Abstract
Angiogenesis has been proposed to play a role in the inflammation observed in Sjögren’s Syndrome (SS). However, no studies have validated the degree of angiogenesis in salivary glands with SS. Therefore, the goal of this study was to determine the presence and localization of angiogenesis and lymphangiogenesis in salivary glands with SS. We used frozen tissue sections from human minor salivary glands (hMSG) with and without SS in our analyses. To investigate signs of angiogenesis, hMSG tissue lysates were used to detect levels of the pro-angiogenic protein vascular endothelial growth factor (VEGF) by western blot analyses. Additionally, we labeled blood vessels using antibodies specific to platelet endothelial cell adhesion molecule-1 (PECAM-1) and von Willebrand Factor (vWF) to determine blood vessel organization and volume fraction using fluorescence microscopy. Lymphatic vessel organization and volume fraction were determined using antibodies specific to lymphatic vessel endothelial hyaluronan receptor (LYVE-1). Our results suggest that expression levels of VEGF are decreased in hMSG with SS as compared with controls. Interestingly, there were no significant differences in blood or lymphatic vessel organization or volume fraction between hMSG with and without SS, suggesting that angiogenesis and lymphangiogenesis have little impact on the progression of SS.
Keywords: lymphatic vessels, minor salivary glands, neovascularization, PECAM-1, rheumatic disease, VEGF, von Willebrand Factor
Introduction
Sjögren’s syndrome (SS) is a chronic inflammatory autoimmune disorder that often results in hyposalivation and affects approximately 0.5% to 3% of the world’s population (Mavragani and Moutsopoulos 2010). Hyposalivation reduces the quality of life for many patients, as it increases the risk of dental caries and oral infections (Lawrence et al. 2008; Sreebny and Valdini 1987; Thomson et al. 2006). Additionally, it causes difficulty in speech, chewing, and swallowing food (Lawrence et al. 2008; Sreebny and Valdini 1987; Thomson et al. 2006). Investigations in SS pathogenesis have predominantly analyzed the lymphocytic infiltration into the salivary glands (Fujihara et al. 1999; Margaix-Muñoz et al. 2009; Pérez et al. 2005; Yamamoto 2003). However, only a few studies have investigated the impact of blood vessel remodeling in salivary glands with SS (Edwards et al. 1993; Saito et al. 1993; Turkcapar et al. 2005).
Proper saliva secretion is dependent upon blood flow to the salivary glands (Smaje and Gamble 1987; Thakor et al. 2003). Interestingly, it has been shown that some patients with SS have increased blood flow (hyperemia) through their major salivary glands, as compared with that of healthy individuals (Chikui et al. 2000; Steiner et al. 1994). This finding is suggestive of blood vessel growth and remodeling (angiogenesis), and has led to numerous studies investigating pro-angiogenic factors in patients with SS (Delaleu et al. 2008; Ohno et al. 2004; Sisto et al. 2012; Szodoray et al. 2004). However, no previous studies have directly analyzed the vascular organization of salivary glands with SS for signs of angiogenesis. This is critically important, as an increase in blood flow may not require angiogenesis, especially within the salivary glands. During maximal stimulation, blood flow to the salivary glands can increase 20-fold (Smaje and Gamble 1987). This observation suggests that a remodeling of the vasculature in the salivary glands may not be necessary to facilitate chronic hyperemia. Additionally, reported findings on protein expression levels of vascular endothelial growth factor (VEGF, an initiator of angiogenesis) have been contradictory, with some studies displaying an increase (Ohno et al. 2004; Sisto et al. 2012) and others no change or a decrease in expression (Delaleu et al. 2008; Szodoray et al. 2004).
Previous studies have associated angiogenesis with autoimmune disorders (Carmeliet 2005; Taylor and Sivakumar 2005). Specifically, studies have observed angiogenesis in the synovium of patients with rheumatoid arthritis (Szekanecz et al. 2005). The aim of this study was to determine the presence, localization, and degree of angiogenesis in human minor salivary glands (hMSG) with SS (given that SS is a rheumatic disorder). We also examined lymphangiogenesis in hMSG with SS, as this process often coincides with angiogenesis (Baluk et al. 2005; Baluk et al. 2009; Cao et al. 2004; Streit and Detmar 2003).
Our investigation determined blood and lymphatic vessel organization, as well as volume fraction (volume of vessels in tissue as a percentage of the total tissue volume) in hMSG tissue sections with and without SS. Additionally, we quantified the expression of protein markers of angiogenesis in tissue lysates with and without SS. Our results suggest that angiogenesis and lymphangiogenesis have little impact on the progression of this disease. Further investigation into the vasculature of SS salivary glands is warranted to determine if chronic hyperemia may be altering endothelial cell physiology.
Materials & Methods
Human Specimens
Frozen labial hMSG were obtained from the SICCA Repository, University of California, San Francisco. These glands were obtained by a lower lip biopsy of female patients with and without SS, and were frozen by snap-freezing in liquid nitrogen. All SS participants were diagnosed with primary SS using the New American College of Rheumatology classification criteria (Shiboski et al. 2012). Participants who did not meet these criteria were used as non-SS controls. The use of the de-identified samples was approved by the Health Sciences Institutional Review Board under the exempt criterion 45 CFR 46.101(b) (4). Histological sections (10 μm) were prepared at The University at Buffalo, Histological Services, Department of Pathology and Anatomical Sciences. Focus scores of the glands can be found in Supplemental Table 1. To assure a proper diagnosis, an oral pathologist evaluated all biopsies using the 2012 American College of Rheumatology Classification Criteria for SS to determine the focus score (the number of focal mononuclear infiltrates with ≥50 mononuclear cells per 4 mm2). Grade was determined by histopathologically examining the tissue and grading for lymphocyte infiltration (focus): grade 0, absent; grade 1, slight; grade 2, moderate, non-focal infiltration; grade 3, 1 focus (> or =50 lymphocytes) per 4 mm2; grade 4, >1 focus. Grade 3 infiltrates correspond to a focus score of 1, which is one of four disease-classifying criteria acknowledged for diagnosis (Krenn et al. 2010; Shiboski et al. 2012). hMSG with a focus score of 0 were used as controls.
