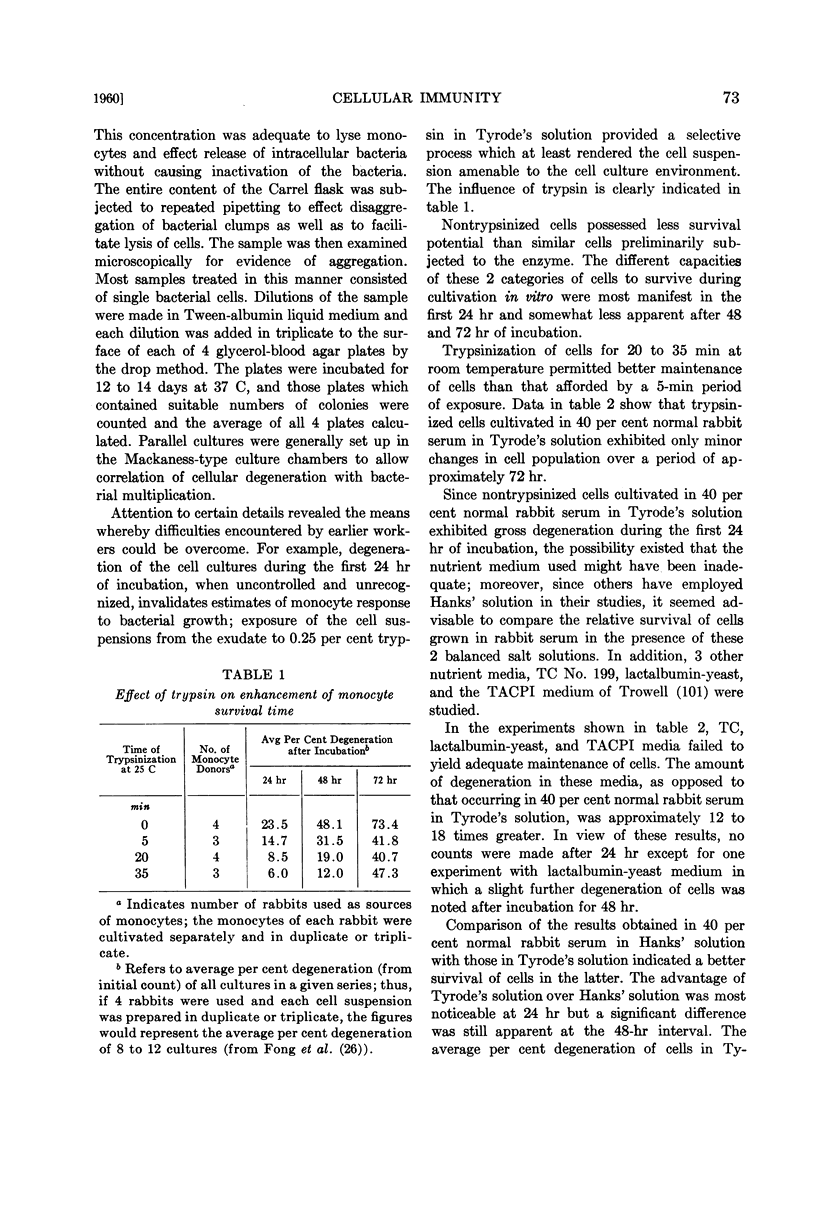
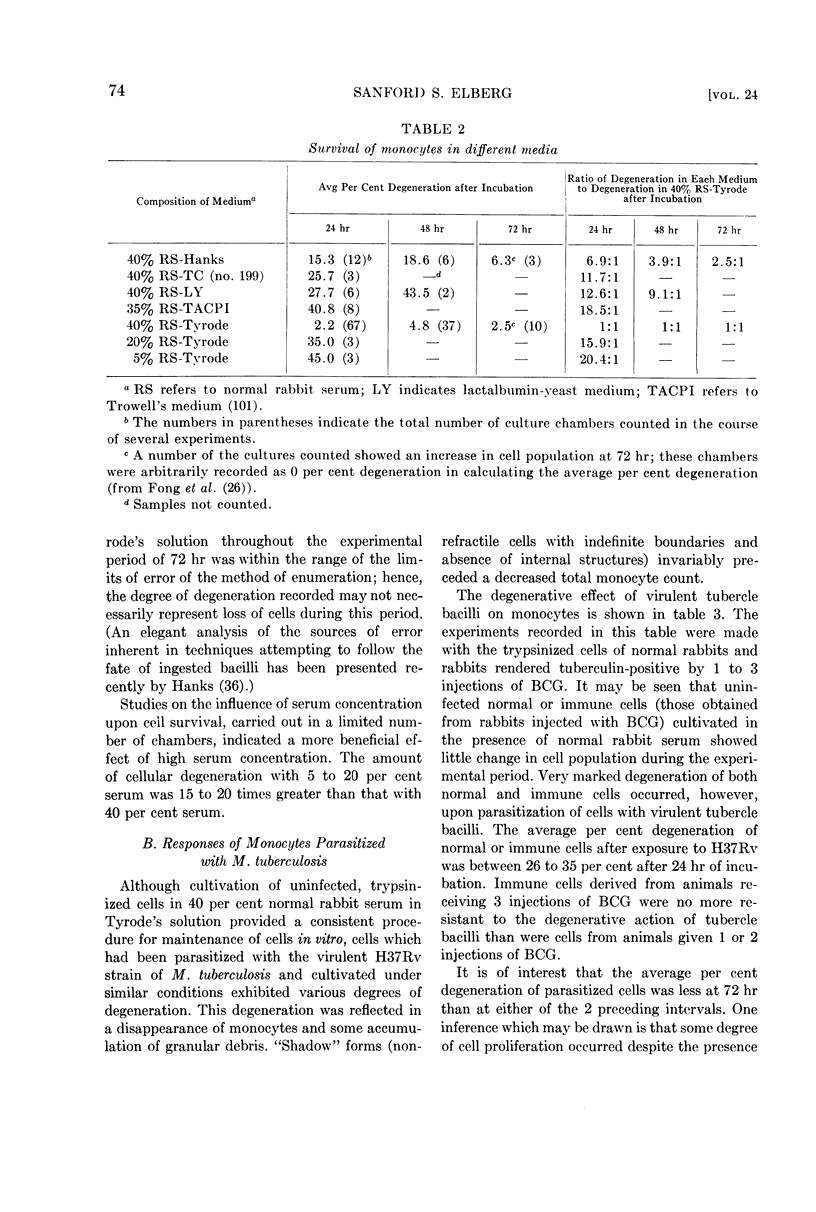
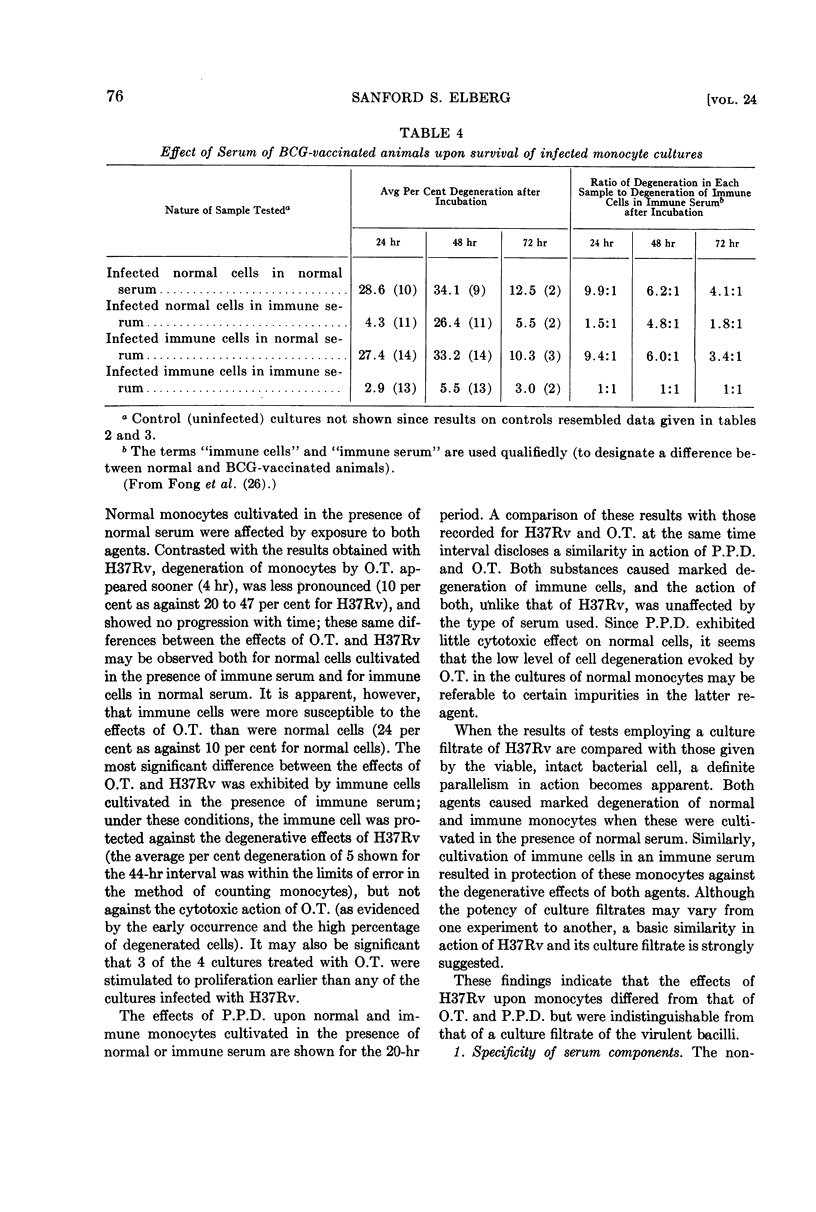
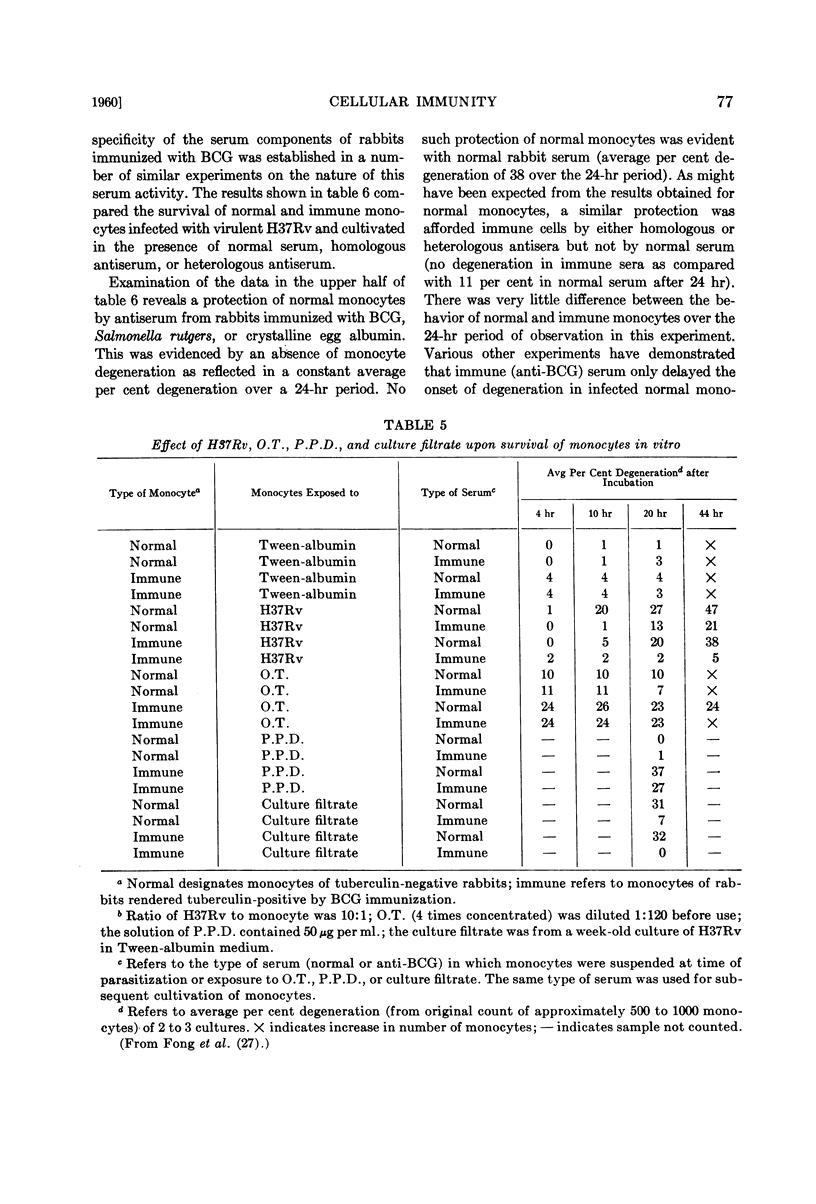
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALONSO D., NUNGESTER W. J. Comparative study of host resistance of guinea pigs and rats. V. The effect of pneumococcal products on glycolysis and oxygen uptake by polymorphonuclear leucocytes. J Infect Dis. 1956 Sep-Oct;99(2):174–181. doi: 10.1093/infdis/99.2.174. [DOI] [PubMed] [Google Scholar]
- BECKER M. E., HARTSELL S. E. The synergistic action of lysozyme and trypsin in bacteriolysis. Arch Biochem Biophys. 1955 Mar;55(1):257–269. doi: 10.1016/0003-9861(55)90563-4. [DOI] [PubMed] [Google Scholar]
- BENEDICT A. A., McFARLAND C. Growth of meningopneumonitis virus in normal and immune guinea pig monocytes. Nature. 1958 Jun 21;181(4625):1742–1743. doi: 10.1038/1811742a0. [DOI] [PubMed] [Google Scholar]
- BESSIS M. Structures cellulaires découvertes par le microscope électronique dans les leucocytes. Rev Hematol. 1956 Jun-Jul;11(3):295–320. [PubMed] [Google Scholar]
- BOEHME D., DUBOS R. J. The effect of bacterial constituents on the resistance of mice to heterologous infection and on the activity of their reticulo-endothelial system. J Exp Med. 1958 Apr 1;107(4):523–536. doi: 10.1084/jem.107.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN W., POMALES-LEBRON A., STINEBRING W. R. Interactions between mononuclear phagocytes and Brucella abortus strains of different virulence. Proc Soc Exp Biol Med. 1958 Feb;97(2):393–397. doi: 10.3181/00379727-97-23752. [DOI] [PubMed] [Google Scholar]
- BRIEGER E. M. New approaches to the study of experimental infection. Bibl Tuberc. 1951;5:236–304. [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. The basis of virulence in Pasteurella pestis: the development of resistance to phagocytosis in vitro. Br J Exp Pathol. 1956 Jun;37(3):286–299. [PMC free article] [PubMed] [Google Scholar]
- CAREY W. F., SPILMAN W., BARON L. S. Protoplast formation by mass adsorption of inactive bacteriophage. J Bacteriol. 1957 Oct;74(4):543–544. doi: 10.1128/jb.74.4.543-544.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLOBERT L. Etude de la lyse de salmonelles pathogènes provoquée par le lysozyme, après délipidation partielle de la paroi externe. Ann Inst Pasteur (Paris) 1958 Aug;95(2):156–167. [PubMed] [Google Scholar]
- DE GREGORIO P. Consumo di O2 dei leucociti e fagocitosi in diverse condizioni sperimentali. Boll Soc Ital Biol Sper. 1956 Jan-Feb;32(1-2):41–45. [PubMed] [Google Scholar]
- ELBERG S. S., FAUNCE K., Jr Immunization against Brucella infection. VI. Immunity conferred on goats by a nondependent mutant from a streptomycin-dependent mutant strain of Brucella melitensis. J Bacteriol. 1957 Feb;73(2):211–217. doi: 10.1128/jb.73.2.211-217.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBERG S. S., FONG J., SCHNEIDER P. Studies on tubercle bacillus-monocyte relationship. I. Quantitative analysis of effect of serum of animals vaccinated with BCG upon bacterium-monocyte system. J Exp Med. 1956 Oct 1;104(4):455–465. doi: 10.1084/jem.104.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBERG S. S., FONG J., SCHNEIDER P. Studies on tubercle bacillus-monocyte relationship. II. Induction of monocyte degeneration by bacteria and culture filtrate: specificity of serum and monocyte effects on resistance to degeneration. J Exp Med. 1957 Jan 1;105(1):25–37. doi: 10.1084/jem.105.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBERG S. S., SCHNEIDER P., FONG J. Cross-immunity between Brucella melitensis and Mycobacterium tuberculosis; intracellular behavior of Brucella melitensis in monocytes from vaccinated animals. J Exp Med. 1957 Oct 1;106(4):545–554. doi: 10.1084/jem.106.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. G., PERKINS F. T. The ability of pertussis vaccine to produce in mice specific immunity of a type not associated with antibody production. Br J Exp Pathol. 1954 Aug;35(4):322–330. [PMC free article] [PubMed] [Google Scholar]
- FISHER T. N., GINSBERG H. S. The reaction of influenza viruses with guinea pig polymorphonuclear leucocytes. III. Studies on the mechanism by which influenza viruses inhibit phagocytosis. Virology. 1956 Oct;2(5):656–664. doi: 10.1016/0042-6822(56)90045-9. [DOI] [PubMed] [Google Scholar]
- FISHMAN M., SILVERMAN M. S. Bactericidal activity of rat leucocytic extracts. I. Antibacterial spectrum and the subcellular localization of the bactericidal activity. J Exp Med. 1957 Jun 1;105(6):521–528. doi: 10.1084/jem.105.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLECK L. La leukergie et son role dans l'immunité. Ann Inst Pasteur (Paris) 1957 Mar;92(3):380–395. [PubMed] [Google Scholar]
- FONG J., CHIN D., AKIYAMA H. J., ELBERG S. S. Studies on tubercle bacillus-monocyte relationship. III. Conditions affecting the action of serum and cells; modification of bacilli in an immune system. J Exp Med. 1959 Jun 1;109(6):523–543. doi: 10.1084/jem.109.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN B. A., VANA L. R. Host-parasite relationships in brucellosis. I. Infection of normal guinea pig macrophages in tissue culture. J Infect Dis. 1958 May-Jun;102(3):258–267. doi: 10.1093/infdis/102.3.258. [DOI] [PubMed] [Google Scholar]
- FURNESS G. Interaction between Salmonella typhimurium and phagocytic cells in cell culture. J Infect Dis. 1958 Nov-Dec;103(3):272–277. doi: 10.1093/infdis/103.3.272. [DOI] [PubMed] [Google Scholar]
- FURNESS G., ROWLEY D. Transduction of virulence within the species Salmonella typhimurium. J Gen Microbiol. 1956 Aug;15(1):140–145. doi: 10.1099/00221287-15-1-140. [DOI] [PubMed] [Google Scholar]
- GINDER D. R. Resistance to fibroma virus infection; the role of immune leukocytes and immune macrophages. J Exp Med. 1955 Jan 1;101(1):43–58. doi: 10.1084/jem.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN M. N., MCCORMICK T. Cytochemical studies during the differentiation of normal human monocytes in vitro. Am J Pathol. 1957 Jul-Aug;33(4):737–747. [PMC free article] [PubMed] [Google Scholar]
- GROGG E., PEARSE A. G. E. The enzymic and lipid histochemistry of experimental tuberculosis. Br J Exp Pathol. 1952 Dec;33(6):567–576. [PMC free article] [PubMed] [Google Scholar]
- HANKS J. H. Assay of the fate of Mycobacteria in cell and tissues cultures. Am Rev Tuberc. 1958 May;77(5):789–801. doi: 10.1164/artpd.1958.77.5.789. [DOI] [PubMed] [Google Scholar]
- HARRIS H., BARCLAY W. R. A method for measuring the respiration of animal cells in vitro, with some observations on the macrophages of the rabbit. Br J Exp Pathol. 1955 Dec;36(6):592–598. [PMC free article] [PubMed] [Google Scholar]
- HENDERSON D. W., LANCASTER M. C., PACKMAN L., PEACOCK S. The influence of a pre-existing respiratory infection on the course of another superimposed by the respiratory route. Br J Exp Pathol. 1956 Dec;37(6):597–611. [PMC free article] [PubMed] [Google Scholar]
- HERZBERG M., ELBERG S. S. Immunization against brucella infection. III. Response of mice and guinea pigs to injection of viable and nonviable suspensions of a streptomycin-dependent mutant of Brucella malitensis. J Bacteriol. 1955 Apr;69(4):432–435. doi: 10.1128/jb.69.4.432-435.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956 May 1;103(5):589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Studies of the bactericidal action of phagocytin. J Exp Med. 1956 May 1;103(5):613–621. doi: 10.1084/jem.103.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., PICKETT M. J. A cellular basis of immunity in experimental Brucella infection. J Exp Med. 1958 Sep 1;108(3):343–360. doi: 10.1084/jem.108.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD D. H. Observations on tissue cultures of mouse peritoneal exudates inoculated with Histoplasma capsulatum. J Bacteriol. 1959 Jul;78(1):69–78. doi: 10.1128/jb.78.1.69-78.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F. Transduction of lysogeny in Escherichia coli. Virology. 1955 Jul;1(2):207–220. doi: 10.1016/0042-6822(55)90017-9. [DOI] [PubMed] [Google Scholar]
- KELLY E. H., GORELICK A. N., SILVERMAN S. J., BRAUN W. Further studies on superinfection in experimental cavine brucellosis. J Infect Dis. 1953 Sep-Oct;93(2):187–191. doi: 10.1093/infdis/93.2.187. [DOI] [PubMed] [Google Scholar]
- KERBY G. P. Release of enzyme from human leukocytes on damage by bacterial derivatives. Proc Soc Exp Biol Med. 1952 Nov;81(2):381–383. doi: 10.3181/00379727-81-19883. [DOI] [PubMed] [Google Scholar]
- LARSH H. W., SHEPARD C. C. HeLa cells and Histoplasma capsulatum; phagocytosis and subsequent intracellular growth. J Bacteriol. 1958 Nov;76(5):557–563. doi: 10.1128/jb.76.5.557-563.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E., REES R. J. Possible site and mode of action of certain lipotropic macromolecules in tuberculosis. Nature. 1955 Jan 22;175(4447):161–163. doi: 10.1038/175161a0. [DOI] [PubMed] [Google Scholar]
- LURIE M. B. Native and acquired resistance to tuberculosis. Am J Med. 1950 Nov;9(5):591–610. doi: 10.1016/0002-9343(50)90210-5. [DOI] [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B., SMITH N., WELLS A. Q. The growth of intracellular tubercle bacilli in relation to their virulence. Am Rev Tuberc. 1954 Apr;69(4):479–494. doi: 10.1164/art.1954.69.4.479. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. The action of drugs on intracellular tubercle bacilli. J Pathol Bacteriol. 1952 Jul;64(3):429–446. doi: 10.1002/path.1700640302. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. The growth of tubercle bacilli in monocytes from normal and vaccinated rabbits. Am Rev Tuberc. 1954 Apr;69(4):495–504. doi: 10.1164/art.1954.69.4.495. [DOI] [PubMed] [Google Scholar]
- MCCUNE R. M., Jr, MCDERMOTT W., TOMPSETT R. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med. 1956 Nov 1;104(5):763–802. doi: 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCUNE R. M., Jr, TOMPSETT R. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med. 1956 Nov 1;104(5):737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCWHORTER A. C., EDWARDS P. R. A new Salmonella serotype (Salmonella rutgers). Cornell Vet. 1956 Oct;46(4):509–510. [PubMed] [Google Scholar]
- MESROBEANU I., MESROBEANU L., MITRICA N., PAPAZIAN E. Gli enzimi leucocitari ed il problema della sopravvivenza degli stafilococchi nei leucociti dopo fagocitosi. Minerva Med. 1957 Apr 18;48(31):1375–1379. [PubMed] [Google Scholar]
- MEYNELL G. G., STOCKER B. A. Some hypotheses on the aetiology of fatal infections in partially resistant hosts and their application to mice challenged with Salmonella paratyphi-B or Salmonella typhimurium by intraperitoneal injection. J Gen Microbiol. 1957 Feb;16(1):38–58. doi: 10.1099/00221287-16-1-38. [DOI] [PubMed] [Google Scholar]
- MIKA L. A., GOODLOW R. J., VICTOR J., BRAUN W. Studies on mixed infections. I. Brucellosis and Q fever. Proc Soc Exp Biol Med. 1954 Dec;87(3):500–507. doi: 10.3181/00379727-87-21424. [DOI] [PubMed] [Google Scholar]
- MUSCHEL L. H., CAREY W. F., BARON L. S. Formation of bacterial protoplasts by serum components. J Immunol. 1959 Jan;82(1):38–42. [PubMed] [Google Scholar]
- MUSCHEL L. H., MUTO T. Bactericidal reaction of mouse serum. Science. 1956 Jan 13;123(3185):62–64. doi: 10.1126/science.123.3185.62. [DOI] [PubMed] [Google Scholar]
- NOSSAL G. J., LEDERBERG J. Antibody production by single cells. Nature. 1958 May 17;181(4620):1419–1420. doi: 10.1038/1811419a0. [DOI] [PubMed] [Google Scholar]
- NYKA W. Enhancement of resistance to tuberculosis in mice experimentally infected with brucella abortus. Am Rev Tuberc. 1956 Feb;73(2):251–265. doi: 10.1164/artpd.1956.73.2.251. [DOI] [PubMed] [Google Scholar]
- POLLACK A. D., VICTOR J. Phagocytosis augmenting factor produced by leucocytes. Proc Soc Exp Biol Med. 1955 Aug;89(4):561–564. doi: 10.3181/00379727-89-21875. [DOI] [PubMed] [Google Scholar]
- RALSTON D. J., BAER B. S., LIEBERMAN M., KRUEGER A. P. Lysis from without of S. aureus K1 by the combined action of phage and virolysin. J Gen Physiol. 1957 Nov 20;41(2):343–358. doi: 10.1085/jgp.41.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALSTON D. J., LIEBERMAN M., BAER B., KRUEGER A. P. Staphylococcal virolysin, a phage-induced lysin; its differentiation from the autolysis of normal cells. J Gen Physiol. 1957 May 20;40(5):791–807. doi: 10.1085/jgp.40.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- ROGERS D. E., TOMPSETT R. The survival of staphylococci within human leukocytes. J Exp Med. 1952 Feb;95(2):209–230. doi: 10.1084/jem.95.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. Bactericidal activity of macrophages in vitro against Escherichia coli. Nature. 1958 Jun 21;181(4625):1738–1739. doi: 10.1038/1811738b0. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SHEPARD C. C. A comparison of the growth of selected mycobacteria in HeLa, monkey kidney, and human amnion cells in tissue culture. J Exp Med. 1958 Feb 1;107(2):237–246. doi: 10.1084/jem.107.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. Phagocytosis by HeLa cells and their susceptibility to infection by human tubercle bacilli. Proc Soc Exp Biol Med. 1955 Nov;90(2):392–396. doi: 10.3181/00379727-90-22043. [DOI] [PubMed] [Google Scholar]
- SKARNES R. C., WATSON D. W. Characterization of leukin: an antibacterial factor from leucocytes active against gram-positive pathogens. J Exp Med. 1956 Dec 1;104(6):829–845. doi: 10.1084/jem.104.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAHELIN H., KARNOVSKY M. L., SUTER E. Studies on the interaction between phagocytes and tubercle bacilli. II. The action of phagocytes upon C14-labelled tubercle bacilli. J Exp Med. 1956 Jul 1;104(1):137–150. doi: 10.1084/jem.104.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAHELIN H., SUTER E., KARNOVSKY M. L. Studies on the interaction between phagocytes and tubercle bacilli. I. Observations on the metabolism of guinea pig leucocytes and the influence of phagocytosis. J Exp Med. 1956 Jul 1;104(1):121–136. doi: 10.1084/jem.104.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STINEBRING W. R., KESSEL R. Continuous growth of Brucella abortus in mononuclear phagocytes of rats and guinea pigs. Proc Soc Exp Biol Med. 1959 Jul;101(3):412–415. doi: 10.3181/00379727-101-24962. [DOI] [PubMed] [Google Scholar]
- SUTER E. Multiplication of tubercle bacilli within mononuclear phagocytes in tissue cultures derived from normal animals and animals vaccinated with BCG. J Exp Med. 1953 Feb 1;97(2):235–245. doi: 10.1084/jem.97.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMPKINS E. H. The monocyte. Ann N Y Acad Sci. 1955 Mar 24;59(5):732–745. doi: 10.1111/j.1749-6632.1955.tb45981.x. [DOI] [PubMed] [Google Scholar]
- TOMPSETT R. Protection of pathogenic staphylococci by phagocytes. Trans Assoc Am Physicians. 1956;69:84–92. [PubMed] [Google Scholar]
- TROWELL O. A. The culture of lymph nodes in synthetic media. Exp Cell Res. 1955 Oct;9(2):258–276. doi: 10.1016/0014-4827(55)90099-9. [DOI] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]
- WEISS L. P., FAWCETT D. W. Cytochemical observations on chicken monocytes macrophages and giant cells in tissue culture. J Histochem Cytochem. 1953 Jan;1(1):47–65. doi: 10.1177/1.1.47. [DOI] [PubMed] [Google Scholar]
- WELSCH M. Formation de protoplastes d'Escherichia coli sous l'influence de la glycine et d'autres acides aminés. Schweiz Z Pathol Bakteriol. 1958;21(3):741–768. [PubMed] [Google Scholar]
- WILEY G. G., WILSON A. T. The ability of group A streptococci killed by heat or mercury arc irradiation to resist ingestion by phagocytes. J Exp Med. 1956 Jan 1;103(1):15–36. doi: 10.1084/jem.103.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON A. T., WILEY G. G., BRUNO P. Fate of non-virulent group A streptococci phagocytized by human and mouse neutrophils. J Exp Med. 1957 Dec 1;106(6):777–786. doi: 10.1084/jem.106.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]


