Abstract
Background
Inflammation is often linked with the progress and poor outcome of lung cancer. The understanding of the relationship between tumor-associated macrophages (TAMs) and lung cancer cells involves in the underlying mechanism of inflammatory cytokine production. Toll-like receptors (TLRs) are engaged in promoting the production of pro-inflammatory cytokines and play an important role in tumor immunology.
Methods
To investigate the mechanisms by which TAMs influence the production of pro-inflammatory cytokines in lung cancer cells, we established an in vitro coculture system using TAMs and human non-small cell lung cancer (NSCLC) cell line SPC-A1. Levels of interleukin (IL)-1β, IL-6 and IL-8 in SPC-A1 were evaluated by RT-PCR and cytometric bead array assay after being cocultured with TAMs. Expression changes of TLRs and TLRs signaling pathway proteins in SPC-A1 were further confirmed by RT-PCR and western blot. The level changes of IL-1β, IL-6 and IL-8 in SPC-A1 were also detected after the stimulation of TLRs agonists.
Results
We found that the phenotype markers of TAMs were highly expressed after stimulating human monocyte cell line THP-1 by phorbol-12-myristate-13-acetate (PMA). Higher mRNA and supernate secretion levels of IL-1β, IL-6 and IL-8 were detected in SPC-A1 after being cocultured with TAMs. We also found that TLR1, TLR6 and TLR7 were up-regulated in SPC-A1 in the coculture system with TAMs. Meanwhile, TLRs signaling pathway proteins were also significantly activated. Moreover, pre-treatment with agonist ligands for TLR1, TLR6 and TLR7 could dramatically promote inductions of IL-1β, IL-6 and IL-8.
Conclusions
These findings demonstrated that TAMs may enhance IL-1β, IL-6 and IL-8 expressions via TLRs signaling pathway. We conclude that TAMs contribute to maintain the inflammation microenvironment and ultimately promote the development and progression of lung cancer.
Keywords: Tumor-associated macrophages (TAMs), Toll-like receptors (TLRs), non-small cell lung cancer (NSCLC), pro-inflammatory cytokines
Introduction
Lung cancer is the first cause of cancer death worldwide and remains a serious public health problem. The overall 5-year survival rate for all stages is 14-17% for non-small cell lung cancer (NSCLC) and 6% for small-cell lung cancer (SCLC) (1). Clinical and epidemiologic studies have suggested a strong connection between inflammation and cancer (2,3). Chronic inflammation predisposes individuals to various types of cancer. It is estimated that underlying infections and inflammatory responses are linked to 15-20% of all deaths from cancer worldwide (4). Inflammatory cytokines are considered key factors promoting tumor progression due to its ability to affect the angiogenesis, proliferation and survival of malignant cells (5). Several pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6 and IL-8 have been implicated to mediate different steps in the pathways leading to the advance of lung cancer (6). Inflammatory stimuli clearly contributes to the development and progression of lung cancer, but the underlying mechanisms of pro-inflammatory cytokine production are not fully understood.
Currently, we have known that within the tumor stroma, tumor-associated macrophages (TAMs) are the pivotal member of inflammatory cells as inflammation microenvironment plays a vital role in tumor progression and metastasis (7). Many evidences have emerged that TAMs participated in tumor-promoting processes such as carcinogenesis, tumor growth and angiogenesis (8). TAMs seem to have M2-polarized subtype, which is alternatively activated in contrast to the classically activated M1 phenotype (9). In a previous research, the function and value of M2-TAMs have been evaluated in development and progression of NSCLC (10).
Toll-like receptors (TLRs) are a family of evolutionally conserved pattern recognition receptors (PRRs). TLRs are included in the type I transmembrane glycoprotein receptor family with N-terminal ligand-recognition, transmembrane and intracellular C-terminal signaling domains (11,12). TLRs are important in innate immunity and are expressed in tumor cells in addition to immunocytes. TLRs are engaged and activated by their respective ligand(s) triggers from a wide range of intercellular signal, ultimately leading to the induction of genes involved in encoding pro-inflammatory cytokines and chemokines (13,14). The main TLRs pathway is dependent on myeloid differentiation factor 88 (MyD88) adaptor proteins. All TLRs commonly use MyD88 as the downstream adapter protein except TLR3. After activation with their individual ligands, TLRs recruit MyD88, resulting in subsequent activation of downstream factors, including nuclear factor (NF)-κB and microtubule-associated proteins (MAPs) kinases, promoting the production of inflammatory cytokines and many other factors (15,16).
