Abstract
Background
Health-related quality of life (HRQOL) heterogeneity among cancer survivors may mask subgroups (classes) with different limitations and long-term outcomes. We determined what HRQOL classes of lung cancer survivors exist, examined transitions among classes over time, and compared survival outcomes of classes present in the initial phase of care.
Methods
Lung cancer survivors in the Cancer Care Outcomes Research and Surveillance Consortium completed EuroQol-5D questionnaires 4.8 months (initial phase) and >1 year (survivorship phase) post-diagnosis (n=1,396). Latent class (LCA) and latent transition analysis (LTA) determined HRQOL classes and transitions across time. Correlates of class membership were tested using multinomial logistic regression. Kaplan-Meier and Cox regression analyses were conducted to compare survival across class membership.
Results
LCA identified four classes at diagnosis and follow-up: (1) poor HRQOL, (2) pain dominant impairment, and (3) mobility/usual activities impairment, (4) good HRQOL. Probabilities of remaining in the same class were 0.87, 0.85, 0.82, and 0.73 for classes 4, 1, 3, and 2, respectively. Younger age, lower income, lower education, comorbidities and a history of depression/emotional problems were associated with higher likelihood of being in classes 1, 2 or 3 at follow-up. Class 1 and 3 had significantly lower median survival estimates than Class 4 (4.8, 3.8, and 5.5 years, respectively, p< 0.001).
Conclusions
Examining the heterogeneity of HRQOL in lung cancer populations allows identification of classes with different limitations and long-term outcomes, and thus guides tailored and patient-centered provision of supportive care.
Keywords: lung cancer, health-related quality of life, transition, latent class, survivor
Introduction
The Institute of Medicine emphasized the increasing complexity of cancer care, the difficulties complexity creates in decision-making for both patients and providers, and the need to adopt patient-centered approaches to inform these decisions.1 Monitoring health-related quality of life (HRQOL) is an important approach to keeping patients’ needs at the forefront, and to evaluating and implementing appropriate cancer treatment strategies in the initial phase of care as well as health care practices in the survivorship phase.2 HRQOL scores can also predict survival in cancer patients and survivors.3 However, overall scores may mask important HRQOL differences and heterogeneity among survivors, or the existence of sub-groups or “classes” of survivors who self-report different types of limitations despite the same overall HRQOL score.4 For example, a class may be characterized by pain-related limitations, while another with the same overall HRQOL score, may be characterized by mobility-related limitations. Moreover, classes may be comprised of survivors with the highest possible or lowest possible HRQOL scores: when these ceiling or floor effects occur, average HRQOL estimates may be inaccurate.5 Therefore, it is important to examine HRQOL at the person-level to better understand self-reported limitations and heterogeneity in survivors.
Examining HRQOL heterogeneity in lung cancer survivors is important for several reasons. Lung cancer survivors consistently report worse HRQOL than other cancer types.7. Examining the specific limitations in survivor HRQOL, and the implications for long-term outcomes, is a fundamental step toward improving HRQOL. Moreover, review studies suggest that, for most lung cancer survivors, the decrease in HRQOL in the initial phase of care due to treatment resolves and survivors return to pre-treatment HRQOL within four to six months.8,9 If heterogeneity exists as described above, it is unclear whether this trend differs for survivors who fall into different HRQOL classes in the initial phase of care. Therefore, identifying the classes of survivors who may be less likely to return to pre-treatment HRQOL has important implications for lung cancer survivor care. Our objective was to examine the HRQOL heterogeneity over time in lung cancer survivors participating in the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium. In particular, we examined HRQOL at a time close to diagnosis when treatment occurs and HRQOL is impacted (initial phase of care), and again when the acute treatment phase has passed (survivorship phase). We first determined what classes of survivors existed based on HRQOL at both time points, then examined how survivors transitioned among classes over time, and what socio-demographic and cancer-related factors affected transitions. Finally, we compared survival for survivors belonging to different HRQOL classes in the initial phase of care.
Methods
First, we applied latent class analysis (LCA) to identify “latent” (not directly observable) classes of survivors in the initial and survivorship phase.10,11 LCA finds groups of individuals that are similar to each other (aggregating into groups or classes) based on measured characteristics. In our case, these characteristics were the HRQOL domains.11,12 LCA allows us to distinguish the heterogeneity among survivors with respect to HRQOL and subsequently identify factors associated with the heterogeneous classes of survivors. We then used latent transition analysis (LTA), a longitudinal extension of LCA, to examine the probability of transitioning from one class to another over time. We then compared survival by HRQOL class membership at the initial phase.
Data
The sample was from CanCORS, established by the National Cancer Institute in 2001 to conduct a survey in five geographically distinct sites, five Cancer Research Network integrated health systems, and 15 Veteran hospitals.13 Lung and colorectal cancer patients were recruited through state cancer registries and health care administrative data (2003 to 2005), an average 4.8 months post-diagnosis. Participants completed initial surveys about treatment, quality of care, and symptoms experienced.14 Racial/ethnic minorities were oversampled. Clinical information was abstracted from medical records. An average of 9 months after the initial survey, participants were contacted for a follow-up survey on symptoms and overall health.. Abbreviated and surrogate surveys were available for patients too ill to complete the full survey or deceased. Vital status information was updated for each participant using national death records and/or managed care plan records up to 9.5 years post diagnosis. Participating institutions received approval from human subjects’ review boards.
Sample selection
There were 2,545 lung cancer patients alive at the initial survey. Exclusion criteria included 1) not completing the full version of the initial survey (n=31) or follow-up survey (n=983, of whom 454 deceased); 2) mixed or other race/ethnicity (n=75); 3) initial survey conducted >1 year from diagnosis (n=7); 4) missing HRQOL data at initial or follow-up survey (n=85). This resulted in a final sample of 1,396 patients. We excluded from the survival analysis 9 participants for whom we did not have vital status information.
HRQOL Measure
The EuroQol-5D (EQ-5D) is a generic, preference-based measure for estimating health utilities. Items measure 5 health domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.15 Response options include: no problems, some problems, and severe problems. For LCA/LTA modeling, “some problems” and “severe problems” were collapsed into one category due to small cell sizes for the severe category (<7%). A score of 1.00 represents perfect health (no problems), zero represents death, and negative scores represent health states valued as worse than death (severe problems on all domains).16 EQ-5D U.S. norms range from 0.87 for those 45-55 to 0.84 for those 75-89 years of age.17 The minimally important difference (MID) for EQ-5D scores in the U.S. has been estimated at 0.04.18
Statistical analysis
Descriptive analyses were conducted for study population characteristics and covariates. Frequencies were obtained for categorical variables and means and standard deviations for continuous variables.
We first used LCA to determine latent classes in the initial and survivorship phase using the responses to the 5 items of the EQ-5D.10 Models with k vs. k+1 classes were tested iteratively until the best data fit was identified (both qualitatively and quantitatively). Model fit was evaluated with Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC, adjusted BIC; lower AIC, BIC and ABIC indicate better fit), likelihood ratio test (LRT), bootstrapped LRT (significant p-values indicate that the larger number of classes is better) defines the sample than the smaller number), and entropy.19 Model identification was determined by fitting the model using multiple sets of random starting values.20 LCA provides the number of classes and the probability of survivors reporting severe problems in each class. For each survivor, a class variable was defined for the initial and the survivorship phase, whose value was equal to k, corresponding to the class the survivor belonged to.
Next, LTA, building off of the LCA model, uses full information maximum likelihood estimation to regress the class variable for the survivorship phase (dependent variable) on the class variable for the initial phase and obtain class prevalence in each phase and a transition probability matrix that describes survivors’ transitions among classes across the two phases21 (Supplementary Figure 1). We tested the fit and consistency of the class structure across our two time points (i.e., measurement invariance) by comparing latent transition models in which parameters were freely estimated with models in which parameters were constrained to be equal across the time points.20 Fit indices such as AIC, BIC, and ABIC were used assess model fit. LCA/LTA procedures were conducted using Mplus V7.1.22
The LTA classification information was used to identify correlates of class transition. Chi-square tests with Bonferroni adjustment for multiple testing were used to evaluate associations between follow-up transition classification and demographic and clinical variables. Demographic characteristics collected at baseline included age (<65 years/≥65 years), race, sex, highest education achieved, and income. Clinical variables include self-report of comorbidity (yes/no for a previous diagnosis of heart disease, pulmonary disease, diabetes, kidney disease, and ever having depression or other emotional problems), cancer stage, time since diagnosis, and treatment received (surgery, chemotherapy, radiation). Significant variables were included in a multinomial logit model23 where the categorical dependent variable was follow-up class membership and class 4 was the reference class.
Survival time was calculated from the date of diagnosis to the date of event, defined as last follow-up/death. Cumulative event rates were calculated (Kaplan and Meier24) and univariate analyses (log-rank test) were conducted to compare differences across classes and relevant covariates (age, race, education, stage at diagnosis, treatment, comorbidities and depression/emotional problems). Cox proportional hazards models25 were developed to test if the hazard of death (hazard ratio; HR) was different across HRQOL initial phase classes controlling for demographic and clinical characteristics (p<.25 for Wald statistic in univariate analysis). If non-proportional hazards were present an interaction term was included (e.g., time*class membership). Model fit was evaluated using the likelihood ratio test.25 All descriptive analyses and survival analyses were conducted using SAS 9.3.26
Results
The majority of the sample was male, White, ≥65 years old and non-small cell survivors (87%) (Table 1). Over one-third of survivors were diagnosed with stage IV disease. Overall, 37% received surgery only and 27% had received surgery, chemotherapy, and radiation.
Table 1.
Sample characteristics (n=1,396)
| No. | %* | |
|---|---|---|
| Race | ||
| White | 1,126 | 80.66 |
| African American | 170 | 12.18 |
| Hispanic | 62 | 4.44 |
| Asian | 38 | 2.72 |
| Age (years) | ||
| ≤54 | 185 | 13.25 |
| 55-64 | 385 | 27.58 |
| 65-74 | 494 | 35.39 |
| ≥75 | 332 | 23.78 |
| Gender | ||
| Male | 722 | 51.72 |
| Female | 674 | 48.28 |
| Income | ||
| <$20,000 | 399 | 30.83 |
| ≥$20,000 to <$40,000 | 393 | 30.37 |
| ≥$40,000 to <$60,000 | 231 | 17.85 |
| ≥$60,000 | 271 | 20.94 |
| Education | ||
| Less than high school | 235 | 16.91 |
| High school graduate/GED | 465 | 33.45 |
| Some college/ vocational school | 408 | 29.35 |
| ≥College degree | 282 | 20.29 |
| Covered by insurance | ||
| No | 15 | 1.08 |
| Yes | 1,379 | 98.92 |
| Comorbid conditions† | ||
| No | 595 | 42.65 |
| Yes | 800 | 57.35 |
| Number of conditions | ||
| Mean(SD) | 0.87 | (0.97) |
| (range) | (0-5) | |
| Depression/emotional problems | ||
| No | 1050 | 75.54 |
| Yes | 340 | 24.46 |
| Cancer type | ||
| Non-small cell | 1224 | 87.68 |
| Small cell | 108 | 7.74 |
| Stage at diagnosis | ||
| Stage I | 563 | 41.98 |
| Stage II | 156 | 11.63 |
| Stage III | 382 | 28.49 |
| Stage IV | 240 | 17.90 |
| Time from diagnosis to initial survey (months) | ||
| Mean(SD) | 4.80 | (1.72) |
| (Range) | (2-12) | |
| Time from diagnosis to follow-up (months) | ||
| Mean(SD) | 13.70 | (3.63) |
| (Range) | (9.13-41.57) | |
| Treatment received by follow-up | ||
| Surgery only | 459 | 37.09 |
| Surgery followed by chemotherapy or | 159 | 11.39 |
| chemotherapy only | ||
| Surgery, chemotherapy and radiation | 386 | 27.65 |
| Chemotherapy and radiation, no surgery | 307 | 21.99 |
| Other† | 85 | 6.09 |
| Initial EQ-5D score | ||
| Mean(SD) | 0.79 | (0.16) |
| (Range) | (0.07-1.00) | |
| Follow-up EQ-5D score | ||
| Mean(SD) | 0.80 | (0.17) |
| (Range) | (0.05-1.00) | |
Percentages may not total 100% due to missing values. Missing was <10%;
Heart attack, heart failure, stroke, diabetes, pulmonary function problems, kidney disease, diabetes;
Radiation only or none
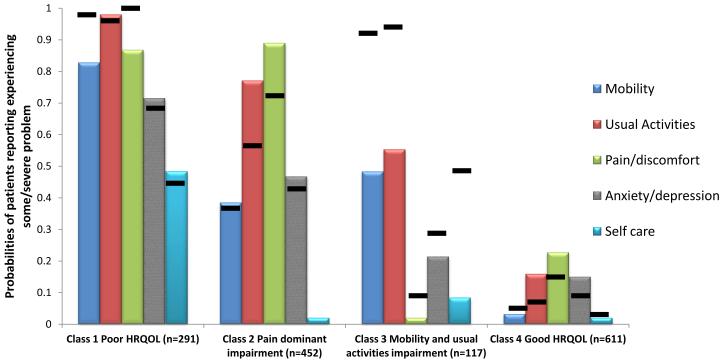
The mean EQ-5D scores were similar in the initial (0.79) and survivorship phase surveys (0.80). Approximately 22% and 26% of survivors had ceiling effects at the initial and survivorship phase respectively. The model fit criteria show that the four-class model fits the data most appropriately for the initial phase (lowest information criterion and LRT p-values, Table 2). These classes were (Figure 1):
Table 2.
Latent class model accuracy and fit parameters* (n=1,396)
| Model | LL | # parameters | AIC | BIC | ABIC | VLRT(p) | LRT(p) | BLRT(p) | Entropy | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| # Classes | ||||||||||
| Baseline | ||||||||||
| 2 | −3810 | 11 | 7643 | 7701 | 7666 | <0.001 | <0.001 | 0.00 | 0.67 | |
| 3 | −3787 | 17 | 7609 | 7699 | 7645 | 0.004 | 0.004 | 0.00 | 0.68 | |
| 4 | −3778 | 23 | 7603 | 7724 | 7651 | 0.03 | 0.03 | 0.02 | 0.59 | |
| 5 | −3774 | 29 | 7605 | 7757 | 7764 | 0.05 | 0.05 | 0.67 | 0.60 | |
| Follow-up | ||||||||||
| 2 | −3770 | 11 | 7562 | 7619 | 7584 | <0.001 | <0.001 | 0.00 | 0.72 | |
| 3 | −3731 | 17 | 7494 | 7583 | 7529 | <0.001 | <0.001 | 0.00 | 0.59 | |
| 4 | −3716 | 23 | 7479 | 7600 | 7527 | 0.001 | 0.001 | 0.00 | 0.66 | |
| 5 | −3714 | 29 | 7487 | 7639 | 7547 | 0.154 | 0.157 | 0.67 | 0.64 | |
Note: LL= loglikelihood; AIC= Akaike Information Criterion; BIC=Bayesian Information Criterion; ABIC=Adjusted BIC; LL= Log Likelihood; VLRT= Vuong, Lo, Mendel & Rubin Test; BLRT= Bootstrap likelihood ratio test; NCVG= Best LL not replicated at maximum random starts
Figure 1.
Poor HRQOL (n=269, 19.3%; EQ-5D=0.56, SD=0.17): high probabilities for “some/severe problems” for each item (0.715-0.980), except for self-care (Supplementary Table1);
Pain-dominant impairment (n=427, 30.6%; EQ-5D=0.76, SD=0.11): high probability (0.885) for “some/severe problems” in pain/discomfort, and lower probabilities (<0.80) of problems in other items;
Mobility/usual activity impairment (n=114, 8.9%; EQ-5D=0.76, SD=0.11): moderate probability (0.488-0.547) of “some/severe problems” in mobility and usual activities and very low probability (<0.250) of other problems; and
Good HRQOL (n=587, 42.0%; EQ-5D=0.92, SD=0.10): low probability (0-0.225) of limitations in all items.
The survivorship phase LCA resulted in a similar four-class model (Figure 1). Item response probabilities and class prevalence were similar to the initial phase (class1: n=345, 24.7%, class 2 n=393, 28.2%, class 3: n=78, 5.6%, and class 4: n=579, 41.5%).
Survivors had high probabilities of remaining within the same class at follow-up (Table 3): for Poor, Pain-dominant, and good HRQOL this probability was greater than 0.80 and for mobility/usual activity impairment greater than 0.70. Good HRQOL and poor HRQOL classes had low probabilities of class transition. Pain-dominant had 0.11 probability of moving to good HRQOL and 0.160 of moving to poor HRQOL. The mobility/usual activity class had only a 0.178 probability of transitioning to good HRQOL. The latent transition model with threshold parameters constrained to be equal across the two time points was the best fitting model (partial measurement invariance; Table 3).
Table 3.
Transition probabilities of staying within class and transitioning out of class at follow-up
| Class membership at follow-up† | ||||
|---|---|---|---|---|
|
| ||||
| Class membership near diagnosis |
Class 1: Poor HRQOL |
Class 2: Pain- dominant impairment |
Class 3: Mobility and usual activity impairment |
Class 4: Good HRQOL |
| Class 1 | 0.851 | 0.069 | 0.023 | 0.057 |
| Class 2 | 0.160 | 0.726 | 0.000 | 0.114 |
| Class 3 | 0.000 | 0.002 | 0.819 | 0.178 |
| Class 4 | 0.045 | 0.010 | 0.077 | 0.868 |
LTA Model fit indices: LL -7222, AIC 14515, BIC 14699, ABIC 14588; entropy 0.742
The multinomial logistic regression model predicting class membership in the survivorship phase included age, education, income, comorbidities, and depression/emotional problems. Non-significant variables excluded were sex, race, cancer stage, time since diagnosis, and treatment received. Model fit statistics indicated the final model had superior fit to the model with no covariates (Table 4). Older survivors were less likely to be in the poor HRQOL and pain-dominant impairment classes, while survivors with lower income were more likely to be in the poor HRQOL class than in the good HRQOL class (Table 4). Survivors with comorbidities were more likely to be in poor HRQOL or mobility/usual activities impairment class than the good HRQOL class. Survivors with depression/emotional problems were significantly more likely to be in any of the three impaired classes than the good HRQOL class.
Table 4.
Patient characteristics* associated with follow-up transition class (reference class=Good HRQOL)
| Poor HRQOL | Pain-dominant impairment | Mobility/Usual Activities impairment | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age (ref=<65) | ||||||
| ≥65years | 0.532 | (0.371-0.764) | 0.534 | (0.375-0.760) | 0.793 | (0.578-1.088) |
| Income (ref=≥60,000) | ||||||
| <$20,000 | 4.350 | (2.398-7.893) | 1.494 | (0.877-2.543) | 1.329 | (0.826-2.139) |
| ≥$20,000 to <$40,000 | 2.226 | (1.285-4.209) | 1.101 | (0.657-1.845) | 1.303 | (0.838-2.028) |
| ≥$40,000 to <$60,000 | 1.629 | (0.854-3.105) | 1.114 | (0.648-1.915) | 1.311 | (0.826-2.082) |
| Education (ref= ≥College degree) | ||||||
| Less than high school | 2.158 | (1.147-4.060) | 2.873 | (1.563-5.282) | 1.546 | (0.888-2.691) |
| High school graduate/GED | 1.681 | (0.975-2.896) | 1.591 | (0.947-2.674) | 1.669 | (1.087-2.561) |
| Some college/ vocational school | 1.333 | (0.771-2.304) | 1.496 | (0.905-2.472) | 1.386 | (0.907-2.117) |
| Comorbid conditions (ref=No) | ||||||
| Yes | 1.696 | (1.188-2.422) | 1.387 | (0.984-1.956) | 1.742 | (1.299-2.354) |
| Depression/emotional problems (ref=No) | ||||||
| Yes | 6.320 | (4.244-9.413) | 4.903 | (3.293-7.299) | 1.745 | (1.162-2.620) |
|
| ||||||
| Model Fit Indices | Intercept only | Intercept with covariates | ||||
| Akaike Information Criterion | 3430 | 3234 | ||||
| −2 log likelihood | 3424 | 3174 | ||||
Variables included based on significant chi-square tests and using adjustment for multiple comparisons
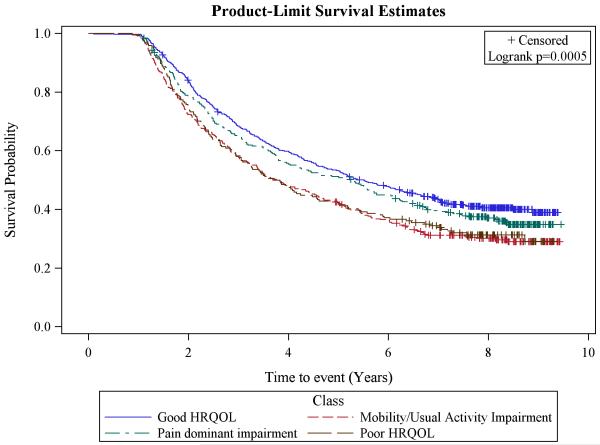
Poor HRQOL (4.8 years) and mobility/usual activities impairment class (3.8 years) had significantly lower median survival estimates than good HRQOL (5.5 years), but not lower survival than the pain-dominant class (5.3 years) (Figure 2). These findings were mostly supported in the Cox regression model adjusted for the interaction of time and class, age, cancer stage, treatment, comorbidities, and depression/emotional problems (results not presented). Compared to Good HRQOL, the HR of death for mobility/usual activities impairment class was 1.37 (CI:1.00,2.79; p=0.029) and for poor HRQOL was 1.47 (95% CI:1.02,1.81; p=0.053). Older survivors, and survivors with higher stage at diagnosis, more extensive treatment (surgery, chemotherapy and radiation), and comorbidities had significantly higher HR than their counterparts. The likelihood ratio test was significant (p<0.0001) and information criterion (−2 log likelihood and AIC) were lower for the model with covariates compared to the model without covariates indicating adequate model fit.
