transient effect on D2Rs (Kapur and Seeman, 2001; Tauscher et al., 1999). Regardless, it must at least in part emerge its response through other mechanisms as individuals meeting criteria for TRS have already demonstrated suboptimal response to standard antipsychotic therapy. This calls into question the role of other receptors and systems, which in the case of clozapine has been postulated to include the glutamatergic system (Abdul-Monim et al., 2006; Abekawa et al., 2006, 2007; Amitai et al., 2012; Amitai et al., 2007; Didriksen et al., 2007; Grayson et al., 2007; Hashimoto et al., 2005; Idris et al., 2005; Lopez-Gil et al., 2007), serotonin 5-HT2A receptors (Nordstrom et al., 1995), and dopamine D1 (Tauscher et al., 2004) or D4 receptors (Nord and Farde, 2011; Suzuki et al., 2011; Yilmaz et al., 2012). At present, though, it remains that we do not understand what accounts for the efficacy of clozapine in TRS. In those with TRS who show suboptimal response to clozapine (i.e. URS), our understanding is even less clear since there are no treatments with established efficacy in these individuals (Cipriani et al., 2009; Sommer et al., 2012).
A host of meta-analyses have been conducted on neuroimaging findings in schizophrenia, although not specifically focused on TRS and URS (Brugger et al., 2011; Ellison-Wright et al., 2008; Howes et al., 2012; Leung et al., 2011; Marsman et al., 2013; Steen et al., 2006; Yao et al., 2013). Accordingly, and in light of how important this topic is, the present article represents a review of the existing literature on treatment resistance (TRS and URS) employing neuroimaging techniques, including arterial spin labeling (ASL), computed tomography (CT), diffusion tensor imaging (DTI), structural magnetic resonance imaging (MRI), functional MRI (fMRI), magnetic resonance spectroscopy (MRS), near infrared spectroscopy, positron emission tomography (PET), and single photon emission tomography (SPECT). The main objective was to elucidate possible neuroimaging characteristics unique to these populations in comparison to those who respond to standard antipsychotic treatment.
2. Experimental/Materials and methods
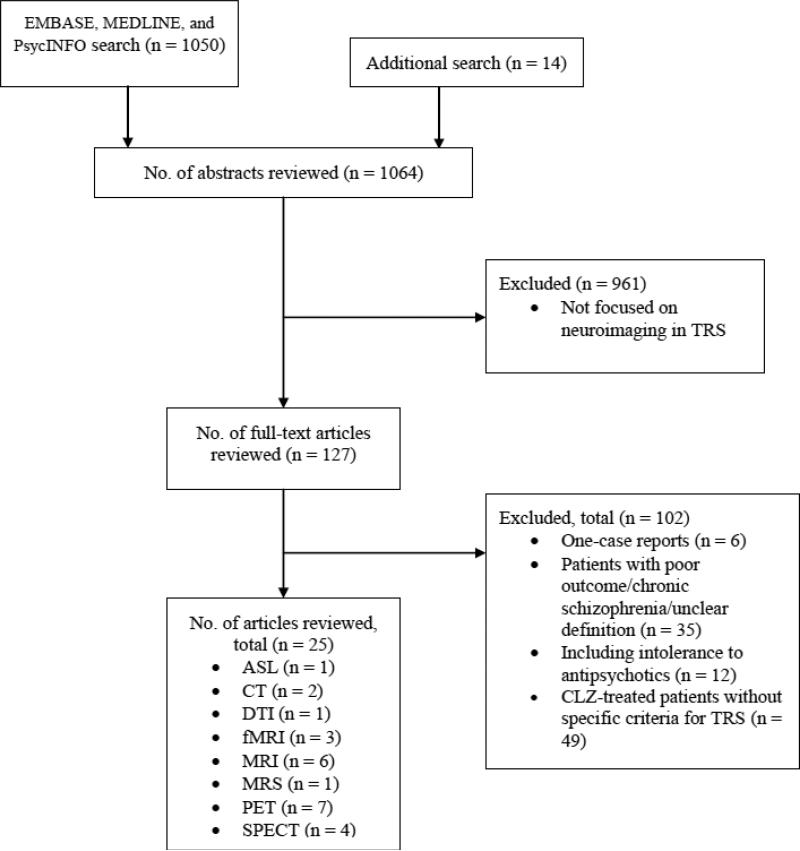
A systematic computerized literature search of EMBASE, MEDLINE, and PsycINFO was conducted. The following search terms were used: (schizophreni*) AND (resistan* OR refractory OR clozapine) AND ((arterial spin labeling) OR ASL OR (computed tomography) OR (computerized tomography) OR CT OR (magnetic resonance) OR MRI OR fMRI OR MRS OR (positron emission tomography) OR PET OR (diffusion tensor imaging) OR DTI OR (single photon emission computed tomography) OR SPECT OR (near infrared spectroscopy) OR NIRS). Only publications written in English pertaining to the focus of this review were selected and no time span was specified for date of publication. The reference sections of the identified studies and previous reviews (Chung and Remington, 2005; Ebdrup et al., 2013; Howes et al., 2009; Howes and Kapur, 2009; Kuroki et al., 2008; Nord and Farde, 2011; Suzuki et al., 2011; Uchida et al., 2011; Yilmaz et al., 2012) were manually searched for additional studies not identified in the computerized search. We excluded case reports including one subject (Conley et al., 2004; Giesel et al., 2012; Jardri et al., 2009; Langguth et al., 2006; Liao et al., 2012; Schreiber et al., 2002). We selected neuroimaging studies on TRS, defined as schizophrenia with a failure to respond to at least two antipsychotic medications. The literature search was conducted independently by two of the authors (S.N. and H.T.). Their disagreement was resolved by consensus. To enhance the quality of reporting in the present systematic review, we followed standardized guidelines (Moher et al., 2009). The last search was conducted on September 24, 2014, and in total yielded 25 articles which formed the empirical basis of this review.
3. Results
3.1. Structural neuroimaging
Structural neuroimaging studies comparing TRS and response to CLZ are summarized in Table 1. Only a few structural neuroimaging studies have investigated TRS, of which one study compared TRS and non-TRS (Zugman et al., 2013). No study has investigated the effects of CLZ on brain structures in TRS. One investigation specifically compared treatment-resistant auditory verbal hallucinations (TR-AVH) and absence of AVH (Kubera et al., 2014). The available structural neuroimaging literature suggests a link between neuroanatomical abnormalities and TRS, including anomalies in the corpus callosum (CC) compared with HC (Holleran et al., 2014; Sun et al., 2009). It remains unclear whether neuroanatomical changes may be related to CLZ response in TRS.
Table 1.
