Abstract
Background and Purpose
The locus coeruleus (LC) is the principal nucleus containing the noradrenergic neurons and is a major endogenous source of pain modulation in the brain. Glial cell line-derived neurotrophic factor (GDNF), a well-established neurotrophic factor for noradrenergic neurons, is a major pain modulator in the spinal cord and primary sensory neurons. However, it is unknown whether GDNF is involved in pain modulation in the LC.
Experimental Approach
Rats with chronic constriction injury (CCI) of the left sciatic nerve were used as a model of neuropathic pain. GDNF was injected into the left LC of rats with CCI for 3 consecutive days and changes in mechanical allodynia and thermal hyperalgesia were assessed. The α2-adrenoceptor antagonist yohimbine was injected intrathecally to assess the involvement of descending inhibition in GDNF-mediated analgesia. The MEK inhibitor U0126 was used to investigate whether the ERK signalling pathway plays a role in the analgesic effects of GDNF.
Key Results
Both mechanical allodynia and thermal hyperalgesia were attenuated 24 h after the first GDNF injection. GDNF increased the noradrenaline content in the dorsal spinal cord. The analgesic effects continued for at least 3 days after the last injection. Yohimbine abolished these effects of GDNF. The analgesic effects of GDNF were partly, but significantly, inhibited by prior injection of U0126 into the LC.
Conclusions and Implications
GDNF injection into the LC exerts prolonged analgesic effects on neuropathic pain in rats by enhancing descending noradrenergic inhibition.
Tables of Links
| LIGANDS |
|---|
| Clonidine |
| Gabapentin |
| GDNF, glial cell line-derived neurotrophic factor |
| Noradrenaline |
| Pregabalin |
| Substance P |
| U0126 |
| Yohimbine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,dAlexander et al., 2013a,b,c,d,,,).
Introduction
Neuropathic pain is caused by a lesion or disease of the somatosensory system, including the peripheral nervous system. This pain syndrome is resistant to conventional analgesics such as opiates and non-steroidal anti-inflammatory drugs (Xu et al., 2012). Instead, antidepressants that potentiate noradrenergic neurotransmission and calcium channel α2δ ligands are recommended as first-line medications, although they have limited effectiveness and substantial side effects (Dworkin et al., 2010). In addition, because neuropathic pain often takes a chronic course, medicines with extended efficacy and fewer adverse effects are needed.
The locus coeruleus (LC), the principal nucleus containing noradrenergic neurons in the brain, is located in the pons and is a major endogenous source of pain modulation in the CNS. Descending noradrenergic neurons originating from the LC inhibit nociceptive transmission in the spinal cord (Millan, 2002; Pertovaara, 2006). Baseline pain sensitivity is only slightly influenced by the noradrenergic system. However, noradrenaline release in the spinal dorsal horn leads to the suppression of pain in pathological conditions (Pertovaara, 2013). Indeed, electrical or chemical stimulation has been shown to be effective in alleviating neuropathic pain in animal models (Pertovaara, 2006; Viisanen and Pertovaara, 2007; Hayashida et al., 2008; Muto et al., 2012). Epidural and intrathecal clonidine, an α2-adrenoceptor agonist, is effective in treating neuropathic pain in humans (Eisenach et al., 1995; Ackerman et al., 2003), although administration of an effective dose results in serious side effects (Weinbroum and Ben-Abraham, 2001). Likewise, the use of antidepressants, including selective noradrenaline and 5-HT reuptake inhibitors, is limited in clinical practice by adverse side effects in spite of their effectiveness against neuropathic pain (O'Connor and Dworkin, 2009). The clinically useful calcium channel α2δ ligand, gabapentin, was reported to alleviate neuropathic pain through stimulation of the LC (Hayashida et al., 2008). Another calcium channel α2δ ligand, pregabalin, also activates descending noradrenergic inhibition in a neuropathic pain state (Takeuchi et al., 2007). Thus, targeting the descending noradrenergic pathway is a promising strategy for treating neuropathic pain.
Glial cell line-derived neurotrophic factor (GDNF) is a potent neurotrophic factor for catecholaminergic neurons, such as dopaminergic and noradrenergic neurons, although the underlying mechanisms of action are still largely unknown (Pascual et al., 2011). The action of GDNF is mainly mediated by a multicomponent receptor complex composed of GFRα-1 and Ret receptor tyrosine kinase or neuronal cell adhesion molecule (NCAM). GDNF signals by binding to its high-affinity receptor, GFRα-1, which subsequently activates transducing receptors, including Ret and NCAM (Pascual et al., 2011). In situ hybridization revealed that GFRα-1 and Ret are expressed by LC noradrenergic neurons (Trupp et al., 1997; Glazner et al., 1998; Golden et al., 1998; Sarabi et al., 2003). NCAM expression is also observed in noradrenergic neurons in the LC (Black et al., 2009). Consistent with its neurotrophic effect, extensive catecholaminergic neuronal death in the LC is observed in the conditional GDNF-deficient mouse (Pascual et al., 2008). In addition, GDNF induces the expression of genes responsible for noradrenaline synthesis, including tyrosine hydroxylase (TH), a rate-limiting enzyme for catecholamine synthesis, and GTP cyclohydrolase-I, a rate-limiting enzyme for the synthesis of tetrahydrobiopterin, an essential cofactor for TH (Arenas et al., 1995; Christophersen et al., 2007). Collectively, these findings suggest that GDNF has significant and important effects on the function of LC noradrenergic neurons.