In the present study, we identified the function of TAMs to the induction of IL-1β, IL-6 and IL-8 from SPC-A1 in the non-contact coculture environment. The underlying TLRs signaling pathway mechanisms that inducing pro-inflammatory cytokines production in the coculture environment would be further investigated.
Materials and methods
Cell lines and culture conditions
The human NSCLC cell line SPC-A1 and human monocyte cell line THP-1 were purchased from ATCC (Manassas, VA, USA). SPC-A1 and THP-1 cells were grown in 5% CO2 at 37 °C in RPMI 1640 medium (Gibco, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA).
THP-1 cells differentiated into M2-type macrophages
We incubated 1×106 of THP-1 cells with 320 nM phorbol-12-myristate-13-acetate (PMA) (Sigma, St. Louis, MO, USA) for 24 h (17,18). After stimulation, THP-1 polarized and differentiated to M2-type macrophages. M2-type macrophages phenotype surface markers of receptors, including CD68 and CD206, would be further detected by direct immunofluorescence and flow cytometry assay.
Direct immunofluorescence assay
Cells were fixed with 4% paraformaldehyde for 1 h and then permeabilized with 0.2% Triton X-100 for 1 h. Blocking buffer (3% BSA) was then added into cells for 1 h at room temperature. FITC-CD206 mAb was used as indicated. Then the slides were washed with PBS. All of the images were captured in microscopic fields (400-fold) under an inverted microscope (Olympus, Tokyo, Japan).
Flow cytometry assay
For CD68 and CD206 staining, the washed cells were incubated with 5 µL of FITC-CD206 mAb (BD, Franklin Lakes, NJ, USA) or 5 µL of PE-CD68 mAb (Miltenyi, Krohne, Genman). The labeled cells were detected by Beckman-coulter Gallios flow cytometer (Beckman-coulter, Brea, CA, USA) and analyzed by Kaluza 2.1 software (Beckman-coulter, Brea, CA, USA).
Coculture of SPC-A1 with M2-type macrophages
After PMA incubation, 1×106 of M2-type macrophages were placed in the upper insert of a six-well non-contact transwell chamber system (Corning Costar, Cambridge, MA, USA). A total of 5×105 of SPC-A1 cells were grown in the bottom of the six-well plates. After the coculture process of 24 and 48 h, SPC-A1 cells were collected to extract RNA and total protein.
Simulation of TLRs with ligands
SPC-A1 were dispensed into a 96-well plate (Corning Costar, Cambridge, MA, USA) at 4×104 cells per well. The cells were incubated with or without TLR ligands. TLR ligands were added at the concentration recommended by the manufacturer as follows: Pam3CysSerLys4 (Pam3CSK4; TLR1 ligand), 100 ng/mL; Fibroblast-stimulating lipopeptide-1 (FSL-1, TLR6 ligand), 100 ng/mL (Invivogen, San Diego, CA, USA); Imiquimod (TLR7 ligand), 250 ng/mL (Invivogen, San Diego, CA, USA). After 6, 12 and 24 h stimulation, SPC-A1 cells were collected and detected for mRNA expressions of IL-1β, IL-6 and IL-8 by RT-PCR.
RNA isolation and RT-PCR
Total RNA was isolated by RNeasy Micro Kit (Qiagen, Hilden, German) and subjected to first-strand cDNA synthesis using PrimeScript RT Master Mix (Takara, Shigaken, Japan). IL-1β, IL-6 and IL-8 mRNA expression levels were measured by using 7500 Real-time PCR detection system (Applied Biosystems/Life Technologies, Grand Island, NY, USA). SYBR Premix DimerEraser (Takara, Shigaken, Japan) was used in the PCR reaction system at the concentration recommended by the manufacturer. Their sequences were as follows, IL-1β primers: 5'-CACGATGCACCTGTACGATCA-3' and 5'-GTTGCTCCATATCCTGTCCCT-3'; IL-6 primers: 5'-AACCTGAACCTTCCAAAGATGG-3' and 5'-TCTGGCTTGTTCCTCACTACT-3'; IL-8 primers: 5'-GCCTTCCTGATTTCTGCAGCT-3' and 5'-TGCACTGACATCTAAGTTCTTTAGCAC-3'; β-actin primers: 5'-TGGCCCCAGCACAATGAA-3' and 5'-CTAAGTCATAGTCCGCCTAGAAGCA-3'. Cycle threshold (CT) values were estimated by normalizing these values against β-action CT values using the 2−ΔΔCt method.
Cytometric bead array assay
The cytokines IL-1β, IL-6 and IL-8 were quantified by using a cytometric bead array assay (CBA) (BD, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. Samples were acquired using BD FACSCalibur flow cytometer (BD, Franklin Lakes, NJ, USA) and analyzed with FCAP Array software v3.0 (BD, Franklin Lakes, NJ, USA).
Western blot analysis
Total proteins of cells were isolated by 1× cell lysis buffer, containing a protease inhibitor mixture (Sigma, St. Louis, MO, USA) and a phosphatase inhibitor mixture (Sigma, St. Louis, MO, USA). Equal amounts of protein per lane were separated by SDS-PAGE and transferred to PVDF membrane (Bio-Rad, Hercules, CA, USA). Membranes were incubated with the appropriate primary antibody (Cell signaling technology, Danvers, MA, USA). After washing, the membranes were reacted with horseradish peroxidase (HRP)-conjugated secondary antibody (ZSGB, Beijing, China). And protein expressions were detected using the enhanced chemiluminescence method (Millipore, Billerica, MA, USA) on X-ray film.
Statistical analyses
Statistical significance between two groups was determined by t-test or between multiple groups by using ANOVA and Tukey’s post hoc test, where P<0.05 was considered significant by using SPSS (version 13.0, Chicago, IL, USA). Data were showed as mean ± SD or standard error (SE). Asterisks in figures represent *P<0.05.
Results
THP-1 cells differentiated into M2-type macrophages
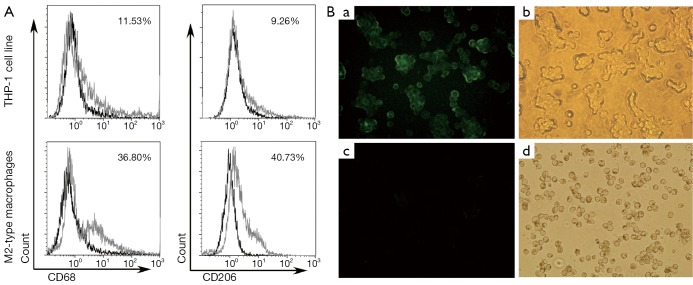
After treatment with 320 nM PMA for 24 h, THP-1 cells quickly stopped proliferating, attached M2-type macrophage phenotypes and differentiated to M2-type macrophages. The PMA-treated THP-1 had significantly enhanced M2-type macrophage phenotype surface markers, including CD68 and CD206. Percentages of CD68+ macrophages and CD206+ macrophages were significantly higher in M2-type macrophages than in untreated THP-1 cell line [(36.22%±5.60%) (mean ± SD) vs. (11.80%±3.57%) (mean ± SD), P<0.05; 40.25%±5.83% vs. 8.80%±4.80%, P<0.05] by flow cytometry assay (Figure 1A). There were also more CD206+ macrophages in PMA-treated macrophage than untreated THP-1 by direct immunofluorescence assay (Figure 1B). Our results showed that THP-1 cells could differentiate and express surface markers of M2-type macrophage in the treatment of PMA.
Figure 1.
THP-1 cell line differentiated into M2-type macrophages. (A) Expressions of CD68 and CD206 in M2-type macrophages by flow cytometry. M2-type macrophage phenotypes CD68 and CD206 were highly expressed in M2-type macrophages; (B) Expression of CD206 in M2-type macrophages by direct immunofluorescence assay: (a,b) M2-type macrophage phenotypes CD206 was highly expressed in M2-type macrophages by fluorescence microscope and ordinary light filming (×400). (c,d) THP-1 cell line did not express CD206 by fluorescence microscope and ordinary light filming (×400).
