Abstract
Pseudomonas aeruginosa is an opportunistic human pathogen causing severe acute and chronic infections. Earlier we have shown that calcium (Ca2+) induces P. aeruginosa biofilm formation and production of virulence factors. To enable further studies of the regulatory role of Ca2+, we characterized Ca2+ homeostasis in P. aeruginosa PAO1 cells. By using Ca2+-binding photoprotein aequorin, we determined that the concentration of free intracellular Ca2+ ([Ca2+]in) is 0.14±0.05 μM. In response to external Ca2+, the [Ca2+]in quickly increased at least 13 fold followed by a multi-phase decline by up to 73%. Growth at elevated Ca2+ modulated this response. Treatment with inhibitors known to affect Ca2+ channels, monovalent cations gradient, or P-type and F-type ATPases impaired [Ca2+]in response, suggesting the importance of the corresponding mechanisms in Ca2+ homeostasis. To identify Ca2+ transporters maintaining this homeostasis, bioinformatic and LC-MS/MS-based membrane proteomic analyses were used. [Ca2+]in homeostasis was monitored for seven Ca2+-affected and eleven bioinformatically predicted transporters by using transposon insertion mutants. Disruption of P-type ATPases PA2435, PA3920, and ion exchanger PA2092 significantly impaired Ca2+ homeostasis. The lack of PA3920 and vanadate treatment abolished Ca2+- induced swarming, suggesting the role of the P-type ATPase in regulating P. aeruginosa response to Ca2+.
Keywords: Calcium homeostasis, ATPase, Ion exchanger, Aequorin, Calcium transporters
INTRODUCTION
Calcium (Ca2+) is a well-known signaling molecule that regulates a number of essential processes in eukaryotes [1]. Abnormalities in cellular Ca2+ homeostasis have been implicated in many human diseases, including diseases associated with bacterial infections, for example, cystic fibrosis (CF) and endocarditis. In addition, Ca2+ plays a regulatory role in innate immune response [2], and its intracellular ([Ca2+]in) and extracellular ([Ca2+]ex) concentrations fluctuate in response to inflammation. For example, the levels of [Ca2+] in pulmonary fluid and nasal secretions of CF patients are increased [3, 4]. Altogether the evidence suggests that cellular Ca2+ balance in a host may provide an environmental cue for opportunistic pathogenic bacteria and trigger their virulence. In support, in prokaryotes, Ca2+ has been implicated in various physiological processes such as spore formation, motility, cell differentiation, transport, and virulence (reviewed in [5]). It has also been shown that Ca2+ modulates bacterial gene expression [6-8], suggesting its regulatory role in prokaryotes. Furthermore, there is growing evidence that Ca2+ plays a signaling role in prokaryotes, which requires a tight control of cellular Ca2+ homeostasis. Several bacteria including Escherichia coli [9], Propionibacterium acnes [10], Streptococcus pneumoniae [11] Bacillus subtilis [12] and cyanobacteria [13] have been shown to maintain intracellular Ca2+ at sub-micromolar levels, and produce Ca2+ transients in response to environmental and physiological conditions [14, 15]. Such responses may play a key role in Ca2+-regulated bacterial physiology and virulence, however, the molecular mechanisms of bacterial Ca2+ homeostasis have not been well characterized. Several studies suggest that bacteria control their [Ca2+]in by using multiple mechanisms of transporting or chelating Ca2+ (reviewed in [5]).
Three major types of Ca2+ transport systems have been described in prokaryotes: gradient driven Ca2+ exchangers, ATP-ases, and non-proteinaceous polyhydroxybutyrate-polyphosphates (PHB-PP) channels. Ca2+ exchangers have been identified in a number of bacterial genera and are thought to serve as a major mechanism for Ca2+ transport in prokaryotes [16]. They are low-affinity Ca2+ transporters that use the energy stored in the electrochemical gradient of ions, and, depending on the gradient, can operate in both directions. The specificity of the transporters may vary. For example, YftkE (ChaA) from B. subtilis [17] as well as ApCAX and SynCAX from cyanobacteria [18] are Ca2+- specific, whereas ChaA from E. coli exhibits Na+/H+ and K+/H+ antiport activity in addition to Ca2+/H+ [19]. Ca2+ exchangers may also play role in cell sensitivity to Ca2+ and salt tolerance, as exemplified by cyanobacterial ApCAX and SynCAX [18]. ATP-ases are mostly high-affinity pumps that export cations from the cytosol by using the energy of ATP. They include P-type and F-type ATPases. Ca2+- translocating P-type ATPases belong to P2A and P2B subgroups, as classified in [20]. The former are similar to mammalian sarco(endo)plasmic reticulum (SERCA) Ca2+ pumps exporting Ca2+ against steep transmembrane gradients, and the latter are similar to plasma membrane (PMCA) calmodulin-binding ATPases. Five characterized prokaryotic P2A-ATPases include PacL from cyanobacteria [21], LMCA1 from Listeria monocytogenes [22], YloB from Bacillus subtilis [23], CaxP from Streptococcus penumoniae [11], and PacL from Flavobacterium odoratum [24]. Most of them were shown to export Ca2+ in membrane vesicles and proposed to play a role in cell protection against high Ca2+. LMCA1 from L. monocytogenes [22] and PacL from F. odoratum [21] were shown to undergo Ca2+-dependent phosphorylation required to transport Ca2+. F-type ATPases, or ATP synthases, are known to synthesize ATP at the expense of transmembrane electrochemical gradient of protons (most commonly). So far, only one F-type ATPase AtpD in E. coli was shown to play role in Ca2+ homeostasis, most likely due to its role in ATP synthesis [25]. Overall, although several prokaryotic gradient- and ATP- driven transporters were shown to translocate Ca2+ in-vitro, only few were tested for their role in cellular Ca2+ homeostasis in-vivo, of which only ion-exchanger SynCAX in Synechocystis sp. PCC6803 was shown to play role in cellular Ca2+ efflux [18]. The difficulty of identifying the roles of Ca2+ transporters in-vivo is likely due to their functional redundancy, the molecular basis of which requires further studies.
Pseudomonas aeruginosa is an opportunistic human pathogen, and a major cause of nosocomial infections and severe chronic infections in endocarditis and in CF patients. Earlier, we showed that growth at high Ca2+ enhances P. aeruginosa biofilm formation and induces biosynthesis of several secreted virulence factors including alginate, extracellular proteases and pyocyanin [6, 7]. However, the molecular mechanisms of Ca2+ regulation are not defined. To enable studies required to uncover such mechanisms, it is necessary to first characterize cellular Ca2+ homeostasis in this organism. Therefore, the aim of this work was to measure the intracellular Ca2+ concentration ([Ca2+]in) in P. aeruginosa cells and characterize its responses to external Ca2+. We employed a recombinant photoprotein aequorin-based reporter system, which has been successfully used to measure [Ca2+]in in live prokaryotic cells [12, 25], and to monitor both short- and long-term [Ca2+]in responses to external Ca2+ transients [9, 12] as well as other environmental and physiological determinants [12, 15]. We also aimed to identify Ca2+ transporters that play role in maintaining cellular Ca2+ homeostasis. The strategy combined bioinformatic and proteomic approaches, followed by characterization of transposon insertion mutants obtained from the University of Washington Genome Center. This study presents the first evidence of Ca2+ homeostasis in P. aeruginosa and identifies several mechanisms and proteins required for maintaining Ca2+ homeostasis and regulating Ca2+-induced swarming motility. In addition, the results provide a basis and excellent tools for further studies of the roles of cellular Ca2+ homeostasis in the regulation of Ca2+-modulated physiology and virulence of this important human pathogen.
MATERIALS AND METHODS
Chemicals used in this study are listed in the supplementary information. Primers were obtained from Integrated DNA Technologies Inc.
Bacterial strains, plasmids, and media
P. aeruginosa strain PAO1, the non-mucoid strain with genome sequence available was used in the study. Biofilm minimal medium (BMM) was made as described in [6]. When required, CaCl2.2H2O was added to final concentration of 1 or 5 mM. For proteomic studies, PAO1 cells were first grown in 5 ml tubes for 16 h (mid-log), and then used to inoculate (0.1 %) 100 ml fresh medium in 250 ml flasks. The cultures were grown to mid-log growth phase and harvested by centrifugation. Transposon insertion mutants were obtained from the University of Washington Two - Allele library and are listed in Table 1. The mutants contained either ISphoA/hah or ISlacZ/hah insertions with tetracycline resistance cassette that disrupted the genes of interest. The mutations were confirmed by two-step PCR: first, transposon flanking primers were used to verify that the target gene is disrupted, and second, transposon-specific primers were used to confirm the transposon insertion. The primer sequence is available at www.gs.washington.edu. For convenience, the mutants were designated as PA:IS, where PA is the identifying number of the disrupted gene from P. aeruginosa PAO1 genome (www.pseudomonas.com).
