Abstract Abstract
Two new Mycetophilidae species, Neuratelia jabalmoussae sp. n. and Neuratelia salmelai sp. n. are described on the basis of material collected from Lebanon, Estonia and Finland. Detailed figures of male terminalia and photographs of general facies are provided along with discussions of their morphological distinction from sibling species. For the first time molecular characters are used to distinguish new fungus gnat species. Molecular analysis relies on cytochrome oxidase subunit one (COI) but has additionally been corroborated by information from the 28S and ITS2 regions of nuclear ribosomal DNA. Situations where morphological and molecular data provide conflicting evidence for species delimitation are discussed. A new country record from Georgia is provided for Neuratelia caucasica.
Keywords: Mycetophilidae, Neuratelia, new species, Western Palaearctic, systematics, molecular analysis, COI, ITS2, 28S
Introduction
The genus Neuratelia Rondani, 1856 forms a well delimited clade in the subfamily Sciophilinae (Mycetophilidae), as sister group to the remaining Sciophilinae (Borkent & Wheeler, 2013). According to Søli et al. (2000) it is characterised by the following combination of characters: laterotergite setose, M and CuA clearly branched but base of M1 obsolete, R5 strongly sinuate, C produced about one fifth of the distance between apex of R5 and apex of M1, and tibia with distinct setae. Very little is known about their biology; according to Laffoon (1965) the larvae of one species were found in moss. This is, however, challenged by Hutson et al. (1980). Altogether 31 extant species are known from across the world including 16 species from the Palaearctic region (Matile 1974, Zaitzev 1994, Sasakawa 2004), 13 species from the Nearctic region (Borkent and Wheeler 2013) and one from both the Neotropical and Oriental regions (Bechev 2000). Additionally, three species have been described from fossils (Evenhuis 2014). Among the Palaearctic species seven are so far known to occur in the Western Palaearctic. There are no keys to cover all described species of the world, of only the Palaearctic region or even just in Europe. For Western Palaearctic species, the most exhaustive one is the key by Zaitzev (1994) that excludes, however, several European species.
So far, alpha-taxonomy of fungus gnats has been carried out using traditional taxonomic methods, primarily morphological examination. Though in recent years nucleotide data have been implemented to address the phylogeny of this group (e.g. Rindal et al. 2009a, 2009b, Ševčík et al. 2013, 2014), to associate sexes of one species (Kurina et al. 2011) and in population genetic studies (Dörge et al. 2014). Hippa and Ševčík (2014) provided mitochondrial 12S and 16S sequences in the description of Nepaletricha sigma. Despite that, no molecular information has so far been utilised for delimitation of a new fungus gnat species. This is surprising, as using a 658-bp fragment from the 5’ end of the mitochondrial cytochrome oxidase gene subunit 1 (COI) – the so-called ‘DNA barcode’ (see Hebert et al. 2003) – has become an increasingly common practice in discriminating insect species during recent years (e. g. Yassin 2008, Huemer and Hebert 2011, Riedel et al. 2013). Sometimes, acquiring additional genetic data from other loci has also been used to corroborate findings discovered by studying DNA barcodes (e.g. Õunap and Viidalepp 2009, Raupach et al. 2010, van Nieukerken et al. 2012).
The aim of this article is to publish taxonomic and faunistic information about Western Palaearctic Neuratelia specimens that the senior author has accumulated over recent years. Both morphological and molecular data were used for species delimitation. This resulted in describing two new species – one from Estonia and Finland and another from Lebanon.
Material and methods
Collection, preparation, illustration and morphological study
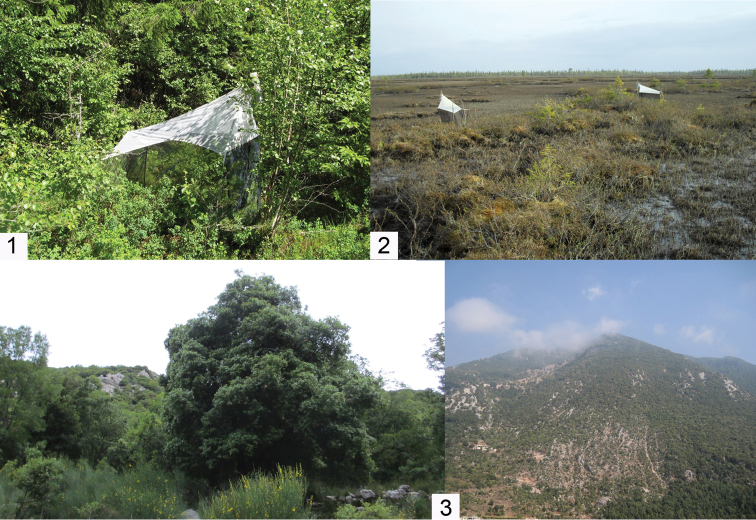
The examined material of two new species was collected from Estonia and Finland using Malaise traps, and from Lebanon by light trapping, respectively. The Estonian locality lies at the herb rich edge of a mixed forest (Fig. 1) while the Finnish localities are predominantly wet fen habitats (Fig. 2) with variable vegetation irrigated by occasional springs. All Finnish localities are from the northern part of the country. In Lebanon, the material was collected from Jabal Moussa Biosphere Reserve, north-east of Beirut, characterised by karstic mountains with evergreen sclerophyllous vegetation (Fig. 3). The additional studied Neuratelia material was collected from Georgia, Greece, Slovakia, Finland and Estonia by sweep netting and Malaise trapping.
Figures 1–3.
Collecting localities of Neuratelia salmelai sp. n. (1, 2) and Neuratelia jabalmoussae sp. n. (3). 1 Palupõhja in Estonia (holotype) 2 Kaita-aapa (Sodankylä) in Finland (a paratype) 3 Mar Elias in Jabal Moussa Biosphere Reserve, Lebanon (holotype).
All specimens were stored initially in ethyl alcohol within which parts of them – after studying under a stereomicroscope Leica S8APO – are still preserved. For more detailed study of male terminalia, they were detached and macerated in a 10% solution of KOH, followed by neutralization and washing in distilled water. The remaining chitinous parts were thereafter inserted into glycerine for study, including black and white illustrations, and preserved as glycerine preparations in polyethylene microvials (see also Kurina 2003). A few specimens including their terminalia were slide mounted in Euparal following the method described by Hippa and Kurina (2012). The current preservation method of each specimen is indicated in the material section. The measurements are given as the range of measured specimens followed by the mean value, while measurements from the holotypes are given in square brackets. The ratios of the three apical palpal segments are given as 3rd:4th:5th. All measurements are taken from specimens in alcohol. Morphological terminology follows Søli et al. (2000).
The habitus photos have been made in an alcohol medium using a Canon 7D camera with a Canon MP-E65 (F2.8 1–5×) lens (see Kurina et al. 2011). The photos of thorax and terminalia were combined using the software LAS V.4.1.0. from multiple gradually focused images taken by a Leica DFC 450 camera attached to a Leica 205C stereomicroscope or Leica DM 6000 B compound microscope, respectively. Adobe Photoshop CS5 was used for editing the figures and compiling the plates. Black and white illustrations of the terminalia were prepared using a U-DA drawing tube attached to an Olympus CX31 compound microscope.
The material is deposited in the Institute of Agricultural and Environmental Sciences, Estonian University of Life Sciences [former Institute of Zoology and Botany], Tartu, Estonia (IZBE), in the Zoological Museum, University of Turku, Finland (ZMUT) and in the personal collection of J. Salmela, Rovaniemi, Finland (JSPC).
Molecular techniques
The genomic DNA was extracted using High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany). Anterior segments of the abdomen that had been stored after dissection of genitalia were crushed and used for the extraction. This process was carried out following the manufacturer’s instructions for extraction of genetic material from mammalian tissue.
In total, one mitochondrial and two nuclear markers were sequenced. A 658-bp ’barcoding’ fragment from close to the 5’ terminus of the mitochondrial gene cytochrome C oxidase subunit 1 (COI), was amplified and sequenced using primers LCO1490 (5’-GGT CAA CAA ATC ATA AAG ATA TTG G-3’) and HCO2198 (5’-TAA ACT TCA GGG TGA CCA AAA AAT CA-3’) (Folmer et al. 1994). A 695-701-bp fragment covering expansion segments D1 and D2 of the nuclear 28S rRNA gene was sequenced using primers D1F (5’-GGG GAG GAA AAG AAA CTA AC-3’) (Abraham et al. 2001) and D2R (5’-TTG GTC CGT GTT TCA AGA CGG G-3’) (Belshaw and Quicke 1997). In the case that this preferable treatment was not successful, the desired part of the 28S was sequenced in two fragments, combining D1F with D1R (5’-CAA CTT TCC CTT ACG GTA CT-3’) (Abraham et al. 2001) and D2R with D2F (5’-AGA GAG AGT TCA AGA GTA CGT G-3’) (Belshaw and Quicke 1997). In addition, a fragment of the internal transcribed spacer 2 region (ITS) located between the 5.8S rRNA and 28S rRNA genes was sequenced using primers ITS2A (5’-TGT GAA CTG CAG GAC ACA T-3’) and ITS2B (5’-TAT GCT TAA ATT CAG GGG GT-3’) (Beebe and Saul 1995). PCR was performed in a total volume of 25 µl, with the reaction mixture containing 1X HOT FIREPol® Blend Master Mix Ready to Load (Solis BioDyne, Tartu, Estonia), 10 pmol of primers and 20-80 ng of purified genomic DNA. PCR was carried out in an Eppendorf Mastercycler epigradient thermocycler (Eppendorf AG, Hamburg, Germany). Its conditions involved an initial denaturation at 95 °C for 15 min, 35 cycles of 30 s at 95 °C, 30 s at 45–60 °C (depending on primers) and 1 min at 72 °C, followed by a final extension at 72°C for 10 min. PCR products were visualised on a 1.2% agarose gel, and 20 μl of the PCR solution was treated with fast alkaline phosphatase and exonuclease I (Thermo Scientific, Pittsburgh, USA). In some cases, direct sequencing from PCR solution was not possible due to multiple products. To sequence these samples, desired products were cut from agarose gel and extracted using a High Pure PCR Product Purification Kit (Roche). DNA cycle sequencing was performed either by Macrogen Europe (Amsterdam, Netherlands) or by the Estonian Biocentre (Tartu, Estonia). Both DNA strands were sequenced for all studied markers.
Phylogenetic analysis
Consensus sequences were created with Geneious R7 (Biomatters Ltd., Auckland, New Zealand) or Sequencher 5.1 (Gene Codes, Ann Arbor, MI, USA). Sequences were double-checked by eye and aligned using ClustalW (Thompson et al. 1994) in BioEdit 7.2.5 (Hall 1999). Two phylogenetic analyses were performed using either only COI or all three regions (COI, 28S, ITS2). Neuratelia minor was used as an outgroup in all phylogenetic analyses.
For COI, a neighbour-joining tree implementing Kimura 2-parameter model (a standard model analysing DNA barcode data, see e.g. Waugh 2007, Õunap and Viidalepp 2009, Hausmann et al. 2013) was constructed in MEGA6 (Tamura et al. 2013). Clade credibilities were assessed by bootstrapping (1000 replications). The tree was visualised using MEGA6.
For the concatenated dataset, data were first divided into three subsets according to the markers used (COI, 28S and ITS). Thereafter, PartitionFinder 1.1.1 (Lanfear et al. 2012) was used to select the most effective partitioning scheme and best substitution model for each partition. Accroding to PartitionFinder results, COI and ITS were treated together as one partition keeping 28S separately for ML analysis with RAxML 7.7.1 (Stamatakis et al. 2008). A GTR+I substitution model was implemented on both partitions. Analysis was run using the default settings of the RAxML online platform (http://embnet.vital-it.ch/raxml-bb/index.php). Ten slow ML searches, one thorough ML search and 100 rapid boostrap replications were performed. The results of bootstrapping were drawn on a single best-scoring ML tree. Phylograms were visualised with FigTree v1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/).
Results
The morphology of studied material distinguished three previously known species of Neuratelia and a group of specimens, clearly delimited by characters of male terminalia. This group, represented by specimens from Estonia and Finland, resembles the widespread Neuratelia nemoralis (Meigen, 1818) and hereafter described and referred to as a new species – Neuratelia salmelai sp. n. In addition, another group of three specimens from different localities in Jabal Moussa Biosphere Reserve (Lebanon) had slight differences from Neuratelia caucasica Zaitzev, 1994 – a species only known from Caucasus. In the latter case, the species is described as Neuratelia jabalmoussae sp. n. but the morphological differences are diminutive underpinning the necessity of including DNA sequence data for species discrimination.
Sequencing the ‘barcode region’ of COI was successful for all specimens included in the current study. The success rate was lower for ITS2 and 28S rDNA, as all attempts to sequence 28S failed for one individual of Neuratelia nemoralis, and for a few specimens, only half of 28S or ITS was obtained (Table 1). GenBank accession numbers for all sequences are presented in Table 1. The NJ tree constructed on the basis of barcodes divided the studied specimens into three well-supported clusters differing from each other by at least 4%. One group comprised only Neuratelia caucasica and another only Neuratelia jabalmoussae, whereas Neuratelia nemoralis and Neuratelia salmelai were intermingled in the third clade (Fig. 4). Studying the concatenated dataset resulted with almost identical results, as Neuratelia jabalmoussae and Neuratelia caucasica remained clearly separate sister taxa with Neuratelia nemoralis and Neuratelia salmelai remaining inseparable on the ML tree (Fig. 4).
Table 1.
Voucher numbers, depositories and GenBank accession codes of studied Neuratelia specimens. COI: cytochrome oxidase subunit I; 28S: 28S rRNA; ITS2: internal transcribed spacer 2.
| Species | Voucher No | Depository | COI | 28S | ITS2 |
|---|---|---|---|---|---|
| Neuratelia minor | IZBE0200274 | IZBE | KP715935 | KP715924 | KP715947 |
| Neuratelia nemoralis | IZBE0200283 | IZBE | KP715936 | KP715925 | KP715948 |
| Neuratelia nemoralis | IZBE0200284 | IZBE | KP715937 | KP715926 | KP715949 |
| Neuratelia nemoralis | MYCE-JS-2013-0095 | ZMUT | KP715938 | KP715927 | KP715950 |
| Neuratelia nemoralis | IZBE0200286 | IZBE | KP715939 | × | KP715951 |
| Neuratelia nemoralis | IZBE0200285 | IZBE | KP715940 | KP715928 | KP715952 |
| Neuratelia nemoralis | MYCE-JS-2013-0235 | ZMUT | KP715941 | KP715929 | KP715953 |
| Neuratelia salmelai | IZBE0200253 | IZBE | KP715942 | KP715930 | KP715954 |
| Neuratelia salmelai | MYCE-NV-2013-0131 | ZMUT | KP715943 | KP715931 | KP715955 |
| Neuratelia jabalmoussae | IZBE0200250 | IZBE | KP715944 | KP715932 | KP715956 |
| Neuratelia caucasica | IZBE0200255 | IZBE | KP715945 | KP715933 | KP715957 |
| Neuratelia caucasica | IZBE0200262 | IZBE | KP715946 | KP715934 | KP715958 |
Figure 4.
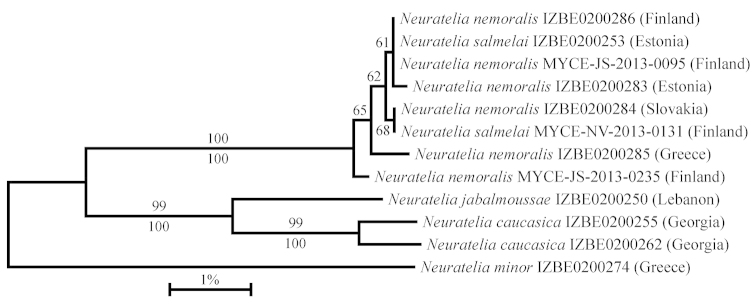
Neighbour-joining tree of the COI ’barcode’ region of Neuratelia spp. Scale bar: Kimura 2-parameter genetic distance. Bootstrap supports are presented above the branches. Maximum likelihood analysis of the concatenated (COI, 28S, ITS2) dataset yielded a tree with similar topology, bootstrap supports for the divergencies obtained in this analysis are given below the branches. Support values inferior to 60 are not shown.
The species
Neuratelia jabalmoussae sp. n.
http://zoobank.org/95794D4E-8664-4BB5-80F2-762C125858BF
Figures 5–6.
Habitus of Neuratelia species. 5 Neuratelia salmelai sp. n., terminalia detached 6 Neuratelia jabalmoussae sp. n. Scale bar = 1 mm.
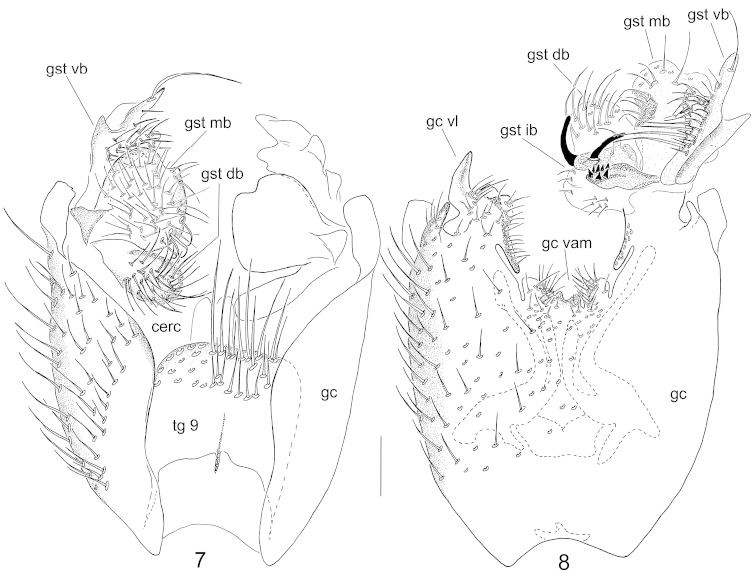
Figures 7–8.
Male terminalia of Neuratelia jabalmoussae sp. n., dorsal view (7) and ventral view (8). Scale bar = 0.1 mm. Abbreviations: cerc = cerci; gc = gonocoxite; gc vam = ventroapical margin of gonocoxite; gc vl = ventroapical lobe of gonocoxite; gst db = dorsal branch of gonostylus; gst ib = internal branch of gonostylus; gst mb = medial branch of gonostylus; gst vb = ventral branch of gonostylus; tg 9 = IX tergite.
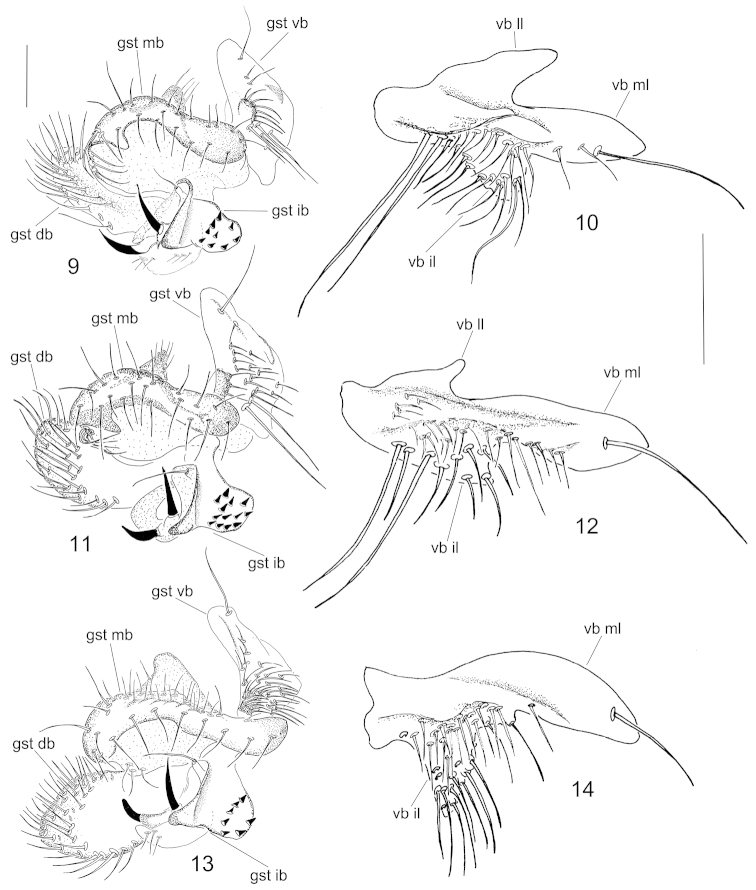
Figures 9–14.
Male terminalia of Neuratelia jabalmoussae sp. n. (9, 10), Neuratelia caucasica Zaitzev, 1994 (11, 12) and Neuratelia minor (Lundström, 1912) (13, 14). 9, 11, 13 internal view of gonostylus 10, 12, 14 ventral view of ventral branch of gonostylus. Scale bars = 0.1 mm. Abbreviations: gst db = dorsal branch of gonostylus; gst ib = internal branch of gonostylus; gst mb = medial branch of gonostylus;gst vb = ventral branch of gonostylus; vb il = internal lobe of ventral branch of gonostylus; vb ll = lateral lobe of ventral branch of gonostylus; vb ml = medial lobe of ventral branch of gonostylus.
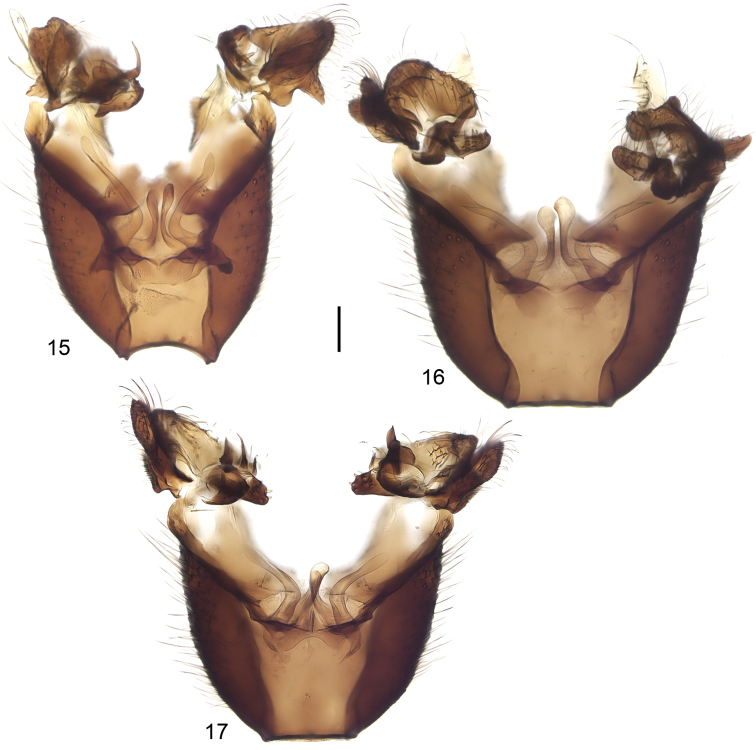
Figures 15–17.
Male terminalia, dorsal view, tergite 9 removed. Neuratelia jabalmoussae sp. n. (15), Neuratelia caucasica Zaitzev, 1994 (16) and Neuratelia minor (Lundström, 1912) (17). Scale bar = 0.1 mm.
Type material.
Holotype. 1♂, LEBANON, Kesrouane Mar Elias, 34°03'06,9"N, 35°46'00,5"E, 1138 m a.s.l., at light, 27.v.–4.vi.2012, J. Kullberg leg. (IZBE0200250, slide mounted in Euparal with terminalia in glycerine). Paratypes. 1♂, LEBANON, Kesrouane Mar Geryes, 34°03'20,9"N, 35°44'28,9"E, 749 m a.s.l., at light, 26.v.–2.vi.2012, J. Kullberg leg. (IZBE0200251, in alcohol with terminalia in glycerine);1♂, LEBANON, Kesrouane Ghbele, 34°03'25,5"N, 35°43'02,5"E, 884 m a.s.l., at light, 26.v.–30.v.2012, J. Kullberg leg. (IZBE0200252, in alcohol).
Description.
Male (Fig. 6). Body length 5.4–5.8, 5.6 [5.6] mm (n=3).
Head dark brown, with numerous pale to yellowish setae. Three ocelli in a shallow and wide triangular arrangement, with laterals separated from eye margins by a distance slightly more than their own diameter. Face conical, about 0.8 times as wide as maximum height; clypeus rectangular, about 0.6 times as wide as high; both brown, setose, with setae on clypeus stronger than those on face. Mouthparts yellow. Palpus five segmented, yellowish with apex of fifth segment brownish. Ratios of three apical palpal segments 1.0: 1.68–1.85, 1.77 [1.85]: 1.97–2.00, 1.98 [2.00]. Scape and pedicel light brown to brown, flagellomeres light brown, with short yellowish setae. First flagellomere yellowish at basal third. Flagellum evenly tapering; first flagellomere clavate, 2.9–3.1 times as long as broad apically, 2–13 flagellomeres cylindrical, fourth flagellomere about 1.7–2.5 times as long as broad, apical flagellomere slightly conical, 4.8–5.4 times as long as broad at base.
Thorax. All parts brown to dark brown, all setae yellow to light brownish. Mesonotum with evenly arranged numerous setae. Scutellum wholly setose with about 10 stronger setae along the margin, not arranged to distinct pairs. Antepronotum with 8–9 and proepisternum with 4–7 setae of unequal size, laterotergite with 22–26 setae and mediotergite with ca 14–16 setae medially on lower part. Other pleural parts bare. Halteres pale yellow, setose.
Legs. All coxae yellow, basally infuscated. All trochanters brown. All femora and tibiae yellow, tarsi seem considerably darker because of dense setae. Foretibia with 1–2 ad, 0–2 d and 2–3 pd. Midtibia with 4–7 a, 2–3 d, 1–2 av and 2–4 pd. Hind tibia with 7–8 a, 1–2 ad (1 at apex), 4–6 d, 0–1 pd, 5 p and with a posterior apical comb of setae. Ratio of femur to tibia for fore-, mid- and hind legs: 0.86–0.94, 0.9 [0.9]; 0.77–0.86, 0.82 [0.77]; 0.71–0.75, 0.73 [0.75]. Ratio of tibia to basitarsus for fore-, mid- and hind legs: 0.91–0.94, 0.93 [0.94]; 1.26–1.3, 1.27 [1.3]; 1.51–1.54, 1,53 [1.51].
Wing hyaline, length 5.0–5.03, 5.02 [5.03] mm (n=3). All veins brown, costal and radial veins somewhat darker. Both surfaces of veins setose, except bare bM-Cu and r-m. Wing membrane with micro- and macrotrichia on both surfaces. Costa reaches very little from R5 to M1. Sc reaches costa at about one sixth between Rs and tip of R1. R5 sinuate. Rs about as long as crossvein r-m. M1 basally obsolete: observable vein begins distally from middle of R1. Cubital fork begins proximally from apex of Sc.
Abdomen with tergites brown and sternites yellowish. Tergites 6–7 somewhat darker. Terminalia (Figs 7, 8, 9, 10, 15) dark brown. Tergite 9 apically almost straight, with wide basal incision about one third of height of tergite. Basolateral portions of tergite 9 narrow and proximally pointed. Setae on tergite 9 similar to these on the gonocoxite, the posteriormost ones slightly stronger than the others. Cerci separated, protruding over tergite 9, with strong apical setae deviating from other setosity. The gonocoxite with a complex ventroapical lobe laterally; ventrobasally with wide shelving incision; ventroapical margin medially with lateral well delimited sub-circular and a medial apically concave setose structures. Dorsomedial margin of the gonocoxite slightly sinuous. The gonostylus with four branches. The dorsal branch simply oval, setose. The ventral branch trifid with 1) internal lobe setose including two stronger internally directed setae, 2) middle lobe elongated with a strong subapical seta, and 3) lateral lobe similar to internal lobe except being bare. Medial branch setose with a well delimited medial hump. Internal branch complex with two strong pointed spines and a lobe bearing 9 short spines ventrally on its apical part. The medial branch of the gonostylus connected with apical part of ventroapical lateral lobe of the gonocoxite. Parameres not protruding over ventroapical margin of gonocoxite.
Female. Unknown.
Biology.
Unknown.
Etymology.
The species is named after the type locality in Jabal Moussa Biosphere Reserve, Lebanon; the specific epithet is a noun in genitive case.
Specific discussion.
Neuratelia jabalmoussae sp. n. is very similar to Neuratelia caucasica, into which it also runs to in the key by Zaitzev (1994) because of having the foretibia slightly shorter than the fore basitarsus. Also the male terminalia of these two species are extremely similar, differ in details as follows: 1) paramers not expanded apically (Fig. 15), while they are well expanded in Neuratelia caucasica (Fig. 16), 2) the lateral lobe of the trifid ventral branch of the gonostylus prominent, about half of the size of medial lobe (Fig. 10), while it is minute in Neuratelia caucasica, about one fifth of the size of medial lobe (Fig. 12), and 3) internal branch of gonostylus has 8 short spines on a separate lobe (Fig. 9), while there are 13 spines in Neuratelia caucasica (Fig. 11). Both species share the general outline of male terminalia also with Western Palaearctic species Neuratelia minor (Lundström, 1912) and with Neuratelia microdigitata Sasakawa, 2004, known from Japan. However, Neuratelia minor has the foretibia slightly longer than fore basitarsus and the ventral branch of gonostylus bifid instead of being trifid. Neuratelia microdigitata has the internal branch of gonostylus with finger-like processes apically on a separate lobe (cf. Sasakawa 2004: fig. 4) instead of short spines as in other three species. All four species have the similar branching of the gonostylus and two strong pointed spines on internal branch of the gonostylus.
Neuratelia caucasica
Zaitzev, 1994
Studied material.
GEORGIA. 2♂♂ 2♀♀, Surami, 42°01'34,2"N, 043°29'52,5"E, 941 m a.s.l., sweeping, O. Kurina leg. 18.v.2012 (IZBE0200255– IZBE0200258, in alcohol); 2♂♂ 1♀, Borjomi, 41°50'9,2"N, 043°19'56,7"E, 936 m a.s.l., sweeping, O. Kurina leg. 21.v.2012 (IZBE0200259, ♂, on pin with terminalia in glycerine; IZBE0200260, IZBE0200261, in alcohol); 3♂♂ 4♀♀, Mtirala NP, near visitor centre, 41°40'20,7"N, 041°52'31,8"E, 465 m a.s.l., sweeping, O. Kurina leg. 20.v.2013 (IZBE0200262, ♂, slidemounted in Euparal with terminalia in glycerine; IZBE0200263–IZBE0200268, in alcohol); 1♂ 2♀♀, Kintrishi NP, 41°45'11,7"N, 041°58'38,4"E, 453 m a.s.l., sweeping, O. Kurina leg. 22.v.2013 (IZBE0200269–IZBE0200271, in alcohol).
Specific discussion.
Having been described from North Caucasus (Krasnodarsk region), the species has not been recorded since and the studied material represents the first records from Georgia. According to male terminalia the species is close to Neuratelia minor and Neuratelia jabalmoussae sp. n.
Neuratelia minor
(Lundström, 1912)
Studied material.
SLOVAKIA. 1♂, NP Muránska planina, Murán 3.5 km NE, sweeping, 48°45'46,5"N, 020°04'55,9"E, 483 m a.s.l. 30.v.2009, O. Kurina leg. (IZBE0200272, on pin with terminalia in glycerine; earlier published in Ševčík and Kurina 2011: 101); 1♂, NP Muránska planina, Šiance, sweeping, 48°46'14,7"N, 020°05'33,0"E, 656 m a.s.l. 30.v.2009, O. Kurina leg. (IZBE0200273, on pin with terminalia in glycerine; earlier published in Ševčík and Kurina 2011: 101). GREECE. 1♂, Central Macedonia, Kerkini lakes area, Vironia village, Beabies site, 41°19'15,4"N, 023°13'39,6"E, 1150 m a.s.l., Malaise trap, 19.–25.v.2008, G. Ramel leg. (IZBE0200274, slide mounted in Euparal).
Specific discussion.
Neuratelia minor was described and figured by Lundström (1912: figs 8, 9) from Romania. Because the type material was subsequently destroyed, Matile designated neotype from Hungary and provided also a new figure of male terminalia (Matile 1974: fig. 6). Both figures are sufficiently detailed, presenting a bifid ventral branch of the gonostylus that clearly discriminates the species morphologically from Neuratelia caucasica and Neuratelia jabalmoussae sp. n. Neuratelia minor has a more eastern distribution in the Western Palaearctic but is also found in France and the Eastern Palaearctic (Chandler 2013).
Neuratelia salmelai sp. n.
http://zoobank.org/1554A8EF-A6FF-484D-9555-4855836A4263
Figs 5 , 18 , 20 , 22 , 24 , 26 , 28
Figure 18.
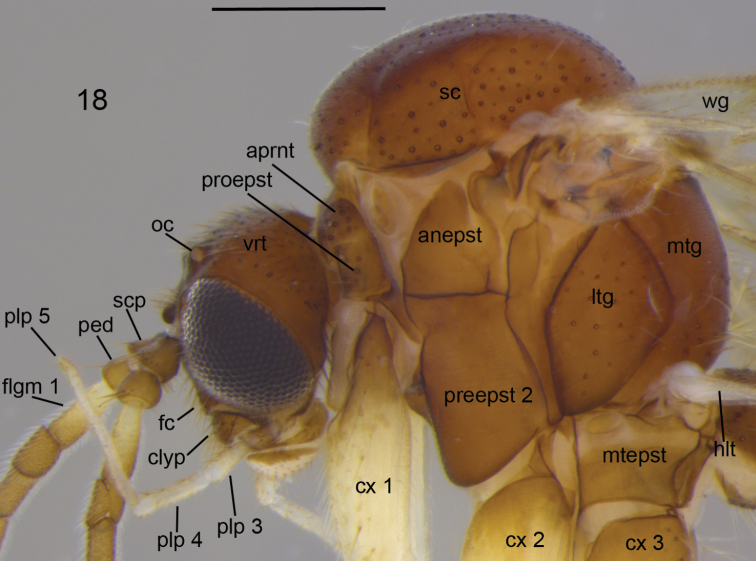
Head and thorax of Neuratelia salmelai sp. n. Scale bar = 0.5 mm. Abbreviations: anepst = anepisternum; aprnt = antepronotum; clyp = clypeus; cx = coxa; fc = face; flgm = flagellar segment; hlt = halter; ltg = laterotergite; mtepst = metepisternum; mtg = mediotergite; oc = ocellus; ped = pedicell; plp = palpal segment; preepst = preepisternum; proepst = proepisternum; sc = scutum; scp = scape; vrt = vertex; wg = wing.
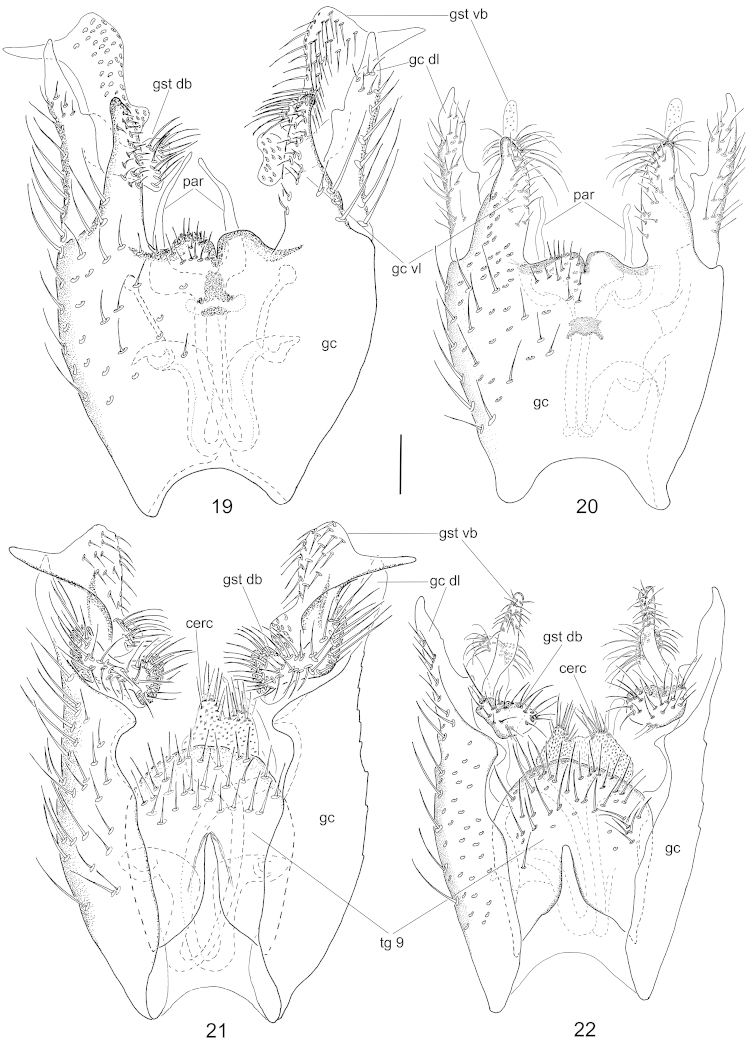
Figures 19–22.
Male terminalia of Neuratelia nemoralis (Meigen, 1818) (19, 21) and Neuratelia salmelai sp. n. (20, 22). Ventral view (19, 20) and dorsal view (21, 22). Scale bar = 0.1 mm. For abbreviations: see Figs 7–8, except: gc dl = dorsoapical lobe of gonocoxite; par = parameres.
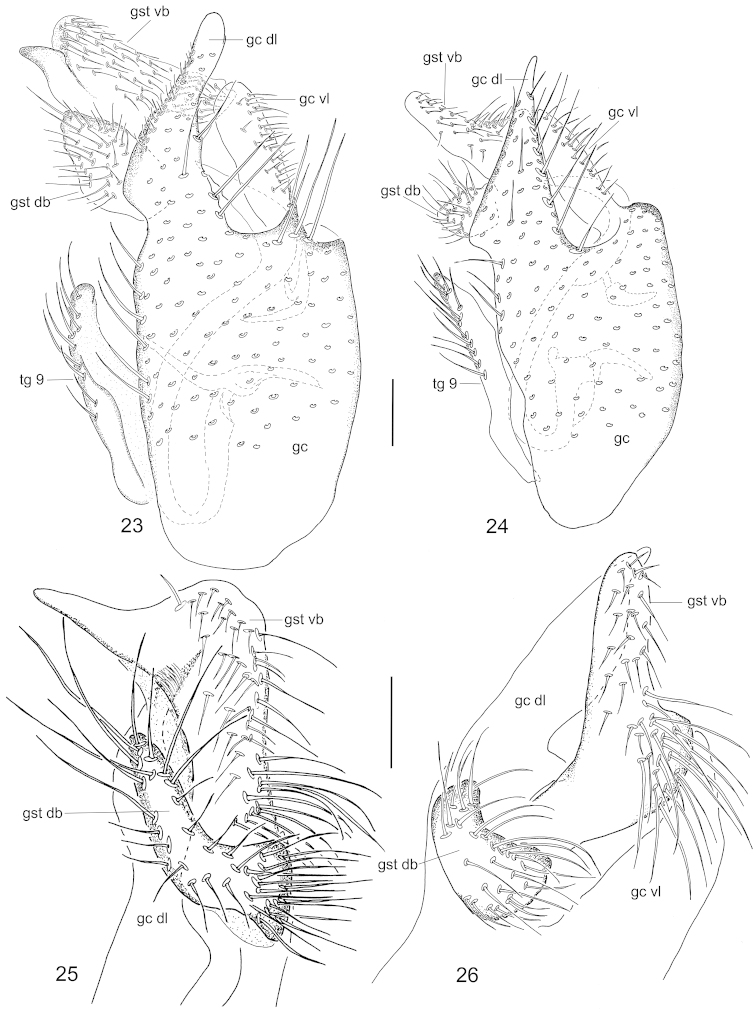
Figures 23–26.
Male terminalia of Neuratelia nemoralis (Meigen, 1818) (23, 25) and Neuratelia salmelai sp. n. (24, 26). Lateral view (23, 24) and internal view of the gonostylus (25, 26). Scale bar = 0.1 mm. For abbreviations: see Figs 7–8 and 19–22.
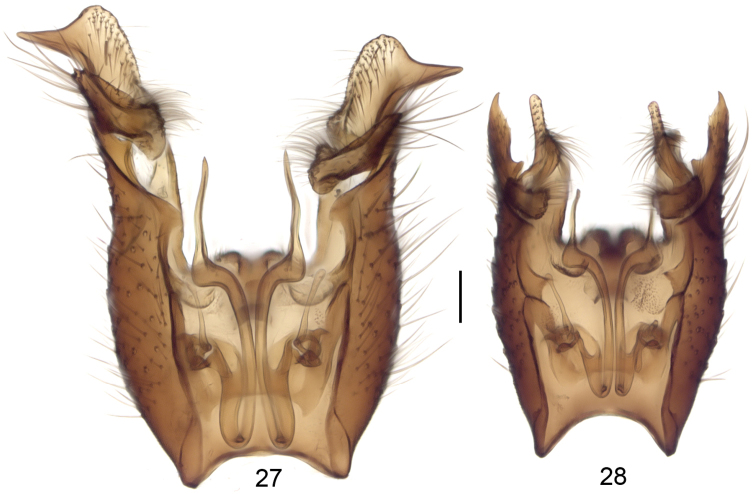
Figures 27–28.
Male terminalia, dorsal view, tergite 9 removed. Neuratelia nemoralis (27) and Neuratelia salmelai sp. n. (28). Scale bar = 0.1 mm.
Type material.
Holotype. 1♂, ESTONIA. Palupõhja, Kaha (ME 57), Malaise trap, 58°25'54,68"N, 026°14'28,90"E, 29.vi.–8.vii.2009, V. Soon leg. (IZBE0200253, slide mounted in Euparal with terminalia in glycerine). Paratypes. 1♂, FINLAND. Lkor: Sodankylä, Kaita-aapa, Malaise trap, 67°50'45,5"N, 026°33'17,6"E, 5.vi.–3.vii.2012, J. Salmela leg. (IZBE0200254, in alcohol with terminalia in glycerine); 1♂, FINLAND. Lkoc: Kittilä, Kielisenpalo, Malaise trap, 68°01'16,6"N, 025°03'46,9"E, 26.vi–24.vii.2007, J. Salmela leg. (MYCE-NV-2013-0093 in ZMUT, in alcohol with terminalia in glycerine); 1♂, FINLAND. Lkoc: Kittilä, Vuotsonperän-jänkä, Malaise trap, 67°37'15,9"N, 025°26'43,6"E, 25.vi.–24.vii.2009, J. Salmela leg. (MYCE-NV-2013-0131 in ZMUT, in alcohol with terminalia in glycerine); 1♂, FINLAND, Lkor: Sodankylä, Pomokaira 67°52'19,2"N, 026°12'46,8"E, 11.6.-10.7.2013, J. Salmela leg. Malaise trap Salix swamp with seepages (DIPT-JS-2014-0199 in JSPC, in alcohol).
Description.
Male (Figs 5, 18). Body length 5.8–6.5, 6.2 [5.8] mm (n=4).
Head (Fig. 18) brown to dark brown, with numerous setae. Three ocelli in a shallow and wide triangular arrangement, with laterals separated from eye margins by a distance about twice of their own diameter. Face conical, about 0.9 times as wide as maximum height; clypeus rectangular, about 0.6 times as wide as high; both brown, setose, with setae on clypeus stronger than those on face. Mouthparts light brown. Palpus five segmented, yellowish brown with second segment and apex of fifth segment darker. Ratios of three apical palpal segments 1.0: 1.37–1.65, 1.52 [1.56]: 1.62–1.82, 1.72 [1.71]. Scape and pedicel brown, pedicel somewhat lighter; flagellomeres light brown, with short pale setae. First flagellomere yellowish at basal half. Flagellum evenly tapering; first flagellomere clavate, 2.5–3.3 times as long as broad apically, 2–13 flagellomeres cylindrical, fourth flagellomere about 2.3–2.6 times as long as broad, apical flagellomere slightly conical, 5.2–6 times as long as broad at base.
Thorax (Fig. 18). All parts brown to dark brown, all setae yellow to light brownish. Mesonotum with evenly arranged numerous setae. Scutellum with about 10 setae along the margin, not arranged to distinct pairs. Antepronotum with 10–13 and proepisternum with 5–8 setae of unequal size, laterotergite with 17–26 setae and mediotergite with ca 12–20 setae medially on lower part. Other pleural parts bare. Halteres yellow, setose.
Legs. All coxae yellow with basal fourths brown. In case of two paratypes, cx3 entirely light brownish with darker basal half. All trochanters brown. All femora and tibiae yellow, tarsi seem darker because of dense setae. Foretibia with 2–3 ad, 1–3 d and 2–3 pd. Midtibia with 6–10 a, 0–4 d, 4–5 av and 2–3 pd. Hind tibia with 8–10 a, 1–2 ad (1 at apex), 7–8 d, 5–7 p and with a posterior apical comb of setae. Ratio of femur to tibia for fore-, mid- and hind legs: 0.86–0.91, 0.88 [0.91]; 0.72–0.87, 0.80 [0.87]; 0.72–0.77, 0.75 [0.72]. Ratio of tibia to basitarsus for fore-, mid- and hind legs: 0.81–1.00, 0.9 [0.9]; 1.22–1.33, 1.27 [1.22]; [1.66].
Wing hyaline, length 4.1–5.0, 4.52 [4.49] mm (n=4). All veins brown, costal and radial veins somewhat darker. Both surfaces of all veins setose. Wing membrane with micro- and macrotrichia on both surfaces. Costa reaches very little from R5 to M1. Sc reaches costa at about quarter between Rs and tip of R1. R5 sinuate. Rs about as long as crossvein r-m. M1 basally obsolete: observable vein begins distally from middle of R1. Cubital fork begins proximally from apex of Sc.
Abdomen with tergites brown to dark brown and with sternites yellow to brownish yellow. Terminalia (Figs 20, 22, 24, 26, 28) dark brown. Tergite 9 apically rounded, with deep and narrow basal incision about half of height of tergite. Basolateral portions of tergite 9 tapering. Setae on tergite 9 similar to these on the gonocoxite. Cerci fused, protruding over tergite 9, with strong apical setae deviating from other setosity. The gonocoxite with elongated dorsoapical and ventroapical lobes. Dorsoapical lobe of the gonocoxite dorsobasally right-angled and apically tapering, both well exposed in lateral view and with subapical medially directed hump. Dorsomedial margin of the gonocoxite slightly undulating. Ventroapical lobe of the gonocoxa apically rounded and subapically somewhat deformed. The gonocoxite ventrobasally with wide shelving incision and ventroapically well sclerotised, with a medial cleft. The gonostylus with two branches. The dorsal branch kidney-shaped, slightly widening towards medial line. The ventral branch elongated, apically evenly rounded, with a clear medial widening which bears strong setae well deviating from other setosity of the branch. The medial widening of the ventral branch of gonostylus connected with apical part of the ventroapical lobe of gonocoxite. Parameres long, sinuous, protruding over ventroapical margin of gonocoxite.
Female. Unknown.
Biology.
Unknown.
Etymology.
The species is named in honour of Dr. Jukka Salmela, who kindly provided us the material collected from Finland.
Specific discussion.
Following the key by Zaitzev (1994) the new species runs to Neuratelia sintenisi Lackschewitz, 1937, as its foretibia is usually shorter than fore-basitarsus. However, this character seems to be variable, as these are of equal length in one paratype, guiding to Neuratelia nemoralis, a species with so far reported Holarctic distribution (Laffoon 1965, Zaitzev 1994, but see specific discussion under the latter). In sharing the general outline of male terminalia, Neuratelia salmelai resembles in addition to Neuratelia nemoralis also to Neuratelia kamijoi Sasakawa, 2004 from Japan. All three species have gonocoxite with protruding lobes dorsoapically and ventroapically, and two-branched gonostylus. Neuratelia kamijoi has the dorsoapical lobe of gonocoxite with clear subapical tooth (cf. Sasakawa 2004: fig. 5) while in the other two species it is more simple. Neuratelia salmelai differs markedly from Neuratelia nemoralis as follows: 1) dorsoapical lobe of gonocoxite apically tapering (apically evenly rounded in Neuratelia nemoralis), 2) dorsal branch of gonostylus kidney-shaped and slightly widening towards medial line (elongated, curved and sharply widening towards medial line in Neuratelia nemoralis), and 3) ventral branch of gonostylus apically evenly rounded with medial widening that bears strong setae deviating well from other setosity of the lobe (ventral branch of gonostylus apically pointed with subapical widening that bears normal setae not deviating from other setosity of the branch in Neuratelia nemoralis).
Neuratelia nemoralis
(Meigen, 1818)
Studied material.
FINLAND. 1♂, Lkor: Savukoski, Törmäoja, Malaise trap, 67°50'48,5"N, 029°28'20,8"E, 14.vi–10.vii.2012, J. Salmela leg. (MYCE-JS-2013-0095 in ZMUT, in alcohol with terminalia in glycerine); 1♂, Lkor: Sodankylä, Tarmpomapää, Malaise trap, 67°59'14,0"N, 025°55'09,4"E, 1.–29.ii.2009, J. Salmela leg. (MYCE-JS-2013-0235 in ZMUT, in alcohol with terminalia in glycerine); 1♂, Ab: Turku, Pomponrahka, Malaise trap, 2011, J. Salmela leg., (IZBE0200286, in alcohol with terminalia in glycerine). ESTONIA. 2♂♂, Matsalu NP, Matsalu village, window trap, 58°44'04"N, 023°42'42"E, 29.v–17.vi.2009, I. Süda leg. (IZBE0200275, IZBE0200276, in alcohol); 7♂♂, Palupõhja, Kaha (ME 57), Malaise trap, 58°25'54,68"N, 026°14'28,90"E, 31.v–15.vi.2009, V. Soon leg. (IZBE0200277– IZBE0200282, in alcohol; IZBE0200283, slide mounted in Euparal). SLOVAKIA. 1♂, NP Slovenský raj, Javorina Mt., sweeping, 48°53'23,1"N, 020°15'20,8"E, 1112 m a.s.l., 4.vi.2009, O. Kurina leg (IZBE0200284, in alcohol with terminalia in glycerine; earlier published in Ševčík and Kurina 2011: 101). GREECE. 1♂, Central Macedonia, Kerkini lakes area, Vironia village, Beabies site, 41°19'15,4"N, 023°13'39,6"E, 1150 m a.s.l., Malaise trap, 19.–25.v.2008, G. Ramel leg. (IZBE0200285, in alcohol with terminalia in glycerine).
Specific discussion.
This is a widely distributed species in the Palaearctic region (Zaitzev 1994, Sasakawa 2004, Chandler 2013), and as far as we know supposed to extend also to North America (Laffoon 1965, Chandler 2013). The species was first reported from the Nearctic region by Coquillett (1900: 391) and thereafter by Johannsen (1911: 264, Fig. 145) and Fisher (1937: 171, Plate 12: Fig. 12), while all subsequently published information is of secondary nature. However, Fisher (1937: 171) already questioned conspecificity of the Nearctic material and as far as we can judge from the figures of both authors, these represent a different species. Thus, the occurrence of Neuratelia nemoralis in the Nearctic region remains open with need for the future study.
Discussion
This study combines for the first time the results of morphological and molecular analyses for delineating species of fungus gnats. As a common practice in insect taxonomy, we relied on characters of male genitalia and the mitochondrial COI barcoding, respectively. In one case, however, these two types of data provided conflicting evidence for species delimitation in the fungus gnat genus Neuratelia. Therefore, additional characters were sought by sequencing also the 28S and ITS2 regions of the nuclear ribosomal DNA. The latter is becoming increasingly applicable in delimitation of insect taxa (e.g. Rokas et al. 2002, Wilkerson et al. 2004, Haarto and Ståhls 2014). While COI has successfully been used in studies on fungus gnat taxonomy (Martinsson et al. 2011, Rindal et al. 2009b, Ševčík et al. 2013, 2014) and ecology (Põldmaa et al. 2015), ITS2 has been incorporated only in a few studies (Ševčík et al. 2013, 2014).
Taxonomic work on insects has mostly been carried out on the basis of morphological examination. In many cases where studying external characters fail to yield unequivocal results, genital morphology has been proven to be valuable source of additional information (Hosken and Stockley 2004). In more complicated cases, however, even the most detailed morphological study can remain inconclusive. One of the ‘classic’ scenarios where morphological examination may produce questionable results is allopatry. Thus, solving the taxonomic status of morphologically similar allopatric populations has for long time been one of the key questions for systematists. There has been no clear practice how to handle such cases, as acquisition of diagnostic characters does not always happen in the same order or at the same rate for different groups of organisms (e. g. Mutanen et al. 2012). Morphological study of Neuratelia caucasica and Neuratelia jabalmoussae presents one more case following the ‘classic’ scenario: these species have clearly separate geographic ranges located no less than a thousand kilometres away from each other but their morphological differences are minute. However, the genetic distance (quantified according to the Kimura 2-parameter model) calculated from the COI barcoding region is only 1.5% between the two specimens of Neuratelia caucasica, but ranges from 4.0% to 4.3% between that species and Neuratelia jabalmoussae (Fig. 4). Many studies have shown that intraspecific genetic distance in the barcode region is several times smaller than the interspecific genetic distance (e. g. Hausmann et al. 2011, Huemer et al. 2014, Pentinsaari et al. 2014). Average intraspecific genetic distance remains under 1% in different insect orders, with only few known exceptions (e. g. Hebert et al. 2010, Park et al. 2011, Pentinsaari et al. 2014). The 4% difference between Neuratelia caucasica and Neuratelia jabalmoussae exceeds usual intraspecific genetic variation in insects for more than 4 times, suggesting these taxa truly are different species. This conclusion is further corroborated by few substitutions and small length variation, both in the ITS2 and 28S regions of rDNA distinguishing the two species. The three gene regions thus provide evidence for considering Neuratelia caucasica and Neuratelia jabalmoussae to represent distinct species.
The situation with Neuratelia salmelai and Neuratelia nemoralis is, however, much more intriguing. Regarding these species, there are five COI barcode haplotypes in our data matrix that have a maximum 1% genetic distance. Specimens of Neuratelia salmelai and Neuratelia nemoralis are impossible to distinguish from each other on the basis of barcode data, as the holotype of Neuratelia salmelai from Estonia carries COI sequence that is identical to that of two specimens of Neuratelia nemoralis from two different regions of Finland. A Finnish paratype of Neuratelia salmelai, on the other hand, has COI sequence identical to that of a specimen of Neuratelia nemoralis from Slovakia. Such a situation has been called ‘barcode sharing’ in literature (e.g. Hausmann et al. 2011, 2013). Additionally, the relatively rapidly evolving sequences of nuclear rDNA 28S and ITS2, did not allow us to distinguish Neuratelia salmelai and Neuratelia nemoralis. 28S was identical in all studied specimens of both species, whereas one individual of Neuratelia nemoralis from Greece had ITS2 haplotype identical to that of both specimens of Neuratelia salmelai, which differ from the remaining specimens of Neuratelia nemoralis by one substitution in ITS2. Thus, delineating Neuratelia salmelai from Neuratelia nemoralis on the basis of current molecular data is not possible regardless of whether a distance-based or character-based (DeSalle et al. 2005) approach is selected.
In contrast to the failure of genetic markers to distinguish Neuratelia salmelai and Neuratelia nemoralis, their male terminalia were remarkably different. The differences are more pronounced than among the other three species included in this study. Most likely these taxa represent recently diverged species that still share the genetic diversity of their common ancestor. The evolution of insect genitalia can be more rapid than diversification of commonly studied markers (Raupach et al. 2010, Hausmann et al. 2013). Another possibility, hybridisation between females of Neuratelia nemoralis and males of some other fungus gnat species, deserves less credit for at least two reasons. First, though interspecific hybridisation sometimes occurs in closely related insects, hybrids usually are confined to clear hybrid zones or exist in particular sympatric populations (Mallet et al. 2011, Sánchez-Guillén et al. 2014). Therefore they constitute only a small proportion of the total population. In the current case the five males of Neuratelia salmelai are 26% of the 19-individual sample of Neuratelia nemoralis group in our study. This is an unrealistically high proportion for hybrids, as material was randomly collected from different parts of these species’ ranges, not concentrating on a particular region where hybridisation could occur. Second, if there really had been hybridisation, it would be natural to assume that putative hybrids (i. e. Neuratelia salmelai) share genetic material with specimens collected from geographically close localities. This is not the case, as no geographic pattern was detected when collecting localities of Neuratelia salmelai and Neuratelia nemoralis were taken into account. Apparently, the Neuratelia salmelai/Neuratelia nemoralis species pair is one of the rare occasions where nucleotide data from common markers and morphological characters do not corroborate each other. Large-scale barcoding projects have shown that such cases usually constitute no more than one or two per cents of the total diversity of insects (e. g. Mutanen et al. 2012, Huemer et al. 2014, Pentinsaari et al. 2014).
Supplementary Material
Acknowledgements
The study was supported by the grants 9174 and 8583 of the Estonian Science Foundation by institutional research funding (IUT21-1, IUT20-30 and IUT20-33) of the Estonian Ministry of Education and Research and the European Union through the European Regional Development Fund (Centre of Excellence FIBIR). We are grateful to Dr. P. Vilkamaa (Helsinki, Finland) and Dr. J. Salmela (Turku, Finland) for providing material collected from Lebanon and Finland, respectively. Prof. T. Saigusa (Fukuoka, Japan) and Dr. J. Kjærandsen (Tromsø, Norway) are thanked for invaluable comments on the species. We are grateful to J. Kullberg (Helsinki, Finland) for collecting the material of Neuratelia jabalmoussae and for sending the photographs of collecting locality in Lebanon and to Prof. T. Tammaru (Tartu, Estonia) for intermediating this. We thank R. Puusepp (Tartu, Estonia) for technical assistance with sequencing. Dr. R. Davis (Tartu, Estonia) kindly checked the English language of the manuscript.
Citation
Kurina O, Õunap E, Põldmaa K (2015) Two new Neuratelia Rondani (Diptera, Mycetophilidae) species from Western Palaearctic: a case of limited congruence between morphology and DNA sequence data. ZooKeys 496: 105–129. doi: 10.3897/zookeys.496.9315
References
- Abraham D, Ryrholm N, Wittzell H, Holloway JD, Scoble MJ, Löfstedt C. (2001) Molecular phylogeny of the subfamilies in Geometridae (Geometroidea: Lepidoptera). Molecular Phylogenetics and Evolution 20: 65–77. doi: 10.1006/mpev.2001.0949 [DOI] [PubMed] [Google Scholar]
- Bechev D. (2000) World distribution of the genera of fungus gnats (Diptera: Sciaroidea, excluding Sciaridae). Studia dipterologica 7: 543–552. [Google Scholar]
- Beebe NW, Saul A. (1995) Discrimination of all members of the Anopheles punctulatus complex by polymerase chain reaction restriction fragment length polymorphism analysis. American Journal of Tropical Medicine and Hygiene 53: 478–481. [DOI] [PubMed] [Google Scholar]
- Belshaw R, Quicke DLJ. (1997) A molecular phylogeny of the Aphidiinae (Hymenoptera, Braconidae). Molecular Phylogenetics and Evolution 7: 281–293. doi: 10.1006/mpev.1996.0400 [DOI] [PubMed] [Google Scholar]
- Borkent CJ, Wheeler TA. (2013) Phylogeny of the tribe Sciophilini (Diptera: Mycetophilidae: Sciophilinae). Systematic Entomology 38: 407–427. doi: 10.1111/syen.12002 [Google Scholar]
- Chandler PJ. (2013) Fauna Europaea: Mycetophilidae. In: Beuk P, Pape T. (Eds) Fauna Europaea: Diptera, Nematocera. Fauna Europaea, version 2.6. http://www.faunaeur.org [accessed 20.v.2014]
- Coquillett DW. (1900) Papers from the Harriman Alaska expedition. IX. Entomological results (3): Diptera. Proceedings of the Washington Academy of Sciences 2: 389–464. [Google Scholar]
- DeSalle R, Egan MG, Siddall M. (2005) The unholy trinity: taxonomy, species delimitation and DNA barcoding. Philosophical Transactions of the Royal Society B-Biological Sciences 360: 1905–1916. doi: 10.1098/rstb.2005.1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörge DD, Zaenker S, Klussmann-Kolb A, Weigand AM. (2014) Traversing worlds - Dispersal potential and ecological classification of Speolepta leptogaster (Winnertz, 1863) (Diptera, Mycetophilidae). Subterranean Biology 13: 1–16. doi: 10.3897/subtbiol.13.6460 [Google Scholar]
- Evenhuis NL. (2014) Catalog of the fossil flies of the world (Insecta: Diptera) (Version 2.0). http://hbs.bishopmuseum.org/fossilcat/fossmyceto.html Version [2.iv.2014] [accessed 19.v.2014]
- Fisher EG. (1937) A comparative study of the male genitalia of the Mycetophilidae of Nearctic America. Unpublished Ph.D. thesis Cornell University. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. (1994) DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299. [PubMed] [Google Scholar]
- Haarto A, Ståhls G. (2014) When mtDNA COI is misleading: congruent signal of ITS2 molecular marker and morphology for North European Melanostoma Schiner, 1860 (Diptera, Syrphidae). ZooKeys 431: 93–134. doi: 10.3897/zookeys.431.7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hausmann A, Haszprunar G, Hebert PDN. (2011) DNA barcoding the geometrid fauna of Bavaria (Lepidoptera): successes, surprises, and questions. PLoS ONE 6(2): . doi: 10.1371/journal.pone.0017134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann A, Godfray HCJ, Huemer P, Mutanen M, Rougerie R, van Nieukerken EJ, Ratnasingham S, Hebert PDN. (2013) Genetic patterns in European geometrid moths revealed by the barcode index number (BIN) system. PLoS ONE 8(12): . doi: 10.1371/journal.pone.0084518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert PD, Cywinska A, Ball SL, deWaard JR. (2003) Biological identifications through DNA barcodes. Proceedings of the Royal Society B: Biological Sciences 270: 313–321. doi: 10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert PDN, deWaard JR, Landry J-F. (2010) DNA barcodes for 1/1000 of the animal kingdom. Biology letters 6: 359–362. doi: 10.1098/rsbl.2009.0848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippa H, Kurina O. (2012) New species and new records of Afrotropical Manota Williston (Diptera, Mycetophilidae), with a key to the species. Zootaxa 3455: 1–48. [Google Scholar]
- Hippa H, Ševčík J. (2014) Notes on Nepaletricha (Diptera: Sciaroidea incertae sedis), with description of three new species from India and Vietnam. Acta Entomologica Musei Nationalis Pragae 54(2): 729–739. [Google Scholar]
- Hosken DJ, Stockley P. (2004) Sexual selection and genital evolution. Trends in Ecology and Evolution 19: 87–93. doi: 10.1016/j.tree.2003.11.012 [DOI] [PubMed] [Google Scholar]
- Huemer P, Hebert PDN. (2011) Cryptic diversity and phylogeography of high alpine Sattleria – a case study combining DNA barcodes and morphology (Lepidoptera: Gelechiidae). Zootaxa 2981: 1–22. [Google Scholar]
- Huemer P, Mutanen M, Sefc KM, Hebert PDN. (2014) Testing DNA barcode performance in 1000 species of European Lepidoptera: large geographic distances have small genetic impacts. PLoS ONE 9(12): . doi: 10.1371/journal.pone.0115774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson AM, Ackland DM, Kidd LN. (1980) Mycetophilidae (Bolitophilinae, Ditomyiinae, Diadocidiinae, Keroplatinae, Sciophilinae and Manotinae) Diptera, Nematocera. Handbooks for the Identification of British Insects, London, 9(3), 111 pp. [Google Scholar]
- Johannsen OA. (1911) The fungus gnats of North America Part III. Maine Agricultural Experiment Station Orono 196: 249–328. doi: 10.5962/bhl.title.86513 [Google Scholar]
- Kurina O. (2003) Notes on the Palaearctic species of the genus Polylepta Winnertz (Diptera: Mycetophilidae) with a new synonymization. Entomologica Fennica 14(2): 91–97. [Google Scholar]
- Kurina O, Õunap E, Ramel G. (2011) Baeopterogyna mihalyii Matile (Diptera, Mycetophilidae): association of sexes using morphological and molecular approaches with the first description of females. ZooKeys 114: 15–27. doi: 10.3897/zookeys.114.1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackschewitz P. (1937) Die Fungivoriden des Ostbaltischen Gebites. Arbeiten des Naturforscher-Vereins zu Riga [N. F. ] 21: 1–47. [Google Scholar]
- Laffoon JL. (1965) Superfamily Mycetophiloidea. In: Stone A, et al. (Eds) A catalog of the Diptera of America north of Mexico. Agricultural Research Service U.S.No 276, 196–129. [Google Scholar]
- Lanfear R, Calcott B, Ho SYW, Guindon S. (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695–1701. doi: 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- Lundström C. (1912) Neue oder wenig bekannte europäische Mycetophilidae II. Annales historico-naturales Musei nationalis hungarici 9: 514–522. [Google Scholar]
- Mallet J, Wynne IR, Thomas CD. (2011) Hybridisation and climate change: brown argus butterflies in Britain (Polyommatus subgenus Aricia). Insect Conservation and Diversity 4: 192–199. doi: 10.1111/j.1752-4598.2010.00122.x [Google Scholar]
- Martinsson S, Kjærandsen J, Sundberg P. (2011) Towards a molecular phylogeny of the fungus gnat genus Boletina (Diptera: Mycetophilidae). Zoologica Scripta 40: 272–281. doi: 10.1111/j.1463-6409.2011.00474.x [Google Scholar]
- Matile L. (1974) Notes sur les Mycetophilidae (Diptera) de la Faune de France. III. Le genre Neuratelia. l’Entomologiste 28(1972): 26–33. [Google Scholar]
- Meigen JW. (1818) Systematische Beschreibung der bekannten europaischen zweiflugeligen Insekten 1 Aachen, xxxvi + 332 + [1] pp., 11 pls. [Google Scholar]
- Mutanen M, Hausmann A, Hebert PDN, Landry J-F, de Waard JR, Huemer P. (2012) Allopatry as a Gordian knot for taxonomists: patterns of DNA barcode divergence in Arctic-Alpine Lepidoptera. PLoS ONE 7(10): . doi: 10.1371/journal.pone.0047214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Õunap E, Viidalepp J. (2009) Description of Crypsiphona tasmanica sp. n. (Lepidoptera: Geometridae: Geometrinae), with notes on limitations in using DNA barcodes for delimiting species. Australian Journal of Entomology 48: 113–124. doi: 10.1111/j.1440-6055.2009.00695.x [Google Scholar]
- Park D-S, Foottit R, Maw E, Hebert PDN. (2011) Barcoding bugs: DNA-based identification of the true bugs (Insecta: Hemiptera: Heteroptera). PLoS ONE 6: . doi: 10.1371/journal.pone.0018749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentinsaari M, Hebert PDN, Mutanen M. (2014) Barcoding beetles: a regional survey of 1872 species reveals high identification success and unusually deep interspecific divergences. PLoS ONE 9(9): . doi: 10.1371/journal.pone.0108651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Põldmaa K, Jürgenstein S, Bahram M, Teder T, Kurina O. (2015) Host diversity and trophic status as determinants of species richness and community composition of fungus gnats. Basic and Applied Ecology 16: 46–53. doi: 10.1016/j.baae.2014.10.004 [Google Scholar]
- Raupach MJ, Astrin JJ, Hannig K, Peters MK, Stoeckle MY, Wägele J. (2010) Molecular species identification of Central European ground beetles (Coleoptera: Carabidae) using nuclear rDNA expansion segments and DNA barcodes. Frontiers in Zoology 7: . doi: 10.1186/1742-9994-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel A, Sagata K, Surbakti S, Tänzler RM. (2013) One hundred and one new species of Trigonopterus weevils from New Guinea. Zookeys 280: 1–150. doi: 10.3897/zookeys.280.3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindal E, Søli GEE, Bachmann L. (2009a) On the systematics of the fungus gnat subfamily Mycetophilinae (Diptera): a combined morphological and molecular approach. Journal of Zoological Systematics and Evolutionary Research 47(3): 227–233. doi: 10.1111/j.1439-0469.2008.00498.x [Google Scholar]
- Rindal E, Søli GEE, Bachmann L. (2009b) Molecular phylogeny of the fungus gnat family Mycetophilidae (Diptera, Mycetophiliformia). Systematic Entomology 34: 524–532. doi: 10.1111/j.1365-3113.2009.00474.x [Google Scholar]
- Rokas A, Nylander JAA, Ronquist F, Stone GN. (2002) A maximum-likelihood analysis of eight phylogenetic markers in gallwasps (Hymenoptera: Cynipidae): implications for insect phylogenetic studies. Molecular Phylogenetics and Evolution 22: 206–219. doi: 10.1006/mpev.2001.1032 [DOI] [PubMed] [Google Scholar]
- Sánchez-Guillén RA, Muñoz J, Hafernik J, Tierney M, Rodriguez-Tapia G, Córdoba-Aguilar A. (2014) Hybridization rate and climate change: are endangered species at risk? Journal of Insect Conservation 18: 295–305. [Google Scholar]
- Sasakawa M. (2004) Fungus Gnats of the Genus Neuratelia Rondani in Japan (Diptera: Mycetophilidae). Esakia 44: 67–79. [Google Scholar]
- Søli GEE, Vockeroth JR, Matile L. (2000) A.4. Families of Sciaroidea. In: Papp L, Darvas B. (Eds) Contribution to a Manual of Palaearctic Diptera. Appendix. Science Herald, Budapest, 49–92. [Google Scholar]
- Ševčík J, Kurina O. (2011) Fungus gnats (Diptera: Sciaroidea) of the Gemer region (Central Slovakia): Part 2 – Mycetophilidae. Časopis Slezského Zemského Muzea (A) 60: 97–126. doi: 10.2478/v10210-011-0011-x [Google Scholar]
- Ševčík J, Kaspřák D, Tóthová A. (2013) Molecular phylogeny of fungus gnats (Diptera: Mycetophilidae) revisited: position of Manotinae, Metanepsiini, and other enigmatic taxa as inferred from multigene analysis. Systematic Entomology 38: 654–660. doi: 10.1111/syen.12023 [Google Scholar]
- Ševčík J, Kaspřák D, Mantič M, Ševčíková T, Tóthová A. (2014) Molecular phylogeny of the fungus gnat family Diadocidiidae and its position within the infraorder Bibionomorpha (Diptera). Zoologica Scripta 43: 370–378. doi: 10.1111/zsc.12059 [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. (2008) A rapid bootstrap algorithm for the RAxML web-servers. Systematic Biology 75: 758–771. doi: 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Molecular Biology and Evolution 30(12): 2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. (1994) ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680. doi: 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieukerken EJ, Doorenweerd C, Stokvis FR, Groenenberg DSJ. (2012) DNA barcoding of the leaf-mining moth subgenus Ectoedemia s. str. (Lepidoptera: Nepticulidae) with COI and EF1-α: two are better than one in recognising cryptic species. Contributions to Zoology 81: 1–24. [Google Scholar]
- Waugh J. (2007) DNA barcoding in animal species: progress, potential and pitfalls. BioEssays 29: 188–197. doi: 10.1002/bies.20529 [DOI] [PubMed] [Google Scholar]
- Wilkerson RC, Reinert JF, Li C. (2004) Ribosomal DNA ITS2 Sequences Differentiate Six Species in the Anopheles crucians Complex (Diptera: Culicidae). Journal of Medical Entomology 41(3): 392–401. doi: 10.1603/0022-2585-41.3.392 [DOI] [PubMed] [Google Scholar]
- Yassin A. (2008) Molecular and morphometrical revision of the Zaprionus tuberculatus species subgroup (Diptera: Drosophilidae), with descriptions of two cryptic species. Annals of the Entomological Society of America 101: 978–988. doi: 10.1603/0013-8746-101.6.978 [Google Scholar]
- Zaitzev AI. (1994) Fungus gnats of the fauna of Russia and adjacent regions. Part 1 Moscow, 288 pp [in Russian, with English summary] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.