Abstract
Breakthrough discoveries identifying common genetic causes for amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) have transformed our view of these disorders. They share unexpectedly similar signatures, including dysregulation in common molecular players including TDP-43, FUS/TLS, ubiquilin-2, VCP, and expanded hexanucleotide repeats within the C9ORF72 gene. Dysfunction in RNA processing and protein homeostasis is an emerging theme. We present the case here that these two processes are intimately linked, with disease-initiated perturbation of either leading to further deviation of both protein and RNA homeostasis through a feed-forward loop including cell-to-cell prion-like spread that may represent the mechanism for relentless disease progression.
Introduction
Amyotrophic lateral sclerosis (ALS, familiarly known in the United States as Lou Gehrig’s disease) was first reported 140 years ago by the great French physician Jean-Martin Charcot. The name describes the key features of the disease: muscle wasting (amyotrophic) due to the degeneration of lower motor neurons and their axons and loss of upper motor neurons and their corticospinal axonal tracts (lateral sclerosis). In contrast to ALS, frontotemporal dementia (FTD) (also known as frontotemporal lobar degeneration (FTLD)) is a progressive neuronal atrophy with loss in the frontal and temporal cortices and characterized by personality and behavioral changes, as well as gradual impairment of language skills. It is the second most common dementia after Alzheimer’s disease (Van Langenhove et al., 2012).
Here, we review the key findings that have revealed a tangled web where multiple pathways are involved in disease initiation and progression in ALS and FTD. RNA and protein homeostasis pathways are intimately linked and their dysfunction is fundamentally involved in disease pathogenesis. Perturbation of either pathway can amplify an initial abnormality through a feed-forward loop, which may underlie relentless disease progression.
Convergence of pathogenic mechanisms of ALS and FTD
Largely indistinguishable, familial (10%) and sporadic (90%) ALS are characterized by premature degeneration of upper and lower motor neurons. Mutations in four genes (C9ORF72, SOD1, TARDBP, and FUS/TLS) account for over 50% of the familial cases (Supplemental table). For FTD, a stronger genetic contribution is reflected by the higher percentage (up to 50%) of patients with a familial history. This includes the first two identified causal genes, the microtubule-associated protein tau (MAPT) (Hutton et al., 1998) and progranulin (PGRN) (Baker et al., 2006; Cruts et al., 2006), which together account for 10–20% of FTD (Van Langenhove et al., 2012). More rarely, mutations in TDP-43 and FUS/TLS are causal for FTD [reviewed in (Lagier-Tourenne et al., 2010; Mackenzie et al., 2010a)]. Recently, hexanucleotide expansion in the C9ORF72 gene was found to be a common genetic cause for ALS and FTD (Dejesus-Hernandez et al., 2011; Gijselinck et al., 2012; Renton et al., 2011) (Supplemental table).
It is estimated that 15% of FTD patients meet ALS criteria (Ringholz et al., 2005), and ALS can be accompanied by cognitive and behavioral impairment, with perhaps as much as 15% of affected individuals also developing symptoms consistent with a typical definition of FTD (Ringholz et al., 2005; Wheaton et al., 2007). ALS and FTD are linked clinically, pathologically and mechanistically, and the diseases are now properly recognized as representatives of a continuum of a broad neurodegenerative disorder, with each presenting in a spectrum of overlapping clinical symptoms (Figure 1).
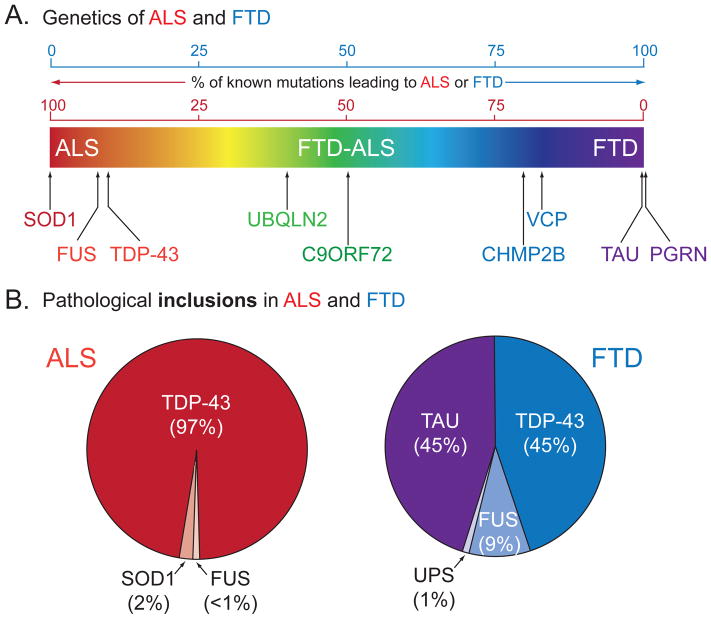
Figure 1. Clinical, genetic and pathological overlap of ALS and FTD.
(A) ALS and FTD represent a continuum of a broad neurodegenerative disorder with each presenting as extremes of a spectrum of overlapping clinical symptoms (ALS in red and FTD in purple). Major known genetic causes for ALS and FTD are plotted according to the ratio of known mutations that give rise to ALS or FTD. (B) Pathological protein inclusions in ALS and FTD, according to the major protein misaccumulated. Inclusions of TDP-43 and FUS/TLS in ALS and FTD reflect the pathological overlap of ALS and FTD.
A breakthrough linking disease mechanisms for ALS and FTD came with the identification of TDP-43 as the major ubiquitinated protein found in both sporadic ALS patients and the most frequent pathological form of FTD (Arai et al., 2006; Neumann et al., 2006). This finding was followed by the discovery of mutations in the gene encoding the RNA binding protein TDP-43 in ~5% of familial ALS cases (Kabashi et al., 2008; Sreedharan et al., 2008; Van Deerlin et al., 2008) and rare patients with FTD (Borroni et al., 2009; Kovacs et al., 2009).
Recognition that errors in RNA binding proteins are causative of ALS and FTD was quickly expanded, with mutations in the fused in sarcoma/translocated in liposarcoma (FUS/TLS) gene shown to account for an additional ~5% of familial ALS and also rare cases of FTD (Kwiatkowski et al., 2009; Vance et al., 2009). Subsequent confirmation that FUS/TLS was present in the pathological inclusions in most of the FTD patients without TDP-43-containing inclusions has led to a proposed reclassification of FTD based on the main protein component accumulated (Mackenzie et al., 2010b; Sieben et al., 2012). These include: FTLD-tau (45%), FTLD-TDP (45%), FTLD-FUS (9%), and a remaining 1% named FTLD-UPS (for ubiquitin-proteasome system) (Figure 1). Altogether, these findings highlight two main discoveries: (1) TDP-43 and FUS/TLS, both RNA binding proteins linked to multiple steps of RNA metabolism, are the major protein components of pathological inclusions observed in over 90% of ALS and over 50% of FTD patients; and (2) errors in RNA processing may be central to ALS and FTD pathogenesis.
A further direct molecular link between ALS and FTD was identification of a large intronic hexanucleotide expansion (~400–1,600 GGGGCC repeats) in the previously uncharacterized gene C9ORF72 (named for its location on chromosome 9, open reading frame 72) in families with either ALS, FTD or both (Dejesus-Hernandez et al., 2011; Gijselinck et al., 2012; Renton et al., 2011). The expanded repeat in C9ORF72 is reminiscent of previously studied repeat expansion diseases (La Spada and Taylor, 2010), especially myotonic dystrophy and fragile X mental retardation syndrome, whose precedents support at least two possible pathogenic mechanisms: RNA-mediated toxicity or haploinsufficiency.
ALS, ALS/dementia and/or FTD causing mutations were also identified in genes involved in protein clearance pathways or maintaining proper protein homeostasis, including ubiquilin-2 (UBQLN2) (Deng et al., 2011), vasolin-containing protein (VCP) (Johnson et al., 2010; Watts et al., 2007), vesicle-associated membrane protein-associated protein B (VAPB) (Nishimura et al., 2004), p62/sequestosome (SQSTM1) (Fecto et al., 2011; Rubino et al., 2012; Teyssou et al., 2013), optineurin (OPTN) (Maruyama et al., 2010), and charged multivesicular body protein 2B or chromatin modifying protein 2B (CHMP2B) (Parkinson et al., 2006; Skibinski et al., 2005). Coupled with protein aggregation as a major pathological hallmark of both ALS and FTD, the genetic discoveries indicate that disruption in protein homeostasis (or proteostasis) is a key characteristic of both diseases.
ALS- and FTD-linked genes disrupt RNA homeostasis
Identification of disease-linked mutations in TDP-43 and FUS/TLS marked the beginning of a paradigm shift, highlighting dysfunctions in RNA metabolism as a central pathogenic pathway in ALS and FTD. TDP-43 and FUS/TLS share similar structural and functional properties with probable involvement in multiple RNA processing steps (Lagier-Tourenne et al., 2010). ALS-linked mutations have been identified in genes encoding TAF15 (TATA-binding protein associated factor 15) (Couthouis et al., 2011; Ticozzi et al., 2011) and EWSR1 (Ewing’s sarcoma breakpoint region 1) (Couthouis et al., 2012), two proteins that are functionally and structurally similar to FUS/TLS, albeit the mutations have not been proven to be causative of disease. Altogether, with additional ALS-linked mutations in the RNA-binding proteins angiogenin (Greenway et al., 2006), senataxin (Chen et al., 2004), and ataxin-2 (Elden et al., 2010), disruption in RNA homeostasis seems highly likely to play a central role in ALS pathogenesis.
TDP-43 and FUS/TLS reshape ALS and FTD
TDP-43 mutation and pathology in ALS and FTD
TDP-43 is a 414 amino acid protein containing two RNA recognition motifs (RRM) followed by a glycine-rich, low sequence complexity prion-like domain (Kato et al., 2012; King et al., 2012). TDP-43 can shuttle between the cytosol and the nucleus (Ayala et al., 2008; Winton et al., 2008), although the majority of TDP-43 appears to be nuclear in most cells at steady state. Pathological inclusions of TDP-43 can be found in the nucleus and cytosol of neurons and glia, with abnormal phosphorylation and ubiquitination of TDP-43 and the presence of truncated C-terminal fragments (Arai et al., 2006; Neumann et al., 2006). More than 40 mutations in sporadic and familial ALS, as well as in rare cases of FTLD [reviewed in (Lagier-Tourenne et al., 2010; Lattante et al., 2013)], are found clustered within a prion-like domain (so named because of its similarity to fungal prions) (Supplemental Figure 1).
In the absence of mutation, TDP-43 pathology can be found in a majority of sporadic ALS patients with the exception of patients with SOD1 mutations (Mackenzie et al., 2007; Tan et al., 2007) and is apparently indistinguishable between patients with or without TDP-43 mutations (Pamphlett et al., 2009). Over 90% of all ALS cases exhibit TDP-43 protein pathology. Cells with TDP-43 aggregates typically have concomitant loss of nuclear TDP-43, indicating loss of nuclear TDP-43 function, while the presence of cytoplasmic protein inclusions suggests gain of one or more toxic properties. Thus, the pathogenic mechanisms for TDP-43 are likely to be a combination of both loss of function and gain of toxic properties.
Normal function of TDP43
TDP-43 was first identified as a protein that bound to the trans-activation response (TAR) element of HIV human immunodeficiency virus and was named TAR DNA-binding protein-43 kDa. TDP-43 can act as a transcriptional repressor and is associated with proteins involved in transcription (Ling et al., 2010; Sephton et al., 2011), including methyl CpG-binding protein 2 (MeCP2) (Sephton et al., 2011), whose mutations are causative for Rett syndrome. Genome-wide approaches are now needed to identify the complete set of genes for which TDP-43 plays a transcriptional role through its direct DNA binding. TDP-43 is involved in many aspects of RNA-related metabolism, including splicing, microRNA (miRNA) biogenesis, RNA transport and translation, and stress granule formation by interacting with numerous hnRNPs, splicing factors, and microprocessor proteins [reviewed in (Buratti and Baralle, 2012; Lagier-Tourenne et al., 2010; Polymenidou et al., 2012)] (Figure 2A),
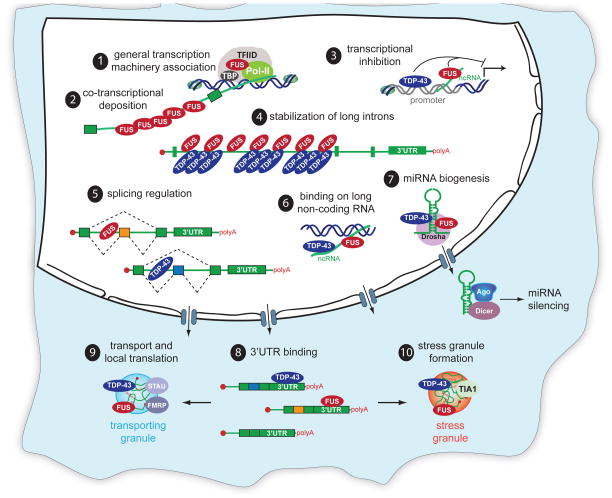
Figure 2. Physiological roles of TDP-43 and FUS/TLS in RNA processing.
Proposed roles for FUS/TLS include 1) association with TBP within the TFIID complex as a participant in the general transcriptional machinery and 2) binding to long introns in a sawtooth-like pattern, consistent with co-transcriptional deposition. Both TDP-43 and FUS/TLS 3) associate with promoter regions. TDP-43 binds single-stranded TG-rich elements in promoter regions thereby blocking transcription of the downstream gene. In response to DNA damage, FUS/TLS is recruited in the promoter region of cyclin D1 (CCND1) by sense and antisense noncoding RNAs (ncRNAs) and represses CCND1 transcription. BothTDP-43 and FUS/TLS 4) bind long intron-containing RNAs, thereby sustaining their levels. 5) TDP-43 and FUS/TLS control the levels of >950 or >370 RNAs, respectively, either via direct binding or indirectly. TDP-43 and FUS/TLS 6) bind long non-coding RNAs, 7) complex with Drosha (consistent with an involvement in miRNA processing), and 8) bind 3′UTRs of a large number of mRNAs. Both TDP-43 and FUS/TLS shuttle between the nucleus and the cytosol and are incorporated into 9) transporting RNA granules and 10) stress granules, in which they form complexes with mRNAs and other RNA binding proteins.
TDP43’s RNA targets
An unbiased genome-wide approach was used to identify the in vivo RNA targets for TDP-43 in mouse (Polymenidou et al., 2011) and human (Tollervey et al., 2011) brain. More conventional methodology has also been used in an effort to identify RNA targets of TDP-43 in rat cortical neurons (Sephton et al., 2011), a mouse NSC-34 cell line (Colombrita et al., 2012), and a human neuroblastoma cell line (Xiao et al., 2011). It is clear that TDP-43 binds to more than 6,000 RNA targets in the brain, roughly 30% of the total transcriptome (Figure 3). The localization of TDP-43’s binding sites across different pre-mRNAs reveals its various roles in RNA maturation. Indeed, intronic binding of TDP-43 on long-intron (>100 kb) containing RNA targets was shown to be required for sustaining their normal levels (Polymenidou et al., 2011). Splice site selection is influenced by TDP-43 binding near exon-intron junctions as well as in the intronic regions far away (<2kb) from the nearest exon (Polymenidou et al., 2011; Tollervey et al., 2011). In addition, TDP-43 binding on the 3′-untranslated regions (3′UTR) of mRNAs may affect their stability or transport, while TDP-43 binding on long non-coding RNAs (ncRNAs) may influence their regulatory roles.
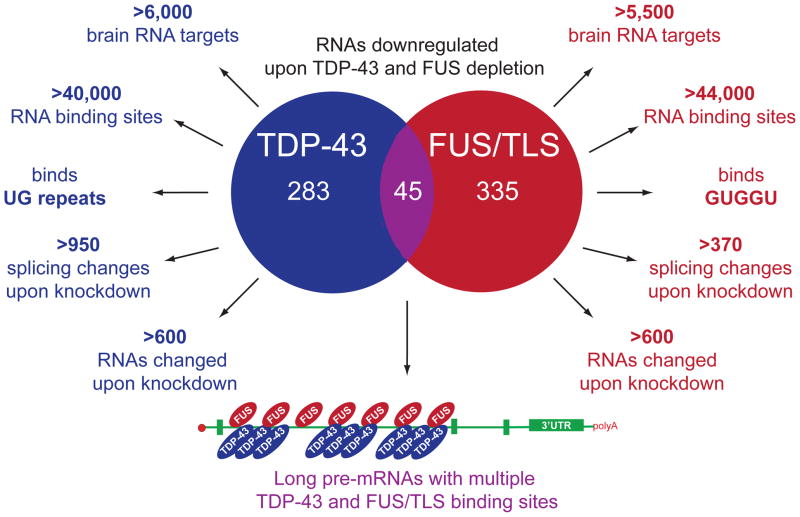
Figure 3. Comparison of TDP-43 and FUS/TLS RNA binding properties.