Increased expressions of pro-inflammatory factors in SPC-A1 cocultured with M2-type macrophages
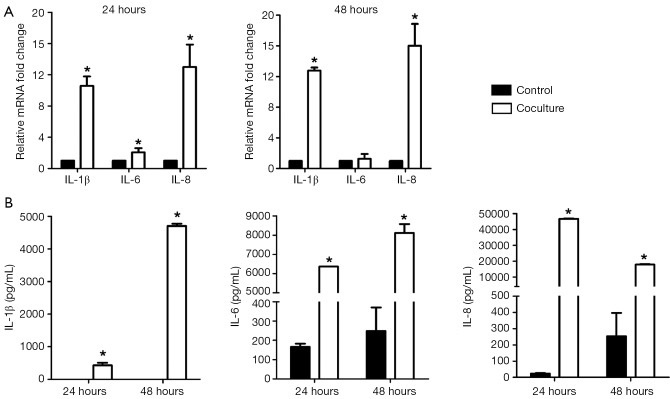
To know the influence of M2-type macrophages to IL-1β, IL-6 and IL-8 expressions in SPC-A1, we detected mRNA and coculture supernate level changes of IL-1β, IL-6 and IL-8 in SPC-A1 after being cocultured with M2-type macrophages by RT-PCR and CBA assay. As shown in Figure 2A, significantly increased IL-1β (10.2-fold, P<0.05 and 12.8-fold, P<0.05), IL-6 (2.0-fold, P<0.05 for 24 h) and IL-8 (12.9-fold, P<0.05 and 15.9-fold, P<0.05) mRNA expressions were evaluated in SPC-A1 after being cocultured with M2-type macrophages for 24 and 48 h. In Figure 2B, in comparison with SPC-A1 cultured alone, IL-1β (427.08±137.99 vs. 0.00±0.00 pg/mL, P<0.05; 4,702.34±126.45 vs. 0.00±0.00 pg/mL, P<0.05), IL-6 (6,369.59±426.20 vs. 166.55±58.23 pg/mL, P<0.05; 8,115.23±457.81 vs. 248.54±211.12 pg/mL, P<0.05) and IL-8 levels (46,767.58±1,378.51 vs. 22.62±5.54 pg/mL, P<0.05; 17,861.19±579.30 vs. 252.81±249.07 pg/mL, P<0.05) were also remarkably up-regulated in coculture supernate for 24 and 48 h. Results demonstrated that coculture system with M2-type macrophages could significantly influence and up-regulate the expressions of pro-inflammatory factor IL-1β, IL-6 and IL-8 in SPC-A1.
Figure 2.
Increased expressions of pro-inflammatory factors in SPC-A1 cocultured with M2-type macrophages. (A) mRNA expressions of IL-1β, IL-6 and IL-8 in SPC-A1 cocultured with M2-type macrophages for 24 and 48 h by RT-PCR. *, P<0.05 vs. SPC-A1 cultured alone for 24 and 48 h; (B) Supernate protein expressions of IL-1β, IL-6 and IL-8 in SPC-A1 cocultured with M2-type macrophages for 24 and 48 h by CBA assay. IL-1β, IL-6 and IL-8 expressions were significantly enhanced in SPC-A1 after being cocultured with M2-type macrophages for 24 and 48 h. *, P<0.05 vs. SPC-A1 cultured alone for 24 and 48 h. IL, interleukin.
Enhanced expressions of TLRs and TLRs signaling pathway proteins in SPC-A1 cocultured with M2-type macrophages
We estimated mRNA and protein levels of TLRs and TLRs signaling pathway proteins in SPC-A1 to confirm that whether TLRs signaling pathway proteins were activated in SPC-A1 after being cocultured with M2-type macrophages. From Figure 3A, we detected and described the expression levels of TLR1~9 in SPC-A1 cultured alone and coculture group. In the present study, we observed that TLR1 (3.5-fold, P<0.05 and 4.1-fold, P<0.05), TLR6 (2.6-fold, P<0.05 and 3.1-fold, P<0.05) and TLR7 (1.9-fold, P<0.05; 2.1-fold, P<0.05) mRNA levels were significantly increased in SPC-A1 after being cocultured with M2-type macrophages for 24 and 48 h than SPC-A1 cultured alone. However, there was no significant difference in expression of TLR2, TLR3, TLR4, TLR5, TLR8 or TLR9. According to Figure 3B, TLRs signaling pathway proteins MyD88, TNF receptor-associated factor 6 (TRAF6) and p-NF-κB p65 were activated in SPC-A1 after being cocultured with M2-type macrophages for 24 or 48 h relative to SPC-A1 cultured alone. We showed that levels of TLR1, TLR6 and TLR7 were up-regulated and TLRs signaling pathway proteins would be activated in SPC-A1 in the coculture system with M2-type macrophages.
Figure 3.