Structural neuroimaging correlates of treatment-resistant schizophrenia and response to clozapine in patients with treatment-resistant schizophrenia
| Year | Author | Type of Neuroimaging | Subjects | Definition of TRS | Treatment for groups with TRS | Controls | Findings |
|---|---|---|---|---|---|---|---|
|
TRS and neuroanatomy
| |||||||
| 2014 | Kubera | MRI | Sz with TR-AVH (n = 10) | Persistent AVH despite ≧2 AP trials (each >6 weeks at adequate dosage of different APs). No pronounced formal thought disorder. Sufficient insight into AVH. | CLZ (monotherapy or combination therapy) | Sz without AVH (n = 10) & HC (n = 14) | Patients with TR-AVH showed lower GM volume in medial and inferior frontal, insular and bilateral temporal regions than those without AVH. Those with TR-AVH showed lower lateral prefrontal GM volume than HC. Negative correlations were found between medial frontal and lateral temporal GMV and symptom duration, location, frequency and intensity in those with TR-AVH. |
| 2014 | Holleran | DTI | CLZ - naïve TRS (n = 19) | Prolonged period of ≧moderate positive symptoms + failure to respond to ≧2 APs, including 1 atypical AP. | Atypical APs other than CLZ (cross-sectional) | HC (n = 19) | Patients with CLZ-naïve TRS showed localized reductions in FA with increases in RD in CC and temporal lobe areas of cortico-cortical WM association tracts. Illness duration was negatively related to FA in splenium of CC in those with CLZ-naïve TRS. |
| 2013 | Zugman | MRI | TRS (n = 61) | Failure to respond to ≧2 different AP trials (each of 4–6 weeks at a CPZ equivalent dose of ≧400 mg/day or 5 mg/day of RIS) + lack of remission (≧4 on PANSS items of delusions, unusual thought content, hallucinatory behavior, mannerisms/posturing, blunted affect, social withdrawal, and lack of spontaneity) for 6 months. | 72.1% of the patients received CLZ (cross-sectional) | Non-TRS (n = 67) & HC (n = 80) | Patients with TRS presented a more widespread reduction in cortical thickness (frontal, parietal, temporal, and occipital regions) than patients with non-TRS (frontal regions) compared with HC. A greater reduction in left DLPFC was found in patients with TRS compared with those with non-TRS. |
| 2009 | Sun | MRI | TRS (n = 42) | Little or no symptomatic response to ≧2 different AP trials (each of ≧6 weeks at therapeutic range doses). | CLZ (n = 11), other atypicals (n = 24), typicals (n = 4) (cross-sectional) | TR MDD (n = 45) & HC (n = 30) | Patients with TRS did not differ from HC in the whole CC area. The mean ICV of patients with TRS was smaller than that of HC. After ICV normalization, splenium of CC was identified as larger in patients with TRS than HC. |
| 2008 | Cachia | MRI | Sz with TR-AVH (n = 30) | Daily AVH despite ≧2 trials (each ≧6 weeks at usual dosages of APs, including ≧1 atypical APs). | APs (cross-sectional) | HC (n = 28) | Patients with TR-AVH had lower global sulcal gyrification in both hemispheres. Local sulcal gyrification decrease was more significant in language-related regions (superior temporal sulcus bilaterally, left middle frontal sulcus and diagonal branch of left sylvian fissure [Broca's area]). |
| Structural change after CLZ administration in TRS | |||||||
| No study | |||||||
|
Prediction of CLZ response in TRS
| |||||||
| 2003 | Arango | MRI | TRS (n = 22) | Minimum level of positive (≧8 on BPRS items for conceptual disorganization, hallucinations, unusual thought content, and suspiciousness or ≧4 on ≧1 of the items) and negative (≧20 on SANS total score or ≧2 on ≧1 SANS global item) symptoms at study entry and retrospective history of residual positive or negative symptoms after ≧2 typical AP trials (each of ≧6 weeks). Failure to respond (<30% improvement in positive or negative symptoms) to fluphenazine (10-30 mg/day, open-label, 6 weeks). | CLZ (prospective) | TRS (n = 23, HAL, prospective) | Larger right prefrontal GM volume was associated with better response (whole and negative symptoms) to CLZ and poorer response to HAL in patients with TRS. Larger right prefrontal GM volume was associated with smaller sensitivity to akathisia by CLZ and greater sensitivity to akathisia by HAL in those with TRS. |
| 2003 | Molina Rodriguez | MRI | TRS (n = 25) | Presence of significant positive or disorganization residual symptoms + failure to respond to ≧2 different-class AP trials (each of ≧6 weeks at a CPZ equivalent dose of ≧800 mg/day) in the preceding 12 months. | CLZ (prospective) | No controls | Improvement in positive symptoms with CLZ was related to temporal GM volume, whereas improvement of disorganization symptoms was inversely related to ICV and hippocampal volume. Improvement in negative symptoms with CLZ was related to DLPFC volume. |
| 2001 | Konicki | CT | CLZ responders with TRS (n = 26) | ≧45 on BPRS-18 total score + ≧4 on BPRS items of conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content + ≧4 on CGI-S + lack of significant symptomatic relief despite 3 AP trials (including 2 different-class AP) trials (each of ≧6 weeks at a CPZ equivalent dose of ≧1000 mg/day) in the preceding 60 months. | CLZ (prospective) | CLZ nonresponders with TRS (n = 10) | CLZ responders with TRS had less prefrontal sulcal widening than nonresponders. Generalized sulcal prominence did not differentiate between them. |
| 1991 | Friedman | CT | TRS (n = 34) | Failure to respond (≧4 on BPRS items of unusual thought content, conceptual disorganization, suspiciousness, or auditory hallucinations) to ≧2 typical AP trials (each of ≧6 weeks at a CPZ equivalent dose of ≧800 mg/day) in the preceding 12 months. | CLZ (prospective) | No controls | CLZ response was related to a smaller prefrontal sulcal prominence reflecting prefrontal atrophy in patients with TRS. |
Abbreviations: AP = antipsychotic, AVH = auditory verbal hallucination, BPRS-18 = the18-items version of the Brief Psychiatric Rating Scale, CC = corpus callosum, CGI-S = the Clinical Global Impression Rating Scales-Severity, CLZ = clozapine, CPZ = chlorpromazine, CT = computed tomography, DLPFC = dorsolateral prefrontal cortex, DTI = diffusion tensor imaging, E-BPRS = the Expanded Brief Psychiatric Rating Scale, FA = fractional anisotropy, GM = gray matter, HC = healthy controls, ICV = intracranial volume, MDD = major depressive disorder, MRI = structural magnetic resonance imaging, PANSS = the Positive and Negative Syndrome Scale, RIS = risperidone, RD = radial diffusivity, SANS = the Scale for the Assessment of Negative Symptoms, Sz = schizophrenia, TR = treatment resistant, TRS = treatment resistant schizophrenia, WM = white matter
3.1.1 Comparison between patients with treatment-resistant schizophrenia and healthy controls
Reductions in cortical thickness have been associated with TRS. Zugman et al. (2013) reported that patients with TRS demonstrated a reduction in cortical thickness in the frontal, parietal, temporal and occipital regions versus HC (Zugman et al., 2013). White matter abnormalities have also been linked to TRS; when compared with HC, patients with CLZ-naïve TRS showed reductions in fractional anisotropy and increases in radial diffusivity in the CC and in the temporal lobe areas of cortico-cortical white matter association tracts, which were negatively related to duration of illness (Holleran et al., 2014). These findings raise the possibility that there exist deficits in the microstructural organization of white matter within these areas in TRS. Furthermore, volumetric studies have reported differences between patients with TRS and HC. Sun et al. (2009) demonstrated that the splenium of the CC was larger in those with TRS than in HC, while no difference in the whole CC was found (Sun et al., 2009). Kubera et al. (2014) noted that patients with TR-AVH showed lower lateral prefrontal GM volume than HC (Kubera et al., 2014) while Cachia et al. (2008) reported that those with TR-AVH had lower global sulcal gyrification in both hemispheres and lower local sulcal gyrification in language-related regions than HC (Cachia et al., 2008).
3.1.2 Comparison between patients with treatment-resistant and non-resistant schizophrenia
Zugman et al. (2013) reported that those with TRS demonstrated more widespread reduction in cortical thickness (frontal, parietal, temporal, and occipital regions) than those with non-TRS (frontal regions) regardless of illness severity, in addition to greater reduction in the left dorsolateral prefrontal cortex (DLPFC) (Zugman et al., 2013). Kubera et al. (2014) reported that those with TR-AVH showed lower lateral medial frontal and lateral temporal GM volume than those without AVH (Kubera et al., 2014). Negative correlations were found between medial frontal and lateral temporal GM volume and symptom duration, location, frequency and intensity in those with TR-AVH.
3.1.3. Clozapine response and structural changes in patients with treatment-resistant schizophrenia
We are not aware of any studies examining structural changes following CLZ treatment in TRS, although several investigations have reported on baseline structural predictors of CLZ response. Friedman et al. (1991) found that CLZ response was positively related to a smaller prefrontal sulcal prominence at baseline, reflecting prefrontal atrophy, in those with TRS (Friedman et al., 1991). Konicki et al. (2001) reported that CLZ responders with TRS had less prefrontal sulcal widening at baseline than nonresponders (Konicki et al., 2001). Arango et al. (2003) noted that larger right prefrontal gray matter (GM) volume was associated with better response in terms of whole and negative symptoms to CLZ and smaller sensitivity to akathisia by CLZ in those with TRS (Arango et al., 2003). Molina Rodriguez et al. (2003) found that clozapine-related improvement in positive symptoms was positively related to baseline temporal gray matter volume, improvement in negative symptoms to baseline DLPFC volume, and improvement in disorganization symptoms inversely related to baseline intracranial volume and hippocampal volume (Molina et al., 2003). These findings suggest that symptomatic improvement with CLZ may be associated with less brain atrophy at baseline, particularly in the prefrontal cortex (PFC). Prospective studies are required, however, to better understand the relationship between concurrent clinical improvement with CLZ and neuroanatomical changes.