Figure 2.
Discussion
We found four distinct classes of lung cancer survivors based on HRQOL both in an initial phase of care close to diagnosis and later at post-treatment follow-up. Approximately 42% of survivors were characterized by good HRQOL. The remaining were classified into one of the three classes with HRQOL impairments in all domains, mainly in pain, or mainly in mobility and usual activities. Survivors were unlikely to transition to a different class at post-treatment follow-up, and the class they belonged to in the initial phase of care had significant implications for longer survival. Younger survivors, those with lower education and income, and those with comorbidities and a history of depression or emotional issues were more likely to be in classes with HRQOL impairments during the survivorship phase.
The distinct classes of pain-dominant and mobility/usual activity impairment support the suggestion that classifying HRQOL as good or poor or just considering an average HRQOL score is limiting.6 We demonstrated that the specific limitations driving HRQOL scores, and thus the heterogeneity among survivors, need to be considered to understand the patient experience and the impact that patterns of HRQOL impairment may have on outcomes, including survival. Importantly, despite similar HRQOL mean scores, the mobility/usual activities impairment and pain-dominant class had quite different survival profiles. Of note, survivors in the pain-dominant class were also more likely to report problems with usual activities and anxiety and depression compared to survivors in the limited mobility/usual activities class at the time closer to diagnosis. Inadequately managed pain may contribute to psychological distress and as a result, interfere with usual activities including work or family obligations.27 Early and targeted supportive care incorporating pharmacologic interventions and other psychosocial therapies to manage pain may be beneficial.28 In the initial phase of care, the mobility/usual activities impairment class was almost exclusively defined by functional limitations, while limitations due to pain were not reported. During the survivorship phase, however, together with an increase of 40% in the probability of mobility, usual activity, and self-care limitations, the probability of reporting pain problems also increased by 10%. Because mobility and usual activity problems are likely related to impaired pulmonary function, therapy incorporating pulmonary rehabilitation,29 physical activity and strength training30 to increase mobility and reduce pain in this survivor class may provide relief.
Consistent with other studies in which younger age, low income, and low education were associated with poor health outcomes in cancer,31,32 these factors predicted membership in a class other than good HRQOL. Younger patients may have greater responsibilities or expectations of their health compared to older patients when they are diagnosed, leading to greater distress if their health status during or post-treatment does not meet these expectations.33 Contrary to some studies where higher cancer stage, treatment type (specifically adjuvant chemotherapy), and surgery were associated with poor outcomes such as post-thoracotomy pain syndromes,34 these factors were not significant predictors of class membership in our study. It may be that we did not have enough variation in stage or treatment to be able to detect this effect, or that we missed important information on specific surgeries that have been shown to be associated with HRQOL. Moreover, it is plausible that, while these factors may affect the HRQOL score, they may have disparate effects on the specific HRQOL limitations that dictate class membership. We also found that a history of depression/emotional problems and comorbidities predicted being in classes other than the good HRQOL class, but only having comorbidities, and not depression/emotional problems, was significantly associated with survival. Others have shown the association of comorbidities with lower HRQOL, disability, and survival in lung cancer.35 Depression and comorbidities may be treatable or optimally managed and thus, may be targeted to improve HRQOL.
Our analytic approach demonstrates an important methodological alternative to summary HRQOL scores by highlighting the heterogeneity and its implications among survivors with respect to HRQOL. This strategy addressed ceiling effects, described class trends for improvement or decline after initial treatment phase, and identified factors associated with classes of survivors.5 Furthermore, the association of specific survivor classes with different survival, our study highlights the clinical importance of early recognition of the variety of HRQOL limitations and not just utilizing average scores to inform care Given that HRQOL classes identified near initial treatment predicted longer-term survival, recent efforts to introduce palliative care early in the cancer continuum are particularly important.1
This study has some limitations. First, while transitions were limited between classes at our study’s measurement points, it is possible that survivors may transition at later times in the cancer trajectory. Second, findings are specific to this sample: analyses should be repeated to validate our results. Moreover, almost all survivors had health insurance, limiting generalizability to larger populations. However, this study incorporates several health care systems and geographically distinct sites. Third, participants unable to complete the full initial survey were not included in our analysis: these may have lower HRQOL, and thus our scores may not reflect the broader population of survivors.. .
Lung cancer survivors were characterized into four different classes based on HRQOL responses and few transitioned to better HRQOL classes. This heterogeneity can provide information to guide personalized care that accounts for specific HRQOL limitations. Comorbidities and depression/emotional problems clearly impact HRQOL and are viable targets for supportive care. Identifying specific HRQOL limitations at the time of treatment, regardless of disease stage, is critical to develop supportive care strategies and improve HRQOL over time.
Supplementary Material
Acknowledgments
Funders: The CanCORS consortium was supported by grants from the National Cancer Institute(NCI) to the Statistical Coordinating Center(U01 CA093344) andfd NCI-supported Primary Data Collection and Research Centers (Dana-Farber Cancer Institute/Cancer Research Network[U01 CA093332], Harvard Medical School/Northern California Cancer Center[U01 CA093324], RAND/UCLA[U01 CA093348], University of Alabama at Birmingham [U01CA093329], University of Iowa[U01CA093339], University of North Carolina[U01 CA 093326] and by Department of Veterans Affairs grant to Durham VA Medical Center[CRS 02-164]; and KK was supported by grant 2 T32 HS013852 from the Agency for Healthcare Research and Quality.
Footnotes
All authors confirm that they have no financial disclosures to report and no conflicts of interest.
References
- 1.Institute of Medicine . Delivering high-quality cancer care: Charting a new course for a system in crisis. National Academies Press; Washington, DC: 2013. [PubMed] [Google Scholar]
- 2.Damm K, Roeske N, Jacob C. Health-related quality of life questionnaires in lung cancer trials: A systematic literature review. Health Econ Rev. 2013;3(1):15-1991–3-15. doi: 10.1186/2191-1991-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li TC, Li CI, Tseng CH, et al. Quality of life predicts survival in patients with non-small cell lung cancer. BMC Public Health. 2012;12:790-2458–12-790. doi: 10.1186/1471-2458-12-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder CF, Garrett-Mayer E, Blackford AL, et al. Concordance of cancer patients' function, symptoms, and supportive care needs. Qual Life Res. 2009;18(8):991–998. doi: 10.1007/s11136-009-9519-6. [DOI] [PubMed] [Google Scholar]
- 5.Huang IC, Frangakis C, Atkinson MJ, et al. Addressing ceiling effects in health status measures: A comparison of techniques applied to measures for people with HIV disease. Health Serv Res. 2008;43(1 Pt 1):327–339. doi: 10.1111/j.1475-6773.2007.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostroff JS, Krebs P, Coups EJ, et al. Health-related quality of life among early-stage, non-small cell, lung cancer survivors. Lung Cancer. 2011;71(1):103–108. doi: 10.1016/j.lungcan.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ R. 2008;29(4):23–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Tanvetyanon T, Soares HP, Djulbegovic B, Jacobsen PB, Bepler G. A systematic review of quality of life associated with standard chemotherapy regimens for advanced non-small cell lung cancer. J Thorac Oncol. 2007;2(12):1091–1097. doi: 10.1097/JTO.0b013e31815cff64. [DOI] [PubMed] [Google Scholar]