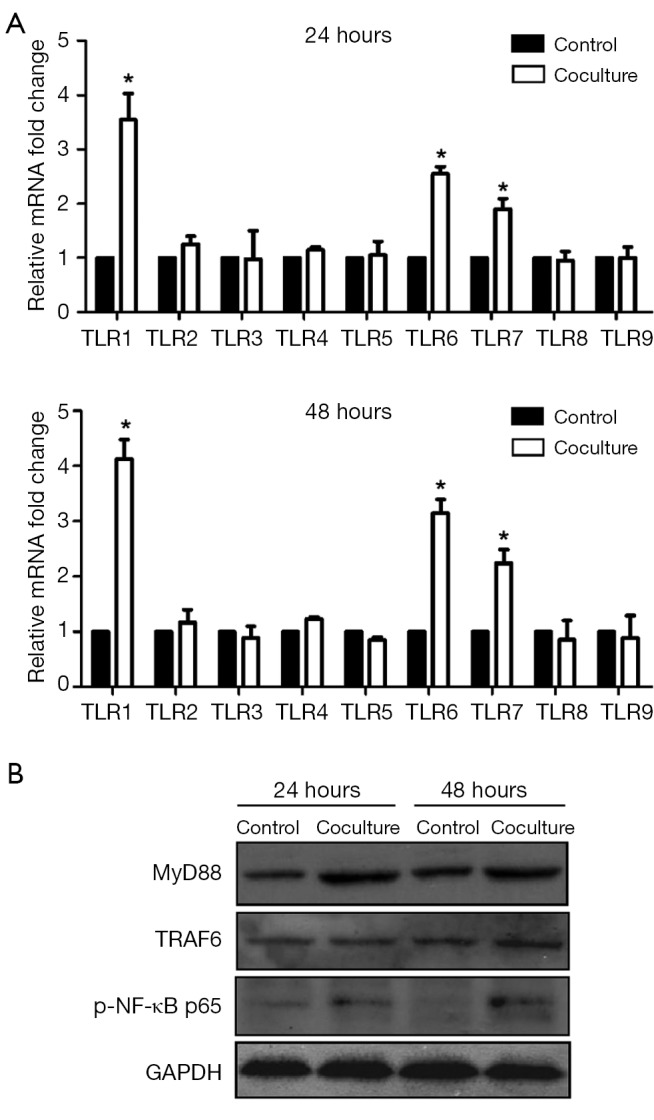
Enhanced expressions of TLRs and TLRs signaling pathway proteins in SPC-A1 cocultured with M2-type macrophages. (A) mRNA levels of TLR1~9 in SPC-A1 after being cocultured with M2-type macrophages for 24 and 48 h by RT-PCR. *, P<0.05 vs. SPC-A1 cultured alone for 24 and 48 h; (B) TLR signaling pathway protein expressions in SPC-A1 after being cocultured with M2-type macrophages for 24 and 48 h by western blot. TLR signaling pathway proteins were activated in SPC-A1 in the coculture system. TLRs, Toll-like receptors.
Increased expressions of inflammatory factors in SPC-A1 stimulated by TLRs agonists
To further reveal whether the activated TLRs were linked with the up-regulated levels of IL-1β, IL-6 and IL-8, we detected the expressions of IL-1β, IL-6 and IL-8 in SPC-A1 after the stimulation of TLRs agonists. As Figure 4 showed, mRNA expression levels of IL-1β, IL-6 and IL-8 in SPC-A1 were enhanced after being stimulated with Pam3CSK4 (TLR1 ligand) (4.2-fold, P<0.05 for 12 h and 7.8-fold, P<0.05 for 24 h in IL-1β; 3.0-fold, P<0.05 for 12 h and 7.0-fold, P<0.05 for 24 h in IL-6; 3.2-fold, P<0.05 for 12 h and 5.9-fold, P<0.05 for 24 h in IL-8), FSL-1 (TLR6 ligand) (2.5-fold, P<0.05 for 12 h and 3.3-fold, P<0.05 for 24 h in IL-1β; 3.1-fold, P<0.05 for 12 h and 5.0-fold, P<0.05 for 24 h in IL-6; 3.3-fold, P<0.05 for 12 h and 4.3-fold, P<0.05 for 24 h in IL-8) and Imiquimod (TLR7 ligand) (2.0-fold, P<0.05 for 12 h and 1.9-fold, P<0.05 for 24 h in IL-1β; 5.9-fold, P<0.05 for 12 h and 4.4-fold, P<0.05 for 24 h in IL-6) compared with 0 h. Nevertheless, Imiquimod was sufficient to enhance IL-8 mRNA expression levels (2.0-fold, P<0.05) in 24 h stimulation relative to 0 h. Results of TLRs agonists stimulation assay showed that the levels of IL-1β, IL-6 and IL-8 would be up-regulated through activation of TLR1, TLR6 or TLR7 signaling in SPC-A1 cells.
Figure 4.
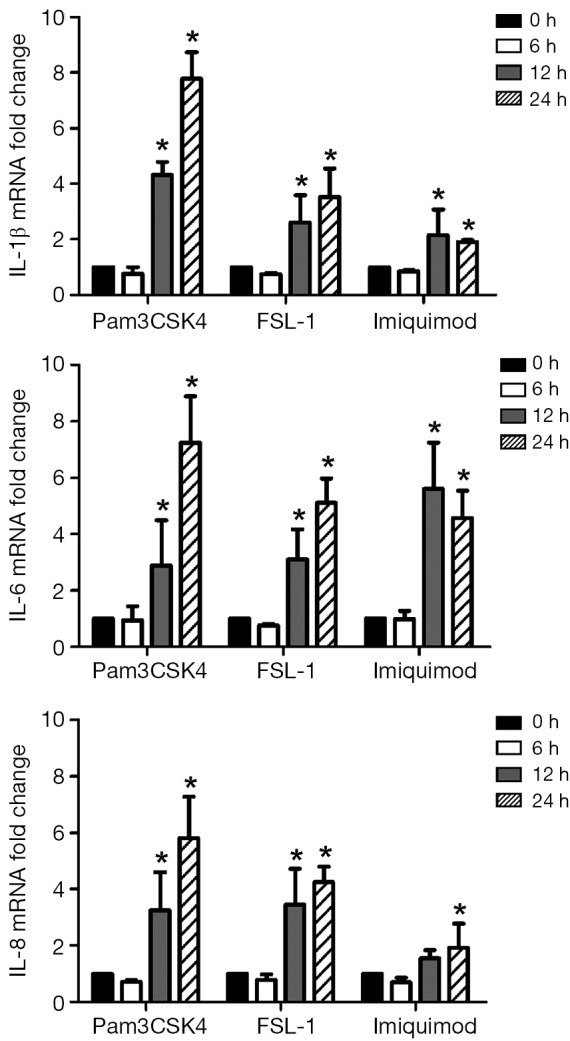
Increased expressions of inflammatory factors in SPC-A1 stimulated by TLRs agonists. mRNA expressions of IL-1β, IL-6 and IL-8 in SPC-A1 were enhanced by stimulation with agonists of TLR1, TLR6 and TLR7 for 12 and 24 h. *, P<0.05 relative to 0 h stimulation time. TLRs, Toll-like receptors; IL, interleukin.
Discussion
As a result of activation by molecular factors in tumor microenvironment, monocytes could differentiate into M1 or M2-polarized macrophages (19). M2-type macrophages highly expressed many receptors, such as mannose receptor (MR, CD206) and scavenger receptor (SR, CD68) and showed phenotype such as IL-10high, IL-12low, IL-23low (18). TAMs have the polarized M2 phenotype and are involved in the regulation of cellular functions including processes associated with cancer advance, such as proliferation, apoptosis and angiogenesis (20,21).
Lung cancer is known to be the most commonly diagnosed cancer, as well as the primary cause of cancer-related mortality for males worldwide and the second leading cause of cancer-related deaths for women (22-24). Increased levels of inflammatory have been observed in different types of cancer, including lung cancer (5). Pro-inflammatory cytokines are fundamental for several biological processes associated malignant tumors. IL-1β, IL-6 and IL-8, known as multifunctional cytokines with high biological activity, participate in physiological and pathological responses such as inflammation, immune response and even tumors. IL-1β was dramatically elevated in patients with NSCLC and implicated in the early stages of tumourigenesis. In vitro studies showed that IL-1β promoted the proliferation and migration of NSCLC cells (25,26). The IL-6 was a potent pleiotropic inflammatory cytokine that was considered a key growth-promoting and antiapoptotic factor. In addition, IL-8 was verified to be involved in growth, proliferation and metastasis of cancer cells (27,28).
To confirm that whether NSCLC cell line could be induced production of pro-inflammation cytokines by TAMs, we established an in vitro coculture system by using THP-1-derived M2-type macrophages and NSCLC cell line SPC-A1. In our research, we found that in the treatment of PMA, THP-1 cells attached specific phenotypes of M2-type macrophages and differentiated to M2-type macrophages. These polarized M2-type macrophages contained phenotype markers and some similar function of TAMs. CD206 was expressed selectively on PMA-treated M2-type macrophages. High expression of CD206 was observed in M2-type macrophages and may be related to angiogenesis, tissue remodeling and repair in tumor tissue. After being cocultured with M2-type macrophages for 24 and 48 h, IL-1β, IL-6 and IL-8 mRNA expressions in SPC-A1 were remarkably up-regulated. IL-1β, IL-6 and IL-8 protein levels in coculture supernate also significantly increased for coculture time of 24 and 48 h. We thought that some mechanisms were involved in this process that made pro-inflammation cytokines from SPC-A1 increase in the influence of M2-type macrophages.
Whether TLRs may participate in this process? Recent researches have reported that TLRs were important signaling regulators in tumor immunology and responded to a lot of products (29). TLRs primarily expressed in immunocytes and tumor cells. TLRs signaling had an active role in inflammation regulation and tumor progression in tumor microenvironment (30). TLRs signaling could activate transcription factors and generate cytokines via intracellular pathways. Of all class of receptors in human, TLR1 and TLR6 are closely connected to inflammation-mediated carcinogenesis and tumor progression. TLR2 preferentially forms heterodimers with either TLR1 or TLR6 (31,32). Recent studies showed that activation of TLR2-TLR6 complexes strongly enhanced growth of Lewis lung carcinoma and generated an inflammatory microenvironment hospitable for metastatic progression (33). TLR7 and/or TLR9 activation underlies systemic autoimmune diseases. TLR7 could also promote tumor progression, chemotherapy resistance and poor clinical outcomes of NSCLC and displayed a crucial role in lung cancer physiopathology (34). Many TLR7 agonists are currently in clinical trials as adjuvants to boost host antitumor responses in cancer patients (35,36). So we further detected TLRs expression levels in SPC-A1 in the coculture system to realize the biological function of TLRs. Our results showed that mRNA levels of TLR1, TLR6 and TLR7 were significantly up-regulated in SPC-A1 after being cocultured with M2-type macrophages for 24 and 48 h. TLRs signaling pathway proteins MyD88, TRAF6 and p-NF-κB p65 were also notably activated in SPC-A1 in the coculture system with M2-type macrophages for 24 and 48 h. From the results, we supposed that TLRs and TLRs signaling pathway proteins MyD88, TRAF6 and p-NF-κB p65 were involved in biological reactions of this coculture process. To further verify the link between TLRs signaling pathway and production of pro-inflammation cytokines, we stimulated TLR1, TLR6 or TLR7 in SPC-A1 with the matching TLR agonists. Our research displayed that the levels of IL-1β, IL-6 and IL-8 would be up-regulated through activation of TLR1, TLR6 or TLR7 in SPC-A1 cells by TLR agonists for the stimulation time of 12 and 24 h. So the up-regulation of IL-1β, IL-6 and IL-8 from SPC-A1 in the influence of M2-type macrophages may be the results of TLRs signaling pathway activation.
In our research, we considered that the mechanism by which TAMs promoted production of pro-inflammation cytokines might contain some specific processes, which stimulated TLR1, TLR6 and TLR7 signaling in tumor cells, to up-regulate IL-1β, IL-6 and IL-8 productions and ultimately induced inflammatory microenvironment of lung cancer. Thus, our findings identify TLRs signaling pathway is a novel regulatory axis that links inflammation signaling to the control of cancer cells development in NSCLC and provides new mechanistic insights into inflammation-promoted tumorigenesis.
Acknowledgements
Funding: We are grateful to the technical support from National Key Clinical Department of Laboratory Medicine of Jiangsu Province Hospital. This work was supported by National Natural Science Foundation of China (No. 81272324, 81371894) and Key Laboratory for Medicine of Jiangsu Province of China (No. XK201114), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure: The authors declare no conflict of interest.
References
- 1.Gomes M, Teixeira AL, Coelho A, et al. The role of inflammation in lung cancer. Adv Exp Med Biol 2014;816:1-23. [DOI] [PubMed] [Google Scholar]
- 2.Coffelt SB, de Visser KE. Cancer: Inflammation lights the way to metastasis. Nature 2014;507:48-9. [DOI] [PubMed] [Google Scholar]
- 3.Qin S, Ma S, Huang X, et al. Th22 cells are associated with hepatocellular carcinoma development and progression. Chin J Cancer Res 2014;26:135-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A.Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [DOI] [PubMed] [Google Scholar]
- 6.Azevedo A, Cunha V, Teixeira AL, et al. IL-6/IL-6R as a potential key signaling pathway in prostate cancer development. World J Clin Oncol 2011;2:384-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoni M, Massari F, Amantini C, et al. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2013;62:1757-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci 2008;13:453-61. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Zhang Y, Zhao J, et al. M2-polarized macrophages contribute to the decreased sensitivity of EGFR-TKIs treatment in patients with advanced lung adenocarcinoma. Med Oncol 2014;31:127. [DOI] [PubMed] [Google Scholar]
- 11.Connolly DJ, O'Neill LA. New developments in Toll-like receptor targeted therapeutics. Curr Opin Pharmacol 2012;12:510-8. [DOI] [PubMed] [Google Scholar]
- 12.Li TT, Ogino S, Qian ZR. Toll-like receptor signaling in colorectal cancer: carcinogenesis to cancer therapy. World J Gastroenterol 2014;20:17699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patten DA, Collett A. Exploring the immunomodulatory potential of microbial-associated molecular patterns derived from the enteric bacterial microbiota. Microbiology 2013;159:1535-44. [DOI] [PubMed] [Google Scholar]
- 14.Du X, Poltorak A, Wei Y, et al. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw 2000;11:362-71. [PubMed] [Google Scholar]
- 15.Chang M, Jin W, Sun SC. Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat Immunol 2009;10:1089-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Xu J, Ke X, et al. Expression and function of Toll-like receptors in peripheral blood mononuclear cells from patients with ovarian cancer. Cancer Immunol Immunother 2015;64:275-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma YY, He XJ, Wang HJ, et al. Interaction of coagulation factors and tumor-associated macrophages mediates migration and invasion of gastric cancer. Cancer Sci 2011;102:336-42. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Zhao X, Wang K, et al. Interaction of monocytes/macrophages with ovarian cancer cells promotes angiogenesis in vitro. Cancer Sci 2013;104:516-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laoui D, Van Overmeire E, De Baetselier P, et al. Functional Relationship between Tumor-Associated Macrophages and Macrophage Colony-Stimulating Factor as Contributors to Cancer Progression. Front Immunol 2014;5:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chanmee T, Ontong P, Konno K, et al. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colvin EK. Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol 2014;4:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Shahrabani F, Vallböhmer D, Angenendt S, et al. Surgical strategies in the therapy of non-small cell lung cancer. World J Clin Oncol 2014;5:595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [DOI] [PubMed] [Google Scholar]
- 24.Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol 2011;6:824-33. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Zhang LF, Wu J, et al. IL-1β-mediated repression of microRNA-101 is crucial for inflammation-promoted lung tumorigenesis. Cancer Res 2014;74:4720-30. [DOI] [PubMed] [Google Scholar]
- 26.Richardson CM, Sharma RA, Cox G, et al. Epidermal growth factor receptors and cyclooxygenase-2 in the pathogenesis of non-small cell lung cancer: potential targets for chemoprevention and systemic therapy. Lung Cancer 2003;39:1-13. [DOI] [PubMed] [Google Scholar]
- 27.Zhang E, Feng X, Liu F, et al. Roles of PI3K/Akt and c-Jun signaling pathways in human papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF, and IL-8 expression and in vitro angiogenesis in non-small cell lung cancer cells. PLoS One 2014;9:e103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes M, Coelho A, Araújo A, et al. IL-6 polymorphism in non-small cell lung cancer: a prognostic value? Tumour Biol 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29.Chen R, Alvero AB, Silasi DA, et al. Cancers take their Toll--the function and regulation of Toll-like receptors in cancer cells. Oncogene 2008;27:225-33. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee S, Crozet L, Damotte D, et al. TLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancer. Cancer Res 2014;74:5008-18. [DOI] [PubMed] [Google Scholar]
- 31.Lim KH, Staudt LM. Toll-like receptor signaling. Cold Spring Harb Perspect Biol 2013;5:a011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinto A, Morello S, Sorrentino R.Lung cancer and Toll-like receptors. Cancer Immunol Immunother 2011;60:1211-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009;457:102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatterjee S, Crozet L, Damotte D, et al. TLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancer. Cancer Res 2014;74:5008-18. [DOI] [PubMed] [Google Scholar]
- 35.Green NM, Marshak-Rothstein A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin Immunol 2011;23:106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov 2010;9:293-307. [DOI] [PubMed] [Google Scholar]