3.2. Functional neuroimaging
Functional neuroimaging correlates of TRS and CLZ response are summarized in Tables 2. Once more, few functional neuroimaging studies have examined TRS; two investigations compared TRS and non-TRS (Demjaha et al., 2014; Demjaha et al., 2012), while a further two studies investigated the neurofunctional effects of CLZ on TRS (Molina et al., 2005; Molina et al., 2008). One investigation specifically compared TR-AVH and absence of AVH (Wolf et al., 2012). Few findings have been replicated at this point, but current evidence suggests that a link exists between TRS and neurofunctional abnormalities, particularly, hypometabolism in the PFC (Molina Rodriguez et al., 1997b; Molina et al., 2007) and hypermetabolism in the basal ganglia (BG) (Molina Rodriguez et al., 1997a; Molina Rodriguez et al., 1997b), as compared to HC. CLZ may increase PFC hypoactivation in patients with TRS (Molina et al., 2005; Molina et al., 2008), although this increase is not necessarily related to clinical improvement (Molina et al., 2005; Molina et al., 2008). Whereas, CLZ response has been related to decreased metabolism in the BG (Molina Rodriguez et al., 1997a; Molina Rodriguez et al., 1996; Molina et al., 2005) and thalamus (Molina Rodriguez et al., 1997a; Molina Rodriguez et al., 1996) in TRS.
Table 2.
Functional neuroimaging correlates of treatment-resistant schizophrenia and response to clozapine in patients with treatment-resistant schizophrenia
| Year | Author | Type of Neuroimaging | Subjects | Definition of TRS | Treatment for groups with TRS | Controls | Findings |
|---|---|---|---|---|---|---|---|
|
TRS and neurofunction
| |||||||
| 2014 | Demjaha | (1)H-MRS | TRS (n = 6) | ≧4 on ≧1 PANSS positive symptom items + ≧75 on PANSS total score + <59 on GAF despite ≧2 sequential AP trials (each of ≧4 weeks at a CPZ equivalent dose of 400–600 mg/day). | APs (cross-sectional) | Sz in remission (n = 8) & HC (n = 10) | Glu levels were elevated in ACC in patients with TRS compared with HC while Glu levels were comparable between TRS and those with Sz in remission. NAA levels were lower in ACC in those with Sz in remission than TRS and HC, which did not differ. |
| 2013 | Klirova | [18F]F DG PET | Sz with TR-AVH (n = 15) | ≧5 episodes/day of AH during the past month + nonresponse to ≧1 typical and 1 atypical AP adequate trials | APs (cross-sectional) | HC (n = 10) | Patients with TR-AVH presented with hypermetabolism in the cluster consisting of bilateral lentiform nucleus, thalamus, and postcentral gyrus, left parahippocampal gyrus, and right superior frontal gyrus, compared with HC. Within the left acoustic-linguistic cortex, hypermetabolism was found in the middle temporal gyrus and TPJ. |
| 2012 | Demjaha | [18F]D OPA PET | TRS (n = 12) | ≧4 on ≧1 PANSS positive symptom items + ≧75 on PANSS total score + <59 on GAF despite ≧2 sequential AP trials (each of ≧4 weeks at a CPZ equivalent dose of 400–600 mg/day). | APs other than CLZ (cross-sectional) | Non-TRS (n = 12) & HC (n = 12) | Patients with TRS showed lower dopamine synthesis capacity in striatum than those with non-TRS. No difference was found in striatal dopamine synthesis capacity between patients with TRS and HC. |
| 2012 | Wolf | ASL | Sz with TR-AVH (n = 10) | Persistent AVH despite ≧2 AP trials (each >6 weeks at adequate dosage of different APs). No pronounced formal thought disorder. Sufficient insight into AVH. | APs (cross-sectional) | Sz without AVH (n = 10) & HC (n = 14) | Patients with TR-AVH showed increased rCBF in left STG and right SMG/TPC than those without AVH. Those with TR-AVH showed increased rCBF in frontotemporal regions than HC. Those with TR-AVH demonstrated positive correlations between AVH severity and rCBF in left STG, ACC, and IFG. |
| 2011 | Wolf | Resting state fMRI | Sz with TR-AVH (n = 10) | Persistent AVH + ≧2 ineffective AP trials (each >6 weeks at adequate dosage of different APs). No pronounced formal thought disorder. Sufficient insight into AVH. | CLZ (monotherapy or combination therapy, cross-sectional) | HC (n = 14) | Within a speech-related network, patients with TR-AVH showed increased connectivity in bilateral temporal regions and decreased connectivity in cingulate cortex compared with HC. Those with TR-AVH exhibited abnormal connectivity in precuneus within attention-related network and right lateral prefrontal areas within executive control network, respectively, compared with HC. Positive correlations were found between AVH severity and functional connectivity of left ACC, left STG, and right lateral PFC. |
| 2010 | Vercammen | Resting state fMRI | Sz with TR-AVH (n = 27) | Daily occurrence of AVH despite ≧2 AP adequate trials. | APs (cross-sectional) | HC (n = 27) | Patients with TR-AVH showed reduced functional connectivity between left TPJ and right hemispheric homotope of Broca's area compared with HC. Those with TR-AVH demonstrated negative correlations between AVH severity and neural coupling between left TPJ and bilateral ACC as well as bilateral amygdala. |
| 2007 | Molina Rodriguez | [18F]F DG PET | TRS with good response to CLZ (n = 23) | ≧4 on ≧1 SAPS psychotic symptom items + ≧4 on CGI-S despite ≧2 different AP treatments (each of ≧4 weeks at a CPZ equivalent dose of ≧800 mg/day) during the previous year. | CLZ (cross-sectional) | Sz (AP naïve, n = 11) & HC (n = 18) | Patients with TRS showed prefrontal (bilateral orbital, medial frontal, left dorsolateral prefrontal) and caudate hypometabolism compared with both HC and AP naïve patients, and lower thalamic activity than HC. |
| 2007 | Fitzgerald | fMRI during word generation task | Sz with TR-AVH (n = 3) | Persistent severe refractory AH + nonresponse to ≧2 adequate AP trials | Atypical APs (CLZ, n = 2, cross-sectional) | HC (n = 4) | Patients with TR-AVH presented with less activation in medial frontal regions (left superior temporal gyrus, left inferior frontal gyrus, right inferior frontal gyrus, anterior cingulate cortex and parietal regions) and greater activation in left caudal precentral gyrus, compared with HC. |
| 2004 | Moresco | [18F]F ESP PET | TRS (n = 6) | ≧27 on BPRS total score despite ≧2 different-class AP treatments (each of ≧6 weeks at a CPZ equivalent dose of ≧500 mg/day). | CLZ (prospective) | TRS (n = 9, OLZ, prospective) | OLZ showed 4-fold D2-like receptor occupancy in striatum (43%) but similar cortical 5-HT2 receptor occupancy (86%) compared with CLZ in patients with TRS, while clinical outcomes were similar. |
| 1997a | Molina Rodriguez | 99mTc-HMPAO SPECT | TRS (n = 36) | Active psychosis despite 2 different-class AP treatments (each of ≧8 weeks at a CPZ equivalent dose of ≧1000 mg/day). | Typical APs | HC (n = 28) | Patients with TRS showed bilateral, but predominantly left-sided, decreased perfusion in frontal and temporal cortices, as well as an increased perfusion in right BG compared with HC. Negative symptoms scores negatively correlated with perfusion in right DLPFC, while parkinsonism positively correlated with activity of primary motor and sensory cortex. |
| 1991 | Coppens | [11C]N MSP PET | TRS (n = 6) | No substantial improvement in major symptoms despite 3 different AP treatments (each of ≧8 weeks at an adequate dose with a therapeutic plasma level range of ≧1 APs). | Typical APs | No controls | There was more than 95% blockade of D2 receptors in the striatum in patients with TRS. |
|
Functional change after CLZ administration in TRS | |||||||
| 2008 | Molina Rodriguez | 99m Tc-HMPAO SPECT during Stroop test | TRS (RIS, n = 10) | Failure to respond to ≧2 different AP treatments (each of ≧4 weeks at a CPZ equivalent dose of ≧800 mg/day). | CLZ (prospective) | HC (n = 10) | Patients with TRS on RIS showed decreased perfusion in medial prefrontal, middle cingulate and insular regions, as well as increased activity in brain stem and posterior hippocampus compared with HC. CLZ normalized a decreased activation of the cingulate and insular cortices, and increased prefrontal hypoactivation. Clinical improvement with CLZ was related to increased activity in thalamus, not changes in limbic perfusion. CLZ responders showed a higher increase than nonresponders in perfusion in medial occipital cortex and caudate head, and greater decrease in perfusion in posterior cingulate and hippocampus. |
| 2005 | Molina Rodriguez | [18F]F DG PET | TRS (HAL, n = 22) | Failure to respond to treatments with RIS and HAL (each of ≧4 weeks at a CPZ equivalent dose of ≧800 mg/day). | CLZ (prospective) | No controls | CLZ decreased PFC and BG activity and increased occipital metabolism, including primary and association visual areas. Change in negative symptoms was related with decrease in BG activity; improvement in disorganization related to metabolic decrease in motor area, and change in positive symptoms was associated with increased activity in visual area. |