Western Blot Analysis
Frozen hMSG tissues were lysed using 2× Laemmli buffer with 10 mM dithiothreitol (DTT) and sonicated for 30 sec with a Fisher Scientific Sonic Dismembrator (model FB-120; microtip; output level, 5; duty cycle, 50%; Thermo Fisher Scientific, Waltham, MA) and boiled for 5 min. The lysates were subjected to 4% to 15% (wt/vol) SDS-PAGE (Bio-Rad; Hercules, CA) on mini-gels and transferred to nitrocellulose membranes. Membranes were blocked for 1 hr with 3% (wt/vol) bovine serum albumin (BSA) in Tris-buffered saline [0.137 M NaCl, 0.025 M Tris (hydroxymethyl)-aminomethane, pH 7.4] containing 0.1% (vol/vol) Tween-20 (TBST) and immunoblotted overnight at 4°C with rabbit-anti-human VEGF (Abcam; Cambridge, MA) diluted 1:500 in TBST containing 3% (wt/vol) BSA. After incubation, membranes were washed three times for 20 min each with TBST and incubated with peroxidase-linked goat-anti-rabbit IgG antibody (Cell Signaling Technology; Danvers, MA) diluted 1:5000 in TBST containing 3% (wt/vol) BSA at room temperature for 1 hr. The membranes were washed three times for 20 min each with TBST and treated with a Bio-Rad Clarity™ detection reagent (Bio-Rad). The protein bands were visualized using a Bio-Rad ChemiDoc™ MP imager and quantification of the bands was performed using Image Lab 4.1 software (Bio-Rad). For signal normalization, membranes were treated with stripping buffer (Thermo Fisher Scientific) and re-probed with rabbit anti-extracellular signal-regulated kinases 1/2 (total Erk-1/2, 1:500; Cell Signaling Technology) followed by incubation with peroxidase-linked goat-anti-rabbit IgG antibody, as described above. Data were expressed as a ratio of normalized values of VEGF band intensity to total Erk-1/2 band intensity.
Immunofluorescence Microscopy
Human frozen tissue sections were fixed in 4% paraformaldehyde for 20 min at room temperature, incubated with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 5 min, and then washed with PBS. Sections were then incubated with 5% serum from the host of the secondary antibodies (Supplemental Table 2) for 1 hr at room temperature, washed three times with PBS, and incubated overnight at 4°C with primary antibodies (Supplemental Table 2). The next day, sections were warmed to room temperature for 20 min and washed three times for 5 min with PBS. Then, they were incubated for 1 hr with secondary antibodies (Supplemental Table 2) and washed three times with PBS. Mosaic images (5×5 tiling; 0.8–0.9 cm2) were obtained using a Leica DMI6000B inverted microscope with attached Hamamatsu Orca-R2 at 40× magnification (Leica Microsystems; Wetzlar, Germany). These images were visually analyzed and compared to previous descriptions of the vasculature and lymphatic vessels of the major salivary glands (Aiyama et al. 2011; Flint 1903).
Analyses of Blood and Lymphatic Vessel Volume Fraction
Immunofluorescent mosaic images were analyzed using the ImageJ software (NIH; Bethesda, MD). In these images, blood vessels were identified by their expression of both platelet endothelial cell adhesion molecule-1 (PECAM-1) and von Willebrand Factor (vWF), or PECAM+/vWF+. The vessels were then highlighted (i.e., pixel value was set to maximum intensity; Supplemental Fig. 1), and processed by a custom application (Supplemental Text 1) to determine the highlighted area as a percentage of total imaged area [this ratio is denoted as blood vessel volume fraction (VB)]. Regions that were not salivary tissue (i.e., surrounding connective tissue or areas without tissue) were set to a pixel intensity value of zero. These zero-intensity pixels were then removed from the total imaged area in the analysis prior to calculating the highlighted area. Similarly, lymphatic vessels were identified by low expression levels of PECAM-1 (relative to vascular PECAM-1) and expression of LYVE-1 (PECAM-1low/LYVE-1+), and then processed in the same manner as the blood vessel images to determine the lymphatic vessel volume fraction (VL).
Statistical Analyses
Data are mean ± SD of the results from three or more experiments. p-values less than 0.05, calculated from a two-tailed t-test, are taken to represent significant differences.
Results
Expression Levels of VEGF Decrease in hMSG with SS
As shown in Figure 1, VEGF was expressed in both SS and non-SS hMSG. Surprisingly, we observed a significant decrease in VEGF expression levels in hMSG with SS as compared to non-SS (0.69 ± 0.16 vs 1.00 ± 0; p=0.027).
Figure 1.
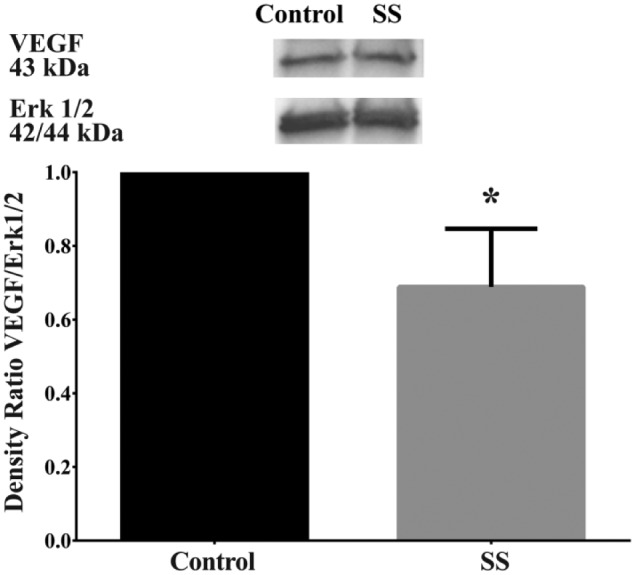
Vascular endothelial growth factor (VEGF) expression was significantly decreased in human minor salivary glands with Sjögren’s syndrome (SS) as compared to controls. Frozen human minor salivary glands were lysed and protein expression was determined by western blot analysis for VEGF and total Erk1/2. Data are expressed as a ratio of VEGF to total Erk1/2 and further normalized to control samples (n=3), where *p<0.05 indicates significant differences from control glands. Results from a representative experiment are shown.
Blood Vessel Organization is Unchanged in SS hMSG
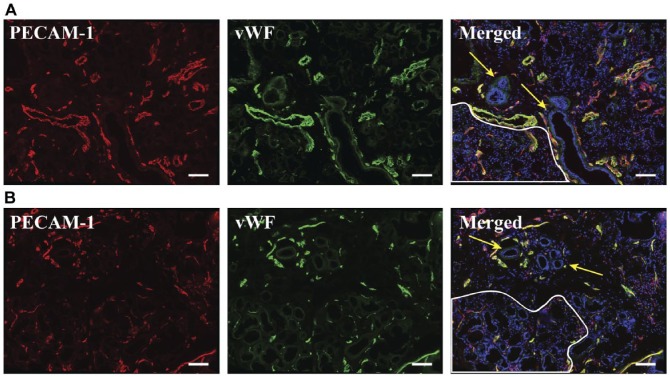
Vasculature was successfully detected by immunofluorescence microscopy using PECAM-1 and vWF as molecular markers. The ducts of the hMSG were encased in numerous capillaries, often observed with at least one larger vessel nearby (Fig. 2; yellow arrows). Acinar regions contained primarily small caliber vasculature (less than 15 µm) diffused throughout the area (Fig. 2; white outline). We also found numerous vessels in the connective tissue and capsule of both hMSG with and without SS (Supplemental Fig. 2). Large lymphocytic foci in SS hMSG were often found to be peri-ductal (Fig. 3), as previously reported (Fox and Speight 1996). The vasculature surrounding the ducts in inflammatory infiltrates appeared similar (i.e., same approximate size and distribution) to the vasculature around ducts in regions without infiltrates and in control glands. Whereas regions of inflammatory infiltrates had no directly comparable region in a control hMSG, they contained sparse small caliber vasculature, similar to acinar regions. It is important to consider that the appearance of the vasculature organization in the images is dependent on the orientation of the salivary structures relative to the cross-sectional plane (ducts and vasculature that are parallel to the cross-section are going to appear larger).
Figure 2.
Blood vessel organization did not appear to be different between non-Sjögren’s syndrome (A) and Sjögren’s syndrome (B) human minor salivary glands. Five-µm frozen human minor salivary gland tissue sections were fixed as described in the Materials & Methods. Expression of PECAM-1 and von Willebrand factor was detected using immunofluorescence microscopy, as follows: mouse-anti-PECAM-1 (red); rabbit-anti-von Willebrand factor (green) and Hoechst nuclear stain (blue). Stained structures (red and green) correspond to salivary gland vasculature. White outlines differentiate acinar regions and yellow arrows indicate ductal structures. The x–y cross section images were obtained using a Leica DMI6000B inverted fluorescence microscope. Images are representative of n=6 experiments. Scale,100 µm.
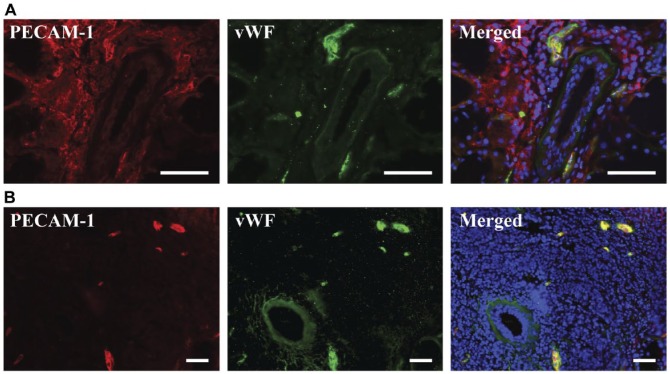
Figure 3.
PECAM-1 is variably expressed on the inflammatory infiltrates of human minor salivary glands. Five-µm frozen Sjögren’s syndrome human minor salivary gland tissue sections were fixed as described in the Materials & Methods. Expression of PECAM-1 and von Willebrand factor was detected using immunofluorescence microscopy, as follows: mouse-anti-PECAM-1 (red); rabbit-anti-von Willebrand factor (green) and Hoechst nuclear stain (blue). Stained structures (red and green) correspond to salivary gland vasculature. The x–y cross section images were obtained using a Leica DMI6000B inverted fluorescence microscope. Presence of PECAM-1 on inflammatory infiltrates was variable, with some infiltrates expressing PECAM-1 (A), and other infiltrates not expressing it (B). PECAM-1 and von Willebrand factor were both expressed on blood vessels (A, B). Scale, 50 µm.
Lymphatic Vessel Organization is Unchanged in SS hMSG
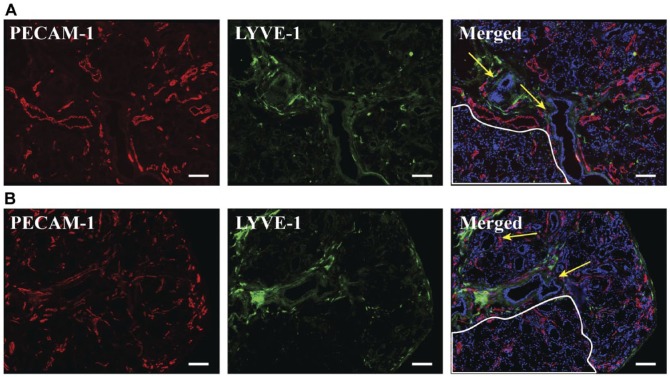
Lymphatic vessels were successfully detected using PECAM-1low and LYVE-1+ as molecular markers. No lymphatic vessels were found within acinar regions in SS or non-SS salivary glands (Fig. 4). We were able to identify connective tissues by differential interference contrast (DIC) microscopy (Supplemental Fig. 3). The connective tissues identified contained a mixture of blood and lymphatic vessels running alongside the edge of the gland and lobes (Fig. 4), often running parallel to ducts. Regions of inflammatory infiltrates in SS hMSG did not contain any lymphatic vessels (Supplemental Fig. 4).
Figure 4.
Lymphatic vessel organization did not appear to be different between non-Sjögren’s syndrome (A) and Sjögren’s syndrome (B) human minor salivary glands. Five-µm frozen human minor salivary gland tissue sections were fixed as described in the Materials & Methods. Expression of PECAM-1 and LYVE-1 was detected using immunofluorescence microscopy, as follows: mouse-anti-PECAM-1 (red); goat-anti-LYVE-1 (green) and Hoechst nuclear stain (blue). Stained structures (green) correspond to salivary gland lymphatic vessels. White outlines differentiate acinar regions and yellow arrows indicate ductal structures. The x–y cross section images were obtained using a Leica DMI6000B inverted fluorescence microscope. Images are representative of n=6 experiments. Scale, 100 µm.
Vascular and Lymphatic Volume Fraction is Unchanged in SS hMSG
Blood vessels in hMSG were identified by immunofluorescence microscopy using PECAM-1 and vWF as molecular markers; this allowed us to perform a volumetric analysis, as described in the Materials & Methods. As shown in Figure 5, VB in hMSG with SS was not significantly different as compared with controls (3.06 ± 0.55 vs 4.00 ± 1.79; p=0.221). This result was independent of the salivary gland focus score. We also determined whether VL was altered, with LYVE-1 used as a marker followed by volumetric analysis, as described in the Materials & Methods. The results were similar to what we observed for blood vessels, as there were no significant differences in VL in hMSG with SS as compared with controls (Fig. 5; 0.44 ± 0.51 vs 0.27 ± 0.25, p=0.481). Please note that the images in Figures 2–4 were selected for their depiction of hMSG blood and lymph vessel organization, and do not necessarily reflect the volume fraction.
Figure 5.
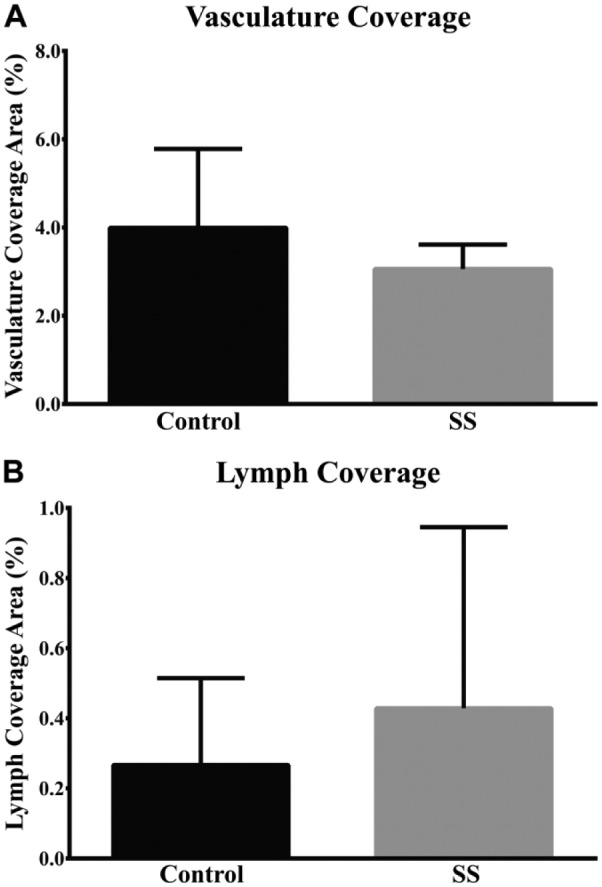
Blood (A) and lymphatic (B) vessel coverage was not significantly different between non-Sjögren’s syndrome and Sjögren’s syndrome human minor salivary glands. Mosaic images, as illustrated in Figs. 2 and 3, were quantified for blood and lymphatic vessel volume fraction as described in the Materials & Methods. Data are expressed as a ratio of blood or lymphatic vessel volume fraction and expressed as the mean ± S.D. (n=6).
Discussion
The vasculature of the salivary glands plays a critical role in proper saliva secretion (Smaje and Gamble 1987; Thakor et al. 2003). Analyzing the changes that occur to the blood vessels during the progression of SS could help us understand why these individuals exhibit hyposalivation. This study attempts to evaluate the presence and degree of angiogenesis as an initial step into looking at possible vascular changes in SS.
The growth factor VEGF is critical for angiogenesis given its role in blood vessel growth and remodeling (Carmeliet 2005). However, few studies of VEGF levels in patients with SS have been found in the literature. One study found that VEGF levels in serum from SS patients were not significantly different from that of controls (Szodoray et al. 2004). Another study using immunohistochemistry showed that VEGF levels appeared upregulated in hMSG with SS (Sisto et al. 2012); however, this analysis was not quantitative. Using western blot pixel density analysis, our results suggest lower expression levels of VEGF in hMSG with SS as compared with controls (Fig. 1). Our results may contrast with the two previous studies due to differences in methodology. Specifically, Szodoray et al. (2004) performed microarrays to analyze serum cytokine levels, which could be influenced more heavily by VEGF originating from other regions of the body, whereas Sisto et al. (2012) used immunohistochemistry to suggest qualitative differences in VEGF expression levels in SS tissue biopsies. Both of these studies employed different classification criteria for SS diagnosis than that used in our study. These results suggest that blood vessel growth and remodeling may not be occurring in salivary glands with SS. Despite these findings, other signaling molecules (such as interleukins and other pro-inflammatory cytokines) have been shown to influence the process of angiogenesis, and may be playing a role (Carmeliet 2003).
SS patients show increased expression levels of many pro-inflammatory cytokines, including interferon (IFN)-γ, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-10, IL-17, IFN-γ-inducible protein-10 (IP-10), and tumor necrosis factor (TNF)-α (Moriyama et al. 2012; Szodoray et al. 2004). Several of these cytokines exhibit pro-angiogenic effects (IL-1β, IL-2, IL-17, and low levels of TNF-α) (Bae et al. 2008; Fajardo et al. 1992; Numasaki et al. 2003; Rosell et al. 2009); however, many others display anti-angiogenic effects (IFN-γ, IL-4, IL-10, IP-10, and high levels of TNF- α) (Angiolillo et al. 1995; Fajardo et al. 1992; Kohno et al. 2003; Lee et al. 2002; Sato et al. 1990). Given this very complex regulatory network for the process of angiogenesis, we felt that analyzing the effects of this process would better determine the presence or absence of angiogenesis in salivary glands with SS.
To elucidate whether angiogenesis is occurring in salivary glands with SS, we examined changes in the vascular organization of hMSG. The vascular organization of the salivary glands has been previously examined (Flint 1903; Lung 1993; Ohtani et al. 1983; Rossi-Schneider et al. 2008; Xu et al. 2011). Although all of the studies had similar findings, the most thorough explanation of the vascular organization in salivary glands was provided by Flint (1903). This study analyzed the complete vascular structure of the submandibular gland (SMG) and found that the submandibular artery enters the gland at the hilus, converging with the excretory duct. The vasculature then parallels the ducts of the SMG, branching in conjunction with the ducts until it reaches the acini, where it wraps around them as capillaries (Flint 1903). These capillaries coalesce into venules, which follow the same path as the arteries back down the hilus (Flint 1903). The mesh of capillaries surrounding the acini is often connected to that of adjacent acini, forming a connected network within a single lobe (Flint 1903). In addition to the meshwork of the acinar region, there are capillaries that surround the ducts of the salivary gland. These capillaries originate from the accompanying artery or arteriole, wrap around the ducts as a mesh and connect to the retrograde vein or venule (Flint 1903). This patterned network also connects to vasculature in the interlobular regions, and to the network of vessels surrounding the capsule of the salivary gland (Flint 1903). In short, blood vessels parallel the majority of the ducts of the SMG and encase the acini. We believe a similar pattern might be true for the hMSG, although no previous studies on this vascular organization were found.
The vascular organization observed in hMSG with and without SS (Fig. 2) appeared similar to each other and to the pattern described previously for the SMG (Flint 1903). One potential difference is that the networks of capillaries in the acinar region of hMSG from both SS and non-SS patients in this study were more sparse than anticipated given the description of the SMG vasculature. However, given the qualitative nature of the descriptions provided in previous text (Flint 1903), it is difficult to make direct comparisons. These results suggest that no vascular re-organization occurs in hMSG with SS.
We also studied the lymph vessel organization of the hMSG, which helped us to evaluate if lymphangiogenesis was occurring in SS. A previous study of the rat major salivary glands and a study of the human parotid gland determined that lymphatic vessels are absent from acinar regions (Aiyama et al. 2011; Marchetti et al. 1989). Lymphatic vessels were only found in the interlobular connective tissue, paralleling the ducts, and within the capsules of the salivary glands (Aiyama et al. 2011; Marchetti et al. 1989). Similarly, we found that the lymphatic vessels of both hMSG with and without SS were only present in the connective tissue, either within the gland, or in the capsule (Fig. 4). This suggests that lymphatic vessel re-organization is not occurring in hMSG with SS.
Blood and lymphatic vessel organization alone may not indicate angiogenesis or lymphangiogenesis. Therefore, we quantified the blood and lymphatic vessel volume fraction in hMSG with and without SS. Given that the blood vessels of the salivary glands are relatively homogenous and randomly oriented with respect to the cross-sectional plane (Clough and Smaje 1984; Flint 1903), these analyses should reflect the volume fraction of the vessels within the salivary gland (Gundersen et al. 1988; Weibel 1979). We found that there were no significant differences between hMSG with and without SS in either blood or lymphatic vessel volume fraction (Fig. 5). Additionally, the values we obtained are consistent with a previous study on volume fraction in the hMSG of healthy subjects (Vered et al. 2000). These results suggest that the blood and lymphatic vessels of hMSG with SS do not increase in diameter and number.
Given our results showing decreased VEGF expression levels along with similar blood and lymphatic vessel organization and volume fraction, it is possible that angiogenesis and lymphangiogenesis do not play a significant role in the progression of SS. This conclusion contrasts with previous results, which stated that angiogenesis was occurring in salivary glands with SS (Sisto et al. 2014). This difference could be attributed to the sole use of PECAM-1 as a marker for endothelial cells, which does not take into account that the inflammatory infiltrates of the salivary glands also express PECAM-1 (Sisto et al. 2014). In fact, we found that many inflammatory infiltrates expressed PECAM-1 (Fig. 3), in agreement with previous studies (Muller et al. 1993; Privratsky et al. 2010; Woodfin et al. 2007), indicating the need for a second marker for endothelial cells. Thus, we used vWF as a secondary marker of endothelial cells for all analyses to avoid miscategorizing leukocytes as endothelial cells.
Previous studies have demonstrated increased blood flow to the salivary glands of SS patients (Chikui et al. 2000; Steiner et al. 1994). This may seem counterintuitive as, typically, blood vessel dilation and remodeling accompanies chronic increases in blood flow (Carmeliet 2005). However, a dramatic increase in blood flow (~20×) was observed during saliva secretion in healthy rabbits (Smaje and Gamble 1987). Therefore, salivary glands may have the capacity to facilitate the hyperemia observed in SS patients without any associated angiogenesis.
In summary, neither signs of angiogenesis nor lymphangiogenesis are apparent in hMSG with SS. However, other changes to the endothelium could be occurring that may impact the process of saliva secretion. In particular, the filtration of plasma into the interstitial fluid is critical for normal continuous saliva secretion (Smaje 1998). Future studies analyzing the endothelium in more detail could greatly improve our understanding of SS.
Supplementary Material
Acknowledgments
Data and specimens used in this study are from the Sjögren’s International Collaborative Clinical Alliance (SICCA), funded under contract HHSN26S201300057C by the National Institute of Dental and Craniofacial Research, with funding support from the National Eye Institute and Office for Research in Women’s Health. The new ACR criteria (Arthritis Care & Research, Vol. 64, No. 4, April 2012, pp. 475-487) were used for sample classification. The authors would like to acknowledge Dr. Alfredo Aguirre, Program Director Oral Pathology, Department of Oral Diagnostic Sciences, The State University of New York at Buffalo (UB), for grade and focus scoring of salivary glands. Any underlying research materials related to this paper (for example data, samples or models) can be accessed.
Footnotes
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by the National Institutes of Health-National Institute of Dental and Craniofacial Research Grants 1RO1DE022971-01, 1RO1DE021697-01A (OJB), 1F31DE024346-01, and 1T32DE023526 (ADM).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by the National Institutes of Health-National Institute of Dental and Craniofacial Research Grants 1RO1DE022971-01, 1RO1DE021697-01A (OJB), 1F31DE024346-01, and 1T32DE023526 (ADM).
References
- Aiyama S, Kikuchi K, Takada K, Ikeda R, Sato S, Kuroki J. (2011). Immunohistochemical study of the lymphatic vessels in major salivary glands of the rat. Okajimas folia anatomica Japonica 87:177-180. [DOI] [PubMed] [Google Scholar]
- Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. (1995). Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med 182:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Park D, Lee YS, Jeoung D. (2008). Interleukin-2 promotes angiogenesis by activation of Akt and increase of ROS. J Microbiol Biotechnol 18:377-382. [PubMed] [Google Scholar]
- Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA. (2005). Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. Journal of Clinical Investigation 115:247-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Yao L-C, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM. (2009). TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. The Journal of clinical investigation 119:2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. (2004). Comparative Evaluation of FGF-2–, VEGF-A–, and VEGF-C–Induced Angiogenesis, Lymphangiogenesis, Vascular Fenestrations, and Permeability. Circulation research 94:664-670. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. (2005). Angiogenesis in life, disease and medicine. Nature 438:932-936. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. (2003). Angiogenesis in health and disease. Nature medicine 9:653-660. [DOI] [PubMed] [Google Scholar]
- Chikui T, Yonetsu K, Izumi M, Eguchi K, Nakamura T. (2000). Abnormal blood flow to the submandibular glands of patients with Sjögren’s syndrome: Doppler waveform analysis. The Journal of rheumatology 27:1222. [PubMed] [Google Scholar]
- Clough G, Smaje L. (1984). Exchange area and surface properties of the microvasculature of the rabbit submandibular gland following duct ligation. The Journal of physiology 354:445-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaleu N, Immervoll H, Cornelius J, Jonsson R. (2008). Biomarker profiles in serum and saliva of experimental Sjogren’s syndrome: associations with specific autoimmune manifestations. Arthritis Research and Therapy 10:R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Wilkinson LS, Speight P, Isenberg DA. (1993). Vascular cell adhesion molecule 1 and alpha 4 and beta 1 integrins in lymphocyte aggregates in Sjögren’s syndrome and rheumatoid arthritis. Annals of the rheumatic diseases 52:806-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. (1992). Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol 140:539-544. [PMC free article] [PubMed] [Google Scholar]
- Flint JM. (1903). The angiology, angiogenesis, and organogenesis of the submaxillary gland. American Journal of Anatomy 2:417-444. [Google Scholar]
- Fox P, Speight P. (1996). Current concepts of autoimmune exocrinopathy: immunologic mechanisms in the salivary pathology of Sjögren’s syndrome. Critical Reviews in Oral Biology & Medicine 7:144-158. [DOI] [PubMed] [Google Scholar]
- Fujihara T, Fujita H, Tsubota K, Saito K, Tsuzaka K, Abe T, Takeuchi T. (1999). Preferential localization of CD8+ αEβ7+ T cells around acinar epithelial cells with apoptosis in patients with Sjögren’s syndrome. The Journal of Immunology 163:2226-2235. [PubMed] [Google Scholar]
- Gundersen H, Bendtsen T, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard J, Pakkenberg B, Sørensen F, Vesterby A. (1988). Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis 96:379-394. [DOI] [PubMed] [Google Scholar]
- Kohno T, Mizukami H, Suzuki M, Saga Y, Takei Y, Shimpo M, Matsushita T, Okada T, Hanazono Y, Kume A, Sato I, Ozawa K. (2003). Interleukin-10-mediated inhibition of angiogenesis and tumor growth in mice bearing VEGF-producing ovarian cancer. Cancer Res 63:5091-5094. [PubMed] [Google Scholar]
- Krenn V, Jakobs M, Kriegsmann J, Krukemeyer MG, Rieger A. (2010). [Is bioptic assurance reasonable in patients with Sjogren’s syndrome? From focus score to diagnosing vasculitides]. Z Rheumatol 69:11-18. [DOI] [PubMed] [Google Scholar]
- Lawrence HP, Thomson WM, Broadbent JM, Poulton R. (2008). Oral health-related quality of life in a birth cohort of 32-year olds. Community dentistry and oral epidemiology 36:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IY, Kim J, Ko EM, Jeoung EJ, Kwon YG, Choe J. (2002). Interleukin-4 inhibits the vascular endothelial growth factor- and basic fibroblast growth factor-induced angiogenesis in vitro. Mol Cells 14:115-121. [PubMed] [Google Scholar]
- Lung MA. (1993). An investigation of the vascular organisation of the canine submandibular gland. J Anat 183 ( Pt 3):619-630. [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Poggi P, Calligaro A, Casasco A. (1989). Microscopic and ultrastructural study of the lymphatic system in the human parotid gland. Acta Anat (Basel) 134:106-112. [DOI] [PubMed] [Google Scholar]
- Margaix-Muñoz M, Bagán JV, Poveda R, Jiménez Y, Sarrión G. (2009). Sjogren’s syndrome of the oral cavity. Review and update. Med Oral Patol Oral Cir Bucal 14:E325-330. [PubMed] [Google Scholar]
- Mavragani CP, Moutsopoulos HM. (2010). The geoepidemiology of Sjögren’s syndrome. Autoimmunity reviews 9:A305-A310. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Hayashida JN, Toyoshima T, Ohyama Y, Shinozaki S, Tanaka A, Maehara T, Nakamura S. (2012). Cytokine/chemokine profiles contribute to understanding the pathogenesis and diagnosis of primary Sjogren’s syndrome. Clin Exp Immunol 169:17-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Weigl S, Deng X, Phillips D. (1993). PECAM-1 is required for transendothelial migration of leukocytes. The Journal of experimental medicine 178:449-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. (2003). Interleukin-17 promotes angiogenesis and tumor growth. Blood 101:2620-2627. [DOI] [PubMed] [Google Scholar]
- Ohno A, Mitsui T, Endo I, Kunishige M, Sigekiyo T, Matsumoto T. (2004). Dermatomyositis associated with Sjögren’s syndrome: VEGF involvement in vasculitis. Clinical neuropathology 23:178. [PubMed] [Google Scholar]
- Ohtani O, Kikuta A, Ohtsuka A, Taguchi T, Murakami T. (1983). Microvasculature as studied by the microvascular corrosion casting/scanning electron microscope method. I. Endocrine and digestive system. Arch Histol Jpn 46:1-42. [DOI] [PubMed] [Google Scholar]
- Pérez P, Kwon YJ, Alliende C, Leyton L, Aguilera S, Molina C, Labra C, Julio M, Leyton C, González MJ. (2005). Increased acinar damage of salivary glands of patients with Sjögren’s syndrome is paralleled by simultaneous imbalance of matrix metalloproteinase 3/tissue inhibitor of metalloproteinases 1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinases 1 ratios. Arthritis & Rheumatism 52:2751-2760. [DOI] [PubMed] [Google Scholar]
- Privratsky JR, Newman DK, Newman PJ. (2010). PECAM-1: conflicts of interest in inflammation. Life sciences 87:69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell A, Arai K, Lok J, He T, Guo S, Navarro M, Montaner J, Katusic ZS, Lo EH. (2009). Interleukin-1beta augments angiogenic responses of murine endothelial progenitor cells in vitro. J Cereb Blood Flow Metab 29:933-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-Schneider TR, Verli FD, Yurgel LS, De Souza MA, Cherubini K. (2008). Contribution to the study of the vasculature of submandibular and sublingual glands and lymph nodes of rats by corrosion cast technique combined with scanning electron microscopy. Microsc Res Tech 71:737-741. [DOI] [PubMed] [Google Scholar]
- Saito I, Terauchi K, Shimuta M, Nishiimura S, Yoshino K, Takeuchi T, Tsubota K, Miyasaka N. (1993). Expression of cell adhesion molecules in the salivary and lacrimal glands of Sjogren’s syndrome. Journal of clinical laboratory analysis 7:180-187. [DOI] [PubMed] [Google Scholar]
- Sato N, Nariuchi H, Tsuruoka N, Nishihara T, Beitz JG, Calabresi P, Frackelton AR., Jr. (1990). Actions of TNF and IFN-gamma on angiogenesis in vitro. J Invest Dermatol 95:85s-89s. [DOI] [PubMed] [Google Scholar]
- Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, Schiodt M, Umehara H, Vivino F, Zhao Y, Dong Y, Greenspan D, Heidenreich AM, Helin P, Kirkham B, Kitagawa K, Larkin G, Li M, Lietman T, Lindegaard J, McNamara N, Sack K, Shirlaw P, Sugai S, Vollenweider C, Whitcher J, Wu A, Zhang S, Zhang W, Greenspan J, Daniels T. (2012b). American College of Rheumatology classification criteria for Sjogren’s syndrome: a data-driven, expert consensus approach in the Sjogren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 64:475-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisto M, Lisi S, Ingravallo G, Lofrumento DD, D‘Amore M, Ribatti D. (2014). Neovascularization is prominent in the chronic inflammatory lesions of Sjögren’s syndrome. International Journal of Experimental Pathology 95:131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisto M, Lisi S, Lofrumento D, D‘Amore M, Frassanito M, Ribatti D. (2012). Sjögren’s syndrome pathological neovascularization is regulated by VEGF-A-stimulated TACE-dependent crosstalk between VEGFR2 and NF-κB. Genes and Immunity 13:411-420. [DOI] [PubMed] [Google Scholar]
- Smaje L. (1998) Capillary Dynamics in Salivary Glands. In Garrett J, Ekström J, Anderson L. eds. Glandular Mechanisms of Salivary Secretion. Basel, Karger, 118-131 [Google Scholar]
- Smaje L, Gamble J. (1987). Transcapillary transport during secretion by the rabbit submandibular salivary gland. Journal of Dental Research 66:564-568. [DOI] [PubMed] [Google Scholar]
- Sreebny LM, Valdini A. (1987). Xerostomia: a neglected symptom. Archives of internal medicine 147:1333. [DOI] [PubMed] [Google Scholar]
- Steiner E, Graninger W, Hitzelhammer J, Lakits A, Petera P, Franz P, Gritzmann N. (1994). Color-coded duplex sonography of the parotid gland in Sjögren’s syndrome. RöFo: Fortschritte auf dem Gebiete der Röntgenstrahlen und der Nuklearmedizin; 160:294. [DOI] [PubMed] [Google Scholar]
- Streit M, Detmar M. (2003). Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene 22:3172-3179. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Gaspar L, Koch AE. (2005). Angiogenesis in rheumatoid arthritis. Front Biosci 10:1739-1753. [DOI] [PubMed] [Google Scholar]
- Szodoray P, Alex P, Brun J, Centola M, Jonsson R. (2004). Circulating cytokines in primary Sjögren’s syndrome determined by a multiplex cytokine array system. Scandinavian journal of immunology 59:592-599. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Sivakumar B. (2005). Hypoxia and angiogenesis in rheumatoid arthritis. Current opinion in rheumatology 17:293-298. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Brown CN, Edwards A. (2003). Effects of prolonged reduction in blood flow on submandibular secretory function in anesthetized sheep. Journal of Applied Physiology 95:751-757. [DOI] [PubMed] [Google Scholar]
- Thomson WM, Lawrence HP, Broadbent JM, Poulton R. (2006). The impact of xerostomia on oral-health-related quality of life among younger adults. Health and Quality of Life outcomes 4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkcapar N, Sak SD, Saatci M, Duman M, Olmez U. (2005). Vasculitis and expression of vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin in salivary glands of patients with Sjögren’s syndrome. The Journal of rheumatology 32:1063-1070. [PubMed] [Google Scholar]
- Vered M, Buchner A, Boldon P, Dayan D. (2000). Age-related histomorphometric changes in labial salivary glands with special reference to the acinar component. Exp Gerontol 35:1075-1084. [DOI] [PubMed] [Google Scholar]
- Weibel ER. (1979) Elementary introduction to stereological principles. In Stereological Methods: Practical methods for biological morphometry. London, Academic Press, 9-62 [Google Scholar]
- Woodfin A, Voisin M-B, Nourshargh S. (2007). PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arteriosclerosis, thrombosis, and vascular biology 27:2514-2523. [DOI] [PubMed] [Google Scholar]
- Xu H, Mao C, Liu JM, Peng X, Zhu ZH, Yu GY. (2011). Microanatomic study of the vascular and duct system of the submandibular gland. J Oral Maxillofac Surg 69:1103-1107. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. (2003). Pathogenesis of Sjögren’s syndrome. Autoimmunity reviews 2:13-18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.