Data are taken from Polymenidou et al (2011), Lagier-Tourenne et al (2012), Tollervey et al (2011), Sephton et al (2011), Colombrita et al (2012), Rogeli et al (2012), Hoell et al (2011), Ishigaki et al (2012), and Nakaya et al (2013).
TDP-43 levels matter greatly for normal RNA maturation. Antisense oligonucleotide mediated reduction of TDP-43 within an otherwise normal mouse nervous system affects the levels of more than 600 mRNAs and the splicing pattern of another ~950 (Polymenidou et al., 2011). TDP-43 also binds to the 3′UTRs of more than 1,000 transcripts (Polymenidou et al., 2011; Tollervey et al., 2011), including its own mRNA, presumably affecting nuclear or cytoplasmic RNA stability. It also has binding sites on many ncRNAs whose functions are not yet clearly defined but include chromatin remodeling, transcription regulation and post-transcriptional processing. Among these, TDP-43 binds to long (>200 base) ncRNAs, including NEAT1 (nuclear-enriched autosomal transcript 1) and MALAT1 (metastasis-associated lung adenocarcinoma transcript 1) (Tollervey et al., 2011). Expression of both NEAT1 and MALAT1 is elevated in FTLD-TDP patients, which correlates with increased TDP-43 association with both ncRNAs (Tollervey et al., 2011). These data suggest that TDP-43 may affect RNA metabolism, including >300 mRNAs without TDP-43 binding sites but whose abundance increases when TDP-43 is reduced (Polymenidou et al., 2011) through an indirect mechanism.
The binding of TDP-43 to small (<200 base) ncRNAs and miRNAs remains largely unexplored. Nonetheless, the association of TDP-43 with Drosha microprocessor (Ling et al., 2010) and Dicer complexes (Freibaum et al., 2010; Kawahara and Mieda-Sato, 2012), is suggestive of a TDP-43 involvement in miRNA biogenesis. Indeed, let-7b miRNA is down-regulated, whereas miR-663 is upregulated after reduction in TDP-43 (Buratti et al., 2010).
FUS/TLS mutation and pathology in ALS and FTD
ALS/FTD-linked mutations in FUS/TLS are clustered into two groups: mutations in the low complexity/prion-like domain and mutations in the C-terminal nuclear localization signal (NLS) (Supplemental Figure 1). Mutations in the latter group typically lead to increased cytoplasmic localization of FUS/TLS (Kwiatkowski et al., 2009; Vance et al., 2009) and several are associated with juvenile onset ALS (Bäumer et al., 2010; Belzil et al., 2012; Huang et al., 2010; Yamashita et al., 2011).
Distinct patterns of FUS pathology have been correlated with disease severity and mutation (Mackenzie et al., 2011). Early-onset ALS cases are characterized by basophilic inclusions and round neuronal cytoplasmic FUS inclusions, whereas late-onset ALS cases are characterized by tangle-like FUS-containing inclusions in both neurons and glial cells. FUS inclusions in the absence of FUS mutations have also been reported in FTD, Huntington’s disease and spinocerebellar ataxia 1 and 2 [reviewed in (Lagier-Tourenne et al., 2010)].
Normal function of FUS/TLS
Similar to TDP-43, FUS/TLS can bind to single- and double-stranded DNA, as well as RNA, and almost certainly participates in a wide range of cellular processes (Lagier-Tourenne et al., 2010; Tan and Manley, 2009).
Transcription
All three members of the FET (FUS/TLS, EWSR1 and TAF-15) family have been shown to associate with RNA polymerase II (RNAP II) and its general transcription factor TFIID. FUS/TLS and TAF15 fractionate with different populations of TFIID complexes, suggesting that they may affect different promoters (Bertolotti et al., 1996). It is likely that FUS/TLS can affect the transcription of specific genes through its association with several nuclear hormone receptors (Powers et al., 1998) and gene-specific transcription factors. Indeed, a recent study identified potential FUS/TLS-response elements of many target genes, indicative of transcriptional activation or repression directly by FUS/TLS (Tan et al., 2012). FUS/TLS can also associate with TBP and TFIIIB to repress transcription by RNAP III, which transcribes small structural and catalytic RNAs (Tan and Manley, 2010).
Splicing
FUS/TLS has been identified as part of the spliceosome machinery in three independent proteomic studies (Hartmuth et al., 2002; Rappsilber, 2002; Zhou et al., 2002). The association of FUS/TLS with the spliceosome and various splicing factors initially implicated FUS/TLS in a co-transcriptional role and/or splicing regulation of pre-mRNAs, a prediction validated by demonstration that about 1,000 RNAs change in splicing pattern or abundance in a FUS/TLS-dependent manner in the mouse brain (Lagier-Tourenne et al., 2012) (Figure 3).
FUS/TLS’s RNA targets
Genome wide approaches (summarized in Figure 3) have identified more than 8,000 in vivo RNA targets for FUS/TLS in mouse (Lagier-Tourenne et al., 2012; Rogelj et al., 2012), 5,500 in human (Lagier-Tourenne et al., 2012), and more than 6,800 in various cell lines (Colombrita et al., 2012; Hoell et al., 2011; Ishigaki et al., 2012; Nakaya et al., 2013). A GUGGU sequence is the most prominent binding motif (Lagier-Tourenne et al., 2012). In addition, AU-rich stem-loops bound by FUS/TLS have also been proposed (Hoell et al., 2011). A sawtooth-like binding pattern to long introns (Lagier-Tourenne et al., 2012; Rogelj et al., 2012) is consistent with co-transcriptional deposition of FUS/TLS and suggests that FUS/TLS remains bound to pre-mRNAs until splicing is completed. In addition, FUS/TLS shows enrichment in binding to 3′-UTRs and exons.
Interestingly, RNAs bound by TDP-43 and FUS/TLS are largely distinct (Lagier-Tourenne et al., 2012; Rogelj et al., 2012). Indeed, depletion of FUS/TLS from an otherwise normal adult mouse nervous system alters levels or splicing of >970 mRNAs, most of which are distinct from RNAs dependent on TDP-43. Remarkably, only 45 RNAs are reduced upon depletion of either TDP-43 or FUS/TLS from mouse brain, including mRNAs transcribed from genes with exceptionally long introns and that encode proteins essential for neuronal integrity (Lagier-Tourenne et al., 2012). A subset of these is significantly lowered after TDP-43 or FUS/TLS depletion in stem cell-derived human neurons and in TDP-43 aggregate-containing motor neurons in sporadic ALS, evidence pointing to a common loss-of-function pathway as one component underlying motor neuron death from misregulation of TDP-43 or FUS/TLS (Lagier-Tourenne et al., 2012).
Cytoplasmic functions of TDP-43 and FUS/TLS
TDP-43 and FUS/TLS in cytoplasmic RNA granules
TDP-43 and FUS/TLS shuttle from the nucleus to the cytosol (Ayala et al., 2008; Zinszner et al., 1997), where they have been associated with cytoplasmic RNA granules that contain non-translating mRNAs. These granules include processing bodies (P-bodies), which contain RNA decay machinery (Buchan and Parker, 2009), stress granules, which contain translation machinery (Anderson and Kedersha, 2009), and transporting RNP granules, which contain RNAs to be locally translated (Kiebler and Bassell, 2006).
TDP-43 and FUS/TLS at the synapse
Deletion of FUS/TLS has produced abnormal dendritic and spine morphology in cultured hippocampal neurons (Fujii et al., 2005). Evidence suggests that FUS/TLS may play an important role in regulating synaptic function, possibly through local transport and translation. In dendrites of cultured hippocampal neurons TDP-43 has been shown to co-localize with fragile X mental retardation protein (FMRP) and staufen, two proteins that mark transporting RNP granules and P-bodies (Wang et al., 2008). Given the evidence that TDP-43 and FUS/TLS bind to many RNA targets important for synaptic function and that TDP-43 and FUS/TLS localize to dendrites in response to neuronal activation (Fujii et al., 2005; Wang et al., 2008), dysfunction of TDP-43 or FUS/TLS is highly likely to alter synaptic function.
Assembly of RNA granules through prion-like domains
Both TDP-43 and FUS/TLS contain low sequence complexity (LC), fungal prion-like domains (King et al., 2012), for which a normal function in RNA granule assembly has recently been proposed (Han et al., 2012b; Kato et al., 2012). Assembly of the LC domain of FUS/TLS produces amyloid-like fibers that, in contrast to pathological amyloid inclusions, are reversible (Kato et al., 2012). Induced assembly of LC domains - along with their linked RNA binding domains - provides a basis for RNA granule assembly and possibly for cell-to-cell spreading.
Disease mechanisms for mutant TDP-43 and FUS/TLS
Evidence for gain of toxicity from mutant TDP-43
Multiple transgenic approaches have been employed to identify properties of mutant TDP-43. We focus here only on mammalian models; readers are directed to excellent reviews elsewhere on yeast, Drosophila, C. elegans and other animals models (Da Cruz and Cleveland, 2011; Joyce et al., 2011; McGoldrick et al., 2013). It should be acknowledged that the multiple efforts that have produced TDP-43 transgenic mice and rats have - for the most part - been disappointing. One effort (with a prion-promoted TDP-43Q331K) did produce age-dependent, mutant-dependent motor neuron disease in which about half of the lower motor neurons died, but disease then plateaued despite continued mutant TDP-43 accumulation at a constant level (Arnold et al., 2013). Mutant TDP-43-dependent degeneration of lower (but not upper) motor neurons occurred without loss of nuclear TDP-43 or accumulation of TDP-43 aggregates, but was accompanied by both loss and gain of splicing function of selected RNA targets at an early disease stage. Thus, disease mechanism is apparently both gain of aberrant property and loss of function. Inexplicably, a similar prion promoted transgenic line (TDP-43A315T) develops disease with very different characteristics: upper motor neuron loss (Wegorzewska et al., 2009) with very modest lower motor neuron disease, prior to death from bowel obstruction (Esmaeili et al., 2013; Guo et al., 2012).
Additional TDP-43 transgenic efforts have established that increased TDP-43 levels (by less than a factor of 2) of either wild type or mutant TDP-43 are highly deleterious (Igaz et al., 2011; Wils et al., 2010). This has revealed a crucial role for an autoregulatory pathway that maintains TDP-43 RNA levels. Evidence for autoregulation of TDP-43 has been repeatedly seen: inactivation of one copy of TDP-43 in mice does not affect either the mRNA or protein level of TDP-43 (Kraemer et al., 2010; Sephton et al., 2010). Autoregulation is mediated, at least in part, by TDP-43-dependent splicing of an intron in the 3′UTR of its own mRNA (Avendano-Vazquez et al., 2012; Ayala et al., 2011b; Polymenidou et al., 2011). Splicing of this intron generates an unstable RNA degraded by nonsense-mediated decay (Polymenidou et al., 2011). An additional proposal is that this TDP-43-dependent 3′UTR splicing event activates a cryptic polyadenylation site whose use leads to nuclear retention of TDP-43 RNA (Avendano-Vazquez et al., 2012).
Increasing TDP-43 levels in mice and rats (by expression of RNAs missing the autoregulatory sequences (Wegorzewska et al., 2009, Wils et al., 2010, Igaz et al., 2011, Arnold et al., 2013) or by disrupting autoregulation (Igaz et al., 2012)) has produced neurodegeneration. The level of expression determines the severity of disease (e.g., Wils et al., 2010, Igaz et al., 2011, Arnold et al, 2013). Mice expressing autoregulated wild type and ALS-linked mutant genomic TDP-43 transgenes develop very mild, late onset cognitive and motor deficits, but without paralysis (Swarup et al., 2011). Age-dependent, mutant-dependent motor neuron disease develops with TDP-43Q331K accumulating to a level similar to the normal level of endogenous TDP-43 (Arnold et al., 2013). Expression of genes missing the autoregulatory 3′UTR - thereby permitting accumulation of mutant TDP-43M337V (to an undetermined level) - drives paralysis in rats within 35 days after inducing transgene expression broadly (Zhou et al., 2010) or within 15 days when the transgene is induced pan-neuronally (Huang et al., 2012).
TDP-43 loss of function in disease
Loss of nuclear function of TDP-43 is clearly a component of the disease process, as nuclear clearing accompanied by cytoplasmic accumulation of TDP-43 has been universally reported in surviving neurons in patients with TDP-43 mutant-mediated ALS (Van Deerlin et al., 2008). Not unexpectedly, TDP-43 is an essential gene in mice, yielding embryonic lethality (Chiang et al., 2010; Kraemer et al., 2010; Sephton et al., 2010; Wu et al., 2010), while TDP-43 heterozygote mice are viable and fertile with autoregulation maintaining nearly normal TDP-43 levels (Kraemer et al., 2010).
Ubiquitous postnatal removal of TDP-43 through conditional TDP-43 gene inactivation produced rapid lethality without motor neuron disease (Chiang et al., 2010). Selective removal of TDP-43 from motor neurons produced age-dependent progressive motor neuron degeneration with ALS-like pathology, although in one study the mice lived a normal life span (Iguchi et al., 2013) and in the other study only the male mice developed pathology and phenotype (Wu et al., 2012). These observations are consistent with the notion that while neuronal loss of function of TDP-43 may contribute to disease development and progression it is insufficient to produce fatal motor neuron disease.
TDP-43’s RNA targets and disease pathogenesis
Among the more than 6,000 RNAs normally bound by TDP-43 – and the 1,500 who are changed in abundance or splicing pattern when nuclear TDP-43 is depleted (Figure 3) - are TDP-43 itself, FUS/TLS, glial excitatory amino acid transporter-2 (EAAT2), amyloid beta precursor protein (APP), presenilin, huntingtin, multiple ataxins, α-synuclein, progranulin, and tau (Polymenidou et al., 2011; Sephton et al., 2011). The most prominently affected class of RNAs are pre-mRNAs with exceptionally long introns (> 100 kb), whose expression is enriched in brain and whose encoded proteins are involved in synaptic activity and functions, including parkin 2 (PARK2), neurexin 1 and 3 (NRXN1 and NRXN3), and neuroligin 1 (NLGN1), whose mutations are associated with various neurological diseases.
Additionally, among the >600 RNAs whose splicing patterns are altered when TDP-43 levels are reduced are FUS/TLS itself and EAAT2, with expression of the latter also reduced in FTLD-TDP brain (Tollervey et al., 2011). Many ALS-linked genes, including Alsin, Chmp2b, Fig4, Vapb, and Vcp are bound by TDP-43, and their expression is modestly altered upon TDP-43 depletion (Polymenidou et al., 2011). TDP-43 also regulates the splicing of sortilin, a tentative receptor for progranulin (Hu et al., 2010), whose mutations are linked to FTD-TDP. Misregulation of sortilin splicing by reduction in TDP-43 affects progranulin metabolism (Prudencio et al., 2012), further suggesting that dysfunction of TDP-43 underlies FTD pathogenesis. Collectively, deregulation of TDP-43 RNA targets supports loss of nuclear TDP-43 function as a plausible contributor to pathogenesis after an initiating stress leading to cytoplasmic TDP-43 accumulation.
Mechanism(s) of ALS-linked FUS/TLS mutants
FUS loss of function in disease
Like TDP-43, loss of nuclear function of FUS/TLS is also a likely component of the disease process, as nuclear clearing accompanied by cytoplasmic accumulation of FUS/TLS was initially reported in surviving neurons of patients with NLS-mutant-mediated FUS/TLS (Kwiatkowski et al., 2009; Vance et al., 2009). Two independent FUS/TLS knockout mouse models have been generated (Kuroda et al., 2000; Hicks et al., 2000). Conflicting results from these models have made it unclear whether FUS/TLS is an essential gene.