| 2003 | Molina Rodriguez | [18F]F DG PET | TRS (n = 25) | Significant positive or disorganization residual symptoms + failure to respond to 2 different-class AP treatments with (each of ≧6 weeks at a CPZ equivalent dose of ≧800 mg/day). | CLZ (prospective) | No controls | Patients with high baseline metabolic activity in DLPFC were more likely to experience improvements in negative symptoms with CLZ. |
| 1997b | Molina Rodriguez | 99mTc-HMPAO SPECT | TRS (n = 39) | Failure to respond to 2 different-class AP treatments with (each of ≧8 weeks at a CPZ equivalent dose of ≧800 mg/day). | CLZ (prospective) | No controls | While taking APs before CLZ, patients with TRS had higher perfusion in the right BG than HC.CLZ responders had higher perfusion in right BG and lower perfusion in left DLPFC than HC. Nonresponders showed higher perfusion in right BG and lower perfusion in thalamus and left BG vs. HC. CLZ responders had higher perfusion than nonresponders in thalamus, BG and PFC at baseline. After receiving CLZ, only CLZ responders showed a decreased perfusion in PFC and thalamus. |
| 1996 | Molina Rodriguez | 99mTc-HMPAO SPECT | TRS (n = 24) | Paranoid symptoms + failure to respond to 2 different-class AP treatments with (each of ≧8 weeks at a CPZ equivalent dose of ≧800 mg/day). | CLZ (prospective) | No controls | While taking AP before CLZ, CLZ responders showed higher perfusion in thalamus, and left BG than HC. CLZ nonresponders showed lower perfusion in right PFC than HC at baseline. After receiving CLZ, only CLZ responders showed a decrease in thalamus and left BG. No changes in perfusion were found in PFC in both responders and nonresponders. |
Abbreviations: ACC = anterior cingulate cortex, AP = antipsychotic, BG = basal ganglia, ASL = arterial spin labeling, AVH = auditory verbal hallucination, BPRS = Brief Psychiatric Rating Scale, CC = corpus callosum, CGI-S = the Clinical Global Impression Rating Scales=Severity, CLZ = clozapine, CPZ = chlorpromazine, DLPFC = dorsolateral prefrontal cortex, FDG = fluoro-deoxyglucose, FESP = fluoro-ethyl-spiperone, fMRI = functional magnetic resonance tomography, GAF = the Global Assessment of Functioning, Glu = glutamate, GM = gray matter, HAL = haloperidol, HC = healthy controls, IFG = inferior frontal gyrus, NAA = N-acetylaspartate, NMSP = 3-N-methylspiperone, OLZ = olanzapine, PANSS = the Positive and Negative Syndrome Scale, PET = positron emission tomography, PFC = prefrontal cortex, rCBF = regional cerebral blood flow, RIS = risperidone, SAPS = the Scale for the Assessment of Positive Symptoms, SMG = supramarginal gyrus, SPECT = single-photon emission computed tomography, STG = superior temporal gyrus, Sz = schizophrenia, 99mTc-HMPAO = echnetium-99m hexamethylpropylene amine oxime, TPC = temporoparietal cortex, TR = treatment-resistant, TRS = treatment resistant schizophrenia
3.2.1 Comparison between patients with treatment-resistant schizophrenia and healthy controls
Functional neuroimaging studies have investigated TRS versus HC with regard to dopamine synthesis capacity, glutamatergic function, and brain metabolism/perfusion. Demjaha et al. (2012) reported that patients with TRS showed no difference in striatal dopamine synthesis capacity in comparison to HC (Demjaha et al., 2012). In contrast, this group also found that glutamate levels were elevated in the anterior cingulate cortex (ACC) in TRS versus HC while NAA levels were comparable (Demjaha et al., 2014). Since the latter study employed subjects who participated in the former study, these data suggest that those with TRS may have normal dopamine synthesis capacity in the striatum and elevated glutamate levels in the ACC, as compared to HC.
Molina Rodriguez et al. (1997) noted that those with TRS had higher perfusion in the right BG than HC (Molina Rodriguez et al., 1997a), This finding was replicated in a second study by this group, in addition to lower perfusion in the prefrontal and temporal cortices; negative symptoms scores negatively correlated with perfusion in the right DLPFC (Molina Rodriguez et al., 1997b). The same investigators also showed that TRS was associated with decreased perfusion in the medial prefrontal, middle cingulate and insular cortices, as well as increased perfusion in the brain stem and the posterior hippocampus in comparison to HC (Molina et al., 2008). Klirova et al. (2013) reported that those with TR-AVH presented with hypermetabolism in the cluster consisting of bilateral lentiform nucleus and thalamus, left parahippocampal gyrus, bilateral postcentral gyrus, and right superior frontal gyrus, compared with HC (Klirova et al., 2013). Within the left acoustic-linguistic cortex, the hypermetabolism was found in the middle temporal gyrus and temporoparietal junction (TPJ). Wolf et al. (2012) reported that those with TR-AVH showed increased regional cerebral blood flow (rCBF) in the frontotemporal regions, compared with HC (Wolf et al., 2012).
Wolf et al. (2011) noted that those with TR-AVH showed increased connectivity in the bilateral temporal regions and decreased connectivity in the cingulate cortex within a speech-related network, compared with HC (Wolf et al., 2011). Further, those with TR-AVH exhibited abnormal connectivity in the precuneus within attention-related network and in the right lateral prefrontal areas within executive control network, respectively. Correlations were found between AVH severity and functional connectivity of the left ACC, left superior temporal gyrus (STG), and right lateral PFC. Vercammen et al. (2010) demonstrated that patients with TR-AVH showed reduced functional connectivity between the left STG and right hemispheric homotope of Broca's area, compared with HC (Vercammen et al., 2010). Negative correlations were found between AVH severity and neural coupling between the left TPJ and bilateral ACC as well as bilateral amygdala in those with TR-AVH. Fitzgerald et al. (2007) noted that those with TRAVH presented with word-generation related hypoactivation in the left superior temporal gyrus, bilateral inferior frontal gyrus, ACC, and parietal regions, compared with HC (Fitzgerald et al., 2007).
In relation to the neural correlates of CLZ response, Molina Rodriguez et al. (2007) demonstrated that those with TRS who responded to CLZ had lower perfusion than HC in the PFC, caudate, and thalamus (Molina et al., 2007). Lastly, while one study has reported that patients with URS (i.e. CLZ resistance) showed lower perfusion in the right PFC than HC (Molina Rodriguez et al., 1996), another study by the same group demonstrated lower perfusion in the thalamus and left BG (Molina Rodriguez et al., 1997a). Summarizing, studies indicate that hypometabolism in the PFC and hypermetabolism in the BG may be associated with TRS; in contrast, findings for URS are both limited and inconsistent.
3.2.2 Comparison between patients with treatment-resistant and non-resistant schizophrenia
Demjaha et al. (2012) noted that patients with TRS showed lower dopamine synthesis capacity in the striatum than those with non-TRS (Demjaha et al., 2012). A subsequent study by this group also found that glutamate levels were comparable in the ACC between those with TRS and those with schizophrenia in remission (numerically higher in TRS), while NAA levels were significantly higher within the ACC in patients with TRS than in those with schizophrenia in remission (Demjaha et al., 2014). Wolf et al. (2012) demonstrated that those with TR-AVH showed increased rCBF in the STG and right supramarginal gyrus/temporoparietal cortex than those without AVH (Wolf et al., 2012). Positive correlations were found between AVH severity and rCBF in the left STG, ACC, and IFG in those with TR-AVH.
3.2.3 Clozapine response and functional imaging in patients with treatment-resistant schizophrenia
3.2.3.1 Change in functional imaging following clozapine treatment
CLZ has been reported to decrease activation in the PFC (Molina et al., 2005; Molina et al., 2008) and BG (Molina et al., 2005), and increase activation of the occipital (Molina et al., 2005), cingulate (Molina et al., 2008), and insular cortices (Molina et al., 2008) in patients with TRS. These studies imply that CLZ may increase hypoactivation in the PFC in those with TRS; however, this does not appear to be related to clinical improvement (Molina et al., 2005; Molina et al., 2008).