Table 1.
Protein prediction and identification. Transposon mutants.
| PA ORFa |
Protein name, gene nameb |
Predicted domain(s)c |
Best characterized hit in the nr NCBI, Accession #, Organism, % identityd |
Transposon mutant PW#e Mutant Genotype |
Mutant Designated Name |
|---|---|---|---|---|---|
| ATP-driven transporters | |||||
| PA1429 | Probable cation transporting P-Type ATPase | E1-E2 ATPase | P-type Na+ ATPase, BAF91372.2, Exiguobacterium aurantiacum, 45% | PW3596 PA1429-E05::ISlacZ/hah | PA1429::IS |
| PA4825 | Probable Mg2+ transport ATPase, P-Type, mgtA | E1-E2 ATPase | Ca2+/Mn2+ P-type ATPase PMR1, CAB87245, Candida albicans, 25% | PW9116 PA4825-F12::ISphoA/hah | PA4825::IS |
| PA3690 | Probable metal-transporting P-type ATPase | E1-E2 ATPase; Heavy metal associated domain | Cd2+ /Zn2+ transporting ATPase, HMA3 P0CW78.1, Arabidopsis thaliana, 32% | PW7241 PA3690-C04::ISlacZ/hah | PA3690::IS |
| PA1634 | K+-transporting ATPase, beta subunit, kdpB | E1-E2 ATPase | NA | PW3911 PA1634-E05::ISlacZ/hah | PA1634::IS |
| PA2435 | Heavy metal translocating P-type ATPase, hmtA | E1-E2 ATPase | PW5099 PA2435-A02::ISphoA/hah | PA2435::IS | |
| PA3920 | Probable metal transporting P-type ATPase | E1-E2 ATPase; Heavy-metal-associated domain | Cation transporting ATPase, NP_440588, 44%, Synechocystis PCC6803, 44% | PW7626 PA3920-G01::ISphoA/hah | PA3920::IS |
| PA1549 | Probable cation-transporting P-type ATPase | E1-E2 ATPase; Heavy-metal-associated domain | CtpA, AAW66130, Rubrivivax gelatinosus, 36% | PW3788 PA1549-G12::ISphoA/hah | PA1549::IS |
| PA5554 | Probable ATPase synthase, beta subunit, atpD | F1 ATP synthase | F1F0-ATP synthase β subunit, Q587Q4, Acidithiobacillus ferroxidans, 77% | PW10412 PA5554-B01::ISlacZ/hah | PA5554::IS |
| PA4496 | Probable ABC transporter, substrate binding subunit | Substrate binding component | Dipeptide binding protein chain A, 1DPP A, E. coli, 50% | PW8565 PA4496-F02::ISphoA/hah | PA4496::IS |
| PA3400 | Hypothetical protein | ABC type transporter | NA | PW6735 PA3400-H11::ISphoA/hah | PA3400::IS |
| Ion gradient driven exchangers | |||||
| PA3963 | Probable Transporter | Cation Efflux Superfamily | Zinc transporter, YiiP, 3H90_A, E.coli K-12, 45% | PW7707 PA3963-E02::ISphoA/hah | PA3963::IS |
| PA2092 | Probable major facilitator superfamily transporter | Major Facilitator Superfamily | Purine efflux pump PbuE, Q0GQS6.1, Bacillus amyloliquefaciens, 35% | PW4602 PA2092-F01::ISlacZ/hah | PA2092::IS |
| PA4292 | Probable Phosphate transporter | PO43-/SO42- permease | NA | PW8230 PA4292-A03::ISphoA/hah | PA4294::IS |
| PA0397 | Probable cation efflux system protein | Cation efflux family | Znt-like transporter 2, Q8NEW0.1, Homo sapiens, 27% | PW1733 PA0397-E03::ISlacZ/hah | PA0397::IS |
| Other transporters | |||||
| PA2999 | Probable Na+-translocating NADH:ubiquin one oxidoreductase subunit, nqrA | NADH-quinone reductase domain | Na+ translocating NADH ubiquinone oxidoreductase subunit A , ZP_08756125.1, Haemophilus pittmaniae HK85,58% | PW6021 PA2999-D10::ISlacZ/hah | PA2999::IS |
| PA4614 | Probable conductance mechanosensitive channel, mscL | Large-conductance mechanosensitive channel | Mechanosensitive channel large, MscL chain A, 3HZQ_A, Staphylococcus aureus, 36% | PW8772 PA4614-B11::ISphoA/hah | PA4614::IS |
| PA5167 | Dicarboxylic acid transporter, dctP | Bacterial extracellular solute binding | PW9688 PA5167-F06::ISphoA/hah | PA5167::IS | |
| PA4016 | Hypothetical protein | Membrane lipoprotein lipid attachment site | NA | PW7791 PA4016-E06::ISphoA/hah | PA4106::IS |
Gene identifier in the PAO1 genome available at www.pseudomonas.com.
As annotated in the PAO1 genome.
Domains were predicted by the algorithms in CDD and PROSITE.
The % identity was calculated over the full length of the proteins using transporter proteins encoded in PAO1 genome as a query.
The mutant strain identifier in the UW library of transposon mutants available at www.gs.washington.edu.
f Proteins were identified by using LC-MS/MS. Protein identification was accepted if the probability threshold was greater than 99% and at least three peptides were identified, each with 95% certainty. Spectral count (SC) was used to estimate the differential protein abundance. Fold difference was calculated as a ratio of the number of the peptides identified at 5 mM vs. 0 mM Ca2+. ND, protein was detected at 0 mM and was not detected at 5 mM Ca2+. New, protein was detected at 5 mM, but was not detected at 0 mM Ca2+. PA number and fold difference (in brackets) are provided for the identified proteins that are encoded within the operonic gene clusters of the predicted proteins.
NA. No characterized hits with greater than 25% amino acid sequence identity were found.
Sequence analyses
To predict Ca2+ transporters in P. aeruginosa PAO1 genome, we applied BLASTp sequence alignments, using the National Centre for Biotechnology Information (NCBI) non-redundant database (GenBank release 160.1), as well as functional domains search using Conserved Domain Database (CDD) and PROSITE. Homologous proteins were selected based on at least 25 % identity over the full length of amino acid sequence. For phylogenetic analyses, amino acid sequences of the functionally characterized transporters of Ca2+ and other cations were aligned with P. aeruginosa putative Ca2+ transporters using ClustalW. Thus obtained multiple sequence alignments were then used to build unrooted phylogenetic tree using Neighbor-joining algorithm in the MEGA 5.1 software.
Proteomic analysis
Membrane proteins were isolated by carbonate extraction as described in [26]. The details are described in supplementary information. Protein concentration was determined using the 2D Quant kit (GE Healthcare). LC-MS/MS-based spectral counting was performed at the OSU Proteomics Facilities using LTQ-OrbitrapXL mass spectrometer. Proteins were identified using Mascot (v.2.2.2 from Matrix Science, Boston, MA, USA) and a database generated by in silico digestion of the P. aeruginosa PAO1 proteome predicted from the genome. Search results were validated using Scaffold (v.3 from Proteome Software Inc., Portland, OR). Proteins were considered identified if the protein probability threshold was greater than 99 % and at least three peptides were identified, each with 95 % certainty.
Expression and reconstitution of aequorin
PAO1 and transposon mutants were transformed with pMMB66EH (courtesy of Dr. Delfina Dominguez), carrying aequorin [27] and carbenicillin resistance genes, using a heat shock method described in [28]. The transformants were selected on Luria bertani (LB) agar containing carbenicillin (300 μg/ml) and, in case of transposon mutants, tetracycline (60 μg /ml) and verified by PCR using aequorin specific primers (For: 5’CTTACATCAGACTTCGACAACCCAAG, Rev: 5’CGTAGAGCTTCTTAGGGCACAG). Aequorin was expressed and reconstituted as described in [9] with modifications. The details are described in supplementary information.