GDNF is also involved in pain processing in the spinal cord and dorsal root ganglia, where cell bodies of primary sensory neurons are located. About half of all nociceptive primary sensory neurons express GDNF receptors and depend on GDNF signalling for cellular development and phenotype acquisition (Airaksinen and Saarma, 2002). In the normal state, GDNF induces hyperalgesia when administered into peripheral tissues (Malin et al., 2006; Bogen et al., 2008). In the neuropathic pain state, intrathecal administration of GDNF exerts potent analgesic effects (Boucher et al., 2000; Sakai et al., 2008), which is possibly mediated via dorsal root ganglion and spinal neurons (Takasu et al., 2011). Intrathecal administration of GDNF prevents neurochemical and gene expression changes in the dorsal root ganglion (Bennett et al., 1998; Bradbury et al., 1998; Boucher et al., 2000; Wang et al., 2003b). Taken together, these findings suggest that GDNF is a major pain modulator in the spinal cord and primary sensory neurons. However, whether GDNF is also involved in pain processing in the brain remains to be established. Here, we have examined the effects of GDNF in the LC in a rat model of neuropathic pain and found that GDNF administration into the LC provides long-lasting pain relief by recruiting descending noradrenergic inhibition.
Methods
Animals
All animal care and experimental procedures were approved by the institutional committee on laboratory animals (approval number 25–126) and performed under the guidelines recommended by the International Association for the Study of Pain (Zimmermann, 1983). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 79 animals were used in these experiments.
Adult male Sprague Dawley rats (7 weeks of age and weighing 210–250 g; Japan SLC, Shizuoka, Japan) were used for all experiments. The rats were individually housed in plastic cages with soft bedding under a 14 h/10 h light/dark cycle at least 3 days before the experiments. Food and water were available ad libitum. All surgical procedures were carried out on rats deeply anaesthetized with intraperitoneal sodium pentobarbital (50 mg·kg−1).
Cannula implantation into the LC
The rats were deeply anaesthetized with pentobarbital and placed in a stereotaxic apparatus (Kopf, Tujunga, CA, USA) for cannula implantation as previously described (Muto et al., 2012). A small burr hole was made in the skull and a stainless steel guide cannula (8.2 mm in length, 26 gauge; AG-8.2, Eicom, Kyoto, Japan) was positioned in the left (ipsilateral) side 1 mm above the LC (anteroposterior, −9.8 mm from the bregma; mediolateral, 1.3 mm from the midline; dorsoventral, 5.8 mm from the brain surface) (Paxinos and Watson, 1998). The guide cannula was secured to the skull with dental cement (Unifast 2, GC Corporation, Tokyo, Japan). A 33 gauge dummy cannula (AD-8.2; Eicom) was placed into the guide cannula to maintain patency. The rats were allowed to recover from surgery for 5–7 days.
At the end of the experiments, the rats were deeply anaesthetized with pentobarbital and transcardially perfused with PBS (pH 7.2) followed by freshly prepared 4% paraformaldehyde in PBS. The pons was removed, post-fixed with the same fixative at 4°C overnight and cryoprotected in 20% sucrose/PBS. The pons was cut into serial transverse cryosections (25 μm in thickness) using a cryostat (Leica, Tokyo, Japan). The most ventral site of the cannula trail was examined under a microscope (Olympus, Tokyo, Japan) and was considered to indicate the position of the cannula tip. We used behavioural data from rats in which the guide cannulae were adequately positioned above the LC. The following key landmarks were used to map the injection sites, as described previously (Brightwell and Taylor, 2009): motor trigeminal nucleus, mesencephalic 5 nucleus, nucleus of the trapezoid body, middle cerebellar peduncle and sensory root of the seventh cranial nerve. The rats in which the cannula tips were incorrectly positioned were excluded from the analysis.
Production of a neuropathic pain model
After guide cannula implantation, chronic constriction injury (CCI) of the sciatic nerve was performed as described previously (Bennett and Xie, 1988). Briefly, the ipsilateral common sciatic nerve was exposed at the level of the middle thigh and loosely ligated with 4-0 silk thread in four regions at intervals of approximately 1 mm. The incision was closed with a 4-0 silk suture. Rats with CCI were considered to exhibit neuropathic pain when they responded to von Frey filaments (Muromachi Kikai, Tokyo, Japan) with less than 7.2 g force, as described below.
Behavioural tests
Two behavioural tests (von Frey and plantar tests) were performed to assess pain during the light cycle. A set of von Frey filaments (Muromachi Kikai) with bending forces ranging from 2.1 to 20.0 g was used to examine mechanical allodynia. Each rat was placed on a metallic mesh floor covered with a plastic box and a von Frey monofilament was applied from underneath the mesh floor to the plantar surface of either the contralateral or ipsilateral hind paw. The weakest force (g) required to induce withdrawal of the stimulated paw at least three times in five trials was referred to as the paw withdrawal threshold. The Plantar Test apparatus (Ugo Basile, Varese, Italy) was used to examine thermal hyperalgesia. Each rat was placed on a glass plate with a radiant heat generator underneath and each hind paw was stimulated twice at 5 min intervals, alternating from side to side. The average time until withdrawal of the hind paw from the thermal stimulus was recorded as the paw withdrawal latency.