3.2.3.2 Symptom improvement and functional imaging following clozapine treatment
Molina Rodriguez et al. (2008) found that clinical improvement in association with CLZ was related to an increase in activity in the thalamus, but not in association with limbic region perfusion (Molina et al., 2008). Looking at specific symptom domain improvement, the same group reported the following: a decrease in BG activity for negative symptoms, reduced metabolism in the motor area for disorganization, and an increase in visual area activity for positive symptoms (Molina et al., 2005). On the other hand, increased hypoactivation in the PFC in those with TRS was not related to clinical improvement in this population (Molina et al., 2005; Molina et al., 2008).
3.2.3.3 Functional imaging as a baseline predictor of clozapine response
Several studies have reported baseline functional predictors for CLZ response. CLZ responders had a higher perfusion in the PFC, BG and thalamus than nonresponders at baseline (Molina Rodriguez et al., 1996; Molina Rodriguez et al., 1997b). It has also been identified that patients with high metabolic activity in the DLPFC at baseline were more likely to experience improvements in their negative symptoms following CLZ administration (Molina et al., 2003).
3.2.3.4 Difference in functional imaging between clozapine responders and nonresponders
Changes in brain activity with CLZ treatment may classify CLZ responders and nonresponders in those with TRS. More specifically, CLZ responders demonstrated greater increases in perfusion in the medial occipital cortex and caudate head, and greater decreases in perfusion in the posterior cingulate and hippocampus when compared with nonresponders (Molina et al., 2008). Molina Rodriguez et al. (1997) also reported that CLZ responders showed decreased perfusion in the PFC and thalamus (Molina Rodriguez et al., 1997b); and in a second study (1996) identified decreased perfusion in the thalamus and left BG of non-responders (Molina Rodriguez et al., 1996) but in this case no change in PFC perfusion in both responders and nonresponders (Molina Rodriguez et al., 1996).
4. Discussion
Despite the importance of TRS clinically, few neuroimaging studies have specifically focused on this population with solid hypothesis. In the few studies designed to contrast those with TRS versus non-TRS, no clear evidence was found in terms of neuroimaging correlates defining treatment resistance. Of note, no study has compared URS and those with TRS who respond to CLZ. There are several potential reasons for the paucity of neuroimaging studies on TRS and URS, including 1) equivocal definition of TRS and 2) current research interests on those at risk mental state and those with first-episode schizophrenia rather than TRS and URS. On the other hand, compared to HC, replicated findings to date include hypometabolism in the PFC (Molina Rodriguez et al., 1997b; Molina et al., 2007), hypermetabolism in the BG (Molina Rodriguez et al., 1997a; Molina Rodriguez et al., 1997b), and structural anomalies in the CC (Holleran et al., 2014; Sun et al., 2009) of patients with TRS. Only a limited number of investigations examined alterations in neuroanatomy and/or neurofunction in response to CLZ treatment. Replicated findings indicate that CLZ may increase PFC hypoactivation, although this does not appear related to clinical improvement; furthermore, CLZ-associated clinical improvement may be related to decreased metabolism in the BG and thalamus in TRS. However, very few studies have compared the effects of clozapine and other antipsychotics on brain metabolism. As such, it remains unclear whether these clozapine-induced metabolic changes are specific to this antipsychotic or are also applicable to antipsychotics in general (Buchsbaum et al., 2009); a direct comparison between them on brain activity could contribute to a better understanding of their respective mechanisms of action. To summarize, further research contrasting TRS, non-TRS, and URS is clearly needed, with particular focus on the frontocortical-basal gangliathalamic circuits implicated in treatment resistance.
5. Limitations
This review has to be considered in light of its limitations. First, few studies specifically compared patients with TRS and non-TRS, which limits the conclusions that can be drawn. Comparisons between TRS and HC may not be specific indicators of treatment-resistance, but rather may also reflect abnormalities found in patients with schizophrenia in general (including non-TRS) or persistent effects of antipsychotics on neurofunction and neuroanatomy (Samaha et al., 2008). Second, few studies employed multi-modal imaging, which offers information both about neurofunction and neuroanatomy in the same sample. Third, most studies employed a cross-sectional design to compare TRS and other populations when a longitudinal design is better suited if we are to elucidate whether TRS is a pre-existing condition or what that evolves across time, or whether antipsychotics or illness progression causes/interferes with what is measured in TRS. Fourth, some studies were excluded from this study because they included patients who did not tolerate antipsychotics. This highlights the importance of common agreement regarding criteria for both TRS and URS. TRS has been defined with positive symptoms rather than negative, cognitive or affective symptoms and response to antipsychotics have been derived from a relative change in representative scales. Thus, it is clearly pertinent to assess response/outcome in the context of multiple symptom domains, in addition to functioning (Suzuki et al., 2012). Fifth, only dopamine and glutamate do not necessarily explain biological mechanisms underlying TRS (Selvaraj et al., 2014). For example, serotonergic dysfunction has been implicated for schizophrenia, including increased 5-HT1A receptors and decreased 5-HT2A receptors in the PFC. Further research is needed to examine relationships between the serotonin system and TRS. Sixth, treatment resistance emerges not only in schizophrenia but also other serious mental illnesses such as bipolar disorders (Arnone et al., 2009). It should be useful to conduct neuroimaging studies to compare TRS and other treatment-resistant mental illnesses to elucidate the biological basis of treatment resistance. Finally, we are not aware of any study that has compared patients with TRS responsive to CLZ and URS, a critical question since the differential response to CLZ supports the position that these two populations are mediated by different underlying mechanisms.
6. Future Directions
The paucity of robust evidence supporting a critical role for the dopaminergic system in TRS clearly argues for lines of investigation that take us beyond dopamine (Selvaraj et al., 2014). While dopamine may still be necessary, it is clearly not sufficient in the case of TRS although differences with non-TRS have been identified. In antipsychotic-free patients with schizophrenia, plasma dopamine metabolite levels at baseline have been shown to be associated with treatment response (Yoshimura et al., 2003). With TRS, dopamine synthesis capacity has been reported to be lower versus patients with non-TRS, but similar to HC (Demjaha et al., 2012), while postmortem brain dopamine levels in the caudate were found to be lower when compared with non-TRS (Roberts et al., 2009).
The glutamate hypothesis represents one of a number of alternative models posited that may complement existing thinking regarding dopamine while acknowledging its limitations in TRS (Cadenhead, 2011; Carlsson and Carlsson, 1990; Javitt and Zukin, 1991; Krystal, 2008; Olney and Farber, 1995). The interaction between glutamate and dopamine has been widely documented (Breier et al., 1998; Cepeda and Levine, 1998; David et al., 2005; Grace, 2000; Kegeles et al., 2000; Kulagina et al., 2001; Levine and Cepeda, 1998; West et al., 2003; Yamamoto et al., 1999), with evidence suggesting that dopaminergic dysregulation may be the final common pathway - a result of enhanced cortical glutamate activity in response to hypofunction of N-methyl D-aspartate (NMDA) receptors in patients with schizophrenia (Sharp et al., 2001). The most convincing link between NMDA receptor function and schizophrenia is the ability of NMDA receptor antagonists to induce not only positive symptoms but also negative and cognitive symptoms in healthy volunteers (Krystal et al., 1994; Lieberman et al., 2008; Malhotra et al., 1996) and to exacerbate psychosis in patients with schizophrenia (Malhotra et al., 1997).
While the aforementioned evidence supports a role for glutamate in schizophrenia, its putative role in TRS remains to be elucidated. Proton magnetic resonance spectroscopy (1H-MRS) permits the non-invasive in vivo study of the glutamatergic system (Abbott and Bustillo, 2006; Di Costanzo et al., 2003; Di Costanzo et al., 2007), which is known to play a crucial role in the frontocortical-basal ganglia-thalamic circuits. To date, only one study has explored glutamate levels in patients with TRS (Demjaha et al., 2014), combined with the previous study by the same group, which examined dopamine synthesis capacity in TRS versus non-TRS, and HC (Demjaha et al., 2012). The limited data available suggest that TRS may be associated with higher glutamate levels and normal dopamine synthesis capacity in comparison with non-TRS and controls. Furthermore, we need clearly established criteria that permit accurate classification (i.e. antipsychotic responsive vs. treatment resistant, further subdivided as CLZ responsive or URS).Thus, given that the dopamine hypothesis may not necessarily explain the biological correlates of TRS, further longitudinal 1H-MRS research is required to explore the glutamatergic dysfunction in the frontocortical-basal ganglia-thalamic circuits comparing TRS, URS, and non-TRS in order to elucidate the mechanisms underlying TRS.
7. Conclusion
In conclusion, treatment resistance represents the greatest unmet need in schizophrenia care at present, associated as it is with worse clinical outcomes, social dysfunction, poorer quality of life, increased use of healthcare resources and greater caregiver burden. As many as 30% of patients with schizophrenia meet criteria for TRS, and while a portion of these individuals will respond to CLZ, a substantial number will not. For these individuals we have no treatments at present, giving rise to a trial-and-error clinical approach that is neither systematic nor particularly effective. Neuroimaging is positioned to play a critical role in this regard and while the work to date is limited, findings suggest that: 1) patients with TRS may present with hypometabolism in the PFC, hypermetabolism in the BG, and structural anomalies in the CC, as compared with HC; 2) CLZ may increase PFC hypoactivation in those with TRS; and 3) CLZ efficacy may be related to decreased metabolism in the BG and thalamus in TRS. It remains, though, that this work is in its earliest stages and considerably more research is required; that no work has yet been carried out involving URS speaks to the considerable gaps in our knowledge. In moving forward, we need clearly established criteria of TRS and URS, a multimodal imaging approach, and longitudinal designs that can complement cross-sectional studies. The fact that we have at least three forms of this illness based on treatment response/nonresponse provides the necessary starting point to use strategies like neuroimaging to elucidate the different underlying mechanisms that ultimately contribute to schizophrenia's clinical heterogeneity.
Figure 1.
Flowchart illustrating literature search and exclusion process (PRISMA diagram).
Abbreviations: ASL = arterial spin labeling, AVH = auditory verbal hallucinations, CLZ = clozapine, CT = computed tomography, DTI = diffusion tensor imaging, MRS = magnetic resonance imaging, fMRI = functional MRI, MRS = magnetic resonance spectroscopy, PET = positron emission tomography, SPECT = single photon emission tomography, TRS = treatment resistant schizophrenia.
Acknowledgments
The work for this review was supported by NARSAD Independent Investigator Grant from the Brain and Behavioural Research Foundation (AG), Ontario Early Researcher Award (AG), and CIHR fellowship award (SN).
Role of Funding Source: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
S. Nakajima, H. Takeuchi, G. Fervaha and A. Graff-Guerrero lead study design, literature review and interpretation and manuscript preparation. E. Plitman, P. Gerretsen, F. Caravaggio, JK Chung, Y Iwata , and G Remington contributed to the interpretation of the results. All authors contributed to and have approved the final manuscript.
Conflict of Interest: Dr. Nakajima has received fellowship grants from the Canadian Institute of Health Research (CIHR), Japan Society for the Promotion of Science, and Nakatomi Foundation, and manuscript fees from Dainippon Sumitomo Pharma and Kyowa Hakko Kirin. Dr. Takeuchi has received fellowship grants from the CIHR, Japanese Society of Clinical Neuropsychopharmacology, Astellas Foundation for Research on Metabolic Disorders, and Centre for Addiction and Mental Health (CAMH) Foundation, and manuscript fees from Dainippon Sumitomo Pharma. Dr. Gerretsen has received fellowship support from the CAMH Foundation, Ontario Mental Health Foundation (OMHF), and CIHR. Dr. Remington has received research support from Novartis, Medicure and Neurocrine Bioscience, as a co-investigator he has received grant support from Pfizer Inc., consultant fees from Laboratorios Farmacéuticos ROVI, Synchroneuron, Novartis, and Roche, and speaker's fees from Novartis. Dr. Graff-Guerrero has received research support from the following external funding agencies: the CIHR, US NIH, OMHF, Brain and Behavior Research Foundation (BBRF), Mexico ICyTDF, CONACyT, and W. Garfield Weston Foundation. Other authors have no financial or other relationship relevant to the subject of this manuscript.
References
- Abbott C, Bustillo J. What have we learned from proton magnetic resonance spectroscopy about schizophrenia? A critical update. Curr Opin Psychiatry. 2006;19(2):135–139. doi: 10.1097/01.yco.0000214337.29378.cd. [DOI] [PubMed] [Google Scholar]
- Abdul-Monim Z, Reynolds GP, Neill JC. The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav Brain Res. 2006;169(2):263–273. doi: 10.1016/j.bbr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Abekawa T, Ito K, Koyama T. Role of the simultaneous enhancement of NMDA and dopamine D1 receptor-mediated neurotransmission in the effects of clozapine on phencyclidine-induced acute increases in glutamate levels in the rat medial prefrontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 2006;374(3):177–193. doi: 10.1007/s00210-006-0115-9. [DOI] [PubMed] [Google Scholar]
- Abekawa T, Ito K, Koyama T. Different effects of a single and repeated administration of clozapine on phencyclidine-induced hyperlocomotion and glutamate releases in the rat medial prefrontal cortex at short- and long-term withdrawal from this antipsychotic. Naunyn Schmiedebergs Arch Pharmacol. 2007;375(4):261–271. doi: 10.1007/s00210-007-0154-x. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155(6):761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agid O, Arenovich T, Sajeev G, Zipursky RB, Kapur S, Foussias G, Remington G. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72(11):1439–1444. doi: 10.4088/JCP.09m05785yel. [DOI] [PubMed] [Google Scholar]
- Amitai N, Kuczenski R, Behrens MM, Markou A. Repeated phencyclidine administration alters glutamate release and decreases GABA markers in the prefrontal cortex of rats. Neuropharmacology. 2012;62(3):1422–1431. doi: 10.1016/j.neuropharm.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology (Berl) 2007;193(4):521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Arango C, Breier A, McMahon R, Carpenter WT, Jr., Buchanan RW. The relationship of clozapine and haloperidol treatment response to prefrontal, hippocampal, and caudate brain volumes. Am J Psychiatry. 2003;160(8):1421–1427. doi: 10.1176/appi.ajp.160.8.1421. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(3):194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Breier A, Adler CM, Weisenfeld N, Su TP, Elman I, Picken L, Malhotra AK, Pickar D. Effects of NMDA antagonism on striatal dopamine release in healthy subjects: application of a novel PET approach. Synapse. 1998;29(2):142–147. doi: 10.1002/(SICI)1098-2396(199806)29:2<142::AID-SYN5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia--a systematic review and meta-analysis. Biol Psychiatry. 2011;69(5):495–503. doi: 10.1016/j.biopsych.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT., Jr. Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry. 1998;155(6):751–760. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Haznedar M, Newmark RE, Chu KW, Dusi N, Entis JJ, Goldstein KE, Goodman CR, Gupta A, Hazlett E, Iannuzzi J, Torosjan Y, Zhang J, Wolkin A. FDG-PET and MRI imaging of the effects of sertindole and haloperidol in the prefrontal lobe in schizophrenia. Schizophr Res. 2009;114(1-3):161–171. doi: 10.1016/j.schres.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Cachia A, Paillere-Martinot ML, Galinowski A, Januel D, de Beaurepaire R, Bellivier F, Artiges E, Andoh J, Bartres-Faz D, Duchesnay E, Riviere D, Plaze M, Mangin JF, Martinot JL. Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage. 2008;39(3):927–935. doi: 10.1016/j.neuroimage.2007.08.049. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS. Startle reactivity and prepulse inhibition in prodromal and early psychosis: effects of age, antipsychotics, tobacco and cannabis in a vulnerable population. Psychiatry Res. 2011;188(2):208–216. doi: 10.1016/j.psychres.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia--implications for schizophrenia and Parkinson's disease. Trends Neurosci. 1990;13(7):272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20(1):1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry. 2001;158(4):518–526. doi: 10.1176/appi.ajp.158.4.518. [DOI] [PubMed] [Google Scholar]
- Chung C, Remington G. Predictors and markers of clozapine response. Psychopharmacology (Berl) 2005;179(2):317–335. doi: 10.1007/s00213-005-2174-x. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Boso M, Barbui C. Clozapine combined with different antipsychotic drugs for treatment resistant schizophrenia. Cochrane Database Syst Rev. 2009;(3):CD006324. doi: 10.1002/14651858.CD006324.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50(11):898–911. doi: 10.1016/s0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- Conley RR, Kelly DL, Beason-Held LL, Holcomb HH, Richardson CM. The effects of clozapine and high-dose olanzapine on brain function in treatment-resistant schizophrenia: a case study. J Psychopharmacol. 2004;18(3):429–431. doi: 10.1177/026988110401800315. [DOI] [PubMed] [Google Scholar]
- Coppens HJ, Slooff CJ, Paans AM, Wiegman T, Vaalburg W, Korf J. High central D2-dopamine receptor occupancy as assessed with positron emission tomography in medicated but therapy-resistant schizophrenic patients. Biol Psychiatry. 1991;29(7):629–634. doi: 10.1016/0006-3223(91)90132-6. [DOI] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Brain Res Rev. 2005;50(2):336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, McGuire PK. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75(5):e11–13. doi: 10.1016/j.biopsych.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine Synthesis Capacity in Patients With Treatment-Resistant Schizophrenia. Am J Psychiatry. 2012 doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- Di Costanzo A, Trojsi F, Tosetti M, Giannatempo GM, Nemore F, Piccirillo M, Bonavita S, Tedeschi G, Scarabino T. High-field proton MRS of human brain. Eur J Radiol. 2003;48(2):146–153. doi: 10.1016/j.ejrad.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Di Costanzo A, Trojsi F, Tosetti M, Schirmer T, Lechner SM, Popolizio T, Scarabino T. Proton MR spectroscopy of the brain at 3 T: an update. Eur Radiol. 2007;17(7):1651–1662. doi: 10.1007/s00330-006-0546-1. [DOI] [PubMed] [Google Scholar]
- Didriksen M, Skarsfeldt T, Arnt J. Reversal of PCP-induced learning and memory deficits in the Morris' water maze by sertindole and other antipsychotics. Psychopharmacology (Berl) 2007;193(2):225–233. doi: 10.1007/s00213-007-0774-3. [DOI] [PubMed] [Google Scholar]
- Ebdrup BH, Norbak H, Borgwardt S, Glenthoj B. Volumetric changes in the basal ganglia after antipsychotic monotherapy: a systematic review. Curr Med Chem. 2013;20(3):438–447. doi: 10.2174/0929867311320030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165(8):1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq S, Agid O, Foussias G, Remington G. Using treatment response to subtype schizophrenia: proposal for a new paradigm in classification. Schizophr Bull. 2013;39(6):1169–1172. doi: 10.1093/schbul/sbt137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Sritharan A, Benitez J, Daskalakis ZJ, Jackson G, Kulkarni J, Egan GF. A preliminary fMRI study of the effects on cortical activation of the treatment of refractory auditory hallucinations with rTMS. Psychiatry Res. 2007;155(1):83–88. doi: 10.1016/j.pscychresns.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Friedman L, Knutson L, Shurell M, Meltzer HY. Prefrontal sulcal prominence is inversely related to response to clozapine in schizophrenia. Biol Psychiatry. 1991;29(9):865–877. doi: 10.1016/0006-3223(91)90053-o. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophr Bull. 2013;39(1):33–42. doi: 10.1093/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesel FL, Mehndiratta A, Hempel A, Hempel E, Kress KR, Essig M, Schroder J. Improvement of auditory hallucinations and reduction of primary auditory area's activation following TMS. Eur J Radiol. 2012;81(6):1273–1275. doi: 10.1016/j.ejrad.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31(2-3):330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res. 2007;184(1):31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Shimizu E, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. Eur J Pharmacol. 2005;519(1-2):114–117. doi: 10.1016/j.ejphar.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hietala J, Syvalahti E, Vilkman H, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, Solin O, Kuoppamaki M, Eronen E, Ruotsalainen U, Salokangas RK. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res. 1999;35(1):41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- Holleran L, Ahmed M, Anderson-Schmidt H, McFarland J, Emsell L, Leemans A, Scanlon C, Dockery P, McCarthy P, Barker GJ, McDonald C, Cannon DM. Altered interhemispheric and temporal lobe white matter microstructural organization in severe chronic schizophrenia. Neuropsychopharmacology. 2014;39(4):944–954. doi: 10.1038/npp.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Egerton A, Allan V, McGuire P, Stokes P, Kapur S. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15(22):2550–2559. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl. 2007;51:s13–18. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology (Berl) 2005;179(2):336–348. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- Jardri R, Delevoye-Turrell Y, Lucas B, Pins D, Bulot V, Delmaire C, Thomas P, Delion P, Goeb JL. Clinical practice of rTMS reveals a functional dissociation between agency and hallucinations in schizophrenia. Neuropsychologia. 2009;47(1):132–138. doi: 10.1016/j.neuropsychologia.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: A new hypothesis. Am J Psychiatry. 2001;158(3):360–369. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, Cooper TB, Carlsson A, Laruelle M. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48(7):627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- Klirova M, Horacek J, Novak T, Cermak J, Spaniel F, Skrdlantova L, Mohr P, Hoschl C. Individualized rTMS neuronavigated according to regional brain metabolism ((18)FGD PET) has better treatment effects on auditory hallucinations than standard positioning of rTMS: a double-blind, sham-controlled study. Eur Arch Psychiatry Clin Neurosci. 2013;263(6):475–484. doi: 10.1007/s00406-012-0368-x. [DOI] [PubMed] [Google Scholar]
- Konicki PE, Kwon KY, Steele V, White J, Fuller M, Jurjus GJ, Jaskiw GE. Prefrontal cortical sulcal widening associated with poor treatment response to clozapine. Schizophr Res. 2001;48(2-3):173–176. doi: 10.1016/s0920-9964(00)00130-4. [DOI] [PubMed] [Google Scholar]
- Krystal JH. Capitalizing on extrasynaptic glutamate neurotransmission to treat antipsychotic-resistant symptoms in schizophrenia. Biol Psychiatry. 2008;64(5):358–360. doi: 10.1016/j.biopsych.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr., Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kubera KM, Sambataro F, Vasic N, Wolf ND, Frasch K, Hirjak D, Thomann PA, Wolf RC. Source-based morphometry of gray matter volume in patients with schizophrenia who have persistent auditory verbal hallucinations. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:102–109. doi: 10.1016/j.pnpbp.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Zigmond MJ, Michael AC. Glutamate regulates the spontaneous and evoked release of dopamine in the rat striatum. Neuroscience. 2001;102(1):121–128. doi: 10.1016/s0306-4522(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Nagao N, Nakahara T. Neuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin-dopamine hypothesis. Prog Brain Res. 2008;172:199–212. doi: 10.1016/S0079-6123(08)00910-2. [DOI] [PubMed] [Google Scholar]
- Langguth B, Eichhammer P, Zowe M, Marienhagen J, Spiessl H, Hajak G. Neuronavigated transcranial magnetic stimulation and auditory hallucinations in a schizophrenic patient: monitoring of neurobiological effects. Schizophr Res. 2006;84(1):185–186. doi: 10.1016/j.schres.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung M, Cheung C, Yu K, Yip B, Sham P, Li Q, Chua S, McAlonan G. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull. 2011;37(1):199–211. doi: 10.1093/schbul/sbp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Cepeda C. Dopamine modulation of responses mediated by excitatory amino acids in the neostriatum. Adv Pharmacol. 1998;42:724–729. doi: 10.1016/s1054-3589(08)60850-9. [DOI] [PubMed] [Google Scholar]
- Liao ZL, Hu SH, Xu Y. A case report on the relationship between treatment-resistant childhood-onset schizophrenia and an abnormally enlarged cavum septum pellucidum combined with cavum vergae. Chin Med J (Engl) 2012;125(7):1349–1351. [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, Aprille JR, Dwyer DS, Li XM, Mahadik SP, Duman RS, Porter JH, Modica-Napolitano JS, Newton SS, Csernansky JG. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60(3):358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Safferman AZ, Pollack S, Szymanski S, Johns C, Howard A, Kronig M, Bookstein P, Kane JM. Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry. 1994;151(12):1744–1752. doi: 10.1176/ajp.151.12.1744. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP. Treatment refractory schizophrenia. Psychiatr Q. 2000;71(4):373–384. doi: 10.1023/a:1004640408501. [DOI] [PubMed] [Google Scholar]
- Lopez-Gil X, Babot Z, Amargos-Bosch M, Sunol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32(10):2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17(3):141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14(5):301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164(9):1411–1417. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Bull. 2013;39(1):120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Burnett S, Bastani B, Ramirez LF. Effects of six months of clozapine treatment on the quality of life of chronic schizophrenic patients. Hosp Community Psychiatry. 1990;41(8):892–897. doi: 10.1176/ps.41.8.892. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina Rodriguez V, Andree RM, Castejon MJ, Zamora ML, Alvaro PC, Delgado JL, Vila FJ. Fronto-striato-thalamic perfusion and clozapine response in treatment-refractory schizophrenic patients. A 99mTc-HMPAO study. Psychiatry Res. 1997a;76(1):51–61. doi: 10.1016/s0925-4927(97)00057-7. [DOI] [PubMed] [Google Scholar]
- Molina Rodriguez V, Montz Andree R, Perez Castejon MJ, Capdevila Garcia E, Carreras Delgado JL, Rubia Vila FJ. SPECT study of regional cerebral perfusion in neuroleptic-resistant schizophrenic patients who responded or did not respond to clozapine. Am J Psychiatry. 1996;153(10):1343–1346. doi: 10.1176/ajp.153.10.1343. [DOI] [PubMed] [Google Scholar]
- Molina Rodriguez V, Montz Andree R, Perez Castejon MJ, Gutierrez Labrador R, Ferre Navarrete F, Carreas Delgado JL, Rubia Vila FJ. Cerebral perfusion correlates of negative symptomatology and parkinsonism in a sample of treatment-refractory schizophrenics: an exploratory 99mTc-HMPAO SPET study. Schizophr Res. 1997b;25(1):11–20. doi: 10.1016/s0920-9964(96)00115-6. [DOI] [PubMed] [Google Scholar]
- Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, Desco M, Palomo T. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl) 2005;178(1):17–26. doi: 10.1007/s00213-004-1981-9. [DOI] [PubMed] [Google Scholar]
- Molina V, Reig S, Sarramea F, Sanz J, Francisco Artaloytia J, Luque R, Aragues M, Pascau J, Benito C, Palomo T, Desco M. Anatomical and functional brain variables associated with clozapine response in treatment-resistant schizophrenia. Psychiatry Res. 2003;124(3):153–161. doi: 10.1016/s0925-4927(03)00108-2. [DOI] [PubMed] [Google Scholar]
- Molina V, Sanz J, Sarramea F, Palomo T. Marked hypofrontality in clozapine-responsive patients. Pharmacopsychiatry. 2007;40(4):157–162. doi: 10.1055/s-2007-984399. [DOI] [PubMed] [Google Scholar]
- Molina V, Tamayo P, Montes C, De Luxan A, Martin C, Rivas N, Sancho C, Dominguez-Gil A. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(4):948–954. doi: 10.1016/j.pnpbp.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Nord M, Farde L. Antipsychotic occupancy of dopamine receptors in schizophrenia. CNS Neurosci Ther. 2011;17(2):97–103. doi: 10.1111/j.1755-5949.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry. 1995;152(10):1444–1449. doi: 10.1176/ajp.152.10.1444. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52(12):998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Remington G, Kapur S, Foussias G, Agid O, Mann S, Borlido C, Richards S, Javaid N. Tetrabenazine augmentation in treatment-resistant schizophrenia: a 12-week, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2012;32(1):95–99. doi: 10.1097/JCP.0b013e31823f913e. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley RR, Lahti AC. Dopaminergic synapses in the caudate of subjects with schizophrenia: relationship to treatment response. Synapse. 2009;63(6):520–530. doi: 10.1002/syn.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Reckless GE, Seeman P, Diwan M, Nobrega JN, Kapur S. Less is more: antipsychotic drug effects are greater with transient rather than continuous delivery. Biol Psychiatry. 2008;64(2):145–152. doi: 10.1016/j.biopsych.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Sato M, Numachi Y, Hamamura T. Relapse of paranoid psychotic state in methamphetamine model of schizophrenia. Schizophr Bull. 1992;18(1):115–122. doi: 10.1093/schbul/18.1.115. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Dannon PN, Goshen E, Amiaz R, Zwas TS, Grunhaus L. Right prefrontal rTMS treatment for refractory auditory command hallucinations - a neuroSPECT assisted case study. Psychiatry Res. 2002;116(1-2):113–117. doi: 10.1016/s0925-4927(02)00065-3. [DOI] [PubMed] [Google Scholar]
- Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci U S A. 2000;97(14):7673–7675. doi: 10.1073/pnas.97.14.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev. 2014;45:233–245. doi: 10.1016/j.neubiorev.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Tomitaka M, Bernaudin M, Tomitaka S. Psychosis: pathological activation of limbic thalamocortical circuits by psychomimetics and schizophrenia? Trends Neurosci. 2001;24(6):330–334. doi: 10.1016/s0166-2236(00)01817-8. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Begemann MJ, Temmerman A, Leucht S. Pharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature review. Schizophr Bull. 2012;38(5):1003–1011. doi: 10.1093/schbul/sbr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Sun J, Maller JJ, Daskalakis ZJ, Furtado CC, Fitzgerald PB. Morphology of the corpus callosum in treatment-resistant schizophrenia and major depression. Acta Psychiatr Scand. 2009;120(4):265–273. doi: 10.1111/j.1600-0447.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Remington G, Mulsant BH, Uchida H, Rajji TK, Graff-Guerrero A, Mimura M, Mamo DC. Defining treatment-resistant schizophrenia and response to antipsychotics: a review and recommendation. Psychiatry Res. 2012;197(1-2):1–6. doi: 10.1016/j.psychres.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Uchida H, Watanabe K, Kashima H. Factors associated with response to clozapine in schizophrenia: a review. Psychopharmacol Bull. 2011;44(1):32–60. doi: 10.64719/pb.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauscher J, Hussain T, Agid O, Verhoeff NP, Wilson AA, Houle S, Remington G, Zipursky RB, Kapur S. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. Am J Psychiatry. 2004;161(9):1620–1625. doi: 10.1176/appi.ajp.161.9.1620. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Kufferle B, Asenbaum S, Fischer P, Pezawas L, Barnas C, Tauscher-Wisniewski S, Brucke T, Kasper S. In vivo 123I IBZM SPECT imaging of striatal dopamine-2 receptor occupancy in schizophrenic patients treated with olanzapine in comparison to clozapine and haloperidol. Psychopharmacology (Berl) 1999;141(2):175–181. doi: 10.1007/s002130050822. [DOI] [PubMed] [Google Scholar]
- Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Predicting dopamine D(2) receptor occupancy from plasma levels of antipsychotic drugs: a systematic review and pooled analysis. J Clin Psychopharmacol. 2011;31(3):318–325. doi: 10.1097/JCP.0b013e318218d339. [DOI] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, Liemburg EJ, den Boer JA, Aleman A. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J Psychiatr Res. 2010;44(11):725–731. doi: 10.1016/j.jpsychires.2009.12.011. [DOI] [PubMed] [Google Scholar]
- West AR, Floresco SB, Charara A, Rosenkranz JA, Grace AA. Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann N Y Acad Sci. 2003;1003:53–74. doi: 10.1196/annals.1300.004. [DOI] [PubMed] [Google Scholar]
- Wolf ND, Gron G, Sambataro F, Vasic N, Frasch K, Schmid M, Thomann PA, Wolf RC. Magnetic resonance perfusion imaging of auditory verbal hallucinations in patients with schizophrenia. Schizophr Res. 2012;134(2-3):285–287. doi: 10.1016/j.schres.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Wolf ND, Sambataro F, Vasic N, Frasch K, Schmid M, Schonfeldt-Lecuona C, Thomann PA, Wolf RC. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J Psychiatry Neurosci. 2011;36(6):366–374. doi: 10.1503/jpn.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kitamura N, Lin XH, Ikeuchi Y, Hashimoto T, Shirakawa O, Maeda K. Differential changes in glutamatergic transmission via N-methyl-D-aspartate receptors in the hippocampus and striatum of rats behaviourally sensitized to methamphetamine. Int J Neuropsychopharmacol. 1999;2(3):155–163. doi: 10.1017/S1461145799001480. [DOI] [PubMed] [Google Scholar]
- Yao L, Lui S, Liao Y, Du MY, Hu N, Thomas JA, Gong QY. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:100–106. doi: 10.1016/j.pnpbp.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Yilmaz Z, Zai CC, Hwang R, Mann S, Arenovich T, Remington G, Daskalakis ZJ. Antipsychotics, dopamine D(2) receptor occupancy and clinical improvement in schizophrenia: A meta-analysis. Schizophr Res. 2012;140(1-3):214–220. doi: 10.1016/j.schres.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Ueda N, Shinkai K, Nakamura J. Plasma levels of homovanillic acid and the response to risperidone in first episode untreated acute schizophrenia. Int Clin Psychopharmacol. 2003;18(2):107–111. doi: 10.1097/00004850-200303000-00008. [DOI] [PubMed] [Google Scholar]
- Zugman A, Gadelha A, Assuncao I, Sato J, Ota VK, Rocha DL, Mari JJ, Belangero SI, Bressan RA, Brietzke E, Jackowski AP. Reduced dorso-lateral prefrontal cortex in treatment resistant schizophrenia. Schizophr Res. 2013;148(1-3):81–86. doi: 10.1016/j.schres.2013.05.002. [DOI] [PubMed] [Google Scholar]