FUS/TLS mutant gain of toxicity
No currently published mouse models stably express ALS-linked mutations in FUS/TLS. However, one study in rats with inducible expression of human wild type or R521C mutant of FUS/TLS reported that postnatal induction (to undetermined levels) in two independent lines of mutant-expressing rats produced paralysis and death by 70 days of age, whereas comparable wild-type human FUS/TLS-expressing rats survived normally (Huang et al., 2011). These findings support a gain of toxicity by mutant FUS/TLS, albeit rats overexpressing wild type FUS/TLS also develop motor and spatial learning deficits accompanied by ubiquitin aggregation by 1 year of age. It should be noted that, similar to the case of TDP-43, increased wild-type FUS/TLS accumulation through homozygous mating in mice is also highly deleterious, driving early lethality (Mitchell et al., 2012). Additional mouse and rat models and further studies are needed to elucidate FUS/TLS-mediated toxicity.
Is TDP-43 and FUS/TLS-mediated toxicity a non-cell-autonomous process?
An increasing body of evidence has established that cell types beyond the target neurons whose dysfunction is responsible for the primary phenotypes also contribute to neurodegeneration, a phenomenon known as non-cell-autonomous toxicity (Garden and La Spada, 2012). Given that TDP-43 and FUS/TLS inclusion can also be found in glia (Mackenzie et al., 2010a), it is conceivable that glia contribute to disease pathogenesis. Indeed, induced pluripotent stem cells (iPSc)-derived astrocytes from patients carrying a familial mutation in TDP-43 (M337V) showed several abnormalities, including increased TDP-43 accumulation and altered subcellular localization (Serio et al., 2013). While these mutant astrocytes did not produce short term toxicity to co-cultured motor neurons, driving expression of the same TDP-43 mutation (M337V) in rats, but only in astrocytes, produced progressive loss of motor neurons and paralysis (Tong et al., 2013). Thus, it is highly plausible that TDP-43 (and possibly FUS/TLS as well) mediated neurodegeneration is a non-cell-autonomous process.
TDP-43, FUS/TLS and a potential link between stress granules and protein inclusion
TDP-43 and FUS/TLS are components of stress granules (Dewey et al., 2012; Li et al., 2013). The main functions of stress granules appear to be in temporally repressing general translation and storage of mRNAs during stress. Importantly, stress granules are disassembled when the stressors are removed (Anderson and Kedersha, 2009).
At least seven independent studies have reported TDP-43 to be localized within stress granules produced in a wide range of cell lines with varying stresses, including oxidative, osmotic, and heat stresses (Ayala et al., 2011a; Colombrita et al., 2009; Dewey et al., 2011; Freibaum et al., 2010; Liu-Yesucevitz et al., 2010; McDonald et al., 2011; Meyerowitz et al., 2011). TDP-43 variants with ALS-linked mutations appear to form larger stress granules with faster kinetics (Dewey et al., 2011; Liu-Yesucevitz et al., 2010) and this requires the prion-like domain (Bentmann et al., 2012; Dewey et al., 2011; Liu-Yesucevitz et al., 2010).
Similarly, FUS/TLS is recruited into stress granules (Andersson et al., 2008) and FUS/TLS with ALS-linked mutations in its NLS show enhanced propensity to associate with stress granules (Bosco et al., 2010a; Dormann et al., 2010; Gal et al., 2011; Ito et al., 2010; Kino et al., 2011). One provocative report claimed that the prion-like domain of FUS/TLS is both necessary and sufficient to form stress granules in cultured cells and to form hydrogels in vitro (Kato et al., 2012). Another report claimed a completely opposite result, with the C-terminal residues together with an ALS-linked mutation (P525L), but not the prion-like domain, required for stress granule formation in cells (Bentmann et al., 2012). The discrepancy remains unresolved.
Nonetheless, the evidence collectively indicates that association of TDP-43 and FUS/TLS into stress granules is a normal physiological response to stress. A tempting speculation is that the association of TDP-43 and FUS/TLS with stress granules may be an initiating event, which following chronic stress eventually leads to irreversible pathological aggregation (Dewey et al., 2012; Li et al., 2013). However, caution is warranted, as these cell culture experiments used overexpression of TDP-43 and FUS/TLS and do not recapitulate one key feature of TDP-43 and FUS/TLS proteionopathies: concomitant loss of nuclear TDP-43 or FUS/TLS with cytoplasmic inclusions (Mackenzie et al., 2010a).
TDP-43 is transiently lost from neuronal nuclei with concomitant accumulation at injury sites in two in vivo experiments in mice using either axotomy or axonal ligation (Moisse et al., 2009; Sato et al., 2009). Interestingly, mutant TDP-43 showed a delayed response in returning to the nucleus during recovery (Swarup et al., 2012). Since current evidence suggests that at disease end stage TDP-43 and FUS/TLS associate with stress granules in ALS and FTD patients (Dormann et al., 2010; Liu-Yesucevitz et al., 2010), future investigation should now focus on how TDP-43 and FUS/TLS switch from reversible association into irreversible pathological inclusions, what the relationship is between this process and the nuclear clearance of TDP-43 and FUS/TLS, and how the combination of pathological inclusions and loss of nuclear TDP-43 and FUS/TLS drives disease progression.
TDP-43, FUS/TLS and SMN, a common pathogenic pathway between ALS and spinal muscular atrophy (SMA)
Spinal Muscular Atrophy (SMA) is a motor neuron disease caused by deficiency in the survival motor neuron (SMN) protein [reviewed in (Burghes and Beattie, 2009)]. SMN is part of a large multi-protein complex that is essential for the biogenesis of spliceosomal-associated small nuclear ribonucleoprotein particles (snRNPs). SMN complexes are found both in the cytoplasm and in nuclear bodies called Gems. Loss of nuclear Gems is a pathological hallmark in SMA. Reduced SMN expression leads to markedly decreased snRNP assembly and reduced snRNA levels in mouse models of SMA and in SMA patients, provoking broad misregulation of RNA splicing.
Recent evidence suggests that perturbation of normal levels of TDP-43 (Shan et al., 2010; Tsuiji et al., 2012) or FUS/TLS (Yamazaki et al., 2012) or expression of ALS-linked mutations in TDP-43 (Yamazaki et al., 2012) and FUS/TLS (Groen et al., 2013; Yamazaki et al., 2012) leads to reduction of nuclear GEM bodies, altered U snRNAs expression and axonal defects, likely through a direct biochemical association between and SMN and TDP-43 (Tsuiji et al., 2012) or FUS/TLS (Groen et al., 2013; Yamazaki et al., 2012). Moreover, these SMN deficits are also found in sporadic ALS patients with TDP-43 inclusions (Ishihara et al., 2013; Tsuiji et al., 2012). Taken together, the collective evidence supports convergent pathways of pathogenesis in SMA and ALS, reinforcing the notion that defects in RNA metabolism may be central mechanistic components in motor neuron disease.
Repeat expansion within C9ORF72
Genome wide association studies (GWAS) of familial ALS patients in the Finish population, as well as in sporadic ALS, demonstrated the presence of a major ALS locus on chromosome 9p21 (Laaksovirta et al., 2010; Shatunov et al., 2010; Van Deerlin et al., 2010; van Es et al., 2009). The minimal region linking all the families was then narrowed down to a 232 kb interval containing only 3 protein-coding genes (MOBKL2B, IFNK, and C9ORF72) (Laaksovirta et al., 2010). Rather than the expected amino-acid substitutions in a protein coding region, a large GGGGCC hexanucleotide repeat expansion (~700–1600 copies) within a non-coding region of a gene (C9ORF72) was found to be causative (Dejesus-Hernandez et al., 2011; Gijselinck et al., 2012; Renton et al., 2011).
Hexanucleotide expansion in C9ORF72 accounts for up to 80% of familial ALS-FTD, 20–50% of familial ALS, 5–20% of sporadic ALS and 10–30% of FTD, making this repeat expansion the most common cause of ALS and FTD (Boeve et al., 2012; Chiò et al., 2012; Cooper-Knock et al., 2012; Hsiung et al., 2012; Mahoney et al., 2012; Simòn-Sánchez et al., 2012; Snowden et al., 2012). Clinically, patients with the C9ORF72 repeat expansion have been reported to have a higher incidence of bulbar-onset ALS, cognitive impairment with earlier disease onset, and accelerated progression compared with patients without the expansion (Byrne et al., 2012; Chiò et al., 2012; Millecamps et al., 2012; Stewart et al., 2012).
Inclusions containing TDP-43 in brain and spinal cord are prevalent in all patients with the repeat expansion. Additionally, there is the presence of TDP-43-negative cytoplasmic or nuclear inclusions containing either p62/SQSTM1 or ubiquilin-2 or both in the cerebellar granular and molecular layers (Brettschneider et al., 2012), where TDP-43 inclusions are absent and neuronal intranuclear and cytoplasmic inclusions in the pyramidal cell layers of the hippocampus where TDP-43 pathology is also less common (Al-Sarraj et al., 2011; Murray et al., 2011; Troakes et al., 2011). Cytoplasmic ubiquilin-2-containing inclusions too have been reported in the cerebellar granular and hippocampal molecular layers (Brettschneider et al., 2012).
Pathogenic mechanisms for the repeat expansion in C9ORF72 gene
The expanded hexanucleotide repeat in the C9ORF72 gene is reminiscent of multiple prior repeat expansion diseases for which three different prototypes of pathogenic mechanisms have been demonstrated: loss of function of the gene containing the repeat (haploinsufficiency), gain of protein toxicity due to the expression of protein containing the repeat expansion (mutant protein), and gain of RNA toxicity due to the production of RNA containing the repeat (mutant RNA) (La Spada and Taylor, 2010). Additional toxic mechanisms can result from complementary repeat-containing RNA produced by bi-directional transcription (Moseley et al., 2006) or repeat associated non-ATG (RAN) translation (Zu et al., 2011), leading to production, respectively, of potentially toxic RNA and protein species. For C9ORF72, because the GGGGCC repeat expansion is located within an alternative non-coding intron 1, the underlying disease pathogenesis may be driven by RNA-mediated or RAN translation-dependent toxicity or haploinsufficiency or any combination of these (Figure 4).
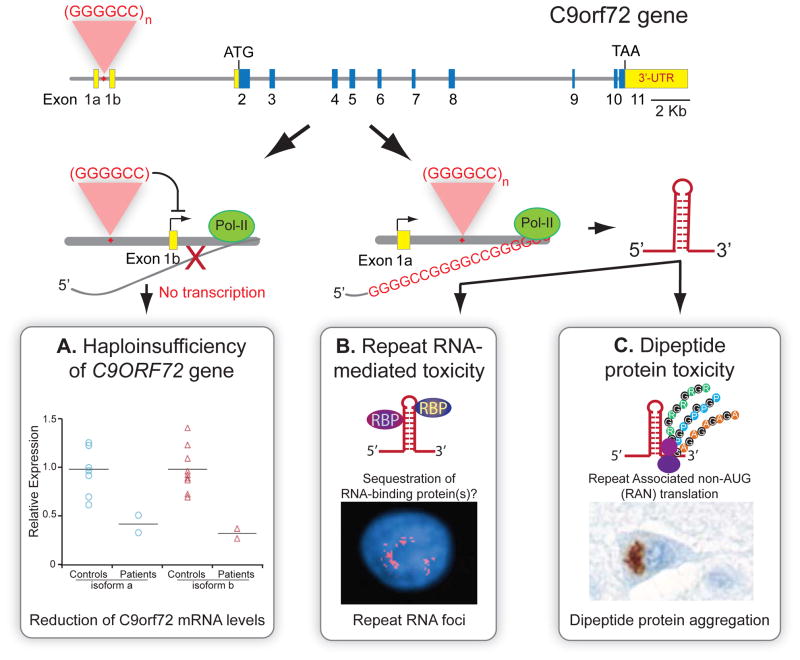
Figure 4. Potential pathogenic mechanisms for GGGGCC repeat expansion within the C9ORF72 gene.
(Top) Schematic representation of the human C9ORF72 gene (yellow: untranslated regions (UTR), blue: coding exons). Hexanucleotide (GGGGCC) repeat expansion is located between alternative exons 1a and 1b. At least three mechanisms may contribute to disease pathogenesis: A) GGGGCC repeat expansions may lead to reduced expression of the allele containing the repeat expansion (haploinsufficiency); B) RNA foci containing transcribed GGGGCC repeats may sequester RNA-binding protein(s); or C) non-AUG (RAN) translation of GGGGCC repeat-containing RNA produces toxic poly-dipeptides in each of three reading frames. Data and images reproduced with permission from A) Gijselinck et al. (2012), B) DeJesus-Hernandez et al. (2011), and C) Mori et al. (2012).
Loss of function and gain of toxicity: lessons from the fragile X locus and myotonic dystrophy
The location of the repeat expansion in intron 1 of C9ORF72 resembles the CGG repeats of the FMR1 (fragile X mental retardation 1) gene, which depending on the size of the repeats yields three different syndromes: fragile X syndrome (>200 repeats), fragile X-associated tremor/ataxia syndrome (50–200 repeats) and premature ovarian insufficiency (50–200 repeats) (Oostra and Willemsen, 2009). Full expansion causes fragile X syndrome (FXS) from loss of FMR1 gene function mediated by hypermethylation of the adjacent FMR1 promoter region and subsequent transcriptional silencing.
FMR1 carriers with CGG repeats between 50–200 develop fragile X-associated tremor/ataxia syndrome (FXTAS) in which the repeats are unmethylated, but produce intention tremor, abnormal gait, peripheral neuropathy and cognitive impairment (Oostra and Willemsen, 2009). In contrast to transcriptional silencing in FXS (Tassone et al., 2000), accumulation of FMR1 mRNA in FXTAS is elevated at least 5-fold, presumably because it is stabilized by binding of hnRNP A2/B1, Pur-α (purine-rich binding protein-α), Sam68, hnRNP-G, along with CUG-binding protein 1 (CUG-BP1) and muscleblind (MBNL1), each of which has been shown either to associate biochemically with the rCGG repeats or colocalize with rCGG RNA foci (Jin et al., 2007; Sellier et al., 2010; Sofola et al., 2007). Furthermore, both Sam68 and hnRNP A2/B1 can be found in the nuclear inclusions of FXTAS patient neurons (Jin et al., 2007; Sellier et al., 2010). While the identities of other RNA-binding proteins potentially trapped in the rCGG foci and the underlying pathogenic mechanisms remain controversial, it is clear that RNA-mediated toxicity is a key component of neurodegeneration in FXTAS. These complications should be borne in mind in considering whether different repeat sizes within the C9ORF72 gene may provide divergent symptoms/ diseases or different severity of phenotypes.
A gain-of-RNA-toxicity mechanism for a repeat expansion disease is best characterized in myotonic dystrophy 1 (DM1), which is caused by up to 2,500 of CTG repeats in the 3′-UTR of the myotonic dystrophy protein kinase (DMPK) gene (Lee and Cooper, 2009). Two proteins, CUG-BP1 and muscleblind, were identified to bind to the CUG repeat-containing RNA (Miller et al., 2000; Timchenko et al., 1996). Of these two proteins, only muscleblind shows repeat-length dependent association and is selectively sequestered into pathogenic RNA foci (Mankodi et al., 2001). Nevertheless, mis-regulation of both muscleblind and CUG-BP1 play roles in DM1 pathogenesis. Indeed, CUG repeats lead to activation of protein kinase C (PKC), which in turn phosphorylates CUG-BP1, whose phosphorylated form has increased activity from increased protein stability, thereby activating multiple splicing changes toward fetal isoforms (Kuyumcu-Martinez et al., 2007; Roberts et al., 1997).
Evidence for C9ORF72 haploinsufficiency
The function of the C9ORF72 gene and its predicted protein product are unknown. Recent bioinfomatical analysis implies a potential involvement of the C9ORF72 protein in membrane trafficking and autophagy (Levine et al., 2013; Zhang et al., 2012), but this remains to be determined. A 50% reduction of mRNA levels corresponding to both short and long mRNA isoforms of C9ORF72 (Dejesus-Hernandez et al., 2011; Gijselinck et al., 2012) has been reported and is consistent with partial or complete silencing of the expanded allele (Figure 4A), although it should be noted that the reduction of the corresponding C9ORF72 proteins has not been demonstrated. Antisense oligonucleotide-mediated reduction of C9ORF72 in zebrafish with produces reduced axon lengths of motor neurons and locomotion deficit (Ciura et al., 2013), consistent with the notion that partial loss of the C9ORF72 gene could contribute to disease pathogenesis.
Evidence for gain of RNA toxicity from C9ORF72 expansion
Intranuclear RNA foci containing the C9ORF72 hexanucleotide repeat have been reported (DeJesus-Hernandez et al., 2011), which may trap one or more RNA-binding proteins thereby inhibiting their functions, especially in RNA processing (Figure 4B). While two RNA-binding proteins, hnRNP-A3 (Mori et al., 2013a) and Pur-α (Xu et al., 2013) have been reported to bind GGGGCC repeats in vitro and both were reported to be components of p62-positive TDP-43-negative inclusions in C9ORF72 patients, their role in pathogenesis is unproven. Neither has been demonstrated to localize at RNA foci formed by the hexanucleotide repeat and the predicted loss of RNA processing function that would follow from sequestration of hnRNP-A3 and Pur-α has not been demonstrated in cells and tissues expressing the hexanucleotide repeat-containing RNA.
Repeat non-ATG translation of C9ORF72 mRNA
Besides the recognized modes of RNA toxicity introduced above, a highly unexpected and potentially toxic mechanism in C9ORF72 has been uncovered: repeat associated RNA-encoded, non-ATG translation (RAN translation). This phenomenon was originally discovered in spinocerebellar ataxia type 8 (SCA8), a progressive neurodegenerative disease caused by a trinucleotide expansion in the bi-directionally transcribed SCA8 gene (Zu et al., 2011). In one direction, the RNA encoding the ataxin 8 (ATXN8) protein contains an in frame CAG-expansion that is translated into polyglutamine. Surprisingly, this RNA is also translated in an ATG-independent manner in all three reading frames of the CAG repeat both in vitro and in SCA8 human cerebellum.
Following from the SCA8 example, two independent studies have now reported translation of the C9ORF72 GGGGCC repeat into polypeptides consisting of repeating di-amino acids: poly-(glycine-alanine, GA), poly-(glycine-proline, GP), and poly-(glycine-arginine, GR) (Figure 4C) that form pathological inclusions in neurons (but not astrocytes) of C9ORF72 patients (Ash et al., 2013; Mori et al., 2013b). Poly GA is apparently the most prevalent form (Mori et al., 2013b). Moreover, an antisense RNA transcript in C9ORF72 patients has also been reported (Mori et al., 2013b), raising the possibility of two additional dipeptide-repeats (poly PR and PA) which may also be generated through RAN translation.
More complexities in repeat–mediated toxicity
If the preceding potential toxicities were not enough, consideration of what is known about SCA8 provides more potential complexities. As mentioned above, the SCA8 locus is bi-directionally transcribed with opposite strand transcription of the CAG repeat producing a non-coding RNA containing a CUG repeat expansion that sequesters muscleblind, leading to splicing changes similar to those observed in DM1 patients (Daughters et al., 2009; Moseley et al., 2006). Added to potential RAN-translation of both repeats, the pathogenic mechanisms include gain-of-function at both the protein and RNA levels.
Disruption of protein homeostasis in ALS and FTD
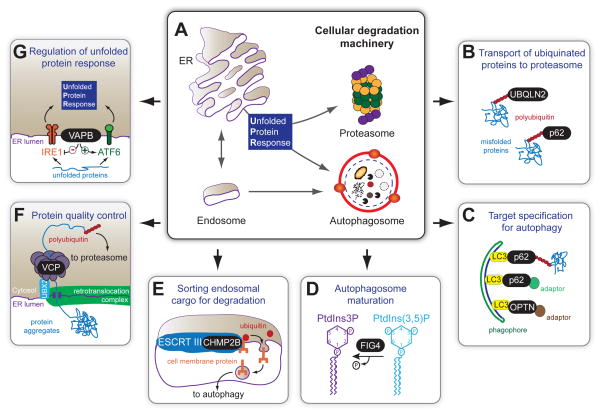
Although the chicken-and-egg question persists for whether protein aggregation per se causes or merely reflects a consequence of neurodegenerative diseases, overwhelming evidence supports protein degradation deficits in a wide range of disorders through disruption of either of the two major protein clearance pathways: the ubiquitin-proteasome system and autophagy. This is certainly true for ALS/FTD, as demonstrated by identification of ALS and FTD-linked mutations in genes affecting protein homeostasis, or proteostasis. These genes include ubiquilin-2 (UBQLN2), p62/SQSTM1 (sequestosome 1), optineurin (OPTN), vasolin-containing protein (VCP), charged multivesicular body protein 2B (CHMP2B), vesicle-associated membrane protein (VAMP)/synatobrevin-associated protein B (VAPB) and Fig4 (Fig4 homolog, SAC1 lipid phosphatase domain containing protein) (Supplemental Figure 2). Among these genes, ubiquilin-2, p62, optineurin, and VCP are directly involved in protein degradation, whereas CHMP2B and Fig4 are required for autophagosome maturation (Figure 5).
Figure 5. ALS/FTD-associated proteins involved in protein homeostasis.
A) Schematic representation of major cellular degradation pathways. Many ALS/FTD-linked proteins are involved in protein clearance pathway, including B) ubiquilin-2 (UBQLN2) and p62/SQSTM1 (which bind to polyubiquitinated proteins and transport them to proteasome for degradation) and C) p62/SQSTM1 and optineurin (OPTN) (which serve as adaptor proteins to bind simultaneously the substrates that are targeted for autophagy and LC3, a component of autophagosomes). D) Fig4 is a lipid phosphatase that converts PI(3,5)P into PI3P. Autophagy is impaired in the neurons and astrocytes missing Fig4, with the disturbance of PIPs expected to disrupt formation or recycling of autolysosomes. E) ALS/FTD-linked mutations in CHMP2B, a core component of endosomal sorting complexes (ESCRT) required for transport, disrupt the normal endosome-lysosome-autophagy morphology and function. ESCRT complexes bind to ubiquitinated cargos and initiate clustering of ubiquitinated cargos and membrane invagination. The internalized cargos, which are cell membrane proteins, are transported into autophagosomes for degradation. F) VCP interacts with a large number of cofactor proteins that act as ubiquitin adaptors, allowing VCP to interact with a large number of ubiquitinated proteins to enable degradation or recycling, including extraction of misfolded proteins from the ER and subsequently targeting them for degradation. G) VAPB is involved in the unfolded protein response (UPR) by modulating activities of different arms of the UPR.
ALS-FTD mutation in Ubiquilin-2 (UBQLN2, ALS-X)
The ubiquilin protein family brings poly-ubiquitinated proteins to the proteasome for degradation, and ubiquilins also function in autophagy. The ALS and ALS/dementia-linked mutations initially identified in UBQLN2 are clustered at or near its proline-rich region, with most altering a conserved proline (P497H, P497S, P506T, P509S, P525S) (Deng et al., 2011; Gellera et al., 2013; Williams et al., 2012). Two additional mutations, S155N and P189T, are located at the N-terminus (Daoud et al., 2012). Experiments in cells transfected to express either of two ALS-linked mutations in ubiquilin-2 (R497H and P506T) suggest that that overall protein degradation is impaired (Deng et al., 2011).
Perhaps not surprisingly, colocalization of ubiquilin-2 and ubiquitin in pathological inclusions is seen in patients with UBQLN2 mutations and these inclusions also contain TDP-43, FUS/TLS and optineurin (Deng et al., 2011; Williams et al., 2012), suggesting that an impaired protein clearance pathway is a pathogenic mechanism (Figure 5B). Furthermore, ubquilin-2 pathology has been reported in a majority of sporadic ALS (Deng et al., 2011) and hexanucleotide repeat expansion in the C9ORF72 genes (Brettschneider et al., 2012). Taken together, mutations in ubiquilin-2 provide a mechanistic link of the protein degradation pathway with neurodegeneration.
p62/SQSTM1 mutations in ALS
Similar to ubiquilin, p62 has been shown to interact with polyubiquitinated proteins (Moscat and Diaz-Meco, 2012) and to interact with LC3, allowing p62 to target polyubiquitinated proteins to the proteasome or autophagy. Therefore, both p62 and ubiquilin-2 link the ubiquitin-proteasome and autophagy pathways (Figure 5B,C). Using a candidate gene approach, sequencing of p62/SQSTM1 in familial and sporadic ALS patients revealed several polymorphisms/mutations scattered throughout the coding regions (Fecto et al., 2011; Rubino et al., 2012; Teyssou et al., 2013), accompanied by TDP-43 inclusions (Teyssou et al., 2013). p62-positive inclusions have also been reported in neurons and glia of a wide array of other neurodegenerative diseases (Brettschneider et al., 2012). Although how these ALS-associated variants in p62 contribute to pathogenesis has not been established, autophagy/proteasome disturbance seems likely to play a role.
ALS mutations in Optineurin (OPTN)
Optineurin is a 577 amino acid multifunctional protein that is able to bind both polyubiquitinated proteins and LC3 (Figure 5C). Indeed, optineurin has been proposed as a receptor for autophagy (Wild et al., 2011). Both nonsense and missense mutations of optineurin have been identified in ALS, accounting ~3% of familial ALS and ~1% of sporadic ALS (Del Bo et al., 2011; Iida et al., 2012a; Iida et al., 2012b; Maruyama et al., 2010; van Blitterswijk et al., 2012). One report has identified OPTN-positive inclusions in patients with OPTN-mutation, with TDP-43 inclusions in sporadic ALS and with SOD1-positive inclusions in patients with SOD1 mutations (Maruyama et al., 2010).
ALS- and FTD-linked mutations in Vasolin-containing protein (VCP)
Mutations in vasolin-containing protein (VCP) were originally identified as causative of inclusion body myopathy with Paget’s disease of bone and of frontotemporal dementia (IBMFTD) (Watts et al., 2004) and later in ALS (Johnson et al., 2010). Some of the same mutations can be found for both IBMFTD and ALS (Supplemental Figure 2). VCP interacts with a large number of ubiquitinated proteins to enable degradation or recycling and functions in multiple protein clearance pathways (Figure 5F), including extracting misfolded proteins from the ER and sorting of endosomal proteins for proper trafficking. Depletion of VCP leads to accumulation of immature autophagosomes, similar to what is observed upon expression of IBMFD-linked mutations (Ju et al., 2009; Tresse et al., 2010), suggesting that VCP is required for proper autophagy. Most intriguingly, TDP-43 is apparently mislocalized to the cytosol upon VCP-mediated autophagic dysfunction (Ju et al., 2009).
FTD- and ALS-linked mutations in CHMP2B (FTD-3)
Charged multivesicular body protein 2B, or chromatin-modifying protein 2B (CHMP2B) mutations were first identified in FTD (termed FTD-3), and additional mutations were identified in different cohorts of FTD patients (Momeni et al., 2006; van der Zee et al., 2008) and in ALS (Cox et al., 2010; Parkinson et al., 2006). CHMP2B is a core component of endosomal sorting complexes (Figure 5E). Multiple studies support mutant CHMP2B-mediated disruption normal endosome-lysosome-autophagy morphology and function (Han et al., 2012a; Urwin et al., 2010; van der Zee et al., 2008). Transgenic mice expressing the intron 5-retention mutant of CHMP2B, but not wild type CHMP2B, develop progressive neurological deterioration accompanied by axonal pathology and early mortality (Ghazi-Noori et al., 2012). Loss of CHMP2B function, on the other hand, after gene disruption in mice produces no phenotype (Ghazi-Noori et al., 2012).
ALS mutations in Fig4
Fig4 encodes a 907 amino acid lipid phosphatase that regulates the abundance of phosphatidyl-inositol-3,5-biphosphate (PI(3,5)P2). Recessive mutation in Fig4 causes severe tremor, abnormal gait, degeneration of sensory and motor neurons and diluted pigmentation in mice. Compound heterozygote mutations, in which a loss-of-function allele combines with a partial loss-of-function mutation, are present in human patients with Charcot-Marie-Tooth disease (CMT4J) (Chow et al., 2007), as are rare, heterozygous variants of Fig4 in ALS (Chow et al., 2009). Fig4 null mice have substantially lowered PI(3,5)P2 levels, which are normally tightly regulated. Not surprisingly, autophagy is impaired in the neurons and astrocytes of mice missing Fig4, with the disturbance of PIPs expected to disrupt formation or recycling of autolysosomes. It is tempting to speculate that ALS-linked variants can tip the balance of phosphoinositide processing and affect autophagic function (Figure 5D).
ALS-mutations in VAPB
Two ALS-linked mutations in the gene encoding the vesicle-associated membrane protein (VAMP)/synatobrevin-associated protein B (VAPB) have been reported (Supplemental Figure 2)(Chen et al., 2010; Funke et al., 2010; Millecamps et al., 2010; Nishimura et al., 2004). Expression of either, but not wild type, in mammalian cell lines produced ER fragmentation and cytoplasmic aggregates of mutant VAPB that also trapped endogenous VAPB (Chen et al., 2010; Kanekura et al., 2006; Nishimura et al., 2004; Teuling et al., 2007). Increased levels of wild type VAPB elicit the unfolded protein response (UPR) (Figure 5G). Reduction in VAPB attenuates it, as do ALS-linked mutants (Chen et al., 2010; Kanekura et al., 2006), probably by interaction with ATF6, one of the three key molecules in initiating the UPR response (Gkogkas et al., 2008). Transgenic mice expressing wild type or mutant VAPB (P56S) cDNA within the nervous system do not, however, develop overt phenotypes nor have reduced survival, but do develop cytoplasmic accumulation of ubiquitin, p62, and TDP-43 at 18 months of age (Qiu et al., 2013; Tudor et al., 2010). Nevertheless, along with ALS-, FTD-, and ALS/FTD-linked mutations in ubiquilin-2, p62, optineuron, VCP, CHMP2B and FIG, the VAPB mutations point to defects in protein clearance as a common component of pathogenesis.
A surprising additional function of VAPB came from study in Drosophila of its MSP (major sperm protein) domain (Tsuda et al., 2008). The MSP domain has been reported to be cleaved and secreted, while the ALS-linked P56S mutant abolished the secretion activity and formed ubiquitinated inclusions. Pathogenic mechanisms may involve aberrant Eph signaling. Biochemically, human MSP interacts with EphA4 (Tsuda et al., 2008), a receptor in the ephrin axonal repellent pathway. Intriguingly, EphA4, has been reported to be a genetic modifier for modulating the vulnerability of motor neurons in ALS (Van Hoecke et al., 2012). How the MSP-like fragment is generated in mammalian system and whether MSP-EphA4 interaction plays a role in modulating ALS disease course will require further investigation.
SOD1: a central component of ALS or an outlier?
Mutations in the copper/zinc superoxide dismutase 1 (SOD1) gene account for 20% of familial cases (Rosen et al., 1993). Mouse models overexpressing ALS-linked mutations in SOD1 recapitulate most features of ALS pathology, which has led to the discovery of two critical features of SOD1-mediated toxicity: (1) mutant SOD1 causes ALS through a gain of toxic property(ies), and (2) pathogenesis of the ubiquitously expressed mutant SOD1 is a non-cell-autonomous process. This latter insight was established by gene excision from selected cell types in transgenic mice otherwise expressing mutant SOD1 ubiquitously, an approach that identified disease onset to be driven by mutant synthesized within motor neurons (Boillee et al., 2006; Wang et al., 2009; Yamanaka et al., 2008) and NG2+ oligodendrocyte precursors (Kang et al., 2013), while mutant SOD1 synthesized within two additional glial cell types [astrocytes (Yamanaka et al., 2008) and microglia (Boillee et al., 2006)] are primary determinants of accelerated disease progression.
A crucial controversy: Is SOD1 a component of sporadic disease?
While ubiquitinated protein aggregates containing SOD1 are a prominent pathological feature in both familial ALS patients with SOD1 mutations and in mice expressing ALS-linked mutations in SOD1 (Bruijn et al., 2004), SOD1-containing inclusions have not been found in most sporadic ALS cases. Nevertheless, early studies hinted that an age-dependent post-translational and non-mutational modification of SOD1 may be able to change the conformation of wild-type SOD1 into the mutant conformation (Bredesen et al., 1997), evidence that these modified forms of wild type SOD1 could be contributors to sporadic ALS. The notion that there is a common pathogenic conformation of wild type and mutant SOD1 has recently made a comeback. Several teams have reported that misfolded SOD1 is present in a portion of sporadic ALS patients (Bosco et al., 2010b; Forsberg et al., 2010; Pokrishevsky et al., 2012). This issue remains highly controversial, with other teams failing to detect misfolded SOD1 in sporadic ALS patients using multiple conformation-specific antibodies (Brotherton et al., 2012; Kerman et al., 2010; Liu et al., 2009).
SOD1 mutant expressing astrocytes are toxic to co-cultured normal motor neurons (Di Giorgio et al., 2008; Di Giorgio et al., 2007; Haidet-Phillips et al., 2011; Marchetto et al., 2008; Nagai et al., 2007). Kaspar and colleagues (Haidet-Phillips et al., 2011) reported the very surprising finding that astrocytes derived from autopsy samples from sporadic ALS patients are also toxic to motor neurons. Most provocatively, this team also reported that non-cell autonomous toxicity to motor neurons from such sporadic ALS-derived astrocytes can be reduced by lowering production of wild type SOD1, thereby implicating wild type SOD1 as a contributing factor in sporadic disease. While replication is needed, these results highlight non-cell autonomous components in ALS pathogenesis and support therapeutic reduction in SOD1 expression in sporadic ALS.
Prion-like spreading for ALS and FTLD
One of the key features of prion diseases is the conformational conversion of a native state to an infectious, misfolded and pathological state of the prion protein. The infectious cycle comes from the perpetuating conversion of the normal prion protein into a pathological conformation and spreading to other cells, a process that has now been demonstrated for neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease (reviewed in Polymenidou and Cleveland, 2012).
Consistent with a prion-like spread, ALS-linked mutant SOD1 can form fibrils (Chattopadhyay et al., 2008) and mutant SOD1 has been shown to possess prion-like aggregation and spreading ability in cultured cells (Grad et al., 2011; Münch et al., 2011), as well as seeding ability using spinal cord homogenate from transgenic animals overexpressing mutant SOD1 (Chia et al., 2010) (Figure 6). Remarkably, increased wild type SOD1 expression (accompanied by its conversion into an insoluble form) is sufficient to accelerate disease course and shorten survival of SOD1 mutant expressing mice (Deng et al., 2006), consistent with a prion-like template-dependent aggregation.
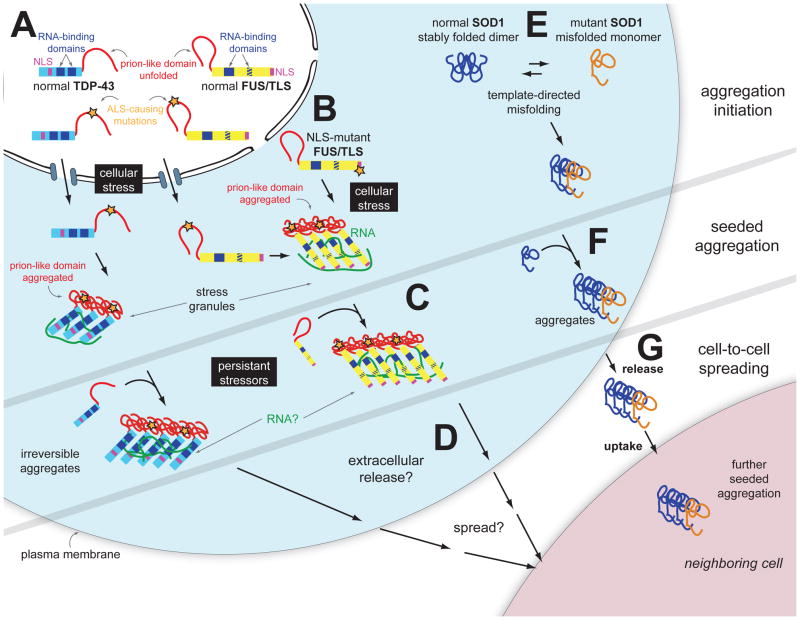
Figure 6. Aggregate assembly and propagation in ALS and FTD.
Prion-like phenomena in ALS may include SOD1, TDP-43 or FUS/TLS seeded aggregation and cell-to-cell spreading. A) TDP-43 and FUS/TLS are both primarily nuclear RNA-binding proteins, whose mutations lead to ALS or FTD. Filled blue boxes on TDP-43 and FUS/TLS molecules indicate RNA-recognition motifs and the striped blue box on FUS/TLS refers to the zinc finger domain that can also bind RNA. B) Cellular stress induces TDP-43 and FUS/TLS incorporation into stress granules, which form through the ordered aggregation of several RNA-binding proteins complexed with RNA molecules. This physiologic reaction to cellular stress may be an initial trigger for pathogenic inclusion formation since the increased local protein concentration and RNA scaffolding molecules may facilitate ordered aggregation of TDP-43 and/or FUS/TLS. C) Upon chronic cellular stress and defects in stress granule disassembly occurring with aging, prion-like conformational changes of TDP-43 and FUS/TLS facilitated by disease causing mutations in them and driven by stress granule formation transform into pathogenic self-perpetuating, irreversible aggregates. D) Possible cell to cell spread (not yet tested for TDP-43 or FUS/TLS) of prion-like aggregates may underlie (at contribute to) disease spread from a focal initiation. E) Self-perpetuating seeding of misfolded, mutant SOD1 has been reported in cell cultures (Grad et al., 2011; Münch et al., 2011). Acquired damage to wild type SOD1 may seed similar self-perpetuating aggregates. F) Mutant, misfolded SOD1 can induce misfolding of wild type SOD1, in a template-directed reaction (Chia et al., 2010; Deng et al., 2006), thereby forming a seed of aggregated protein. G) SOD1 aggregates transfer from cell-to-cell to initiate misfolding and aggregation of wild type or mutant SOD1 in neighboring cells (Münch et al., 2011).
Furthermore, both TDP-43 and FUS/TLS contain prion-like domains, which may facilitate seeding and aggregation (Figure 6). Indeed, a recent study reported that intracellular aggregation of TDP-43 can be triggered in cultured cells by transduction of fibrillar aggregates prepared in vitro (Furukawa et al., 2011). In addition, disease-linked mutations in prion-like domains in hnRNP-A2B1 and hnRNP-A1 increase their propensity to form self-seeding fibrils and cross-assemble with wild type counterparts (Kim et al., 2013). Altogether, along with recognition that the initial symptoms of ALS are typically confined to a particular region, followed by an orderly spread that might be predicted for prion-like propagation, the evidence suggests that a prion-like seeding and spreading mechanism could underlie TDP-43 and FUS/TLS-mediated disease.
Feed forward loops: Converging disruptions in RNA and protein homeostasis
One of the most devastating features of ALS is the relentless progression and spread of degeneration, and here, we attempt to provide a molecular basis for this phenomenon. The recent discovery of how RNA granules can form through a low complexity/prion-like domain in TDP-43, FUS/TLS and hnRNP A2/B1 (Han et al., 2012b; Kato et al., 2012) has fueled an attractive hypothesis in which prion-like spreading of aggregated SOD1, TDP-43, or FUS/TLS could contribute to ALS pathogenesis (Polymenidou and Cleveland, 2011).
ALS-linked mutations in protein clearance pathways can lead to TDP-43 aggregation
Both TDP-43 and FUS/TLS are intrinsically aggregation-prone in vitro (Johnson et al., 2009; Sun et al., 2011), which may predispose them to formation of pathological inclusions through their prion-like domains (Kato et al., 2012, Han et al., 2012, Kim et al., 2013), independent of any proposed progression from an initiating stress granule complex (Dewey et al., 2012). Not surprisingly, both ubiquitin-proteasome and autophagy pathways are used for TDP-43 clearance (Brady et al., 2011; Urushitani et al., 2010; Wang et al., 2010). Mutations or disruption of many of ALS-linked genes involved in protein homeostasis pathways (VCP, ubiquilin-2, p62, and CHMP2B) lead to TDP-43 aggregation.
Down-regulation of VCP or expression of disease-linked mutations of VCP generate cytosolic TDP-43 aggregations (Gitcho et al., 2009; Ju et al., 2009; Ritson et al., 2010), autophagy defects (Ju et al., 2009), and decreased proteasomal activity (Gitcho et al., 2009). Similarly, reduction of CHMP2B and expression of FTD-linked mutations in CHMP2B inhibit the maturation of autolysosomes, which in turn lead to accumulation of cytosolic TDP-43 aggregates (Filimonenko et al., 2007). Patients with ALS-FTD-linked mutations in ubiliqulin-2, which appear to inhibit proteasome activity, develop TDP-43 proteinopathy (Deng et al., 2011). Ubiquilin-1 interacts with TDP-43 and overexpression of ubiquilin-1 can recruit TDP-43 into cytoplasmic aggregates that co-localize with autophagosomes in cultured cells (Kim et al., 2009). Finally, p62/sequestosome-1 is misaccumulated in both ALS and FTD (Seelaar et al., 2007) along with TDP-43 (Tanji et al., 2012), while increased expression of it reduces TDP-43 aggregates in cultured cells (Brady et al., 2011). Taken together, these findings indicate that ALS/FTD-linked mutations in genes that are involved in protein homeostasis can directly contribute to TDP-43 proteinopathy.
Except for ubquilin-2 mutations (Deng et al., 2011; Williams et al., 2012), inclusion of FUS/TLS has not been reported in response to mutations or disruption of ALS-linked genes involved in the protein homeostasis pathways. However, as described above, one class of ALS-linked mutations disrupts nuclear localization signals, producing higher cytosolic accumulation of FUS/TLS (Dormann et al., 2010, Bosco et al., 2010). This relocalization of FUS/TLS may be a primary cause for initiating FUS/TLS proteinopathies.
TDP-43 regulates expression of ALS-linked genes involved in protein clearance
TDP-43 affects levels of RNAs that encode proteins involved in protein homeostasis, including CHMP2B, Fig4, OPTN, VAPB, and VCP (Polymenidou et al., 2011). Additionally, TDP-43 has been shown to bind the pre-mRNA of the autophagy-related 7 (Atg7) gene essential for autophagy, with reduction of TDP-43 down-regulating Atg7, thereby impairing autophagy (Bose et al., 2011). It is worth mentioning that mice lacking Atg5 and Atg7 in the nervous system exhibit neurodegeneration (Hara et al., 2006; Komatsu et al., 2006), strongly suggesting not unexpectedly - that autophagy is essential for normal neuronal function. Altogether, these results suggest an intricate regulatory network in which TDP-43 can regulate the expression of the very gene(s) that participate in TDP-43 clearance, providing an additional mechanism of regulating TDP-43 abundance (the other being the autoregulation of TDP-43 by binding to its own mRNA), while TDP-43 also indirectly affects global protein clearance pathways by regulating the expression of key components in autophagy.
Similarly, FUS/TLS binds to the mRNAs encoding optineurin (Lagier-Tourenne et al., 2012; Colombrita et al., 2012), ubiquilin-2 (Lagier-Tourenne et al., 2012; Hoell et al., 2011), VAPB (Lagier-Tourenne et al., 2012; Hoell et al., 2011), and VCP (Lagier-Tourenne et al., 2012; Colombrita et al., 2012; Hoell et al., 2011), although reduction of FUS/TLS in the mouse central nervous system does not significantly alter their expression levels (Lagier-Tourenne et al., 2012). In a motoneuron-like cell line, FUS/TLS has been argued to be preferentially bound to cytoplasmic mRNAs that are involved in the ubiquitin-proteasome pathway, in particular the cullin-RING E3 ubiquitin ligases (Colombrita et al., 2012). Perhaps most importantly, the endoplasmic reticulum or ubiquitin-proteasome pathways are overrepresented in the mRNA targets bound by ALS-linked mutations in FUS/TLS (Hoell et al., 2011). Altogether, the evidence strongly suggests that, similar to the case for TDP-43, mutation or nuclear loss of function of FUS/TLS affects protein clearance pathways by regulating expression levels of genes in the pathway.
Unifying underlying pathogenic pathways in ALS and FTD
We propose that converging pathogenic mechanisms underlying ALS and FTD are disruption of both RNA and protein homeostasis and disturbed homeostasis that produces a feed-forward loop that drives disease progression (Figure 7). In this model, the initiating event that triggers disease initiation can occur at multiple points in either protein or RNA homeostasis pathways, including genetic mutations that predispose one pathway to be more error-prone or other non-genetic factors, such as aging, in which proteostasis decline is well documented.
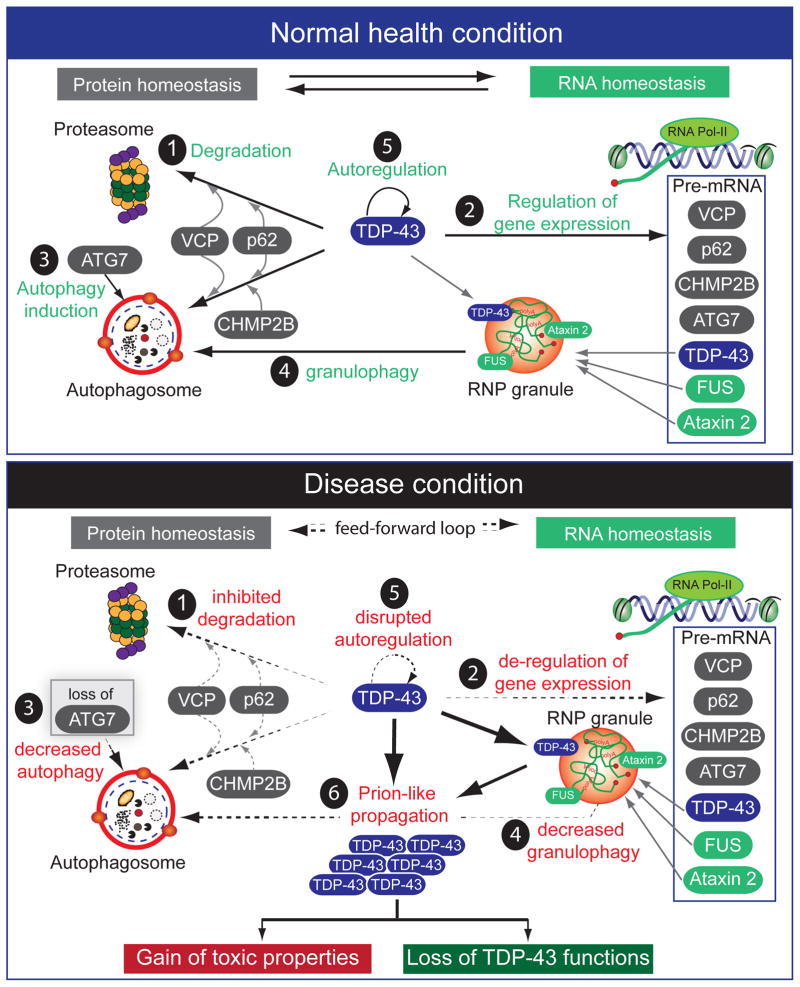
Figure 7. Molecular interplay of TDP-43 with other ALS- and FTD-linked genes in protein and RNA homeostasis.
Proposed pathogenic mechanisms in TDP-43-mediated neurodegneration converge onto disruption of protein and RNA homeostasis. Proteins involved in protein and RNA homeostasis are labeled as grey and green, respectively. (Upper panel) Normal functions of ALS/FTD-linked proteins. 1) Three ALS-linked genes (VCP, p62 and CHMP2B) are required for proper turnover of TDP-43, which is degraded both by the proteasome and by autophagy. 2) TDP-43 regulates the expression of the same ALS-linked genes that are required for its own degradation as well as 3) a key autophagy induction protein, ATG7. TDP-43 also regulates two other RNA-binding proteins linked to ALS (FUS/TLS and Ataxin-2). TDP-43, FUS/TLS and Ataxin-2 form RNA-protein granules that are degraded through 4) autophagy/granulophagy. Thus, TDP-43 governs both protein and RNA homeostasis and 5) its own level is tightly maintained. (Lower panel) Disrupted protein and RNA homeostasis that fuels a feed-forward loop driving disease progression. An initiating event that triggers disease initiation can occur at multiple points in either protein or RNA homeostasis pathways, including genetic mutations that predispose one pathway to be more error-prone or other non-genetic factors, such as aging. The well-documented 1) decline in proteostasis during aging may lead to elevated accumulation of TDP-43. Subsequently, 2) the genes that are controlled by TDP-43 become de-regulated, including 3) loss of expression of ATG7, which in turn reduces 4) autophagy (and granulophagy). The net result of this produces 5) disrupted autoregulation of TDP-43 with an increased cytoplasmic concentration of TDP-43, which provokes 6) prion-like templating of it followed by propagation and spread. These gain-of-toxic properties induce over-production of “nonfunctional prion-like” TDP-43 that leads to further loss of TDP-43 function. Similar scenarios could operate for prion-like domain-containing RNA binding proteins, such as FUS/TLS and hnRNP A2/B1.
More provocatively, prion-domain-containing RNA-binding proteins may also be predisposed to self-promoting aggregation and spread, which could explain the seemingly sporadic nature of many instances of both diseases. Subsequent disease progression may be amplified by failure in cross-regulation among multiple proteins/genes, with several ALS-linked genes [including VCP, p62/SQSTM1, and CHMP2B] required for TDP-43 degradation, whereas TDP-43 regulates expression of VCP and CHMP2B. In addition, not only does TDP-43 bind to its own mRNA, which is essential for its autoregulation, but TDP-43 also binds to several ALS-linked genes involved in RNA homeostasis, including Ang1 (angiogenin), Atxn2 (ataxin-2), and FUS/TLS. Similar mechanisms could exist for FUS/TLS.
Once initiated, errors in RNA and protein homeostasis accumulate, which eventually lead to failure in autoregulation, deregulation of ALS-linked genes, proteotoxic stress, and loss of neuroprotection. The failure to maintain proper protein and RNA homeostasis is highly likely to drive a feed-forward cycle, leading to a snowballing effect perturbating many aspects of protein and RNA function. Subsequent propagation and spreading of TDP-43 and FUS/TLS aggregates into neighboring cells could drive spread from a focal initiation site.
Prospects for therapies in ALS/FTD
Following Jean-Martin Charcot’s initial description of ALS, he made the grim statement regarding therapy: “The prognosis, up to the present, is of the gloomiest. There does not exist, so far as I am aware, a single example of a case where, the group of symptoms just described having existed, recovery followed”. Sadly, 140 years has passed and ALS remains the same devastating and lethal disease. There is currently only one FDA-approved drug, riluzole, an inhibitor of presynaptic glutamate release, which only extends the survival of the patients for 2–3 months. In the past two decades, many potential therapeutic interventions have been attempted but none have been successful [reviewed in (Zinman and Cudkowicz, 2011)].
Therapies by lowering synthesis of a toxic species
For disease from mutant SOD1 (and if wild type SOD1 is confirmed to be a contributor to sporadic disease), therapy lowering the synthesis of either would be directly on disease mechanism. Indeed, reducing SOD1 expression has been reported to slow disease progression of transgenic mice and rats expressing human mutant SOD1 (Ralph et al., 2005; Raoul et al., 2005; Smith et al., 2006). A further glimmer of hope has emerged from a successful phase I safety trial using antisense oligonucleotides against SOD1 in patients carrying mutant SOD1 (Miller et al., 2013). A similar strategy targeting the toxic RNA species can be envisioned for the more frequent instances of disease from hexanucleotide expansion in C9ORF72.
Therapy design by improving protein homeostasis
Several lines of evidence indicate that broad defects in protein homeostasis may contribute to ALS pathogenesis: (1) All ALS patients have one of the following protein inclusions in affected motor neurons: TDP-43, FUS/TLS or SOD1; (2) ALS-linked mutations are identified in several genes involved in ER stress, autophagy and the ubiquitin-proteasome pathway; (3) ALS-linked mutations in ubiquilin-2, CHMP2B and VCP can lead to TDP-43 aggregation; (4) Dysfunctions in ERAD and autophagy are observed in mouse models expressing mutant SOD1; (5) Autophagy appears to be activated and upregulated in motor neurons of sporadic ALS patients.
It is not clear how a decline in general protein degradation machinery might cause aggregation of specific proteins in different neurodegenerative diseases. However, it is conceivable that increasing (or delaying age-dependent decline in) proteostasis could, in principle, prevent or slow down the formation of protein inclusions – or at least accumulation of some or all of the toxic protein species. Initial hints that this approach could be beneficial came from report of modest delay in disease progression following treatment of a very small number of mice with arimoclomol, an inducer of heat shock proteins HSP70 and HSP90 (Kieran et al., 2004). Phase 2/3 clinical trials are currently underway for this approach. Dampening the unfolded protein response (UPR) by deleting a downstream X-box binding protein (XBP-1) was reported to provide a modest survival benefit (~20 days) to a small cohort (N =7) of SOD1G86R mice, but the apparent benefit was disappointingly found only in female mice (Hetz et al., 2009). Finally, pharmacological activation of autophagy was reported in another small cohort of mice (N=10 per drug treatment) to improve cognitive and motor phenotype in male mice overexpressing wild type TDP-43 (Wang et al., 2012).
Independent replications of the above experiments with larger cohorts that are powered to provide statistical significance - extended to multiple ALS/FTD mouse models - are now needed to validate the therapeutic potential of these approaches. Moreover, enthusiasm for the rationale of autophagy induction must be tempered by recognition that simply activating the autophagy pathway may cause cytosolic depletion of essential organelles [reviewed in (Wong and Cuervo, 2010)], and it should be recognized that autophagy is a double-edged sword.
Concluding remarks
With recent advances in identifying major common genetic causes and the identities of major components in the pathological aggregates for ALS and FTLD, perturbation of both RNA and protein homeostasis is a convergent molecular feature with a probable feed-forward loop driving the failure in maintaining RNA and protein homeostasis as a central underlying mechanism for the relentless deterioration of neurons. There is probably no silver bullet for curing all sporadic cases. However, with knowledge of genetic causes and molecular players, it is the most exciting time for discovery in ALS and FTD. Much remains still to be learned, bearing in mind Charcot’s charge from 140 years ago: “Let us keep searching. It is indeed the best method of finding and perhaps thanks to our efforts, the verdict we will give such a patient (with ALS) tomorrow will not be the same we must give this man today.”
Supplementary Material
Acknowledgments
We apologize to all whose work cannot be cited because of space restrictions. We thank Drs. Dara Ditsworth, Holly Kordasiewicz and Clotilde Lagier-Tourenne for helpful comments and Dr. Meredith LeMasurier for editorial revisions. This work was supported by a grant from the NIH (R01-NS27036) to D.W.C. and (K99-NS075216) to M.P. D.W.C receives the salary support from the Ludwig Institute for Cancer Research. S.-C. L. was a recipient of a National Institute of Aging training grant (T32 AG 000216). M.P. was the recipient of a long-term fellowship from the international Human Frontier Science Program Organization.
References
- Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobágyi T, Shaw CE. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122:691–702. doi: 10.1007/s00401-011-0911-2. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10:430–436. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- Andersson MK, Ståhlberg A, Arvidsson Y, Olofsson A, Semb H, Stenman G, Nilsson O, Aman P. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008;9:37. doi: 10.1186/1471-2121-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Arnold ES, Ling SC, Huelga SC, Lagier-Tourenne C, Polymenidou M, Ditsworth D, Kordasiewicz HB, McAlonis-Downes M, Platoshyn O, Parone PA, et al. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci USA. 2013;110:E736–745. doi: 10.1073/pnas.1222809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash PEA, Bieniek KF, Gendron TF, Caulfield T, Lin W-L, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul I, Joseph W, Rademakers R, et al. Unconventional Translation of C9ORF72 GGGGCC Expansion Generates Insoluble Polypeptides Specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avendano-Vazquez SE, Dhir A, Bembich S, Buratti E, Proudfoot N, Baralle FE. Autoregulation of TDP-43 mRNA levels involves interplay between transcription, splicing, and alternative polyA site selection. Genes Dev. 2012;26:1679–1684. doi: 10.1101/gad.194829.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala V, Granado-Serrano AB, Cacabelos D, Naudí A, Ilieva EV, Boada J, Caraballo-Miralles V, Lladó J, Ferrer I, Pamplona R, et al. Cell stress induces TDP-43 pathological changes associated with ERK1/2 dysfunction: implications in ALS. Acta Neuropathol. 2011a;122:259–270. doi: 10.1007/s00401-011-0850-y. [DOI] [PubMed] [Google Scholar]
- Ayala YM, De Conti L, Avendaño-Vázquez SE, Dhir A, Romano M, D’Ambrogio A, Tollervey J, Ule J, Baralle M, Buratti E, et al. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011b;30:277–288. doi: 10.1038/emboj.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YM, Zago P, D'Ambrogio A, Xu YF, Petrucelli L, Buratti E, Baralle FE. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. doi: 10.1242/jcs.038950. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Bäumer D, Hilton D, Paine SML, Turner MR, Lowe J, Talbot K, Ansorge O. Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations. Neurology. 2010;75:611–618. doi: 10.1212/WNL.0b013e3181ed9cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzil VV, Langlais JS, Daoud H, Dion PA, Brais B, Rouleau GA. Novel FUS Deletion in a Patient With Juvenile Amyotrophic Lateral Sclerosis. Arch Neurol. 2012;69:653–656. doi: 10.1001/archneurol.2011.2499. [DOI] [PubMed] [Google Scholar]
- Bentmann E, Neumann M, Tahirovic S, Rodde R, Dormann D, Haass C. Requirements for stress granule recruitment of fused in Sarcoma (FUS) and TAR DNA binding protein of 43 kDa (TDP-43) J Biol Chem. 2012;287:23079–23094. doi: 10.1074/jbc.M111.328757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A, Lutz Y, Heard DJ, Chambon P, Tora L. hTAF(II)68, a novel RNA/ssDNA-binding protein with homology to the pro-oncoproteins TLS/FUS and EWS is associated with both TFIID and RNA polymerase II. EMBO J. 1996;15:5022–5031. [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Boylan KB, Graff-Radford NR, Dejesus-Hernandez M, Knopman DS, Pedraza O, Vemuri P, Jones D, Lowe V, Murray ME, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–783. doi: 10.1093/brain/aws004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Borroni B, Bonvicini C, Alberici A, Buratti E, Agosti C, Archetti S, Papetti A, Stuani C, Di Luca M, Gennarelli M, et al. Mutation within TARDBP leads to frontotemporal dementia without motor neuron disease. Hum Mutat. 2009;30:E974–983. doi: 10.1002/humu.21100. [DOI] [PubMed] [Google Scholar]
- Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ, Sapp P, McKenna-Yasek D, Brown RH, Hayward LJ. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet. 2010a;19:4160–4175. doi: 10.1093/hmg/ddq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, Goolsby H, Fontaine BA, Lemay N, McKenna-Yasek D, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010b;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose JK, Huang CC, Shen CKJ. Regulation of Autophagy by Neuropathological Protein TDP-43. J Biol Chem. 2011;286:44441–44448. doi: 10.1074/jbc.M111.237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady OA, Meng P, Zheng Y, Mao Y, Hu F. Regulation of TDP-43 aggregation by phosphorylation and p62/SQSTM1. J Neurochem. 2011;116:248–259. doi: 10.1111/j.1471-4159.2010.07098.x. [DOI] [PubMed] [Google Scholar]
- Bredesen DE, Ellerby LM, Hart PJ, Wiedau-Pazos M, Valentine JS. Do posttranslational modifications of CuZnSOD lead to sporadic amyotrophic lateral sclerosis? Ann Neurol. 1997;42:135–137. doi: 10.1002/ana.410420202. [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Van Deerlin VM, Robinson JL, Kwong L, Lee EB, Ali YO, Safren N, Monteiro MJ, Toledo JB, Elman L, et al. Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta Neuropathol. 2012;123:825–839. doi: 10.1007/s00401-012-0970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton TE, Li Y, Cooper D, Gearing M, Julien JP, Rothstein JD, Boylan K, Glass JD. Localization of a toxic form of superoxide dismutase 1 protein to pathologically affected tissues in familial ALS. Proc Natl Acad Sci USA. 2012;109:5505–5510. doi: 10.1073/pnas.1115009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratti E, Baralle FE. TDP-43: gumming up neurons through protein-protein and protein-RNA interactions. Trends Biochem Sci. 2012;37:237–247. doi: 10.1016/j.tibs.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Buratti E, De Conti L, Stuani C, Romano M, Baralle M, Baralle F. Nuclear factor TDP-43 can affect selected microRNA levels. FEBS J. 2010;277:2268–2281. doi: 10.1111/j.1742-4658.2010.07643.x. [DOI] [PubMed] [Google Scholar]
- Burghes AHM, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci. 2009;10:597–609. doi: 10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, Heverin M, Jordan N, Kenna K, Lynch C, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol. 2012;11:232–240. doi: 10.1016/S1474-4422(12)70014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Durazo A, Sohn SH, Strong CD, Gralla EB, Whitelegge JP, Valentine JS. Initiation and elongation in fibrillation of ALS-linked superoxide dismutase. Proc Natl Acad Sci USA. 2008;105:18663–18668. doi: 10.1073/pnas.0807058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Anagnostou G, Chai A, Withers J, Morris A, Adhikaree J, Pennetta G, de Belleroche JS. Characterization of the properties of a novel mutation in VAPB in familial amyotrophic lateral sclerosis. J Biol Chem. 2010;285:40266–40281. doi: 10.1074/jbc.M110.161398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YZ, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, Dierick I, Abel A, Kennerson ML, Rabin BA, et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4) Am J Hum Genet. 2004;74:1128–1135. doi: 10.1086/421054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia R, Tattum MH, Jones S, Collinge J, Fisher EMC, Jackson GS. Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PLoS ONE. 2010;5:e10627. doi: 10.1371/journal.pone.0010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang PM, Ling J, Jeong YH, Price DL, Aja SM, Wong PC. Deletion of TDP-43 down-regulates Tbc1d1, a gene linked to obesity, and alters body fat metabolism. Proc Natl Acad Sci USA. 2010;107:16320–16324. doi: 10.1073/pnas.1002176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiò A, Borghero G, Restagno G, Mora G, Drepper C, Traynor BJ, Sendtner M, Brunetti M, Ossola I, Calvo A, et al. Clinical characteristics of patients with familial amyotrophic lateral sclerosis carrying the pathogenic GGGGCC hexanucleotide repeat expansion of C9ORF72. Brain. 2012;135:784–793. doi: 10.1093/brain/awr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CY, Landers JE, Bergren SK, Sapp PC, Grant AE, Jones JM, Everett L, Lenk GM, McKenna-Yasek DM, Weisman LS, et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet. 2009;84:85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, Li J, Zhang X, et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, Lattante S, Le Ber I, Latouche M, Tostivint H, Brice A, Kabashi E. Loss of function of C9orf72 causes motor deficits in a zebrafish model of Amyotrophic Lateral Sclerosis. Ann Neurol. 2013 doi: 10.1002/ana.23946. [DOI] [PubMed] [Google Scholar]
- Colombrita C, Onesto E, Megiorni F, Pizzuti A, Baralle FE, Buratti E, Silani V, Ratti A. TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post-transcriptional fate in motoneuron-like cells. J Biol Chem. 2012;287:15635–15647. doi: 10.1074/jbc.M111.333450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, Silani V, Ratti A. TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem. 2009;111:1051–1061. doi: 10.1111/j.1471-4159.2009.06383.x. [DOI] [PubMed] [Google Scholar]
- Cooper-Knock J, Hewitt C, Highley JR, Brockington A, Milano A, Man S, Martindale J, Hartley J, Walsh T, Gelsthorpe C, et al. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain. 2012;135:751–764. doi: 10.1093/brain/awr365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couthouis J, Hart MP, Erion R, King OD, Diaz Z, Nakaya T, Ibrahim F, Kim HJ, Mojsilovic-Petrovic J, Panossian S, et al. Evaluating the role of the FUS/TLS-related gene EWSR1 in amyotrophic lateral sclerosis. Hum Mol Genet. 2012;21:2899–2911. doi: 10.1093/hmg/dds116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couthouis J, Hart MP, Shorter J, Dejesus-Hernandez M, Erion R, Oristano R, Liu AX, Ramos D, Jethava N, Hosangadi D, et al. Feature Article: A yeast functional screen predicts new candidate ALS disease genes. Proc Natl Acad Sci USA. 2011;108:20881–20890. doi: 10.1073/pnas.1109434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LE, Ferraiuolo L, Goodall EF, Heath PR, Higginbottom A, Mortiboys H, Hollinger HC, Hartley JA, Brockington A, Burness CE, et al. Mutations in CHMP2B in lower motor neuron predominant amyotrophic lateral sclerosis (ALS) PLoS ONE. 2010;5:e9872. doi: 10.1371/journal.pone.0009872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Da Cruz S, Cleveland DW. Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr Opin Neurobiol. 2011;21:904–919. doi: 10.1016/j.conb.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud H, Suhail H, Szuto A, Camu W, Salachas F, Meininger V, Bouchard JP, Dupré N, Dion PA, Rouleau GA. UBQLN2 mutations are rare in French and French-Canadian amyotrophic lateral sclerosis. Neurobiol Aging. 2012;33:2230. doi: 10.1016/j.neurobiolaging.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Daughters RS, Tuttle DL, Gao W, Ikeda Y, Moseley ML, Ebner TJ, Swanson MS, Ranum LPW. RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet. 2009;5:e1000600. doi: 10.1371/journal.pgen.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bo R, Tiloca C, Pensato V, Corrado L, Ratti A, Ticozzi N, Corti S, Castellotti B, Mazzini L, Sorarù G, et al. Novel optineurin mutations in patients with familial and sporadic amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatr. 2011;82:1239–1243. doi: 10.1136/jnnp.2011.242313. [DOI] [PubMed] [Google Scholar]
- Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477:211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci USA. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey CM, Cenik B, Sephton CF, Dries DR, Mayer P, Good SK, Johnson BA, Herz J, Yu G. TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol Cell Biol. 2011;31:1098–1108. doi: 10.1128/MCB.01279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey CM, Cenik B, Sephton CF, Johnson BA, Herz J, Yu G. TDP-43 aggregation in neurodegeneration: Are stress granules the key? Brain Res. 2012;1462:16–25. doi: 10.1016/j.brainres.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IRA, Capell A, Schmid B, et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 2010;29:2841–2857. doi: 10.1038/emboj.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili MA, Panahi M, Yadav S, Hennings L, Kiaei M. Premature death of TDP-43 (A315T) transgenic mice due to gastrointestinal complications prior to development of full neurological symptoms of amyotrophic lateral sclerosis. Int J Expt Pathol. 2013;94:56–64. doi: 10.1111/iep.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecto F, Yan J, Vemula SP, Liu E, Yang Y, Chen W, Zheng JG, Shi Y, Siddique N, Arrat H, et al. SQSTM1 Mutations in Familial and Sporadic Amyotrophic Lateral Sclerosis. Arch Neurol. 2011;68:1440–1446. doi: 10.1001/archneurol.2011.250. [DOI] [PubMed] [Google Scholar]
- Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerød L, Fisher EMC, Isaacs A, Brech A, Stenmark H, Simonsen A. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol. 2007;179:485–500. doi: 10.1083/jcb.200702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg K, Jonsson PA, Andersen PM, Bergemalm D, Graffmo KS, Hultdin M, Jacobsson J, Rosquist R, Marklund SL, Brännström T. Novel antibodies reveal inclusions containing non-native SOD1 in sporadic ALS patients. PLoS ONE. 2010;5:e11552. doi: 10.1371/journal.pone.0011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freibaum BD, Chitta RK, High AA, Taylor JP. Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery. J Proteome Res. 2010;9:1104–1120. doi: 10.1021/pr901076y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii R, Okabe S, Urushido T, Inoue K, Yoshimura A, Tachibana T, Nishikawa T, Hicks GG, Takumi T. The RNA binding protein TLS is translocated to dendritic spines by mGluR5 activation and regulates spine morphology. Curr Biol. 2005;15:587–593. doi: 10.1016/j.cub.2005.01.058. [DOI] [PubMed] [Google Scholar]
- Funke AD, Esser M, Krüttgen A, Weis J, Mitne-Neto M, Lazar M, Nishimura AL, Sperfeld AD, Trillenberg P, Senderek J, et al. The p.P56S mutation in the VAPB gene is not due to a single founder: the first European case. Clin Genet. 2010;77:302–303. doi: 10.1111/j.1399-0004.2009.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J Biol Chem. 2011;286:18664–18672. doi: 10.1074/jbc.M111.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal J, Zhang J, Kwinter DM, Zhai J, Jia H, Jia J, Zhu H. Nuclear localization sequence of FUS and induction of stress granules by ALS mutants. Neurobiol Aging. 2011;32:2323, e2327–2340. doi: 10.1016/j.neurobiolaging.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, La Spada AR. Intercellular (Mis)communication in Neurodegenerative Disease. Neuron. 2012;73:886–901. doi: 10.1016/j.neuron.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellera C, Tiloca C, Del Bo R, Corrado L, Pensato V, Agostini J, Cereda C, Ratti A, Castellotti B, Corti S, et al. Ubiquilin 2 mutations in Italian patients with amyotrophic lateral sclerosis and frontotemporal dementia. J Neurol Neurosurg Psychiatr. 2013;84:183–187. doi: 10.1136/jnnp-2012-303433. [DOI] [PubMed] [Google Scholar]
- Ghazi-Noori S, Froud KE, Mizielinska S, Powell C, Smidak M, Fernandez de Marco M, O'Malley C, Farmer M, Parkinson N, Fisher EMC, et al. Progressive neuronal inclusion formation and axonal degeneration in CHMP2B mutant transgenic mice. Brain. 2012;135:819–832. doi: 10.1093/brain/aws006. [DOI] [PubMed] [Google Scholar]
- Gijselinck I, Van Langenhove T, van der Zee J, Sleegers K, Philtjens S, Kleinberger G, Janssens J, Bettens K, Van Cauwenberghe C, Pereson S, et al. A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol. 2012;11:54–65. doi: 10.1016/S1474-4422(11)70261-7. [DOI] [PubMed] [Google Scholar]
- Gitcho MA, Strider J, Carter D, Taylor-Reinwald L, Forman MS, Goate AM, Cairns NJ. VCP mutations causing frontotemporal lobar degeneration disrupt localization of TDP-43 and induce cell death. J Biol Chem. 2009;284:12384–12398. doi: 10.1074/jbc.M900992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkogkas C, Middleton S, Kremer AM, Wardrope C, Hannah M, Gillingwater TH, Skehel P. VAPB interacts with and modulates the activity of ATF6. Hum Mol Genet. 2008;17:1517–1526. doi: 10.1093/hmg/ddn040. [DOI] [PubMed] [Google Scholar]
- Grad LI, Guest WC, Yanai A, Pokrishevsky E, O’Neill MA, Gibbs E, Semenchenko V, Yousefi M, Wishart DS, Plotkin SS, et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc Natl Acad Sci USA. 2011;108:16398–16403. doi: 10.1073/pnas.1102645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway MJ, Andersen PM, Russ C, Ennis S, Cashman S, Donaghy C, Patterson V, Swingler R, Kieran D, Prehn J, et al. ANG mutations segregate with familial and sporadic amyotrophic lateral sclerosis. Nat Genet. 2006;38:411–413. doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- Groen EJ, Fumoto K, Blokhuis AM, Engelen-Lee J, Zhou Y, van den Heuvel DM, Koppers M, van Diggelen F, van Heest J, Demmers JA, et al. ALS-associated mutations in FUS disrupt the axonal distribution and function of SMN. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt222. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang Q, Zhang K, An T, Shi P, Li Z, Duan W, Li C. HO-1 induction in motor cortex and intestinal dysfunction in TDP-43 A315T transgenic mice. Brain Res. 2012;1460:88–95. doi: 10.1016/j.brainres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nature Biotech. 2011;29:824–828. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Ryu HH, Jun MH, Jang DJ, Lee JA. The functional analysis of the CHMP2B missense mutation associated with neurodegenerative diseases in the endo-lysosomal pathway. Biochem Biophys Res Commun. 2012a;421:544–549. doi: 10.1016/j.bbrc.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, et al. Cell-free Formation of RNA Granules: Bound RNAs Identify Features and Components of Cellular Assemblies. Cell. 2012b;149:768–779. doi: 10.1016/j.cell.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Hartmuth K, Urlaub H, Vornlocher HP, Will CL, Gentzel M, Wilm M, LUhrmann R. Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc Natl Acad Sci USA. 2002;99:16719–16724. doi: 10.1073/pnas.262483899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GG, Singh N, Nashabi A, Mai S, Bozek G, Klewes L, Arapovic D, White EK, Koury MJ, Oltz EM, et al. Fus deficiency in mice results in defective B-lymphocyte development and activation, high levels of chromosomal instability and perinatal death. Nat Genet. 2000;24:175–179. doi: 10.1038/72842. [DOI] [PubMed] [Google Scholar]
- Hoell JI, Larsson E, Runge S, Nusbaum JD, Duggimpudi S, Farazi TA, Hafner M, Borkhardt A, Sander C, Tuschl T. RNA targets of wild-type and mutant FET family proteins. Nat Struct Mol Biol. 2011;18:1428–1431. doi: 10.1038/nsmb.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung GYR, DeJesus-Hernandez M, Feldman HH, Sengdy P, Bouchard-Kerr P, Dwosh E, Butler R, Leung B, Fok A, Rutherford NJ, et al. Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain. 2012;135:709–722. doi: 10.1093/brain/awr354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Padukkavidana T, Vægter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM. Sortilin-Mediated Endocytosis Determines Levels of the Frontotemporal Dementia Protein, Progranulin. Neuron. 2010;68:654–667. doi: 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tong J, Bi F, Zhou H, Xia XG. Mutant TDP-43 in motor neurons promotes the onset and progression of ALS in rats. J Clin Invest. 2012;122:107–118. doi: 10.1172/JCI59130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Zhou H, Tong J, Chen H, Liu YJ, Wang D, Wei X, Xia XG. FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. PLoS Genet. 2011;7:e1002011. doi: 10.1371/journal.pgen.1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Zhang J, Geser F, Trojanowski JQ, Strober JB, Dickson DW, Brown RH, Jr, Shapiro BE, Lomen-Hoerth C. Extensive FUS-Immunoreactive Pathology in Juvenile Amyotrophic Lateral Sclerosis with Basophilic Inclusions. Brain Pathol. 2010;20:1069–1076. doi: 10.1111/j.1750-3639.2010.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Lee EB, Chen-Plotkin A, Swanson E, Unger T, Malunda J, Xu Y, Winton MJ, Trojanowski JQ, et al. Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J Clin Invest. 2011;121:726–738. doi: 10.1172/JCI44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi Y, Katsuno M, Niwa JI, Takagi S, Ishigaki S, Ikenaka K, Kawai K, Watanabe H, Yamanaka K, Takahashi R, et al. Loss of TDP-43 causes age-dependent progressive motor neuron degeneration. Brain. 2013;136:1371–1382. doi: 10.1093/brain/awt029. [DOI] [PubMed] [Google Scholar]
- Iida A, Hosono N, Sano M, Kamei T, Oshima S, Tokuda T, Kubo M, Nakamura Y, Ikegawa S. Optineurin mutations in Japanese amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatr. 2012a;83:233–235. doi: 10.1136/jnnp.2010.234963. [DOI] [PubMed] [Google Scholar]
- Iida A, Hosono N, Sano M, Kamei T, Oshima S, Tokuda T, Nakajima M, Kubo M, Nakamura Y, Ikegawa S. Novel deletion mutations of OPTN in amyotrophic lateral sclerosis in Japanese. Neurobiol Aging. 2012b;33:1843 e1819–1824. doi: 10.1016/j.neurobiolaging.2011.12.037. [DOI] [PubMed] [Google Scholar]
- Ishigaki S, Masuda A, Fujioka Y, Iguchi Y, Katsuno M, Shibata A, Urano F, Sobue G, Ohno K. Position-dependent FUS-RNA interactions regulate alternative splicing events and transcriptions. Sci Rep. 2012;2:529. doi: 10.1038/srep00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Ariizumi Y, Shiga A, Kato T, Tan CF, Sato T, Miki Y, Yokoo M, Fujino T, Koyama A, et al. Decreased number of Gemini of coiled bodies and U12 snRNA level in amyotrophic lateral sclerosis. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt262. [DOI] [PubMed] [Google Scholar]
- Ito D, Seki M, Tsunoda Y, Uchiyama H, Suzuki N. Nuclear transport impairment of amyotrophic lateral sclerosis-linked mutations in FUS/TLS. Ann Neurol. 2010;69:152–162. doi: 10.1002/ana.22246. [DOI] [PubMed] [Google Scholar]
- Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST. Pur α Binds to rCGG Repeats and Modulates Repeat-Mediated Neurodegeneration in a Drosophila Model of Fragile X Tremor/Ataxia Syndrome. Neuron. 2007;55:556–564. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem. 2009;284:20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, Gibbs JR, Brunetti M, Gronka S, Wuu J, et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–864. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce PI, Fratta P, Fisher EMC, Acevedo-Arozena A. SOD1 and TDP-43 animal models of amyotrophic lateral sclerosis: recent advances in understanding disease toward the development of clinical treatments. Mamm Genome. 2011;22:420–448. doi: 10.1007/s00335-011-9339-1. [DOI] [PubMed] [Google Scholar]
- Ju JS, Fuentealba RA, Miller SE, Jackson E, Piwnica-Worms D, Baloh RH, Weihl CC. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J Cell Biol. 2009;187:875–888. doi: 10.1083/jcb.200908115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Kanekura K, Nishimoto I, Aiso S, Matsuoka M. Characterization of amyotrophic lateral sclerosis-linked P56S mutation of vesicle-associated membrane protein-associated protein B (VAPB/ALS8) J Biol Chem. 2006;281:30223–30233. doi: 10.1074/jbc.M605049200. [DOI] [PubMed] [Google Scholar]
- Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nature Neurosci. 2013;16:571–579. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, et al. Cell-free Formation of RNA Granules: Low Complexity Sequence Domains Form Dynamic Fibers within Hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Mieda-Sato A. TDP-43 promotes microRNA biogenesis as a component of the Drosha and Dicer complexes. Proc Natl Acad Sci USA. 2012;109:3347–3352. doi: 10.1073/pnas.1112427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman A, Liu HN, Croul S, Bilbao J, Rogaeva E, Zinman L, Robertson J, Chakrabartty A. Amyotrophic lateral sclerosis is a non-amyloid disease in which extensive misfolding of SOD1 is unique to the familial form. Acta Neuropathol. 2010;119:335–344. doi: 10.1007/s00401-010-0646-5. [DOI] [PubMed] [Google Scholar]
- Kiebler MA, Bassell GJ. Neuronal RNA granules: movers and makers. Neuron. 2006;51:685–690. doi: 10.1016/j.neuron.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Kieran D, Kalmar B, Dick JRT, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, Maclea KS, Freibaum B, Li S, Molliex A, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Shi Y, Hanson KA, Williams LM, Sakasai R, Bowler MJ, Tibbetts RS. Potentiation of amyotrophic lateral sclerosis (ALS)-associated TDP-43 aggregation by the proteasome-targeting factor, ubiquilin 1. J Biol Chem. 2009;284:8083–8092. doi: 10.1074/jbc.M808064200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King OD, Gitler AD, Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012;1462:61–80. doi: 10.1016/j.brainres.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino Y, Washizu C, Aquilanti E, Okuno M, Kurosawa M, Yamada M, Doi H, Nukina N. Intracellular localization and splicing regulation of FUS/TLS are variably affected by amyotrophic lateral sclerosis-linked mutations. Nucleic Acids Res. 2011;39:2781–2798. doi: 10.1093/nar/gkq1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J-i, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Murrell JR, Horvath S, Haraszti L, Majtenyi K, Molnar MJ, Budka H, Ghetti B, Spina S. TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord. 2009;24:1843–1847. doi: 10.1002/mds.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer BC, Schuck T, Wheeler JM, Robinson LC, Trojanowski JQ, Lee VMY, Schellenberg GD. Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol. 2010;119:409–419. doi: 10.1007/s00401-010-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Sok J, Webb L, Baechtold H, Urano F, Yin Y, Chung P, de Rooij DG, Akhmedov A, Ashley T, et al. Male sterility and enhanced radiation sensitivity in TLS(−/−) mice. EMBO J. 2000;19:453–462. doi: 10.1093/emboj/19.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu-Martinez NM, Wang GS, Cooper TA. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell. 2007;28:68–78. doi: 10.1016/j.molcel.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- La Spada AR, Taylor JP. Repeat expansion disease: progress and puzzles in disease pathogenesis. Nature Rev Genet. 2010;11:247–258. doi: 10.1038/nrg2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksovirta H, Peuralinna T, Schymick JC, Scholz SW, Lai SL, Myllykangas L, Sulkava R, Jansson L, Hernandez DG, Gibbs JR, et al. Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol. 2010;9:978–985. doi: 10.1016/S1474-4422(10)70184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19:R46–R64. doi: 10.1093/hmg/ddq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Polymenidou M, Hutt KR, Vu AQ, Baughn M, Huelga SC, Clutario KM, Ling SC, Liang TY, Mazur C, et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nature Neurosci. 2012;15:1488–1497. doi: 10.1038/nn.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattante S, Rouleau GA, Kabashi E. TARDBP and FUS Mutations Associated with Amyotrophic Lateral Sclerosis: Summary and Update. Hum Mutat. 2013;34:812–826. doi: 10.1002/humu.22319. [DOI] [PubMed] [Google Scholar]
- Lee JE, Cooper TA. Pathogenic mechanisms of myotonic dystrophy. Biochem Soc Trans. 2009;37:1281. doi: 10.1042/BST0371281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine TP, Daniels RD, Gatta AT, Wong LH, Hayes MJ. The product of C9orf72, a gene strongly implicated in neurodegeneration, is structurally related to DENN Rab-GEFs. Bioinformatics. 2013;29:499–503. doi: 10.1093/bioinformatics/bts725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. J Cell Biol. 2013;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SC, Albuquerque CP, Han JS, Lagier-Tourenne C, Tokunaga S, Zhou H, Cleveland DW. ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proc Natl Acad Sci USA. 2010;107:13318–13323. doi: 10.1073/pnas.1008227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderweyde T, Vanderwyde T, Citro A, Mehta T, Zaarur N, McKee A, Bowser R, et al. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS ONE. 2010;5:e13250. doi: 10.1371/journal.pone.0013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HN, Sanelli T, Horne P, Pioro EP, Strong MJ, Rogaeva E, Bilbao J, Zinman L, Robertson J. Lack of evidence of monomer/misfolded superoxide dismutase-1 in sporadic amyotrophic lateral sclerosis. Ann Neurol. 2009;66:75–80. doi: 10.1002/ana.21704. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010a;9:995–1007. doi: 10.1016/S1474-4422(10)70195-2. [DOI] [PubMed] [Google Scholar]
- Mackenzie IRA, Ansorge O, Strong M, Bilbao J, Zinman L, Ang LC, Baker M, Stewart H, Eisen A, Rademakers R, et al. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: two distinct patterns correlating with disease severity and mutation. Acta Neuropathol. 2011;122:87–98. doi: 10.1007/s00401-011-0838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IRA, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- Mackenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010b;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, Yeatman T, Warrington EK, Schott JM, Fox NC, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135:736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankodi A, Urbinati CR, Yuan QP, Moxley RT, Sansone V, Krym M, Henderson D, Schalling M, Swanson MS, Thornton CA. Muscleblind localizes to nuclear foci of aberrant RNA in myotonic dystrophy types 1 and 2. Hum Mol Genet. 2001;10:2165–2170. doi: 10.1093/hmg/10.19.2165. [DOI] [PubMed] [Google Scholar]
- Marchetto MCN, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, Suzuki H, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- McDonald KK, Aulas A, Destroismaisons L, Pickles S, Beleac E, Camu W, Rouleau GA, Vande Velde C. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet. 2011;20:1400–1410. doi: 10.1093/hmg/ddr021. [DOI] [PubMed] [Google Scholar]
- McGoldrick P, Joyce PI, Fisher EM, Greensmith L. Rodent models of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2013;1832:1421–1436. doi: 10.1016/j.bbadis.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Meyerowitz J, Parker SJ, Vella LJ, Ng DC, Price KA, Liddell JR, Caragounis A, Li QX, Masters CL, Nonaka T, et al. C-Jun N-terminal kinase controls TDP-43 accumulation in stress granules induced by oxidative stress. Mol Neurodegener. 2011;6:57. doi: 10.1186/1750-1326-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecamps S, Boillee S, Le Ber I, Seilhean D, Teyssou E, Giraudeau M, Moigneu C, Vandenberghe N, Danel-Brunaud V, Corcia P, et al. Phenotype difference between ALS patients with expanded repeats in C9ORF72 and patients with mutations in other ALS-related genes. J Med Genet. 2012;49:258–263. doi: 10.1136/jmedgenet-2011-100699. [DOI] [PubMed] [Google Scholar]
- Millecamps S, Salachas F, Cazeneuve C, Gordon P, Bricka B, Camuzat A, Guillot-Noël L, Russaouen O, Bruneteau G, Pradat PF, et al. SOD1, ANG, VAPB, TARDBP, and FUS mutations in familial amyotrophic lateral sclerosis: genotype-phenotype correlations. J Med Genet. 2010;47:554–560. doi: 10.1136/jmg.2010.077180. [DOI] [PubMed] [Google Scholar]
- Miller JW, Urbinati CR, Teng-Umnuay P, Stenberg MG, Byrne BJ, Thornton CA, Swanson MS. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J. 2000;19:4439–4448. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TM, Pestronk A, David W, Rothstein J, Simpson E, Appel SH, Andres PL, Mahoney K, Allred P, Alexander K, et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol. 2013;12:435–442. doi: 10.1016/S1474-4422(13)70061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JC, McGoldrick P, Vance C, Hortobágyi T, Sreedharan J, Rogelj B, Tudor EL, Smith BN, Klasen C, Miller CCJ, et al. Overexpression of human wild-type FUS causes progressive motor neuron degeneration in an age- and dose-dependent fashion. Acta Neuropathol. 2012;125:273–288. doi: 10.1007/s00401-012-1043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisse K, Mepham J, Volkening K, Welch I, Hill T, Strong MJ. Cytosolic TDP-43 expression following axotomy is associated with caspase 3 activation in NFL−/− mice: support for a role for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009;1296:176–186. doi: 10.1016/j.brainres.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Momeni P, Rogaeva E, Van Deerlin V, Yuan W, Grafman J, Tierney M, Huey E, Bell J, Morris CM, Kalaria RN, et al. Genetic variability in CHMP2B and frontotemporal dementia. Neurodegener Dis. 2006;3:129–133. doi: 10.1159/000094771. [DOI] [PubMed] [Google Scholar]
- Mori K, Lammich S, Mackenzie IRA, Forné I, Zilow S, Kretzschmar H, Edbauer D, Janssens J, Kleinberger G, Cruts M, et al. hnRNP A3 binds to GGGGCC repeats and is a constituent of p62-positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol. 2013a;125:413–423. doi: 10.1007/s00401-013-1088-7. [DOI] [PubMed] [Google Scholar]
- Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, et al. The C9orf72 GGGGCC Repeat Is Translated into Aggregating Dipeptide-Repeat Proteins in FTLD/ALS. Science. 2013b;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci. 2012;37:230–236. doi: 10.1016/j.tibs.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley ML, Zu T, Ikeda Y, Gao W, Mosemiller AK, Daughters RS, Chen G, Weatherspoon MR, Clark HB, Ebner TJ, et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet. 2006;38:758–769. doi: 10.1038/ng1827. [DOI] [PubMed] [Google Scholar]
- Münch C, O'Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci USA. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, Dejesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, Wszolek ZK, Ferman TJ, Josephs KA, Boylan KB, et al. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya T, Alexiou P, Maragkakis M, Chang A, Mourelatos Z. FUS regulates genes coding for RNA-binding proteins in neurons by binding to their highly conserved introns. RNA. 2013;19:498–509. doi: 10.1261/rna.037804.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nishimura AL, Mitne-Neto M, Silva HCA, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JRM, Gillingwater T, Webb J, et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75:822–831. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra BA, Willemsen R. FMR1: a gene with three faces. Biochim Biophys Acta. 2009;1790:467–477. doi: 10.1016/j.bbagen.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamphlett R, Luquin N, McLean C, Jew SK, Adams L. TDP-43 neuropathology is similar in sporadic amyotrophic lateral sclerosis with or without TDP-43 mutations. Neuropathol Appl Neurobiol. 2009;35:222–225. doi: 10.1111/j.1365-2990.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- Parkinson N, Ince PG, Smith MO, Highley R, Skibinski G, Andersen PM, Morrison KE, Pall HS, Hardiman O, Collinge J, et al. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B) Neurology. 2006;67:1074–1077. doi: 10.1212/01.wnl.0000231510.89311.8b. [DOI] [PubMed] [Google Scholar]
- Pokrishevsky E, Grad LI, Yousefi M, Wang J, Mackenzie IR, Cashman NR. Aberrant Localization of FUS and TDP43 Is Associated with Misfolding of SOD1 in Amyotrophic Lateral Sclerosis. PLoS ONE. 2012;7:e35050. doi: 10.1371/journal.pone.0035050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M, Cleveland DW. The Seeds of Neurodegeneration: Prion-like Spreading in ALS. Cell. 2011;147:498–508. doi: 10.1016/j.cell.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M, Cleveland DW. Prion-like spread of protein aggregates in neurodegeneration. J Exp Med. 2012;209:889–893. doi: 10.1084/jem.20120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Bennett CF, Cleveland DW, Yeo GW. Misregulated RNA processing in amyotrophic lateral sclerosis. Brain Res. 2012;1462:3–15. doi: 10.1016/j.brainres.2012.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling S-C, Sun E, Wancewicz E, Mazur C, et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011 doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers CA, Mathur M, Raaka BM, Ron D, Samuels HH. TLS (translocated-in-liposarcoma) is a high-affinity interactor for steroid, thyroid hormone, and retinoid receptors. Mol Endocrinol. 1998;12:4–18. doi: 10.1210/mend.12.1.0043. [DOI] [PubMed] [Google Scholar]
- Prudencio M, Jansen-West KR, Lee WC, Gendron TF, Zhang YJ, Xu YF, Gass J, Stuani C, Stetler C, Rademakers R, et al. Misregulation of human sortilin splicing leads to the generation of a nonfunctional progranulin receptor. Proc Natl Acad Sci USA. 2012;109:21510–21515. doi: 10.1073/pnas.1211577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Qiao T, Beers M, Tan W, Wang H, Yang B, Xu Z. Widespread aggregation of mutant VAPB associated with ALS does not cause motor neuron degeneration or modulate mutant SOD1 aggregation and toxicity in mice. Mol Neurodegener. 2013;8:1. doi: 10.1186/1750-1326-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DCP, Wong LF, Bilsland LG, Greensmith L, Kingsman SM, et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–433. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, Henderson CE, Aebischer P. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- Rappsilber J. Large-Scale Proteomic Analysis of the Human Spliceosome. Genome Res. 2002;12:1231–1245. doi: 10.1101/gr.473902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, et al. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65:586–590. doi: 10.1212/01.wnl.0000172911.39167.b6. [DOI] [PubMed] [Google Scholar]
- Ritson GP, Custer SK, Freibaum BD, Guinto JB, Geffel D, Moore J, Tang W, Winton MJ, Neumann M, Trojanowski JQ, et al. TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. J Neurosci. 2010;30:7729–7739. doi: 10.1523/JNEUROSCI.5894-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, Timchenko NA, Miller JW, Reddy S, Caskey CT, Swanson MS, Timchenko LT. Altered phosphorylation and intracellular distribution of a (CUG)n triplet repeat RNA-binding protein in patients with myotonic dystrophy and in myotonin protein kinase knockout mice. Proc Natl Acad Sci USA. 1997;94:13221–13226. doi: 10.1073/pnas.94.24.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelj B, Easton LE, Bogu GK, Stanton LW, Rot G, Curk T, Zupan B, Sugimoto Y, Modic M, Haberman N, et al. Widespread binding of FUS along nascent RNA regulates alternative splicing in the brain. Sci Rep. 2012;2:603. doi: 10.1038/srep00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rubino E, Rainero I, Chiò A, Rogaeva E, Galimberti D, Fenoglio P, Grinberg Y, Isaia G, Calvo A, Gentile S, et al. SQSTM1 mutations in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Neurology. 2012;79:1556–1562. doi: 10.1212/WNL.0b013e31826e25df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Takeuchi S, Saito A, Ding W, Bamba H, Matsuura H, Hisa Y, Tooyama I, Urushitani M. Axonal ligation induces transient redistribution of TDP-43 in brainstem motor neurons. Neuroscience. 2009;164:1565–1578. doi: 10.1016/j.neuroscience.2009.09.050. [DOI] [PubMed] [Google Scholar]
- Seelaar H, Schelhaas HJ, Azmani A, Kusters B, Rosso S, Majoor-Krakauer D, de Rijk MC, Rizzu P, ten Brummelhuis M, van Doorn PA, et al. TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain. 2007;130:1375–1385. doi: 10.1093/brain/awm024. [DOI] [PubMed] [Google Scholar]
- Sellier C, Rau F, Liu Y, Tassone F, Hukema RK, Gattoni R, Schneider A, Richard S, Willemsen R, Elliott DJ, et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29:1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton CF, Cenik C, Kucukural A, Dammer EB, Cenik B, Han Y, Dewey CM, Roth FP, Herz J, Peng J, et al. Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. J Biol Chem. 2011;286:1204–1215. doi: 10.1074/jbc.M110.190884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton CF, Good SK, Atkin S, Dewey CM, Mayer P, Herz J, Yu G. TDP-43 is a developmentally regulated protein essential for early embryonic development. J Biol Chem. 2010;285:6826–6834. doi: 10.1074/jbc.M109.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio A, Bilican B, Barmada SJ, Ando DM, Zhao C, Siller R, Burr K, Haghi G, Story D, Nishimura AL, et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc Natl Acad Sci USA. 2013;110:4697–4702. doi: 10.1073/pnas.1300398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Chiang PM, Price DL, Wong PC. Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc Natl Acad Sci USA. 2010;107:16325–16330. doi: 10.1073/pnas.1003459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatunov A, Mok K, Newhouse S, Weale ME, Smith B, Vance C, Johnson L, Veldink JH, van Es MA, van den Berg LH, et al. Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol. 2010;9:986–994. doi: 10.1016/S1474-4422(10)70197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieben A, Van Langenhove T, Engelborghs S, Martin JJ, Boon P, Cras P, De Deyn PP, Santens P, Van Broeckhoven C, Cruts M. The genetics and neuropathology of frontotemporal lobar degeneration. Acta Neuropathol. 2012;124:353–372. doi: 10.1007/s00401-012-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón-Sánchez J, Dopper EGP, Cohn-Hokke PE, Hukema RK, Nicolaou N, Seelaar H, de Graaf JRA, de Koning I, van Schoor NM, Deeg DJH, et al. The clinical and pathological phenotype of C9orf72 hexanucleotide repeat expansions. Brain. 2012;135:723–735. doi: 10.1093/brain/awr353. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- Smith RA, Miller TM, Yamanaka K, Monia BP, Condon TP, Hung G, Lobsiger CS, Ward CM, McAlonis-Downes M, Wei H, et al. Antisense oligonucleotide therapy for neurodegenerative disease. J Clin Invest. 2006;116:2290–2296. doi: 10.1172/JCI25424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AMT, Jones M, Gerhard A, Davidson YS, Robinson A, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135:693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofola OA, Jin P, Qin Y, Duan R, Liu H, de Haro M, Nelson DL, Botas J. RNA-Binding Proteins hnRNP A2/B1 and CUGBP1 Suppress Fragile X CGG Premutation Repeat-Induced Neurodegeneration in a Drosophila Model of FXTAS. Neuron. 2007;55:565–571. doi: 10.1016/j.neuron.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart H, Rutherford NJ, Briemberg H, Krieger C, Cashman N, Fabros M, Baker M, Fok A, Dejesus-Hernandez M, Eisen A, et al. Clinical and pathological features of amyotrophic lateral sclerosis caused by mutation in the C9ORF72 gene on chromosome 9p. Acta Neuropathol. 2012;123:409–417. doi: 10.1007/s00401-011-0937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Diaz Z, Fang X, Hart MP, Chesi A, Shorter J, Gitler AD. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol. 2011;9:e1000614. doi: 10.1371/journal.pbio.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup V, Audet JN, Phaneuf D, Kriz J, Julien JP. Abnormal regenerative responses and impaired axonal outgrowth after nerve crush in TDP-43 transgenic mouse models of amyotrophic lateral sclerosis. J Neurosci. 2012;32:18186–18195. doi: 10.1523/JNEUROSCI.2267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup V, Phaneuf D, Bareil C, Robertson J, Rouleau GA, Kriz J, Julien JP. Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments. Brain. 2011;134:2610–2626. doi: 10.1093/brain/awr159. [DOI] [PubMed] [Google Scholar]
- Tan AY, Manley JL. The TET Family of Proteins: Functions and Roles in Disease. J Mol Cell Biol. 2009;1:82–92. doi: 10.1093/jmcb/mjp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AY, Manley JL. TLS inhibits RNA polymerase III transcription. Mol Cell Biol. 2010;30:186–196. doi: 10.1128/MCB.00884-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AY, Riley TR, Coady T, Bussemaker HJ, Manley JL. TLS/FUS (translocated in liposarcoma/fused in sarcoma) regulates target gene transcription via single-stranded DNA response elements. Proc Natl Acad Sci USA. 2012;109:6030–6035. doi: 10.1073/pnas.1203028109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A, Nishizawa M, Kakita A, