Abstract
Because of its favorable anatomical and immunological characteristics, the eye has been at the forefront of translational gene therapy. Dozens of promising proofs of concept have been obtained in animal models of inherited retinal degenerations (IRDs), and some of them have been relayed to the clinic. The results from the first clinical trials for a congenital form of blindness have generated great interest and have demonstrated the safety and efficacy of intraocular administrations of viral vectors in humans. However, this progress has also generated new questions and posed challenges that need to be addressed to further expand the applicability of gene therapy in the eye, including safe delivery of viral vectors to the outer retina, treatment of dominant IRDs as well as of IRDs caused by mutations in large genes, and, finally, selection of the appropriate IRDs and patients to maximize the efficacy of gene transfer. This review summarizes the strategies that are currently being exploited to overcome these challenges and drive the clinical development of retinal gene therapy.
Clinical Trials of Gene Therapy for IRDs: Success and Challenges Ahead
Inherited retinal degenerations (IRDs), a major cause of severe vision impairment, affect more than 2 million people worldwide.1 IRDs are a group of diseases with high genetic heterogeneity and differences in inheritance patterns, age of onset, and severity of visual dysfunction.2,3 Mutations in more than 200 genes mainly expressed in photoreceptors (PR) (Fig. 1B), and to a lesser extent in the retinal pigment epithelium (RPE) (Fig. 1B), cause IRDs.4,5 Sight-restoring therapy for many IRDs is still a major unmet medical need. However, in the last decades, the identification of many IRD-causing genes has paved the way for the development of gene-based therapies.
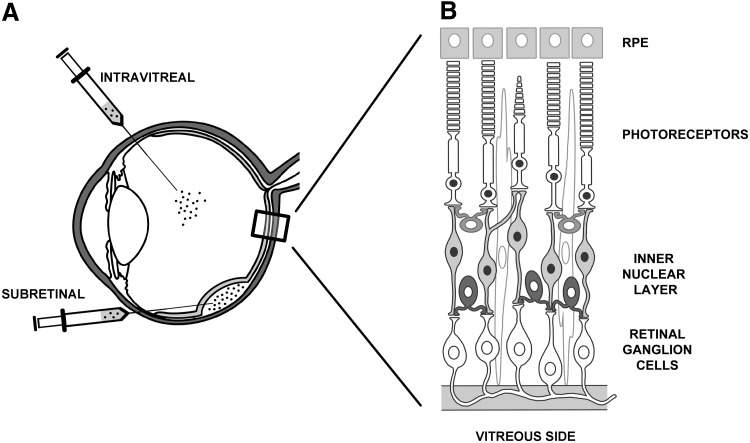
FIG. 1.
Schematic representation of intraocular injection routes (A) and retinal layers (B).
The eye has been at the forefront of translational gene therapy because of its small, enclosed structure, immune privilege, and easy accessibility. In addition, the availability of various animal models, along with in vivo imaging techniques, allows for noninvasive and consistent monitoring of the effects of gene delivery; outcomes may be compared with disease progression in the contralateral control eye.6,7 Leber congenital amaurosis type 2 (LCA2) is the first IRD to have been treated with retinal gene therapy in phase I/II clinical trials, the results of which represent the most successful example of ocular gene therapy, to date. LCA2, an autosomal recessive IRD, is caused by mutations in RPE65, an essential gene in the retinal pathway. RPE65 encodes an isomerase protein expressed in the RPE that promotes visual chromophore recycling. LCA2 is an ideal candidate disease for gene therapy because RPE65 deficiency causes defective visual cycle and poor visual function early in life8,9; LCA2 retinal structure is left fairly intact until the second to third decade of life, the period in which progressive PR degeneration becomes evident.8,10 After subretinal administration of a gene therapy vector based on adeno-associated virus (AAV) type 2 (AAV2/2) was shown to induce therapeutic effects in small and large LCA2 animal models, three independent clinical trials were launched (NCT00516477; NCT00643747; NCT00481546). The longest comprehensive follow-up of treated patients reported to date is 3 years.11,12
Although it is difficult to directly compare the results of the three studies because of their several variables, data gathered from these clinical trials collectively indicate that gene therapy is both safe and effective.11,13–19 Patients from all trials exhibited improved retinal and visual function, though to different extents, and reactivation of the visual cortex has been described in one trial.20 Maximal efficacy was obtained in the youngest LCA2 patients, who presumably had better retinal preservation and function to begin with.17 Importantly, injection of the vector in the contralateral eyes of three LCA2 patients who had been previously treated with the same vector was shown to be safe and effective.21 This implies that subretinal readministration of AAV2/2 is feasible, even in the case of preexisting immunity to the vector, a criterion that has been used to exclude patients from gene therapy trials involving systemic vector administrations.22,23