- 9.Bottomley A, Efficace F, Thomas R, Vanvoorden V, Ahmedzai SH. Health-related quality of life in Non–Small-cell lung cancer: Methodologic issues in randomized controlled trials. J Clin Oncol. 2003;21(15):2982–2992. doi: 10.1200/JCO.2003.01.203. [DOI] [PubMed] [Google Scholar]
- 10.Collins LM, Lanza ST. Wiley; Hoboken, N.J.: 2010. Latent class and latent transition analysis: With applications in the social behavioral, and health sciences; p. 285. [Google Scholar]
- 11.McCutcheon AL. Latent class analysis. Sage Publications; Beverly Hills, CA: 1987. [Google Scholar]
- 12.Muthen B, Muthen LK. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24(6):882–891. [PubMed] [Google Scholar]
- 13.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The cancer care outcomes research and surveillance consortium. J Clin Oncol. 2004;22(15):2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients' experience and outcomes: Development and pilot study of the cancer care outcomes research and surveillance patient survey. Support Care Cancer. 2006;14(8):837–848. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 15.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 16.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: Development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the national health measurement study. Med Care. 2007;45(12):1162–1170. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care. 2010;48(4):365–371. doi: 10.1097/mlr.0b013e3181c162a2. [DOI] [PubMed] [Google Scholar]
- 19.Vermunt JK. Latent class modeling with covariates: Two improved three-step approaches. Political Analysis. 2010;18(4):450–469. [Google Scholar]
- 20.Nylund K. Latent transition analysis: Modeling extensions and an application to peer victimization. [Doctoral disseration]. University of California, Los Angeles. 2007 [Google Scholar]
- 21.Asparaouhov T, Muthen B. 2011 C on c and X. Technical Appendix. [Google Scholar]
- 22.Muthen B, Muthen LK. Mplus version. 2012 Jan 7;:7. [Google Scholar]
- 23.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd John Wiley and Sons; Hoboken, NJ: 2000. [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53(282):457–481. [Google Scholar]
- 25.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 26.SAS Institute SAS 9.3. 2012:9.3. [Google Scholar]
- 27.Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399–409. doi: 10.1016/s0006-3223(03)00545-6. [DOI] [PubMed] [Google Scholar]
- 28.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic Non–Small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 29.Glattki GP, Manika K, Sichletidis L, Alexe G, Brenke R, Spyratos D. Pulmonary rehabilitation in non-small cell lung cancer patients after completion of treatment. Am J Clin Oncol. 2012;35(2):120–125. doi: 10.1097/COC.0b013e318209ced7. [DOI] [PubMed] [Google Scholar]
- 30.Andersen AH, Vinther A, Poulsen LL, Mellemgaard A. Do patients with lung cancer benefit from physical exercise? Acta Oncol. 2011;50(2):307–313. doi: 10.3109/0284186X.2010.529461. [DOI] [PubMed] [Google Scholar]
- 31.Larsson M, Ljung L, Johansson BBK. Health-related quality of life in advanced non-small cell lung cancer: Correlates and comparisons to normative data. Eur J Cancer Care (Engl) 2012;21(5):642–649. doi: 10.1111/j.1365-2354.2012.01346.x. [DOI] [PubMed] [Google Scholar]
- 32.Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psychooncology. 2013;22(4):745–755. doi: 10.1002/pon.3055. [DOI] [PubMed] [Google Scholar]
- 33.Gotay CC. Trial-related quality of life: Using quality-of-life assessment to distinguish among cancer therapies. J Natl Cancer Inst Monogr. 1996;2020:1–6. [PubMed] [Google Scholar]
- 34.Karmakar MK, Ho AMH. Postthoracotomy pain syndrome. Thoracic Surgery Clinics. 2004;14(3):345–352. doi: 10.1016/S1547-4127(04)00022-2. [DOI] [PubMed] [Google Scholar]
- 35.Moller A, Sartipy U. Associations between changes in quality of life and survival after lung cancer surgery. J Thorac Oncol. 2012;7(1):183–187. doi: 10.1097/JTO.0b013e3182340abb. [DOI] [PubMed] [Google Scholar]
- 36.Grutters JPC, Joore MA, Wiegman EM, et al. Health-related quality of life in patients surviving non-small cell lung cancer. Thorax. 2010;65(10):903–907. doi: 10.1136/thx.2010.136390. [DOI] [PubMed] [Google Scholar]
- 37.Yang P. Epidemiology of lung cancer prognosis: Quantity and quality-of-life. Methods Mol Biol. 2009;471:469–486. doi: 10.1007/978-1-59745-416-2_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trippoli S, Vaiani M, Lucioni C, Messori A. Quality-of-life and utility in patients with non-small cell lung cancer. Quality-of-life study group of the Master 2 Project in pharmacoeconomics. Pharmacoeconomics. 2001;19(8):855–863. doi: 10.2165/00019053-200119080-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.