Luminescence measurements and estimation of free intracellular calcium
Cells with reconstituted aequorin were aliquoted (100 μl) in 96 well plate (Griener bio one, Lumitrack 600) and, when required, treated with inhibitors (2, 4 di-nitrophenol at 0.5, 1, or 2 mM; LaCl3 at 300 or 600 μM; gramicidin D at 1 or 10 μg/ml; calcimycin at 5 μM; or vanadate at 2 mM) for 10 min in the dark at room temperature without shaking. Gramicidin D and calcimycin were dissolved in 50 % and 3 % DMSO, respectively. To ensure penetration of gramicidin D and calcimycin through the bacterial outer membrane, we added 10 μg/ml of compound 48/80, known to permeabilize the outer membrane of gram negative bacteria without affecting the cytoplasmic membrane [29]. In this case, the corresponding amounts of DMSO and 48/80 were used to treat cells as a negative control. Luminescence was measured at 25 °C using Synergy Mx Multi-Mode Microplate Reader (Biotek). To measure the basal level of [Ca2+]in, the measurements were recorded for 1 min at 4 sec interval, then the cells were challenged with 1 or 5 mM Ca2+ (final concentration) injected by using the internal Synergy injectors, mixed for 1 sec, and the luminescence was recorded for 20 min at 4-5 sec interval. Injection of buffer alone was used as a negative control, and did not cause any significant fluctuations in [Ca2+]in. [Ca2+]in was calculated by using the formula pCa= 0.612 (−log10k) + 3.745, where k is a rate constant for luminescence decay (s−1) [9]. The excel-based template incorporating the formula and the aequorin standard curve was generously shared by Dr. Anthony Campbell. The results were normalized against the total amount of available aequorin, which was estimated by summing the light detected during an entire experiment and the light detected during discharge. The discharge was performed by permeabilizing cells with 2 % Nonidet 40 (NP40) in the presence of 12.5 mM CaCl2. The luminescence released during the discharge was monitored for 5 min at 4-5 sec interval. The estimated remaining available aequorin was at least 10 % of the total aequorin, unless mentioned otherwise. To control possible cell lysis and aequorin leakage during the procedure, following the luminescence measurements, the cells were collected by centrifugation, and luminescence was reported for both the supernatants and the cell pellets resuspended in buffer (25 mM HEPES, 1 mM MgCl2, 125 mM NaCl; pH 7.5). The experimental conditions reported here were optimized to prevent any significant cell lysis. The responses to Ca2+ challenges were characterized and validated by using curve fitting analysis with IGOR PRO software v.6.3.1.2. (WaveMetrics).
Ca2+ Tolerance Assay
To test cell tolerance to high Ca2+, PAO1 and mutants were grown at 5 or 100 mM Ca2+. Mid-log cultures grown in 5-ml BMM were inoculated (1 %) into 200 μl of fresh BMM (no added, 5 or 100 mM Ca2+) in 96 well plates. The plates were incubated for 12, 24 h or 48 h, and the OD600 was measured using Synergy Mx Multi-Mode Microplate Reader (Biotek). The tolerance to Ca2+ was calculated as a ratio of OD600 of the cultures grown at elevated Ca2+ to the cultures grown at no added Ca2+. For the wild type PAO1, this ratio equals one.
Swarming Assay
Swarming motility was assayed as described in [30]. PAO1 and mutants were grown in BMM at 0 mM or 5 mM Ca2+. 2 μl of the mid log cultures normalized to the OD600 of 0.3 were spot inoculated onto the surface of swarming agar [30]. When needed, inhibitors (10 μg/ml gramicidin D, 2 mM vanadate, or 600 μM LaCl3) were added. Gramicidin D was dissolved in DMSO and added with compound 48/80 (10 μg/ ml). After inoculation, the plates were incubated for 16 h and the colony diameters were measured. The effect of Ca2+ was calculated as a fold difference (ratio) between the diameters of the colonies grown at 5 mM and 0 mM Ca2+. The mutants and treatments were compared to their corresponding controls using the mean percentage of fold difference from at least three independent experiments.
RESULTS
P. aeruginosa maintains [Ca2+]in homeostasis
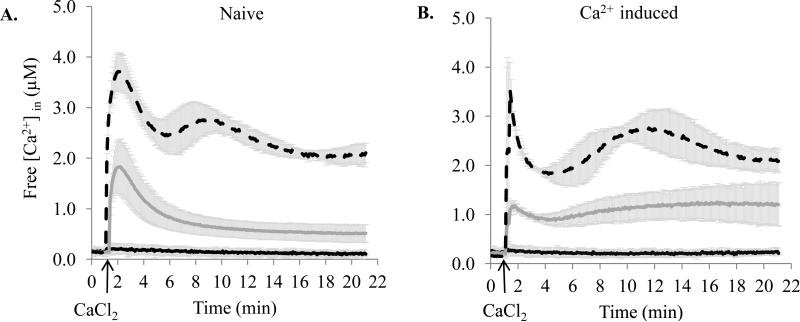
To study Ca2+ homeostasis in P. aeruginosa PAO1, we first measured the basal level of free intracellular Ca2+ ([Ca2+]in), and then monitored the changes in [Ca2+]in in response to externally added 1 and 5 mM Ca2+. Cells grown at no added Ca2+ (naïve) or 5 mM Ca2+ (induced) were compared. In naïve cells, the [Ca2+]in was 0.14 ± 0.05 μM (Fig. 1A). The addition of 1 mM Ca2+ caused a 13 fold increase of [Ca2+]in to 1.86 ± 0.53 μM within 0.6 min (2.92 μM/min) followed by a two-phase decline, validated by the curve fitting analysis. The first, fast, phase proceeded at a rate 0.75 μM/min, lasted for ~2 min, and accounted for 38 % of [Ca2+]in reduction. During the second, slow, phase, [Ca2+]in reduced for another 35% to 0.5 μM at a rate 0.04 μM/min. When the cells were transferred to a fresh buffer containing no Ca2+, a complete recovery to the basal [Ca2+]in level was observed (data not shown). We also monitored [Ca2+]in for additional 70 min and detected a very slow decrease at a rate 1.43 nM/min (data not shown). Considering the minor changes during this extended incubation, [Ca2+]in was monitored during 21 min in all further experiments. Challenging naïve cells with 5 mM Ca2+ caused 28 fold increase in [Ca2+]in to 3.93 ± 0.37 μM within 0.92 min (3.88 μM/min) followed by a multi-phase decrease. First, [Ca2+]in dropped similarly to naïve cells by 38 %, but at a lower rate 0.46 μM/min. Then it increased to 3.01 ± 0.34 μM and slowly declined to 2.22 μM, showing a total decrease by 44 %.
Figure 1.
The effect of externally added Ca2+ on [Ca2+]in in P. aeruginosa PAO1. Cells were grown in BMM with no added Ca2+ (A) or in the presence of 5 mM Ca2+ (B), and challenged with of 0 mM (black), 1mM (dark grey), and 5 mM (dash) Ca2+. The basal level of luminescence was monitored for 1 min. 1 mM or 5 mM CaCl2 was added at the time indicated by the arrow, followed by luminescence for 20 min. Changes in free [Ca2+]in were calculated as described in the Methods section. The averages of at least three independent experiments are plotted.
In induced cells, the basal [Ca2+]in was 0.22 ± 0.04 μM, which is 57 % higher than in naïve cells (Fig. 1B). Upon exposure to 1 mM Ca2+, the induced cells increased their [Ca2+]in at a rate (3.12 μM/min) similar to naïve cells, but only fivefold (to 1.19 ± 0.11 μM) and during a shorter period of time (0.3 min). The following decrease proceeded with a much lower rate (0.16 μM/min), lasted for ~1.5 min, and accounted for 21 %. In contrast to naïve cells, during the second phase, [Ca2+]in slowly increased with a rate 0.04 μM/min to 1.23 ± 0.45 μM. The response to 5 mM Ca2+ in induced cells also showed a multi-phase pattern. Although the first rapid increase brought [Ca2+]in to a similar to naïve cells level (3.66 ± 0.44 μM), it was only 17 fold higher than the basal level. This increase occurred very quickly within 0.08 min at a rate 42.62 μM/min. The following decline was threefold faster than in naïve cells (1.6 μM/min). The second increase was similar to the one in naïve cells elevating [Ca2+]in to 2.9 ± 0.45 μM, followed by a decline to 2.13 ± 0.27 μM with a total decrease by 42 %.
The PAO1 genome contains multiple homologs of Ca2+ transporting ATPases and gradient driven exchangers
Homology searches in the PAO1 genome (www.pseudomonas.com) using amino acid sequences of the characterized prokaryotic Ca2+ transporters revealed 18 genes encoding different types of transporters (Table 1). Based on sequence similarity and predicted conserved domains, ten were predicted to encode P-, F-type, and ABC-type ATPases, and eight to encode ion exchangers and other types of transporters. They include two earlier characterized proteins: heavy metal translocating P-type ATPase (PA2435) and dicarboxylic acid transporter (PA5167) [31, 32]. The comparative sequence analyses revealed that seven predicted P-type ATPases share 13 - 39 % sequence identity and do not have other paralogs in the PAO1 genome. Phylogenetic analysis using functionally characterized prokaryotic P-type ATPases showed that the PAO1 proteins can be grouped into four clades, which coincide with ion specificity: 1) Ca2+ (PA1429); 2) Mg2+ (PA4825); 3) K+ (PA1634); and 4) Pb2+, Cd2+, Zn2+, CO2+, Cu2+ (PA2435, PA3920, PA1549, and PA3690) (Fig. S1). Further sequence comparison revealed that PA1429 together with five prokaryotic Ca2+-translocating P-type ATPases in clade 1 are more closely related to the human Ca2+ translocating ATPase SERCA than to the human calmodulin-binding ATPase PMCA1 (data not shown). This supports their classification into the subgroup of P2A-ATPases [20]. Interestingly, clade 2, including PA4825 and Mg2+-translocating MgtB from S. typhimurium, is closely related to PMCA1 (data not shown). Furthermore, considering that PA2435 was shown to uptake both Cu2+ and Zn2+ [32], we combined Cu2+ and heavy metal- translocating proteins into one clade (4). As a result, PA1549, earlier grouped into functionally uncharacterized subfamily FUPA27 [33], was clustered with clade 4 proteins, suggesting its possible involvement in translocation of these ions. Other predicted transporters have one to four paralogs in the PAO1 genome and share 28 % – 82 % amino acid sequence identity. All the predicted transporters are conserved among ten sequenced pseudomonads and share 19 % – 100 % identity with their corresponding homologs.
Growth at elevated Ca2+ alters abundance of PAO1 membrane proteins
The modulated [Ca2+]in response in Ca2+ induced cells suggested the involvement of transporters, whose expression is affected by Ca2+. To identify such transporters, membrane proteins from PAO1 cells grown at no added or 5 mM Ca2+ were extracted and subjected to LC-MS/MS spectral counting - based comparative analyses. In total, about 500 proteins were identified, of which more than 80 % were transmembrane or membrane-associated proteins. These included seven bioinformatically predicted putative Ca2+ transporters, and ten proteins encoded within the same apparent operons (Table 1). To estimate the effect of Ca2+, for every protein we calculated a ratio of the total number of fragmentation spectra that map to the peptides of the protein in the samples collected at 5 mM vs. no added Ca2+. The results suggest that six predicted Ca2+ transporters (shown in bold in Table 1) increased abundance at least twofold in response to growth at elevated Ca2+. Considering that apparent operonic genes may have similar expression profile, the results also suggest Ca2+ induction for PA3400, PA5167, PA4016, and PA1549. However, PA4496 and PA4497 showed decreased abundance in response to 5 mM Ca2+.
Ca2+ homeostasis in PAO1 involves multiple transporters of different types
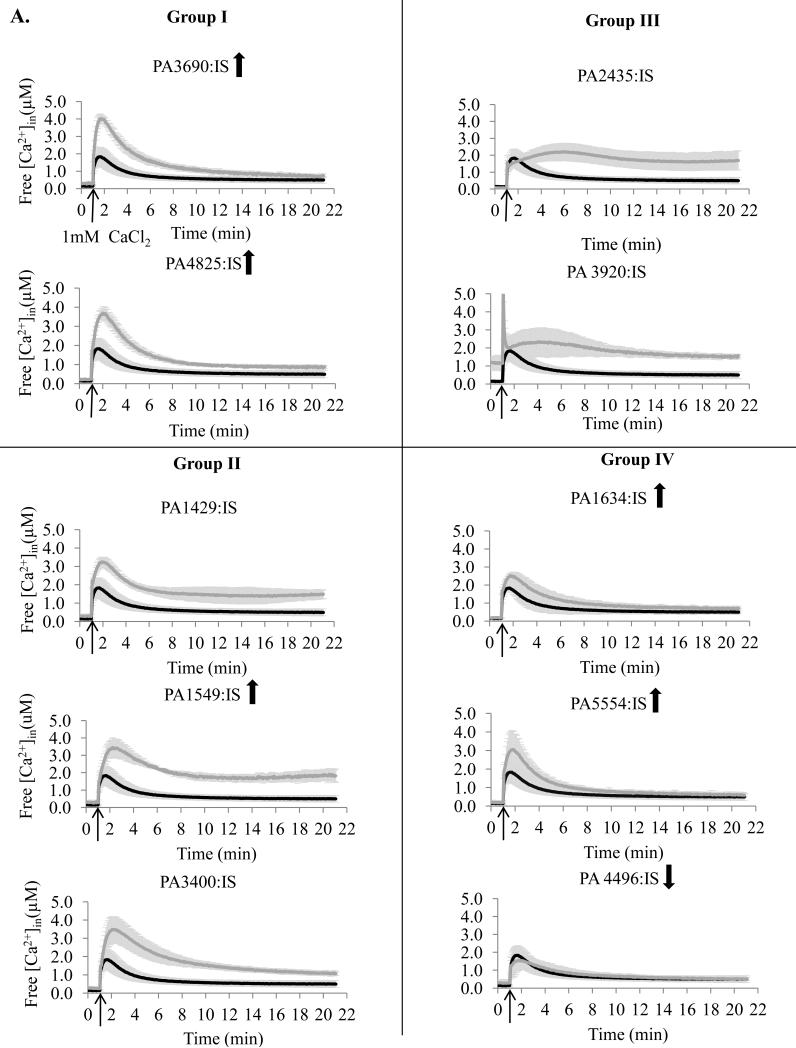
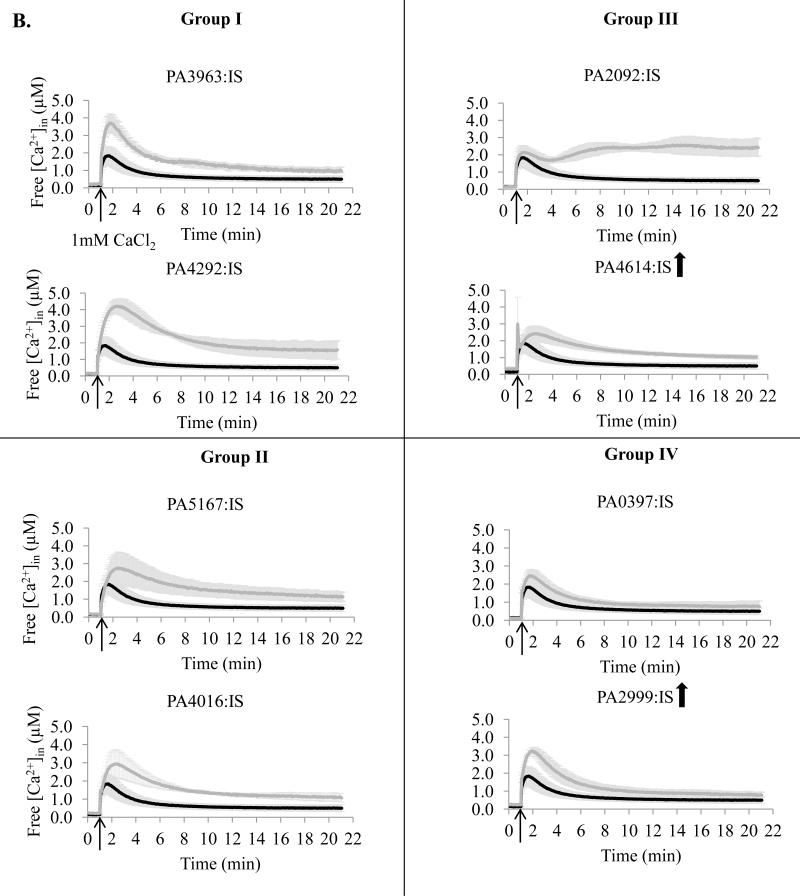
To study the role of the predicted transporters in Ca2+ homeostasis, we obtained 18 mutants, each with one predicted transporter encoding gene disrupted with either ISphoA/hah or ISlacZ/hah transposon insertion. The mutants were monitored for their [Ca2+]in response to 1 mM Ca2+ and compared to the wild type (WT) PAO1 cells. Based on the results they were grouped into four groups (Fig 2). Group I (four mutants) showed the initial rapid increase in [Ca2+]in at least twofold higher than in WT cells followed by the recovery to approximately the WT level [Ca2+]in. Group II (five mutants) failed the recovery to the WT level [Ca2+]in during 21 min monitoring, and had the remaining [Ca2+]in at the levels at least twofold higher than in PAO1 cells. Group III (four mutants) showed two peaks of [Ca2+]in increase and the remaining [Ca2+]in at the level at least twofold higher than in WT cells. The [Ca2+]in changes less than twofold vs. PAO1 were considered not significant (five mutants in group IV).
Figure 2.
Free [Ca2+]in profiles of transposon mutants with disrupted ATP-dependent transporters (A) or ion exchange transporters (B). The mutants were obtained from the University of Washington Two - Allele library. Cells were grown in BMM media with no added Ca2+. The basal level of luminescence was monitored for 1 min. 1 mM CaCl2 was added at the time indicated by the arrow, followed by luminescence measurements for 20 min. Changes in free [Ca2+]in were calculated as described in the Methods section. PA numbers represent the open reading frames in PAO1 genome. Black, PAO1 wild type; grey, transposon mutant. The data is an average of at least three independent experiments. Upward and downward arrows indicate that the protein abundance was increased and decreased, correspondingly, during growth at 5 mM CaCl2.
Among the ten examined mutants with disrupted putative ATP-ases, seven showed significant changes in [Ca2+]in response (Fig 2A). They included mutants lacking six P-type ATPases: PA3690, PA4825 (Group I), PA1429, PA1549 (Group II), PA2435, PA3920 (Group III), and ABC transporter PA3400 (Group II). The group III mutants showed the most drastic changes in [Ca2+]in response, and together with PA1549-lacking mutant failed to significantly decrease the elevated [Ca2+]in. The remaining three ATP-ases: P-type PA1634, F-type PA5554, and ABC transporter PA4496 showed no role in maintaining [Ca2+]in homeostasis (Group IV). Among the eight tested putative ion exchangers and other transporters, disruption of six showed significant changes in [Ca2+]in response (Fig. 2B). They included: three ion exchangers PA3963, PA4292 (Group I), and PA2092 (Group III), dicarboxylic acid transporter PA5167, hypothetical protein PA4016 (Group II), and mechanosensitive channel PA4614 (Group III). Among these proteins, the most significant effect was observed for the mutant lacking a major facilitator type transporter PA2092, that was unable to reduce [Ca2+]in after the initial increase. Ion exchanger PA0397 and probable Na+ translocating oxidoreductase PA2999 showed no role in Ca2+ homeostasis.
Multiple mechanisms are involved in Ca 2+ homeostasis in PAO1
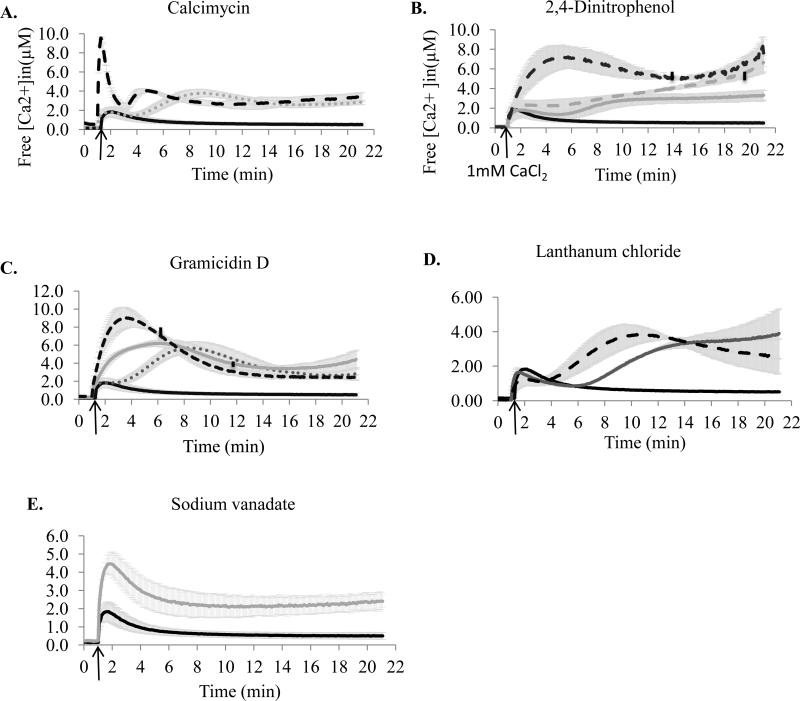
To identify the role of different types of transporters in Ca2+ homeostasis and better understand the mutants [Ca2+]in profiles, we examined the effect of several inhibitors specifically affecting different mechanisms associated with ion transport, on the maintenance of [Ca2+]in. For this, PAO1 cells were treated with the selected inhibitors and monitored for their [Ca2+]in response to 1 mM Ca2+. First, calcimycin, a Ca2+ ionophore known to carry Ca2+ into the cells [34], was used to test the response of PAO1 cells to Ca2+ influx (Fig. 3A). Treatment with 5 μM calcimycin caused a rapid (36 μM/min) uptake of Ca2+ and increase of [Ca2+]in to 9.54 ± 0.15 μM followed by an immediate but slower (4.1 μM/min) decline to 2.36 ± 0.33 μM. The second increase in [Ca2+]in , although appearing 4 min earlier than in the DMSO control, is most likely due to the presence of DMSO (used to dissolve calcimycin).
Figure 3.
Effect of inhibitors on free [Ca2+]in in P. aeruginosa PAO1. Cells grown in BMM media containing no added Ca2+ were treated with inhibitors for 10 min at room temperature. The basal level of luminescence was monitored for 1 min. 1 mM CaCl2 was added at the time indicated by the arrow, followed by luminescence measurements for 20 min. Changes in free [Ca2+]in were calculated as described in the Methods section. A. Calcimycin. Black, 0 mM; dashed black, 5 μM; small dotted grey, solvent control. B. Cells challenged with 2, 4 dinitrophenol. Black, 0mM; grey, 0.5 mM; dashed grey, 1 mM; dashed black, 2 mM. C. Gramicidin D. Black, 0 μg/ml; small dotted grey, 1 μg/ml; dashed black, 10 μg/ml; and grey, solvent control. Vertical lines on the plots indicate the points, at which the remaining available aequorin reached 10 % of the estimated total aequorin. D. LaCl3. Black, 0 μM; grey, 300 μM, and dashed black, 600 μM. E. Vanadate (2 mM, pH 7.5). The data were averaged from at least three independent experiments.
To test the role of a proton gradient across the cytoplasmic membrane and intracellular ATP in [Ca2+]in response, 2,4-Dinitrophenol (DNP), a proton ionophore known to disrupt a proton gradient and uncouple oxidative phosphorylation [25], was used (Fig. 3B). In a concentration dependent manner, DNP caused a second peak of [Ca2+]in to increase, which at higher levels of DNP appeared to merge with the first peak and reached 7.28 ± 0.74 μM. This level of [Ca2+]in was reached at the rate of 22 fold slower than in calcimycin-treated cells, and was followed by a 32 % decrease. The remaining [Ca2+]in was higher with increasing dose of DNP, and at 2 mM DNP, reached 8.39 ± 1.35 μM.
To examine the role of monovalent cation exchangers in PAO1 Ca2+ homeostasis, gramicidin D treatment was used (Fig. 3C). Gramicidin D is known to form channels across the cytoplasmic membrane and dissipate the gradients of H+, Na+, and K+ [35], which may also affect the intracellular ATP pool [36]. In a concentration-dependent manner, gramicidin D treatment elevated the initial increase in [Ca2+]in up to 9.06 ± 1.15 μM, which was followed by a decline to 2.49 ± 0.23 μM. The rates of [Ca2+]in increase and decrease were at least twofold higher at 10 μg/ml than at 1 μg/ml gramicidin D. The [Ca2+]in recovery levels were similar in the treated cells and at least fivefold higher than in the untreated cells. The effect of gramicidin D on [Ca2+]in also included the effect caused by DMSO.
Lanthanum (III) is a Ca2+ antagonist, known to block Ca2+ channels [37]. It may also arrest Ca2+-binding ATPases in a phosphorylated form and prevent further conformational changes required for Ca2+ translocation [38]. The effect of LaCl3 on the level of [Ca2+]in showed two distinct concentration-dependent patterns: a decrease in the initial rise in [Ca2+]in and appearance of the second peak of [Ca2+]in (Fig. 3D). The remaining level of Ca2+in was at least fivefold higher in the LaCl3 treated cells than in the untreated.
We also tested the effect of vanadate on Ca2+ homeostasis in PAO1 (Fig. 3E). Vanadate is known to inhibit P-type [39] and ABC [40] ATPases, and therefore is expected to block Ca2+ uptake or efflux mediated by these transporters. Vanadate treatment increased the initial rise of [Ca2+]in by twofold, and the remaining level of [Ca2+]in by fourfold.
Ca2+ homeostasis is not involved in PAO1 tolerance to external Ca2+
To examine whether the putative Ca2+ transporters play a role in cell tolerance to high Ca2+, thirteen mutants from groups I - III were grown in the presence of 5 or 100 mM Ca2+ and compared to WT. The results showed that at 5 mM Ca2+, neither PAO1 nor mutants showed any growth defects. Similarly, the addition of 100 mM Ca2+, although introduced a 13 h lag phase, did not significantly affect growth of the wild type or the mutants (data not shown).
Ca2+ homeostasis is involved in regulating Ca2+-induced swarming motility in PAO1
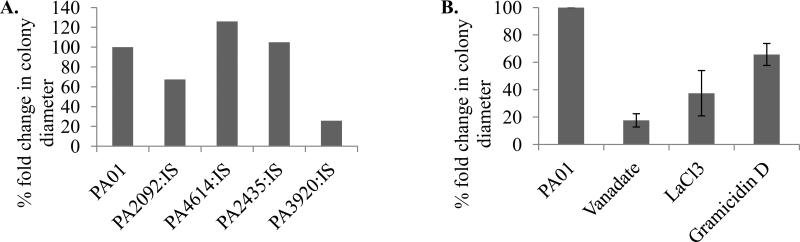
To determine the role of Ca2+ homeostasis in Ca2+- induced swarming motility in PAO1, the group III mutants with abolished ability to maintain Ca2+ homeostasis were tested for swarming motility at 5 mM Ca2+ vs. no added Ca2+ (Fig. 4 A). The presence of Ca2+ induced swarming in PAO1 by sixfold. When this induction was taken as 100 %, two mutants PA3920:IS, with disrupted P-type ATPase, and PA2092:IS, with disrupted major facilitator protein, showed only 27 % and 67 % of Ca2+ induction, respectively. PA2435:IS, with disrupted P-type ATPase, showed no significant difference in swarming, and PA4614:IS, with disrupted mechanosensitive channel, swarmed 26 % further in the presence of Ca2+ than PAO1. We also tested the effect of inhibitors targeting P-type and ABC ATPases (vanadate), Ca2+ channels (LaCl3), or dissipating H+, Na+, and K+ gradients (gramicidin D) on Ca2+-induced swarming motility (Fig. 4 B). All three inhibitors reduced the effect of Ca2+ on swarming. The most significant reduction was observed in the presence of 2 mM vanadate, where Ca2+ induction reached only 17 % of the induction in untreated PAO1. The presence of 10 μg/ml gramicidin D or 600 μM LaCl3 decreased Ca2+ induction by 34% and 63%, respectively. The results for LaCl3 should, however, be taken with a caution, since it precipitated during preparation.
Figure 4.
Swarming motility. Cells were grown on swarming agar containing 0 mM or 5 mM Ca2+. A. Group III mutants. B. PAO1 cells. Inhibitors: 2 mM vanadate, 600 μm LaCl3, or 10 μg/ml gramicidin D were added to the medium prior to plating. Colony diameters were measured, and fold differences (5 mM vs. 0 mM) were calculated using the corresponding controls. The percentage of fold changes was calculated considering that the fold difference in untreated PAO1 was 100 %. The averages of at least three biological replicates were used to calculate the percentage of the fold changes. To avoid bias, in case of mutants, colony diameters were first averaged, and then the fold differences between the mutants and WT were calculated. The standard deviation between replicates was below 10 %.
DISCUSSION
This study establishes that Pseudomonas aeruginosa PAO1, a human pathogen with Ca2+- induced virulence, maintains free intracellular Ca2+ at sub-micromolar level, which rapidly increases and slowly restores in response to external Ca2+. This process is impaired by blocking monovalent cation gradient or modulating Ca2+ uptake, and requires several transporters from the superfamilies of P-type ATPases and ion exchangers. These proteins will make an excellent tool for studying Ca2+ regulation and signaling in this organism. Finally, the study showed the role of Ca2+ homeostasis in Ca2+- induced swarming motility, suggesting its regulatory role in P. aeruginosa response to Ca2+.
The intracellular level of [Ca2+]in (0.1 – 0.2 μM) and the magnitude (5-28 fold) of the initial response to external Ca2+ (1 - 5 mM) places P. aeruginosa among three other bacteria E. coli JM109, Anabaena sp. PCC7120, and B. subtilis [9, 12, 13], whose intracellular Ca2+ levels have been characterized. In contrast, Propionibacterium acne, although has a similar basal level of [Ca2+]in, responds to 1mM Ca2+ by only twofold increase in [Ca2+]in [10]. The results illustrate that P. aeruginosa is able to maintain sub-micromolar levels of intracellular Ca2+ in the presence of millimolar levels of external Ca2+, suggesting its ability to (1) block Ca2+ uptake, (2) pump out Ca2+ from the cells against the steep concentration gradient, or (3) chelate Ca2+ inside the cells. The first two abilities in prokaryotes were attributed to the function of several mechanisms including ion channels, P- and F-type ATPases and ion gradient driven transporters that were identified using inhibitors [13, 25] or individual proteins [22].
The detected two-phase [Ca2+]in recovery (short-rapid and long-slow) following the challenge with 1 mM Ca2+ may suggest the function of distinct efflux mechanisms of high and low efficiency. The following fluctuations of [Ca2+]in in the presence of 5 mM Ca2+ may be a result of continuous cumulative effect of both influx and efflux mechanisms eventually resulting in the recovery of the [Ca2+]in basal level. Comparison of Ca2+-induced and naïve cells revealed multiple differences in [Ca2+]in responses. Lower initial [Ca2+]in responses with elevated rates of the [Ca2+]in initial increase and decrease in the presence of 5 mM Ca2+ in induced cells suggest that Ca2+ induces adaptation mechanisms. However, the elevated basal level of [Ca2+]in in induced cells and their inability to significantly reduce the increased [Ca2+]in at 1 mM Ca2+ indicate that growth at elevated Ca2+ possibly sensitizes cells to external Ca2+, as was proposed in E. coli [9]. Overall, these observations suggest the presence of multiple mechanisms for controlling Ca2+ homeostasis in P. aeruginosa and their complex regulation in response to different levels of Ca2+ in the environment.
Among several types of transporters determined to be involved in the maintenance of [Ca2+]in homeostasis, the most numerous were P-type ATPases. This superfamily of transporters has long been established to transport metals, but was not initially considered to play a major role in Ca2+ transport in prokaryotes. Later, several reports described both Ca2+-dependent P-type ATPase activity and ATP-dependent Ca2+ transport or transporters, most of which were shown to efflux Ca2+ in-vitro in membrane vesicles [21, 41]. However their role in maintaining cellular Ca2+ homeostasis in-vivo has not been tested. In this study, out of the seven P-type ATPases predicted in the PAO1 genome, four were induced by Ca2+, and six played a role in [Ca2+]in maintenance. These six were phylogenetically related to divalent cation translocating ATPases, whereas PA1634 clustered with K+-translocating KdpB from E. coli, showed no effect on [Ca2+]in homeostasis. P-type ATPases are known to be inhibited by vanadate, functioning as a phosphate analog [39]. In agreement, four mutants with disrupted P-type ATPases showed a [Ca2+]in profile similar to the profile of vanadate treated PAO1 cells with increased [Ca2+]in or lowered ability of its recovery. The disruption of two other P-type ATPases PA2435 and PA3920 showed the most significant effect and abolished the ability of PAO1 to reduce [Ca2+]in below 1.5 μM in the presence of 1mM Ca2+. PA2435 has been shown earlier to translocate Cu2+and Zn2+ [32], but in our phylogenetic analysis, it clustered closer to heavy metal-translocating ATPases, whereas PA3920 was more closely related to Cu2+-translocating transporters. Noteworthy, these two proteins were not detected to be induced by Ca2+, and therefore, likely do not contribute to the increased rate of Ca2+ efflux in Ca2+-induced cells. Since the identified P-type ATPases contributing to the detected changes in the intracellular Ca2+ do not contain typical Ca2+-binding domains, they are not likely to play a direct role in translocating Ca2+. The ATPases may be involved in controlling transition of metals important for Ca2+ translocation or generating an ion gradient that serves as an energy source for Ca2+ transporters, as was proposed in A. vinelandii [42].
To characterize the contribution of F-type ATPases (ATP synthases) in Ca2+ homeostasis, we treated cells with a protonophore 2,4 DNP, known to inhibit ATP synthesis by uncoupling oxidative phosphorylation [25]. This treatment raised the level of [Ca2+]in and impaired the ability of PAO1 to decrease it, most likely due to the lack of ATP. The role of ATP in Ca2+ efflux has been described in E. coli, where the lack of F-type ATP synthase AtpD decreased cellular ATP content and impaired [Ca2+]in efflux [25]. However, the disruption of PA5554, the AtpD homolog with 82 % amino acid sequence identity, although induced by Ca2+, did not affect Ca2+ homeostasis. It is possible that two paralogs of PA5554 in the genome: PA1697 and PA1104, with 24 and 28 % amino acid sequence identity, correspondingly, could compensate for the absence of PA5554 and provide ATP required for Ca2+ efflux.
Gradient driven Ca2+ exchangers have been considered as a major mechanism in bacterial Ca2+ efflux that most commonly employs H+ and Na+ as coupling ions. In agreement, treating PAO1 with gramicidin D, known to dissipate monovalent ion gradients across the cytoplasmic membrane [34], caused a significant initial accumulation of [Ca2+]in, which decreased over time, suggesting the function of alternative efflux mechanisms. We predicted and tested the role of four putative gradient driven exchangers in [Ca2+]in homeostasis, three of which showed a significant impact on Ca2+ homeostasis. The disruption of PA2092 showed the most prominent effect, as the mutant was not able to decrease the elevated [Ca2+]in in the presence of 1mM Ca2+. None of the predicted ion exchangers were induced by Ca2+, suggesting that, similarly to P-type ATPases PA2435 and PA3920, they do not play role in the differences between naïve and induced cells. The results suggest that Ca2+ translocation may be coordinated by the combined action of both P-type ATPases and ion exchangers as was illustrated for SERCA or PMCA ATPases and Na+/Ca2+ exchangers in mammalian systems [43, 44].
Treating PAO1 cells with a Ca2+ channel blocker LaCl3 [37] reduced the initial uptake of Ca2+, confirming the role of La3+ sensitive channels in PAO1 Ca2+ influx, as has been shown in other bacteria [9, 13]. The antagonistic effect of La3+ on Ca2+ influx in E. coli was associated with poly-3-hydroxybutyrate/polyphosphate (PHB-PP) channels [9]. However, search of the PAO1 genome for homologs of the functionally defined PHB-PP genes from Ralstonia [45] returned only scattered homologous genes. This may indicate the presence of a non- or low-homology PHB-PP biosynthetic pathway or a different type of La3+ sensitive channels in PAO1. Interestingly, in addition to blocking Ca2+ influx, LaCl3 treatment caused a secondary [Ca2+]in increase and a plateau at about 3 μM. This suggests that La3+ also inhibits Ca2+ efflux, which is consistent with the known inhibition effect of La3+ on P-type ATPases via blocking phosphorylation events [20, 38]. Furthermore, we detected that the disruption of PA4614 encoding Ca2+-induced probable mechanosensitive channel MscL caused a very quick uptake of Ca2+ followed by a second increase and a slow recovery of [Ca2+]in. In E. coli, although the expression of the homologous gene was also affected by Ca2+, its disruption showed no effect on Ca2+ homeostasis [25]. Mechanosensitive channels are known to open in response to membrane tension and protect cells against hypoosmotic shock as reviewed in [46]. It is not clear why the lack of the MscL protein caused the increase in Ca2+ uptake. We hypothesize that Ca2+-induced MscL may contribute to limiting Ca2+ uptake when in a closed state under the tested physiological conditions, and when mscL is disrupted, cells become more permeable to Ca2+ influx.
Finally, we determined that elevated Ca2+ induced swarming motility in PAO1, whereas treatment with vanadate, LaCl3 and gramicidin D decreased this induction. The disruption of P-type ATPase PA3920 and ion exchanger PA2092 also significantly diminished the inducing effect of Ca2+ on swarming. The effect of Ca2+ on swarming motility varies in different bacteria, for example, Ca2+ has been shown to induce swarming in Vibrio parahaemolyticus [47], but inhibited this type of motility in fluorescent pseudomonads [48]. This study shows for the first time that Ca2+ homeostasis plays an important role in P. aeruginosa Ca2+ induced swarming, a complex physiological phenomenon known to modulate virulence and antibiotic resistance in this organism [49].
Overall, this study reports that P. aeruginosa PAO1 maintains a sub-micromolar basal level of [Ca2+]in using multiple transport mechanisms that most likely require ATP and monovalent cation gradient. Involvement of multiple transport systems in Ca2+ influx and efflux has been suggested in E. coli [9, 50], and may be differentially regulated by growth conditions. This functional redundancy was also illustrated by the high tolerance of PAO1 cells to external Ca2+, and reflects the physiological importance of the controlled cellular Ca2+ homeostasis. The two major aspects of such importance include protecting cells against the toxicity of high [Ca2+] and maintaining low basal level of [Ca2+]in required for Ca2+ to play a signaling role. The transient changes in [Ca2+]in (magnitude, length, and frequency) may function to relate external signal(s) to cellular response(s), as has been established in eukaryotes [1] and proposed in prokaryotes [5]. We identified at least three transporters that play a major role in [Ca2+]in homeostasis, and will be used in further studies required for experimental confirmation of signaling role of Ca2+ in P. aeruginosa.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Anthony Campbell from the School of Pharmacy and Pharmaceutical Sciences, Cardiff University in UK for sharing his expertise and the templates for calculating [Ca2+]in. We thank Dr. Delfina Dominguez from The University of Texas at El Paso for sharing E. coli strain carrying pMMB66EH. We thank Dr. Wouter Hoff from Oklahoma State University for critical review of the manuscript and insightful discussions. We thank Dr. Masato Kumauchi from Oklahoma State University for curve-fitting analysis. Mass spectrometry analyses were performed in the DNA/Protein Resource Facility at Oklahoma State University, using resources supported by the NSF MRI and EPSCoR programs (Award DBI/0722494). This work was supported by the Grant-in-Aid from American Heart Association (Award 09BGIA2330036) and the Research Grant from OCAST (Award HR12-167). Transposon mutants were obtained from the University of Washington Two - Allele library (grant # NIH P30 DK 089507).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Ridefelt P, Hakansson L, Venge P. Regulation of human eosinophil migration across lung epithelial monolayers by distinct calcium signaling mechanisms in the two cell types. Journal of immunology (Baltimore, Md. 1999;163:5649–5655. [PubMed] [Google Scholar]
- 3.Halmerbauer G, Arri S, Schierl M, Strauch E, Koller DY. The relationship of eosinophil granule proteins to ions in the sputum of patients with cystic fibrosis. Clin Exp Allergy. 2000;30:1771–1776. doi: 10.1046/j.1365-2222.2000.00988.x. [DOI] [PubMed] [Google Scholar]
- 4.Lorin MI, Gaerlan PF, Mandel ID, Denning CR. Composition of nasal secretion in patients with cystic fibrosis. The Journal of laboratory and clinical medicine. 1976;88:114–117. [PubMed] [Google Scholar]
- 5.Dominguez DC. Calcium signalling in bacteria. Molecular microbiology. 2004;54:291–297. doi: 10.1111/j.1365-2958.2004.04276.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarkisova S, Patrauchan MA, Berglund D, Nivens DE, Franklin MJ. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. Journal of bacteriology. 2005;187:4327–4337. doi: 10.1128/JB.187.13.4327-4337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrauchan MA, Sarkisova SA, Franklin MJ. Strain-specific proteome responses of Pseudomonas aeruginosa to biofilm-associated growth and to calcium. Microbiology+ 2007;153:3838–3851. doi: 10.1099/mic.0.2007/010371-0. [DOI] [PubMed] [Google Scholar]
- 8.Domníguez DC LR, Holland IB, Campbell AK. Proteome analysis of B. subtilis in response to calcium. J Anal Bioanal Techniques. 2011;S6 [Google Scholar]
- 9.Jones HE, Holland IB, Baker HL, Campbell AK. Slow changes in cytosolic free Ca2+ in Escherichia coli highlight two putative influx mechanisms in response to changes in extracellular calcium. Cell calcium. 1999;25:265–274. doi: 10.1054/ceca.1999.0028. [DOI] [PubMed] [Google Scholar]
- 10.Futsaether CM, Johnsson A. Using fura-2 to measure intracellular free calcium in Propionibacterium acnes. Canadian journal of microbiology. 1994;40:439–445. doi: 10.1139/m94-072. [DOI] [PubMed] [Google Scholar]
- 11.Rosch JW, Sublett J, Gao G, Wang YD, Tuomanen EI. Calcium efflux is essential for bacterial survival in the eukaryotic host. Molecular microbiology. 2008;70:435–444. doi: 10.1111/j.1365-2958.2008.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbaud ML, Guiseppi A, Denizot F, Haiech J, Kilhoffer MC. Calcium signalling in Bacillus subtilis. Bba-Mol Cell Res. 1998;1448:212–226. doi: 10.1016/s0167-4889(98)00145-1. [DOI] [PubMed] [Google Scholar]
- 13.Torrecilla I, Leganes F, Bonilla I, Fernandez-Pinas F. Use of recombinant aequorin to study calcium homeostasis and monitor calcium transients in response to heat and cold shock in cyanobacterial. Plant Physiol. 2000;123:161–175. doi: 10.1104/pp.123.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leganes F, Forchhammer K, Fernandez-Pinas F. Role of calcium in acclimation of the cyanobacterium Synechococcus elongatus PCC 7942 to nitrogen starvation. Microbiol-Sgm. 2009;155:25–34. doi: 10.1099/mic.0.022251-0. [DOI] [PubMed] [Google Scholar]
- 15.Campbell AK, Naseern R, Holland IB, Matthews SB, Wann KT. Methylglyoxal and other carbohydrate metabolites induce lanthanum-sensitive Ca2+ transients and inhibit growth in E.coli. Arch Biochem Biophys. 2007;468:107–113. doi: 10.1016/j.abb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Rosen BP. Bacterial calcium transport. Biochimica et biophysica acta. 1987;906:101–110. doi: 10.1016/0304-4157(87)90007-4. [DOI] [PubMed] [Google Scholar]
- 17.Fujisawa M, Wada Y, Tsuchiya T, Ito M. Characterization of Bacillus subtilis YfkE (ChaA): a calcium-specific Ca2+/H+ antiporter of the CaCA family. Arch Microbiol. 2009;191:649–657. doi: 10.1007/s00203-009-0494-7. [DOI] [PubMed] [Google Scholar]
- 18.Waditee R, Hossain GS, Tanaka Y, Nakamura T, Shikata M, Takano J, Takabe T, Takabe T. Isolation and functional characterization of Ca2+/H+ antiporters from cyanobacteria. Journal of Biological Chemistry. 2004;279:4330–4338. doi: 10.1074/jbc.M310282200. [DOI] [PubMed] [Google Scholar]
- 19.Radchenko MV, Tanaka K, Waditee R, Oshimi S, Matsuzaki Y, Fukuhara M, Kobayashi H, Takabe T, Nakamura T. Potassium/proton antiport system of Escherichia coli. Journal of Biological Chemistry. 2006;281:19822–19829. doi: 10.1074/jbc.M600333200. [DOI] [PubMed] [Google Scholar]
- 20.Palmgren MG, Nissen P. P-type ATPases. Annual review of biophysics. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 21.Berkelman T, Garretengele P, Hoffman NE. The Pacl gene of Synechococcus Sp strain Pcc-7942 encodes a Ca2+-transporting Atpase. Journal of bacteriology. 1994;176:4430–4436. doi: 10.1128/jb.176.14.4430-4436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faxen K, Andersen JL, Gourdon P, Fedosova N, Morth JP, Nissen P, Moller JV. Characterization of a Listeria monocytogenes Ca2+ pump a SERCA-type ATPase with only one Ca2+-binding site. Journal of Biological Chemistry. 2011;286:1609–1617. doi: 10.1074/jbc.M110.176784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raeymaekers L, Wuytack EY, Willems I, Michiels CW, Wuytack F. Expression of a P-type Ca2+-transport ATPase in Bacillus subtilis during sporulation. Cell calcium. 2002;32:93–103. doi: 10.1016/s0143-4160(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 24.Desrosiers MG, Gately LJ, Gambel AM, Menick DR. Purification and characterization of the Ca2+-ATPase of Flavobacterium odoratum. The Journal of biological chemistry. 1996;271:3945–3951. doi: 10.1074/jbc.271.7.3945. [DOI] [PubMed] [Google Scholar]
- 25.Naseem R, Wann KT, Holland IB, Campbell AK. ATP regulates calcium efflux and growth in E. coli. J Mol Biol. 2009;391:42–56. doi: 10.1016/j.jmb.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 26.Nouwens AS, Cordwell SJ, Larsen MR, Molloy MP, Gillings M, Willcox MD, Walsh BJ. Complementing genomics with proteomics: the membrane subproteome of Pseudomonas aeruginosa PAO1. Electrophoresis. 2000;21:3797–3809. doi: 10.1002/1522-2683(200011)21:17<3797::AID-ELPS3797>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Campbell AK S-NG. Bioluminescent and chemiluminescent indicators for molecular signalling and function in living cells. In: WT M, editor. Flourescent and Luminescent probes for biological activity. Academic Press; New York: 1993. pp. 58–82. [Google Scholar]
- 28.Irani VR, Rowe JJ. Enhancement of transformation in Pseudomonas aeruginosa PAO1 by Mg2+ and heat. BioTechniques. 1997;22:54–56. doi: 10.2144/97221bm09. [DOI] [PubMed] [Google Scholar]
- 29.Katsu T, Kobayashi H, Fujita Y. Mode of Action of Gramicidin S on Escherichia coli Membrane. Biochimica et biophysica acta. 1986;860:608–619. doi: 10.1016/0005-2736(86)90560-2. [DOI] [PubMed] [Google Scholar]
- 30.Overhage J, Lewenza S, Marr AK, Hancock REW. Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini-Tn5-lux mutant library. Journal of bacteriology. 2007;189:2164–2169. doi: 10.1128/JB.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentini M, Storelli N, Lapouge K. Identification of C(4)-dicarboxylate transport systems in Pseudomonas aeruginosa PAO1. Journal of bacteriology. 2011;193:4307–4316. doi: 10.1128/JB.05074-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewinson O, Lee AT, Rees DC. A P-type ATPase importer that discriminates between essential and toxic transition metals. P Natl Acad Sci USA. 2009;106:4677–4682. doi: 10.1073/pnas.0900666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan H, Babayan V, Blyumin E, Gandhi C, Hak K, Harake D, Kumar K, Lee P, Li TT, Liu HY, Lo TC, Meyer CJ, Stanford S, Zamora KS, Saier MH., Jr. The p-type ATPase superfamily. Journal of molecular microbiology and biotechnology. 2010;19:5–104. doi: 10.1159/000319588. [DOI] [PubMed] [Google Scholar]
- 34.Pressman BC. Biological application of ionophores. Annual Review of Biochemistry. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- 35.Burkhart BM, Langs DA, Pangborn WA, Duax WL, Pletnev V, Gassman RM. Gramicidin D conformation, dynamics and membrane ion transport. Biopolymers. 1999;51:129–144. doi: 10.1002/(SICI)1097-0282(1999)51:2<129::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 36.Jarrell KF, Hamilton EA. Effect of gramicidin on methanogenesis by various methanogenic bacteria. Applied and environmental microbiology. 1985;50:179–182. doi: 10.1128/aem.50.1.179-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reusch RN, Huang RP, Bramble LL. Poly-3-hydroxybutyrate polyphosphate complexes from voltage activated Ca2+ channels in the plasma membranes of Escherichia coli. Biophys J. 1995;69:754–766. doi: 10.1016/S0006-3495(95)79958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luterbacher S, Schatzmann HJ. The site of action of La3+ in the reaction cycle of the human red cell membrane Ca2+-pump ATPase. Experientia. 1983;39:311–312. doi: 10.1007/BF01955322. [DOI] [PubMed] [Google Scholar]
- 39.Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Bio. 2004;5:282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- 40.Pezza RJ, Villarreal MA, Montich GG, Argarana CE. Vanadate inhibits the ATPase activity and DNA binding capability of bacterial MutS. A structural model for the vanadate-MutS interaction at the Walker A motif. Nucleic acids research. 2002;30:4700–4708. doi: 10.1093/nar/gkf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gambel AM, Desrosiers MG, Menick DR. Characterization of a P-type Ca(2+)-ATPase from Flavobacterium odoratum. The Journal of biological chemistry. 1992;267:15923–15931. [PubMed] [Google Scholar]
- 42.Bhattacharyya P, Barnes EM., Jr. ATP-dependent calcium transport in isolated membrane vesicles from Azotobacter vinelandii. The Journal of biological chemistry. 1976;251:56–14-59. [PubMed] [Google Scholar]
- 43.Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure - Roles of diastolic leak and Ca2+ transport. Circulation Research. 2003;93:487–490. doi: 10.1161/01.RES.0000091871.54907.6B. [DOI] [PubMed] [Google Scholar]
- 44.Magyar CE, White KE, Rojas R, Apodaca G, Friedman PA. Plasma membrane Ca2+-ATPase and NCX1 Na+/Ca2+ exchanger expression in distal convoluted tubule cells. American journal of physiology. Renal physiology. 2002;283:F29–40. doi: 10.1152/ajprenal.00252.2000. [DOI] [PubMed] [Google Scholar]
- 45.Budde CF, Mahan AE, Lu J, Rha C, Sinskey AJ. Roles of multiple acetoacetyl coenzyme A reductases in polyhydroxybutyrate biosynthesis in Ralstonia eutropha H16. Journal of bacteriology. 2010;192:5319–5328. doi: 10.1128/JB.00207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Booth IR, Edwards MD, Black S, Schumann U, Miller S. Mechanosensitive channels in bacteria: signs of closure? Nature reviews. Microbiology. 2007;5:431–440. doi: 10.1038/nrmicro1659. [DOI] [PubMed] [Google Scholar]
- 47.Gode-Potratz CJ, Chodur DM, McCarter LL. Calcium and iron regulate swarming and type III secretion in Vibrio parahaemolyticus. Journal of bacteriology. 2010;192:6025–6038. doi: 10.1128/JB.00654-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakai M, Futamata H, Kanazawa S. Effects of high concentrations of inorganic salts on swarming ability in fluorescent Pseudomonas strains. Bioscience Biotechnology and Biochemistry. 2003;67:1479–1484. doi: 10.1271/bbb.67.1479. [DOI] [PubMed] [Google Scholar]
- 49.Yeung AT, Torfs EC, Jamshidi F, Bains M, Wiegand I, Hancock RE, Overhage J. Swarming of Pseudomonas aeruginosa is controlled by a broad spectrum of transcriptional regulators, including MetR. Journal of bacteriology. 2009;191:5592–5602. doi: 10.1128/JB.00157-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ambudkar SV, Zlotnick GW, Rosen BP. Calcium efflux from Escherichia coli. Evidence for two systems. The Journal of biological chemistry. 1984;259:6142–6146. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.