The biotechnology company Spark Therapeutics is now testing AAV2/2-RPE65 gene therapy for LCA2 in an advanced phase III clinical trial, a study including patients as young as 3 years of age (NCT00999609). The objective of the study is to file for regulatory approval in the United States as early as 2016.24 An alternative gene therapy approach for LCA2 treatment is now under evaluation in a clinical trial that uses the RPE65 promoter in combination with AAV2/4 (NCT01496040) that targets transgene expression specifically to the RPE. This may increase the specificity and efficacy of the therapy.25 Importantly, successful LCA2 clinical trials have encouraged broader application of gene therapy to IRDs due to mutations in genes expressed in various retinal layers, such as MERTK, expressed in the RPE and mutated in retinitis pigmentosa type 38 (RP38; NCT01482195)26; ABCA4, expressed in PR and whose deficiency leads to Stargardt disease (STGD1; NCT01367444)27,28; MYO7A, expressed in both RPE and PR and found mutated in individuals with Usher syndrome type 1B (USH1B; NCT01505062)29; ND4, expressed in retinal ganglion cells and mutated in Leber hereditary optic neuropathy (NCT01267422, NCT02064569)30,31; and CHM1, expressed in multiple retinal cell types and mutated in choroideremia (CHM, NCT01461213).32 The initial results of the phase I/II CHM clinical trial have been recently published and have confirmed that subretinal administration of AAV2/2 is well tolerated in humans.32 In addition, recovery of visual acuity and improvement in maximal retinal sensitivity in treated eyes were observed despite retinal detachment.
Although preliminary data from these first clinical trials are extremely promising and bode well for further development of retinal gene therapy, some issues have been raised. Subretinal administration of the vector-containing solution which causes transient retinal detachment13,16,18 in the LCA2 parafoveal region, in some cases has resulted in permanent retinal damage.11,18 This raises concerns about using this surgical procedure in retinal tissues diminished by degenerative processes. In addition, in one trial, despite observed visual improvement, the rate of progression of retinal degeneration in the vector-treated retina was reported to be similar to that in the contralateral untreated eye,33 raising concerns about the longevity of the effects. Finally, unlike what was observed in LCA2 dogs,25,34–36 no improvement in the full-field electroretinogram has thus far been reported in LCA2 patients treated with AAV. Interspecies differences in either levels of RPE65 expression or AAV retinal transduction efficiency may explain this and suggest that there is room for improvement in LCA2 retinal gene therapy.
Some of the challenges that the retinal gene therapy field is facing after these trials are discussed below along with strategies undertaken to overcome them.
Safe and Effective Delivery of Vectors to the Outer Retina: Are We There Yet?
Subretinal injections, which release the vector into the subretinal space (Fig. 1A), transduce PR and RPE, the two cell types in which the majority of genes mutated in IRD are expressed,37 most effectively thus far. However, subretinal injections are technically challenging, and concerns about their invasiveness, especially in patients with diseased retinas, have been raised in some of the LCA2 clinical trials in which foveal thinning, macular holes, choroidal effusions, and ocular hypo- and hypertension have been reported.11,18 Indeed, the interaction between foveal PR and the underlying RPE in the primate retina is very strong; processes emerging from the apical surface of the RPE form a multilaminar sheath that wraps around the outer segment tips.38 This may explain why foveal reattachment is more complex than extrafoveal reattachment. In fact, subretinal injections in extrafoveal regions exhibiting greater outer nuclear layer thickness than the fovea are being considered in light of the generation of new “pseudofoveas” resulting from vector transduction15,32,39 (see below). Alternative, less invasive administration, such as intravitreal injection (Fig. 1A), would broadly distribute the vector throughout the retina without causing risky retinal detachment. However, when injected intravitreally, most viral vectors, including the majority of AAV serotypes, do not transduce the retina with the exception of AAV2/2, and to some extent AAV2/6 and AAV2/8, whose transduction is, however, mainly restricted to retinal ganglion and Müller cells in the inner retina.6,40 The failure of vectors delivered intravitreally to transduce PR and RPE in the outer retina appears to be caused by the presence of physical barriers, such as the inner limiting membrane, which is particularly thick in large animals, as well as the relative abundance of AAV receptors that capture vectors after intravitreal administration.41 Indeed, if retinal architecture is altered by a degenerative process42–44 or by enzymatic digestion,41 the diffusion of AAV viral particles to the outer retina from the vitreous side increases.