Injection of drugs into the LC
All injections were performed under light sedation with isoflurane (1.5–2%). The drugs were injected into the LC through a 33 gauge stainless steel injection cannula (AMI-9.2, Eicom) that extended 1 mm below the tip of the guide cannula. Injections were performed using a 10 μL Hamilton syringe (Hamilton Company, Reno, NY, USA) connected to the injection cannula by a polytetrafluoroethylene tube (JT-10, Eicom). The drugs were slowly administered at a dose of 0.5 μL over a period of 30 s. Recombinant human GDNF (R&D Systems, Minneapolis, MN, USA) was dissolved in saline and saline was used as a control. GDNF was injected three times, with a 24 h interval between each injection, from days 7 to 9 after CCI, and behavioural tests were performed before GDNF injection. The MEK (MAPK/ERK) inhibitor U0126 (Sigma-Aldrich, St. Louis, MO, USA) (Davies et al., 2000) was dissolved in 20% dimethyl sulfoxide in saline. The solvent (vehicle) was used as a control for U0126. U0126 (1 μg) in a volume of 0.5 μL was injected into the LC 30 min before GDNF injection every day from days 7 to 9 after CCI.
Quantification of noradrenaline content
Noradrenaline content in the dorsal spinal cord was assayed with a HPLC system (Eicom). The ipsilateral fifth lumbar segment of the dorsal spinal cord was removed 24 h after first injection of GDNF or saline into CCI rats on day 7. Tissues were sonicated in 0.17 M perchloric acid containing 100 μM EDTA and 160 mM sodium acetate and incubated on ice for 30 min. The homogenates were centrifuged at 20 000 g, 0°C, for 15 min. The supernatants were filtered through a 0.45 μm filter (Merck Millipore, Billerica, MA, USA) and 10 μL of filtrate was applied to the HPLC system. The system had a 150 × 3 mm octadecyl silane column (SC-5ODS, Eicom) and an electrochemical detector (HTEC-500, Eicom) set to an applied potential of +750 mV versus an Ag/AgCl reference analytical electrode. The change in electric current (nA) was recorded using a computer interface. The mobile phase was composed of aceto-citric acid buffer (pH 3.5, 0.1 M), methanol, sodium-1-octane sulfonate (0.46 M) and disodium EDTA (0.015 mM). The flow rate was 0.5 mL·min−1.
Intrathecal administration of the α2-adrenoceptor antagonist
On the same day, the guide cannula was inserted into the LC, a polyethylene catheter (PE-10; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) filled with saline was inserted under anaesthesia with pentobarbital as previously described (Yaksh et al., 1985). The catheter was passed 8.0 cm in the subarachnoid space from the atlanto-occipital membrane to the level of the lumbar enlargement. Only rats without apparent neurological dysfunction after catheter insertion were used for subsequent experiments. Yohimbine (Sigma-Aldrich), an α2-adrenoceptor antagonist, was dissolved in saline. The rats were intrathecally injected with 10 μL of yohimbine (20 μg) or saline as control for yohimbine, followed by 10 μL of saline. Yohimbine was administered in rats on day 10 after CCI, with repeated GDNF treatment from days 7 to 9. Behavioural tests were performed before and 20 min after yohimbine injection.
Data analysis
Values are expressed as means ± SEM. Differences in paw withdrawal threshold values between drug and control treatments were evaluated using the Mann–Whitney U-test with Bonferroni correction. Differences in paw withdrawal latency values between drug and control treatments were assessed with two-way repeated-measures anova followed by unpaired t-test with Bonferroni correction. The Kruskal–Wallis test followed by the Steel test and one-way anova followed by Dunnett's post hoc comparison were used to compare the threshold and latency values, respectively, obtained with the various GDNF doses. A value of P < 0.05 was considered to indicate statistical significance.
Results
Alleviation of neuropathic pain by GDNF injection into the LC
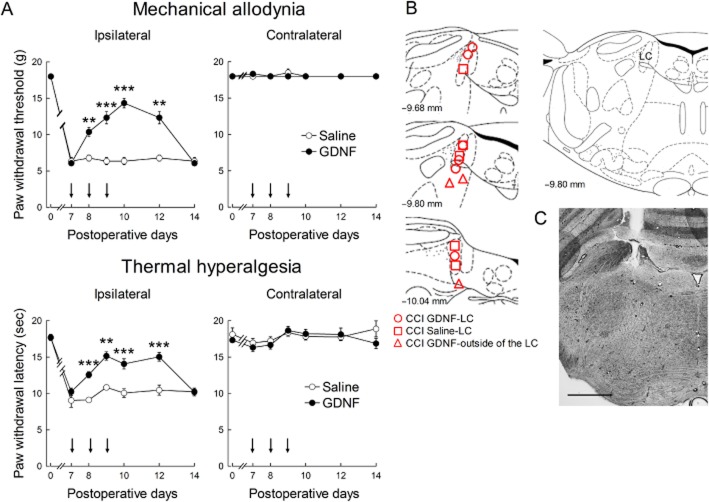
Seven days after the CCI operation, paw withdrawal threshold and latency to mechanical and thermal stimuli, respectively, were decreased on the ipsilateral side, but not on the contralateral side (Figure 1A). On days 7, 8 and 9 after CCI, GDNF (1.5 μg) was injected once per day into the ipsilateral LC because most descending LC neurons predominantly innervate the ipsilateral spinal cord (Clark and Proudfit, 1991; Howorth et al., 2009). As shown by the representative results in Figure 1B and C, most of the cannula tips were correctly positioned in the LC. After GDNF injection, the paw withdrawal threshold and latency (F(1,8) = 47.526, P < 0.001 by two-way repeated-measures anova) were increased compared with saline injection (Figure 1A). In cases where the guide cannula was incorrectly positioned, the GDNF injection had no analgesic effect (data not shown). The analgesic effects were maintained for at least 3 days after the last GDNF injection and wore off in 6 days (Figure 1A). The paw withdrawal thresholds and latencies on the contralateral side were unchanged by the GDNF injection (Figure 1A). In contrast to the long-term analgesic effects, GDNF had no acute analgesic effect on neuropathic pain, requiring 24 h for pain relief to manifest (Figure 2A; F(1,8) = 11.073, P = 0.002 by two-way repeated-measures anova for thermal hyperalgesia). At this time point, noradrenaline content was increased in the dorsal spinal cord (Figure 2B). We further investigated the dose dependency of the analgesic effects of GDNF on day 10 after CCI, when the analgesic effects were most obvious at a dose of 1.5 μg. As shown in Figure 3, GDNF attenuated both mechanical allodynia (P < 0.001 by Kruskal–Wallis test) and thermal hyperalgesia (F(3,22) = 5.110, P = 0.008 by one-way anova) in a dose-dependent manner.
Figure 1.
Effects of GDNF injection into the LC on neuropathic pain. (A) Mechanical allodynia and thermal hyperalgesia were assessed on the ipsilateral and contralateral hind paws of CCI rats. GDNF (1.5 μg) or saline was repetitively administered into the ipsilateral LC as indicated by arrows. **P < 0.01, ***P < 0.001: compared with saline treatment. Paw withdrawal threshold was assessed using the Mann–Whitney U-test with Bonferroni correction, while latency was evaluated using the unpaired t-test with Bonferroni correction; n = 4–6. (B) Schematic depiction of the microinjection sites. The numbers on the left of the coronal sections represent the distances from the bregma. The different symbols represent the tip sites in CCI rats injected with GDNF or saline and in CCI rats injected with GDNF outside the LC. (C) A representative image of the cannula track in the LC. Scale bar, 100 μm.
Figure 2.
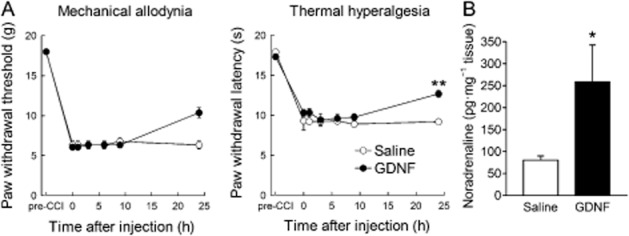
Acute effects of GDNF injection into the LC on neuropathic pain. (A) Mechanical allodynia and thermal hyperalgesia were assessed on the ipsilateral hind paw of CCI rats. A single dose of GDNF (1.5 μg) or saline was injected into the ipsilateral LC on day 7 after CCI. **P < 0.01; compared with saline treatment; unpaired t-test with Bonferroni correction; n = 4–6. (B) Noradrenaline content was assayed in the fifth lumbar dorsal spinal cord of CCI rats 24 h after GDNF or saline injection. *P < 0.05; compared with saline treatment; unpaired t-test; n = 3–6.
Figure 3.
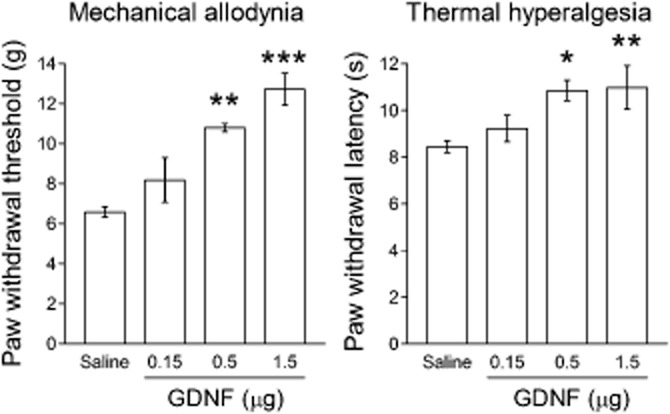
Dose-dependent analgesic effects of GDNF. GDNF or saline was administered once a day from day 7 to 9 after CCI. Mechanical allodynia and thermal hyperalgesia were assessed on the ipsilateral hind paw of CCI rats on day 10. *P < 0.05, **P < 0.01, ***P < 0.001; compared with saline. Differences in paw withdrawal threshold and latency were evaluated using the Steel test and Dunnett's test, respectively; n = 4–11.
Neuropathic pain is alleviated by GDNF via the spinal α2-adrenoceptor
It is well known that noradrenergic neurons in the LC suppress nociceptive transmission via activation of α2-adrenoceptors in the spinal cord (Pertovaara, 2006). Therefore, we investigated whether the analgesic effects of GDNF in the LC was mediated by spinal noradrenergic transmission. GDNF was injected once a day on days 7 to 9 after CCI and then yohimbine was intrathecally injected on day 10. The analgesic effects of GDNF on mechanical allodynia and thermal hyperalgesia (F(1,13) = 7.184, P = 0.019 by two-way repeated-measures anova) were abolished in the CCI rats 20 min after the intrathecal administration of yohimbine, compared with saline (Figure 4).
Figure 4.
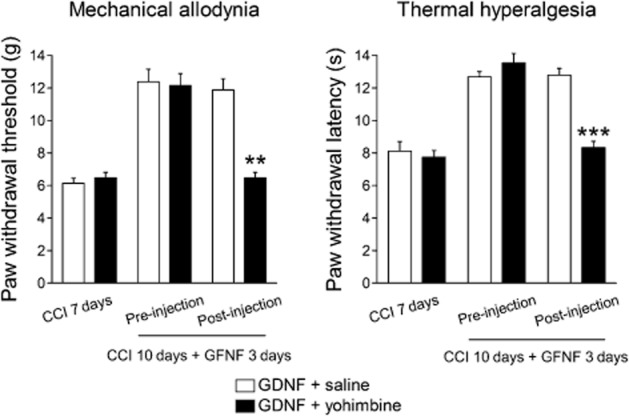
Effects of intrathecal administration of the α2-adrenoceptor antagonist yohimbine on the analgesic effects of GDNF. Yohimbine or saline was intrathecally administered on day 10 in CCI rats after three GDNF injections. Mechanical allodynia and thermal hyperalgesia were assessed on the ipsilateral hind paw 20 min after the yohimbine injection. **P < 0.01, ***P < 0.001; compared with saline treatment. Paw withdrawal threshold was evaluated using the Mann–Whitney U-test with Bonferroni correction, while latency was assessed using the unpaired t-test with Bonferroni correction; n = 7–8.
GDNF alleviates neuropathic pain via ERK signalling
Because ERK is a major intracellular effector mediating the trophic effects of GDNF (Peterziel et al., 2002), we investigated ERK involvement in the analgesic effects of GDNF in the LC. To block ERK activation, U0126, an inhibitor of MEK which phosphorylates ERK, was injected into the LC 15 min prior to each GDNF injection on days 7 to 9 after CCI. U0126 injection partially, but significantly, suppressed the analgesic effect of GDNF on mechanical allodynia and thermal hyperalgesia (F(1,12) = 36.096, P < 0.001 by two-way repeated-measures anova) compared with vehicle (Figure 5).
Figure 5.
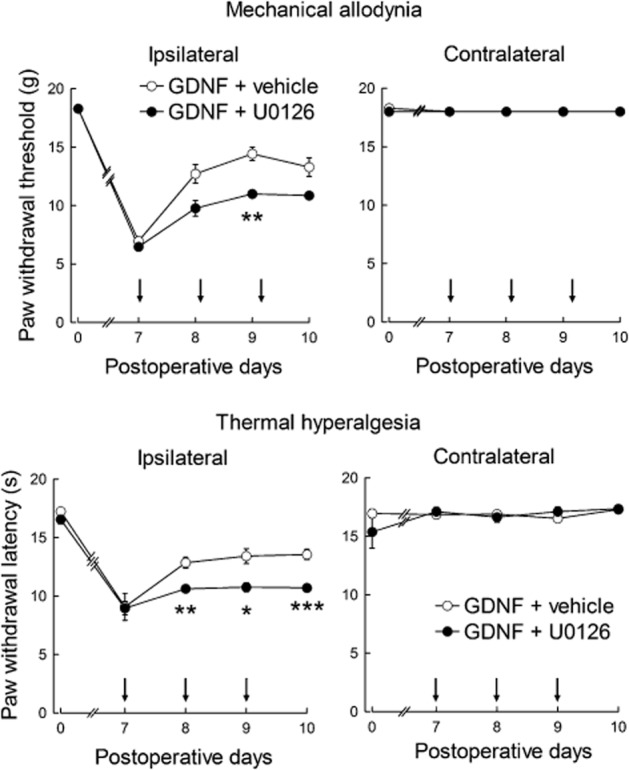
Effects of the MEK inhibitor U0126 on the analgesic effects of GDNF. U0126 or vehicle was injected into the LC 30 min prior to GDNF injection. Both U0126 and GDNF were injected once a day on days 7 to 9 as indicated by the arrows. *P < 0.05, **P < 0.01, ***P < 0.001; compared with vehicle. Paw withdrawal threshold was evaluated using the Mann–Whitney U-test with Bonferroni correction, while latency was assessed using the unpaired t-test with Bonferroni correction; n = 7.
Discussion and conclusions
In the present study, GDNF injection into the LC exerted potent and prolonged analgesic effects on intractable neuropathic pain in rats. Because GDNF injections that missed the LC had no analgesic effect, the LC is most likely site responsible for the effects of GDNF. Consistent with our results, the GDNF receptor components GFRα-1, Ret and NCAM are all expressed by noradrenergic neurons in the LC (Trupp et al., 1997; Glazner et al., 1998; Golden et al., 1998; Sarabi et al., 2003; Black et al., 2009). The GDNF-induced analgesia was blocked by the spinal administration of the α2-adrenoceptor antagonist yohimbine in the present study. It has been reported that stimulation of the LC induces release of noradrenaline in the spinal cord (Hentall et al., 2003) and that antinociception induced by LC activation is attenuated by spinal administration of α2-adrenoceptor antagonists (Jones and Gebhart, 1986; West et al., 1993). LC activation has been reported to exert analgesic effects on neuropathic pain (Pertovaara, 2006; Viisanen and Pertovaara, 2007), although it has also been shown that inhibition of LC neuronal activity suppresses neuropathic pain (Brightwell and Taylor, 2009).
Although a noradrenaline-induced change in the control of motor output may change pain-related behaviours, spinal noradrenergic transmission substantially suppresses nociception (Pertovaara, 2006). In addition, electrical stimulation of the LC is reported to augment, rather than suppress, somatomotor output (Fung et al., 1991). However, for as long as we observed the animals, the motor function of rats given GDNF injections into the LC was comparable with that of rats given saline. Therefore, the analgesic effects of GDNF were more likely to have resulted from inhibition of spinal nociceptive transmission via activation of descending noradrenergic LC neurons. Nonetheless, it remains unknown whether endogenous GDNF physiologically or pathologically modulates pain sensation under normal or neuropathic conditions. GDNF mRNA is reportedly not detectable in the LC (Arenas et al., 1995; Trupp et al., 1997; Golden et al., 1998). However, because LC neurons express GDNF receptor components (GFRα-1, Ret and NCAM) (Trupp et al., 1997; Glazner et al., 1998; Golden et al., 1998; Sarabi et al., 2003; Black et al., 2009), exogenous GDNF and GDNF receptor agonists could produce analgesia in the LC as well as in the spinal cord and primary sensory neurons (Boucher et al., 2000; Sakai et al., 2008; Takasu et al., 2011).
GDNF-induced analgesia may have a different mode of action from that after direct excitation of LC neurons, as shown in previous reports. Activation of the LC with electrical stimulation attenuates neuropathic pain (Viisanen and Pertovaara, 2007). However, while the antinociceptive effect of electrical stimulation is rapid in onset, it only manifests during stimulation (Jones and Gebhart, 1986). The analgesic effect of substance P injection into the LC disappears within 15 min (Muto et al., 2012). Gabapentin injection into the LC exerts a somewhat persistent analgesic effect, but the effect peaks rapidly, that is within 30 min (Hayashida et al., 2008). Consistent with these transient analgesic effects of LC activation, spinal activation of α2-adrenoceptors results in short-term analgesia (Feng et al., 2009). In marked contrast, GDNF-induced analgesia in the LC exhibits a slow onset and long duration, which may reflect the need for gene expression and/or protein synthesis. Indeed, our present findings suggest that GDNF might increase noradrenaline synthesis because GDNF injection into the LC increased noradrenaline content. The activity of TH, a rate-limiting enzyme in noradrenaline synthesis, is mainly regulated by changes in its protein levels and phosphorylation. GDNF is reported to induce TH expression (Christophersen et al., 2007). Our present findings indicate that ERK plays a role in GDNF-induced analgesia in the LC. ERK is a major regulator of TH activity and is involved in TH induction (Guo et al., 1998; Takekoshi et al., 2001). In addition, ERK phosphorylates TH at Ser31 to increase enzymic activity (Daubner et al., 2011). Interestingly, ERK phosphorylation is reported to be decreased in LC neurons after CCI (Borges et al., 2013). The reduced phosphorylation of ERK may lead to impaired noradrenaline synthesis in neuropathic pain. Furthermore, GDNF induces the expression of GTP cyclohydrolase-I (Christophersen et al., 2007), a rate-limiting enzyme that synthesizes tetrahydrobiopterin, an essential cofactor for TH. Therefore, GDNF may enhance noradrenergic transmission in the spinal cord by increasing noradrenaline synthesis in the descending pathways originating from the LC.
GDNF may also exert its analgesic effects by increasing the basal activity of LC neurons. Although the effects of GDNF on LC neuronal activity are unknown, GDNF can potentiate, acutely, the excitability of midbrain dopaminergic neurons and trigeminal ganglion neurons (Yang et al., 2001; Takeda et al., 2010). GDNF increases the amplitude of spontaneous and evoked excitatory currents in dopaminergic neurons (Wang et al., 2003a). LC neuronal activity is reported to change in neuropathic pain states. For example, noxious stimulation-evoked responses in LC neurons are enhanced, while basal tonic activity is unaffected (Viisanen and Pertovaara, 2007; Alba-Delgado et al., 2012; Bravo et al., 2013). Furthermore, it was reported that descending noradrenergic inhibition originating from the LC is suppressed by tonic inhibition from the A7 noradrenergic cell group, promoting neuropathic hypersensitivity (Wei and Pertovaara, 2013). In addition, descending analgesic activity is impaired or enhanced in the neuropathic pain state (Saadé and Jabbur, 2008). Therefore, GDNF may exert its analgesic effects by enhancing or normalizing the excitability of LC neurons.
In conclusion, in the present study, GDNF injection into the LC exerted potent and prolonged analgesic effects on intractable neuropathic pain in rats. GDNF also enhanced descending noradrenergic inhibition in the spinal cord over an extended period of time. These analgesic effects of GDNF were mediated partly by ERK activation in the LC. These results suggest that strategies that target LC neurons may have therapeutic potential in the treatment of chronic neuropathic pain.
Acknowledgments
We thank Drs. Masatoshi Nagano and Motoyo Maruyama for technical assistance in the HPLC assay and Dr. Yumi Muto for histological preparation. This work was supported by a Grant-in-Aid for Encouragement of Young Scientists (B) (22791457 to A.S.) from the Japan Society for the Promotion of Science and a Ministry of Education, Culture, Sports, Science and Technology-Supported Program for the Strategic Research Foundation at Private Universities, 2008–2012 (S0801035 to H.S.), Japan.
Glossary
Abbreviations
- CCI
chronic constriction injury
- GDNF
glial cell line-derived neurotrophic factor
- LC
locus coeruleus
- MEK
MAPK/ERK
- NCAM
neuronal cell adhesion molecule
- TH
tyrosine hydroxylase
- U0126
1,4-diamino-2,3-dicyano-1,4-bis(ο-aminophenylmercapto)butadiene
Conflict of interest
None.
References
- Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage. 2003;26:668–677. doi: 10.1016/s0885-3924(03)00144-1. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Alba-Delgado C, Mico JA, Sánchez-Blázquez P, Berrocoso E. Analgesic antidepressants promote the responsiveness of locus coeruleus neurons to noxious stimulation: implications for neuropathic pain. Pain. 2012;153:1438–1449. doi: 10.1016/j.pain.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic Receptors. Br J Pharmacol. 2013c;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013d;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas E, Trupp M, Åkerud P, Ibáñez CF. GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo. Neuron. 1995;15:1465–1473. doi: 10.1016/0896-6273(95)90024-1. [DOI] [PubMed] [Google Scholar]
- Bennett DLH, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Black MA, Deurveilher S, Seki T, Marsh DR, Rutishauser U, Rafuse VF, et al. Role of polysialylated neural cell adhesion molecule in rapid eye movement sleep regulation in rats. Eur J Neurosci. 2009;30:2190–2204. doi: 10.1111/j.1460-9568.2009.07000.x. [DOI] [PubMed] [Google Scholar]
- Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCγ, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008;28:12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges GS, Berrocoso E, Ortega-Alvaro A, Mico JA, Neto FL. Extracellular signal-regulated kinase activation in the chronic constriction injury model of neuropathic pain in anaesthetized rats. Eur J Pain. 2013;17:35–45. doi: 10.1002/j.1532-2149.2012.00181.x. [DOI] [PubMed] [Google Scholar]
- Boucher TJ, Okuse K, Bennett DLH, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Bravo L, Alba-Delgado C, Torres-Sanchez S, Mico JA, Neto FL, Berrocoso E. Social stress exacerbates the aversion to painful experiences in rats exposed to chronic pain: the role of the locus coeruleus. Pain. 2013;154:2014–2023. doi: 10.1016/j.pain.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Brightwell JJ, Taylor BK. Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience. 2009;160:174–185. doi: 10.1016/j.neuroscience.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophersen NS, Grønborg M, Petersen TN, Fjord-Larsen L, Jørgensen JR, Juliusson B, et al. Midbrain expression of Delta-like 1 homologue is regulated by GDNF and is associated with dopaminergic differentiation. Exp Neurol. 2007;204:791–801. doi: 10.1016/j.expneurol.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. The projection of locus coeruleus neurons to the spinal cord in the rat determined by anterograde tracing combined with immunocytochemistry. Brain Res. 1991;538:231–245. doi: 10.1016/0006-8993(91)90435-x. [DOI] [PubMed] [Google Scholar]
- Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85:S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D. Epidural clonidine analgesia for intractable cancer pain. Pain. 1995;61:391–399. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- Feng X, Zhang F, Dong R, Li W, Liu J, Zhao X, et al. Intrathecal administration of clonidine attenuates spinal neuroimmune activation in a rat model of neuropathic pain with existing hyperalgesia. Eur J Pharmacol. 2009;614:38–43. doi: 10.1016/j.ejphar.2009.04.044. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Manzoni D, Chan JY, Pompeiano O, Barnes CD. Locus coeruleus control of spinal motor output. Prog Brain Res. 1991;88:395–409. doi: 10.1016/s0079-6123(08)63825-x. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Mu X, Springer JE. Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol. 1998;391:42–49. doi: 10.1002/(sici)1096-9861(19980202)391:1<42::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, et al. Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–150. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Guo Z, Du X, Iacovitti L. Regulation of tyrosine hydroxylase gene expression during transdifferentiation of striatal neurons: changes in transcription factors binding the AP-1 site. J Neurosci. 1998;18:8163–8174. doi: 10.1523/JNEUROSCI.18-20-08163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Obata H, Nakajima K, Eisenach JC. Gabapentin acts within the locus coeruleus to alleviate neuropathic pain. Anesthesiology. 2008;109:1077–1084. doi: 10.1097/ALN.0b013e31818dac9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentall ID, Mesigil R, Pinzon A, Noga BR. Temporal and spatial profiles of pontine-evoked monoamine release in the rat's spinal cord. J Neurophysiol. 2003;89:2943–2951. doi: 10.1152/jn.00608.2002. [DOI] [PubMed] [Google Scholar]
- Howorth PW, Teschemacher AG, Pickering AE. Retrograde adenoviral vector targeting of nociresponsive pontospinal noradrenergic neurons in the rat in vivo. J Comp Neurol. 2009;512:141–157. doi: 10.1002/cne.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Characterization of coeruleospinal inhibition of the nociceptive tail-flick reflex in the rat: mediation by spinal α2-adrenoceptors. Brain Res. 1986;364:315–330. doi: 10.1016/0006-8993(86)90844-9. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, et al. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Muto Y, Sakai A, Sakamoto A, Suzuki H. Activation of NK1 receptors in the locus coeruleus induces analgesia through noradrenergic-mediated descending inhibition in a rat model of neuropathic pain. Br J Pharmacol. 2012;166:1047–1057. doi: 10.1111/j.1476-5381.2011.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R, López-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Gómez-Díaz R, López-Barneo J. GDNF and protection of adult central catecholaminergic neurons. J Mol Endocrinol. 2011;46:R83–R92. doi: 10.1530/JME-10-0125. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth edn. San Diego: Academic Press; 1998. [Google Scholar]
- Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol. 2013;716:2–7. doi: 10.1016/j.ejphar.2013.01.067. [DOI] [PubMed] [Google Scholar]
- Peterziel H, Unsicker K, Krieglstein K. TGFβ induces GDNF responsiveness in neurons by recruitment of GFRα1 to the plasma membrane. J Cell Biol. 2002;159:157–167. doi: 10.1083/jcb.200203115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadé NE, Jabbur SJ. Nociceptive behavior in animal models for peripheral neuropathy: spinal and supraspinal mechanisms. Prog Neurobiol. 2008;86:22–47. doi: 10.1016/j.pneurobio.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Sakai A, Asada M, Seno N, Suzuki H. Involvement of neural cell adhesion molecule signaling in glial cell line-derived neurotrophic factor-induced analgesia in a rat model of neuropathic pain. Pain. 2008;137:378–388. doi: 10.1016/j.pain.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Sarabi A, Hoffer BJ, Olson L, Morales M. Glial cell line neurotrophic factor-family receptor α-1 is present in central neurons with distinct phenotypes. Neuroscience. 2003;116:261–273. doi: 10.1016/s0306-4522(02)00559-6. [DOI] [PubMed] [Google Scholar]
- Takasu K, Sakai A, Hanawa H, Shimada T, Suzuki H. Overexpression of GDNF in the uninjured DRG exerts analgesic effects on neuropathic pain following segmental spinal nerve ligation in mice. J Pain. 2011;12:1130–1139. doi: 10.1016/j.jpain.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Takeda M, Kitagawa J, Nasu M, Takahashi M, Iwata K, Matsumoto S. Glial cell line-derived neurotrophic factor acutely modulates the excitability of rat small-diameter trigeminal ganglion neurons innervating facial skin. Brain Behav Immun. 2010;24:72–82. doi: 10.1016/j.bbi.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Takekoshi K, Ishii K, Kawakami Y, Isobe K, Nanmoku T, Nakai T. Ca2+ mobilization, tyrosine hydroxylase activity, and signaling mechanisms in cultured porcine adrenal medullary chromaffin cells: effects of leptin. Endocrinology. 2001;142:290–298. doi: 10.1210/endo.142.1.7914. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Takasu K, Ono H, Tanabe M. Pregabalin, S-(+)-3-isobutylgaba, activates the descending noradrenergic system to alleviate neuropathic pain in the mouse partial sciatic nerve ligation model. Neuropharmacology. 2007;53:842–853. doi: 10.1016/j.neuropharm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibáñez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-α indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viisanen H, Pertovaara A. Influence of peripheral nerve injury on response properties of locus coeruleus neurons and coeruleospinal antinociception in the rat. Neuroscience. 2007;146:1785–1794. doi: 10.1016/j.neuroscience.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen G, Lu B, Wu CP. GDNF acutely potentiates Ca2+ channels and excitatory synaptic transmission in midbrain dopaminergic neurons. Neurosignals. 2003a;12:78–88. doi: 10.1159/000071817. [DOI] [PubMed] [Google Scholar]
- Wang R, Guo W, Ossipov MH, Vanderah TW, Porreca F, Lai J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003b;121:815–824. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- Wei H, Pertovaara A. Regulation of neuropathic hypersensitivity by α2-adrenoceptors in the pontine A7 cell group. Basic Clin Pharmacol Toxicol. 2013;112:90–95. doi: 10.1111/j.1742-7843.2012.00930.x. [DOI] [PubMed] [Google Scholar]
- Weinbroum AA, Ben-Abraham R. Dextromethorphan and dexmedetomidine: new agents for the control of perioperative pain. Eur J Surg. 2001;167:563–569. doi: 10.1080/110241501753171146. [DOI] [PubMed] [Google Scholar]
- West WL, Yeomans DC, Proudfit HK. The function of noradrenergic neurons in mediating antinociception induced by electrical stimulation of the locus coeruleus in two different sources of Sprague-Dawley rats. Brain Res. 1993;626:127–135. doi: 10.1016/0006-8993(93)90571-4. [DOI] [PubMed] [Google Scholar]
- Xu B, Descalzi G, Ye HR, Zhuo M, Wang YW. Translational investigation and treatment of neuropathic pain. Mol Pain. 2012;8:15. doi: 10.1186/1744-8069-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Dirksen R, Harty GJ. Antinociceptive effects of intrathecally injected cholinomimetic drugs in the rat and cat. Eur J Pharmacol. 1985;117:81–88. doi: 10.1016/0014-2999(85)90474-1. [DOI] [PubMed] [Google Scholar]
- Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, et al. GDNF acutely modulates excitability and A-type K+ channels in midbrain dopaminergic neurons. Nat Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]