Recent efforts have been focused on engineering the AAV capsid to favor its diffusion from the vitreous side. Quadruple and pentuple tyrosine mutant AAV2/2 vectors provided the first proof of concept of the feasibility of outer retina transduction from the vitreous in mice.45,46 More recently, Dalkara and co-workers used an in vivo directed evolution approach to select an AAV2/2 variant (7m8) that was able to transduce mouse PR and RPE following intravitreal injection.47 However, intravitreal injection of both tyrosine and 7m8 AAV2/2 mutants in larger animal models failed to reproduce the outer retina transduction levels observed in mice, presumably because of more pronounced physical barriers in larger animals than in mice.47,48 Further understanding of retinal barriers inhibiting transduction as well as in vivo directed evolution performed directly in large animals may lead in future to more efficient ways to target the primate retina via diffusion from the vitreous. However, until this is the case, subretinal delivery remains the most efficient administration route to target RPE and PR.
Overcoming the Challenge of Delivering Large Genes to the Retina
Despite the popularity gained by AAV vectors, one of the main obstacles to their widespread application is their packaging capacity of ∼5 kb, precluding them from being used to treat IRDs like STGD and USH1B, which are caused by mutations in genes whose coding sequence exceeds 5 kb. Thus, several investigators are exploring alternative vectors with larger cloning capacities than AAV, such as adenoviral (Ad) and lentiviral (LV) vectors, as well as DNA nanoparticles (NP). Ad were the first viral vectors to be tested successfully in the retina.49 However, Ad vectors efficiently transduce the RPE but less robustly adult mouse PR.6,50,51 Similarly, LV vectors have been successfully used to transduce PR in newborn rat and mouse retinas and thus, to effectively improve the phenotype of animal models of IRDs.27,29,52–56 Although recent reports suggest that LV vectors transduce adult nonhuman primate PR,28,29 RPE transduction has been primarily observed in adult animals.51,52,57–60 The thicker and more developed physical barriers, which are found in adult as opposed to newborn mouse retina, have been hypothesized to limit adult PR transduction by large particles such as Ad and LV.61–63 Indeed, the efficiency of PR transduction in adult animals can be improved by enzymatic disruption of the inter-PR matrix or by advanced retinal degeneration.61–63 Despite the drawbacks of LV for PR transduction, two recent phase I/II clinical trials in STGD1 and USHIB patients (NCT01367444 and NCT01505062, respectively) may provide alternative platforms for gene therapy of IRDs caused by mutations in large genes. Nonviral vectors with large cargo capacity, such as polyethylene glycol-substituted 30-mer lysine peptides (CK30-PEG)-compacted DNA NP, have been shown to safely deliver genes, including those exceeding 5 kb in size, to PR and RPE.64–67 Thus, compacted DNA NP are a potentially viable option for delivery of large genes to PR. However, limited experience with these vectors, along with the need to clarify their transduction characteristics in large animal models, including nonhuman primates, prompts further testing before compacted DNA NP can be used to deliver large genes to human PR.
As an alternative platform has yet to convincingly match the PR transduction ability of AAV, considerable interest has been directed toward expanding AAV cargo capacity. Efforts at packaging large genes into single AAV particles have resulted in the generation of oversized AAV vectors that, although able to transduce large genes in PR,68,69 contain genomes highly heterogenous in size,70–74 potentially posing a major safety concern for their further clinical development. Alternatively, the inherent ability of AAV genomes to concatemerize75 has been exploited to generate dual AAV vectors, each containing one of two halves of a large gene expression cassette. Different dual AAV strategies (referred to as trans-splicing,76 overlapping,77 and hybrid dual-vector strategies78) have been used to efficiently deliver large genes to various tissues, including the retina.76,78–88 Various groups have independently reported that dual AAV trans-splicing and hybrid vectors efficiently reconstitute large genes in mouse81,82,89,90 and pig PR.82,83 Although the levels of PR transduction achieved with dual AAV vectors are lower than those achieved with a single AAV vector (∼4% in mice and 40% in pigs82,83), subretinal administration of dual AAV vectors has been shown to significantly improve the phenotype of mouse models of STGD1 and USH1B.81,82 Current efforts aim at improving the levels of transgene expression from dual AAV while reducing those of shorter proteins produced from either the 5′ and 3′ half vector of dual AAV.82,90 Considering the low levels of PR transduction achieved by vectors with high cargo capacity and the improved cargo capacity offered by dual AAV vectors in the retina, they may be the preferred platform for delivery of large genes to PR in IRDs.
Suppressing and Replacing Rhodopsin: Easier Said than Done
One-third of IRD patients with a recognizable pattern of inheritance are affected by dominant forms of the disease.2 Many cases are because of mutations resulting in toxic gain-of-function effects.3 In these cases, reducing the toxic product rather than adding a correct copy of the gene is required to provide significant benefits. Given that more than 150 different mutations associated with dominant retinitis pigmentosa have been described in the rhodopsin (RHO) gene alone, most of the gene therapy efforts to date have been directed to silence RHO mutations. Allele-specific catalytic RNAs, including ribozymes91,92 and short hairpin RNA,93 have been explored with variable degrees of efficacy. However, the most efficient silencing has been obtained by targeting both the wild-type and mutant RHO alleles.94–100 As doing so results in robust RHO suppression, the simultaneous addition of a RHO copy resistant to silencing is required as part of the so-called suppression and replacement therapeutic approach to dominant IRD. Alternatively, zinc-finger transcriptional repressors have been designed to silence RHO at the level of its locus,101 a strategy that may theoretically overcome the challenge of silencing this very abundant protein that accounts for >70% of the total rod PR outer segments protein content.102 New-generation customized DNA-binding modules, including TALE and CRISPR-inactive CAS9,103 provide new tools to design effective RHO transcriptional repressors. While RHO expression has been successfully suppressed to therapeutically relevant levels in animal models by several groups,94,97,98,101 its replacement to sufficient levels (at least 50% of endogenous) appears challenging given RHO's high expression levels in PR. However, this is necessary to avoid converting an often mild dominant disease into a more severe recessive condition.
The Importance of Selecting the Right Disease and Patients
Optimal vectors and delivery routes do not guarantee the success of gene therapy. As gene transfer efficacy relies on viable target cells, identifying patients early enough in the course of their condition is crucial to reap the benefits of gene therapy. Conditions like LCA2, LCA1,104 or achromatopsia105 exhibit preserved retinal structure for decades after the diagnosis despite severe visual impairment. These forms could be considered as ideal candidates for gene therapy, not only from a therapeutic perspective, but also from a clinical development standpoint, as the rescue of the functional component of the disease can be tested in a reasonable time frame in the context of clinical trials. Instead, many forms of retinitis pigmentosa show mild phenotypes because of a slowly progressive retinal degeneration. While from a therapeutic perspective these are also good targets for gene therapy, given the wide window of time for therapeutic intervention, clinical development of novel therapies for such conditions appears challenging because preventing degeneration becomes the main endpoint of clinical trials; this requires long-term observation and a detailed knowledge of the natural history of the disease. For many conditions that exhibit quick degeneration in combination with a functional defect (i.e., STGD1 in which transport of retinal is impaired,106 or LCA4 in which key enzymes in the phototransduction cascade are destabilized107), early gene therapy may be predicted to both prevent retinal degeneration and restore visual function in transduced PR, which could be addressed in a reasonable time frame in the context of clinical trials. Incidental findings in LCA2 and CHM clinical trials that perimacular areas transduced by viral vectors are converted into functional “pseudofoveas”15,32,39 are a promising sign that gene therapy can be effectively applied to conditions with advanced stage degeneration should areas with spared PR still be available. In summary, timely intervention, which requires early clinical and molecular diagnosis in combination with a well-characterized natural history of the disease, will be required to maximize efficacy of gene therapy for IRDs. Ultimately, retinal gene therapy is more likely to reach the final stages of clinical development for those conditions deriving from a functional defect that can be reverted by gene delivery than for purely degenerative diseases in which efficacy of gene delivery is predominantly determined by the extent of prevention of further degeneration.
Future Considerations
Recent advancements in high-throughput genotyping techniques for accurate noninvasive in vivo monitoring of retinal and visual function and development of safer and more efficient vectors for retinal gene therapy are factors improving our ability to treat genetic blindness. With these approaches we are able to (1) diagnose conditions with the highest genetic heterogeneity in humans at the molecular level, (2) provide an early and accurate picture of the retinal statuses of these patients, which can be followed up over time, and (3) use gene transfer tools with maximal therapeutic efficacy and minimal local toxicity. The next challenge for the widespread application of gene therapy to many IRD is one that involves funding and infrastructure for clinical translation. Research funding is always a challenge, especially for rare diseases. While preclinical studies can generally be funded through traditional avenues, clinical translation needs more substantial investments for clinical-grade vector production, nonclinical safety testing, regulatory filing, and clinical studies. U.S. and E.U. funding agencies are increasing available funding allocated to support the development of new therapies for rare diseases. Growing budgets are prompting the establishment of collaborations between academic centers, where initial preclinical proof-of-concept studies are performed, and industries, which nurture clinical development by providing the appropriate infrastructures that are required for market approval. On the other hand, biotechs and pharmaceutical companies are beginning to look at rare diseases and gene therapy with more interest than ever before, as the latter has reached the first clinical successes and market approval, and the former are ideal targets to test the safety and efficacy of novel therapeutic platforms. Yet, to develop and test a gene-based treatment for each and every one of the hundreds of different genes involved in IRDs would not be feasible, especially for those rarely mutated in patients. Standardizing production and reagents for those vectors most commonly used in clinical trials has been proposed as a first step in improving the efficiency of translational research.108 In parallel, data sharing (including cross-reference of Investigational New Drug application/Investigational Medicinal Product Dossier) from pharm/tox studies and long-term follow-up in large animal models may promote more efficient development of new approaches.108 In conclusion, translating retinal gene therapy from animal research into clinical trials is still a lengthy process, but the field is actively working on defining the missing points that will allow translation of many promising approaches from bench to bedside.
Acknowledgments
We thank Alexis Burton and Graciana Diez-Roux (Scientific Office, TIGEM, Pozzuoli, Naples, Italy) for the critical reading of this article. The following funding support is gratefully acknowledged: the European Research Council/ERC Grant Agreement No. 282085 “RetGeneTx”; the European Research/ERC Grant Agreement No. 311682 “Allelechoker”; the NIH Grant R24 EY019861-01A; and the Italian Telethon Foundation Grant TGM11MT1.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sahel JA, Marazova K, Audo I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb Perspect Med 2014;5:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res 2010;29:335–375 [DOI] [PubMed] [Google Scholar]
- 3.Dalkara D, Sahel JA. Gene therapy for inherited retinal degenerations. C R Biol 2014;337:185–192 [DOI] [PubMed] [Google Scholar]
- 4.Dryja TP. Retinitis pigmentosa and stationary night blindness. In: OMMBID: The Online Metabolic and Molecular Bases of Inherited Disease. Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson KM. and Mitchell G, eds. (McGraw-Hill Companies, New York, NY: ). Chap. 235, pp. 1–71 [Google Scholar]
- 5.Lewis RA, Allikmets R, Lupski JR. Inherited macular dystrophies and susceptibility to degeneration. In: OMMBID: The Online Metabolic and Molecular Bases of Inherited Disease. Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson KM. and Mitchell G, eds. (McGraw-Hill Companies, New York, NY: ). Chap. 243, pp. 1–35 [Google Scholar]
- 6.Trapani I, Puppo A, Auricchio A. Vector platforms for gene therapy of inherited retinopathies. Prog Retin Eye Res 2014;43:108–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahel JA, Roska B. Gene therapy for blindness. Annu Rev Neurosci 2013;36:467–488 [DOI] [PubMed] [Google Scholar]
- 8.Jacobson SG, Aleman TS, Cideciyan AV, et al. . Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc Natl Acad Sci USA 2005;102:6177–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung DC, Traboulsi EI. Leber congenital amaurosis: clinical correlations with genotypes, gene therapy trials update, and future directions. J AAPOS 2009;13:587–592 [DOI] [PubMed] [Google Scholar]
- 10.Simonelli F, Ziviello C, Testa F, et al. . Clinical and molecular genetics of Leber's congenital amaurosis: a multicenter study of Italian patients. Invest Ophthalmol Vis Sci 2007;48:4284–4290 [DOI] [PubMed] [Google Scholar]
- 11.Jacobson SG, Cideciyan AV, Ratnakaram R, et al. . Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol 2012;130:9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Testa F, Maguire AM, Rossi S, et al. . Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology 2013;120:1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bainbridge JW, Smith AJ, Barker SS, et al. . Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 14.Cideciyan AV, Hauswirth WW, Aleman TS, et al. . Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther 2009;20:999–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cideciyan AV, Hauswirth WW, Aleman TS, et al. . Vision 1 year after gene therapy for Leber's congenital amaurosis. N Engl J Med 2009;361:725–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauswirth WW, Aleman TS, Kaushal S, et al. . Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maguire AM, High KA, Auricchio A, et al. . Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 2009;374:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maguire AM, Simonelli F, Pierce EA, et al. . Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonelli F, Maguire AM, Testa F, et al. . Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 2010;18:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashtari M, Cyckowski LL, Monroe JF, et al. . The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Invest 2011;121:2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett J, Ashtari M, Wellman J, et al. . AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med 2012;4:120ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.High KA. The gene therapy journey for hemophilia: are we there yet? Hematology 2012;2012:375–381 [DOI] [PubMed] [Google Scholar]
- 23.Nathwani AC, Tuddenham EG, Rangarajan S, et al. . Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lok C. Curing blindness: vision quest. Nature 2014;513:160–162 [DOI] [PubMed] [Google Scholar]
- 25.Le Meur G, Stieger K, Smith AJ, et al. . Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther 2007;14:292–303 [DOI] [PubMed] [Google Scholar]
- 26.Conlon TJ, Deng WT, Erger K, et al. . Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Dev 2013;24:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong J, Kim SR, Binley K, et al. . Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther 2008;15:1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binley K, Widdowson P, Loader J, et al. . Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors: safety and biodistribution of StarGen for Stargardt disease. Invest Ophthalmol Vis Sci 2013;54:4061–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zallocchi M, Binley K, Lad Y, et al. . EIAV-based retinal gene therapy in the shaker1 mouse model for usher syndrome type 1B: development of UshStat. PLoS One 2014;9:e94272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koilkonda RD, Yu H, Chou TH, et al. . Safety and effects of the vector for the Leber hereditary optic neuropathy gene therapy clinical trial. JAMA Ophthalmol 2014;132:409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cwerman-Thibault H, Augustin S, Ellouze S, et al. . Gene therapy for mitochondrial diseases: Leber hereditary optic neuropathy as the first candidate for a clinical trial. C R Biol 2014;337:193–206 [DOI] [PubMed] [Google Scholar]
- 32.Maclaren RE, Groppe M, Barnard AR, et al. . Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet 2014;383:1129–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cideciyan AV, Jacobson SG, Beltran WA, et al. . Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA 2013;110:E517–E525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acland GM, Aguirre GD, Ray J, et al. . Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 2001;28:92–95 [DOI] [PubMed] [Google Scholar]
- 35.Annear MJ, Bartoe JT, Barker SE, et al. . Gene therapy in the second eye of RPE65-deficient dogs improves retinal function. Gene Ther 2011;18:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narfstrom K, Katz ML, Bragadottir R, et al. . Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci 2003;44:1663–1672 [DOI] [PubMed] [Google Scholar]
- 37.Rattner A, Sun H, Nathans J. Molecular genetics of human retinal disease. Annu Rev Genet 1999;33:89–131 [DOI] [PubMed] [Google Scholar]
- 38.Anderson DH, Fisher SK. The relationship of primate foveal cones to the pigment epithelium. J Ultrastruct Res 1979;67:23–32 [DOI] [PubMed] [Google Scholar]
- 39.Cideciyan AV, Aguirre GK, Jacobson SG, et al. . Pseudo-fovea formation after gene therapy for RPE65-LCA. Invest Ophthalmol Vis Sci 2015;56:526–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stieger K, Cronin T, Bennett J, et al. . Adeno-associated virus mediated gene therapy for retinal degenerative diseases. Methods in Mol Biol 2011;807:179–218 [DOI] [PubMed] [Google Scholar]
- 41.Dalkara D, Kolstad KD, Caporale N, et al. . Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Molecular Ther 2009;17:2096–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park TK, Wu Z, Kjellstrom S, et al. . Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther 2009;16:916–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolstad KD, Dalkara D, Guerin K, et al. . Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Hum Gene Ther 2010;21:571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vacca O, Darche M, Schaffer DV, et al. . AAV-mediated gene delivery in Dp71-null mouse model with compromised barriers. Glia 2014;62:468–476 [DOI] [PubMed] [Google Scholar]
- 45.Kay CN, Ryals RC, Aslanidi GV, et al. . Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS One 2013;8:e62097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrs-Silva H, Dinculescu A, Li Q, et al. . Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther 2011;19:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalkara D, Byrne LC, Klimczak RR, et al. . In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 2013;5:189ra176. [DOI] [PubMed] [Google Scholar]
- 48.Mowat FM, Gornik KR, Dinculescu A, et al. . Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Ther 2014;21:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett J, Wilson J, Sun D, et al. . Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci 1994;35:2535–2542 [PubMed] [Google Scholar]
- 50.Kumar-Singh R. Barriers for retinal gene therapy: separating fact from fiction. Vis Res 2008;48:1671–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puppo A, Cesi G, Marrocco E, et al. . Retinal transduction profiles by high-capacity viral vectors. Gene Ther 2014;21:855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyoshi H, Takahashi M, Gage FH, et al. . Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA 1997;94:10319–10323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi M, Miyoshi H, Verma IM, et al. . Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol 1999;73:7812–7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimoto T, Gibbs D, Lillo C, et al. . Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B. Gene Ther 2007;14:584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis RJ, Tosi J, Janisch KM, et al. . Functional rescue of degenerating photoreceptors in mice homozygous for a hypomorphic cGMP phosphodiesterase 6 b allele (Pde6bH620Q). Invest Ophthalmol Vis Sci 2008;49:5067–5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicoud M, Kong J, Iqball S, et al. . Development of photoreceptor-specific promoters and their utility to investigate EIAV lentiviral vector mediated gene transfer to photoreceptors. J Gene Med 2007;9:1015–1023 [DOI] [PubMed] [Google Scholar]
- 57.Auricchio A, Kobinger G, Anand V, et al. . Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet 2001;10:3075–3081 [DOI] [PubMed] [Google Scholar]
- 58.Bainbridge JW, Stephens C, Parsley K, et al. . In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther 2001;8:1665–1668 [DOI] [PubMed] [Google Scholar]
- 59.Bemelmans AP, Bonnel S, Houhou L, et al. . Retinal cell type expression specificity of HIV-1-derived gene transfer vectors upon subretinal injection in the adult rat: influence of pseudotyping and promoter. J Gene Med 2005;7:1367–1374 [DOI] [PubMed] [Google Scholar]
- 60.Balaggan KS, Binley K, Esapa M, et al. . Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J Gene Med 2006;8:275–285 [DOI] [PubMed] [Google Scholar]
- 61.Gruter O, Kostic C, Crippa SV, et al. . Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther 2005;12:942–947 [DOI] [PubMed] [Google Scholar]
- 62.Pang J, Cheng M, Haire SE, et al. . Efficiency of lentiviral transduction during development in normal and rd mice. Mol Vis 2006;12:756–767 [PubMed] [Google Scholar]
- 63.Pang J, Cheng M, Stevenson D, et al. . Adenoviral-mediated gene transfer to retinal explants during development and degeneration. Exp Eye Res 2004;79:189–201 [DOI] [PubMed] [Google Scholar]
- 64.Adijanto J, Naash MI. Nanoparticle-based technologies for retinal gene therapy. Eur J Pharm Biopharm 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han Z, Conley SM, Makkia RS, et al. . DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J Clin Invest 2012;122:3221–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai X, Conley SM, Nash Z, et al. . Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J 2010;24:1178–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai X, Nash Z, Conley SM, et al. . A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS One 2009;4:e5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allocca M, Doria M, Petrillo M, et al. . Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest 2008;118:1955–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopes VS, Boye SE, Louie CM, et al. . Retinal gene therapy with a large MYO7A cDNA using adeno-associated virus. Gene Ther 2013;20:824–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong B, Nakai H, Xiao W. Characterization of genome integrity for oversized recombinant AAV vector. Mol Ther 2010;18:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirsch ML, Agbandje-McKenna M, Samulski RJ. Little vector, big gene transduction: fragmented genome reassembly of adeno-associated virus. Mol Ther 2010;18:6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirsch ML, Li C, Bellon I, et al. . Oversized AAV transduction is mediated via a DNA-PKcs-independent, Rad51C-dependent repair pathway. Mol Ther 2013;21:2205–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lai Y, Yue Y, Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome>or=8.2 kb. Mol Ther 2010;18:75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging. Mol Ther 2010;18:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duan D, Sharma P, Yang J, et al. . Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol 1998;72:8568–8577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan Z, Zhang Y, Duan D, et al. . Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc Natl Acad Sci USA 2000;97:6716–6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duan D, Yue Y, Engelhardt JF. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol Ther 2001;4:383–391 [DOI] [PubMed] [Google Scholar]
- 78.Ghosh A, Yue Y, Lai Y, et al. . A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol Ther 2008;16:124–130 [DOI] [PubMed] [Google Scholar]
- 79.Halbert CL, Allen JM, Miller AD. Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat Biotechnol 2002;20:697–701 [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Yue Y, Li L, et al. . Dual AAV therapy ameliorates exercise-induced muscle injury and functional ischemia in murine models of Duchenne muscular dystrophy. Hum Mol Genet 2013;22:3720–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lopes VS, Boye SE, Louie CM, et al. . Retinal gene therapy with a large MYO7A cDNA using adeno-associated virus. Gene Ther 2013;20:824–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trapani I, Colella P, Sommella A, et al. . Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med 2014;6:194–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colella P, Trapani I, Cesi G, et al. . Efficient gene delivery to the cone-enriched pig retina by dual AAV vectors. Gene Ther 2014;21:450–456 [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Duan D. Novel mini-dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum Gene Ther 2012;23:98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lai Y, Yue Y, Liu M, et al. . Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat Biotechnol 2005;23:1435–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lostal W, Bartoli M, Bourg N, et al. . Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer. Hum Mol Genet 2010;19:1897–1907 [DOI] [PubMed] [Google Scholar]
- 87.Odom GL, Gregorevic P, Allen JM, et al. . Gene therapy of mdx mice with large truncated dystrophins generated by recombination using rAAV6. Mol Ther 2011;19:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chao H, Sun L, Bruce A, et al. . Expression of human factor VIII by splicing between dimerized AAV vectors. Mol Ther 2002;5:716–722 [DOI] [PubMed] [Google Scholar]
- 89.Reich SJ, Auricchio A, Hildinger M, et al. . Efficient trans-splicing in the retina expands the utility of adeno-associated virus as a vector for gene therapy. Hum Gene Ther 2003;14:37–44 [DOI] [PubMed] [Google Scholar]
- 90.Dyka FM, Boye SL, Chiodo VA, et al. . Dual adeno-associated virus vectors result in efficient in vitro and in vivo expression of an oversized gene, MYO7A. Hum Gene Ther Methods 2014;25:166–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LaVail MM, Yasumura D, Matthes MT, et al. . Ribozyme rescue of photoreceptor cells in P23H transgenic rats: long-term survival and late-stage therapy. Proc Natl Acad Sci USA 2000;97:11488–11493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lewin AS, Drenser KA, Hauswirth WW, et al. . Ribozyme rescue of photoreceptor cells in a transgenic rat model of autosomal dominant retinitis pigmentosa. Nat Med 1998;4:967–971 [DOI] [PubMed] [Google Scholar]
- 93.Tessitore A, Parisi F, Denti MA, et al. . Preferential silencing of a common dominant rhodopsin mutation does not inhibit retinal degeneration in a transgenic model. Mol Ther 2006;14:692–699 [DOI] [PubMed] [Google Scholar]
- 94.Gorbatyuk M, Justilien V, Liu J, et al. . Preservation of photoreceptor morphology and function in P23H rats using an allele independent ribozyme. Exp Eye Res 2007;84:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gorbatyuk M, Justilien V, Liu J, et al. . Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery. Vis Res 2007;47:1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gorbatyuk MS, Pang JJ, Thomas J, Jr., et al. , Knockdown of wild-type mouse rhodopsin using an AAV vectored ribozyme as part of an RNA replacement approach. Mol Vis 2005;11:648–656 [PubMed] [Google Scholar]
- 97.Chadderton N, Millington-Ward S, Palfi A, et al. . Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol Ther 2009;17:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O'Reilly M, Palfi A, Chadderton N, et al. . RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet 2007;81:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mao H, Gorbatyuk MS, Rossmiller B, et al. . Long-term rescue of retinal structure and function by rhodopsin RNA replacement with a single adeno-associated viral vector in P23H RHO transgenic mice. Hum Gene Ther 2012;23:356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Millington-Ward S, Chadderton N, O'Reilly M, et al. . Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa. Mol Ther 2011;19:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mussolino C, Sanges D, Marrocco E, et al. . Zinc-finger-based transcriptional repression of rhodopsin in a model of dominant retinitis pigmentosa. EMBO Mol Med 2011;3:118–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roof DJ, Korenbrot JI, Heuser JE. Surfaces of rod photoreceptor disk membranes: light-activated enzymes. J Cell Biol 1982;95:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kabadi AM, Gersbach CA. Engineering synthetic TALE and CRISPR/Cas9 transcription factors for regulating gene expression. Methods 2014;69:188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacobson SG, Cideciyan AV, Peshenko IV, et al. . Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human Leber congenital amaurosis en route to therapy: residual cone-photoreceptor vision correlates with biochemical properties of the mutants. Hum Mol Genet 2013;22:168–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lipinski DM, Thake M, MacLaren RE. Clinical applications of retinal gene therapy. Prog Retin Eye Res 2013;32:22–47 [DOI] [PubMed] [Google Scholar]
- 106.Molday RS, Zhong M, Quazi F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta 2009;1791:573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramamurthy V, Niemi GA, Reh TA, et al. . Leber congenital amaurosis linked to AIPL1: a mouse model reveals destabilization of cGMP phosphodiesterase. Proc Natl Acad Sci USA 2004;101:13897–13902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O'Reilly M, Kohn DB, Bartlett J, et al. . Gene therapy for rare diseases: summary of a National Institutes of Health workshop, September 13, 2012. Hum Gene Ther 2013;24:355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]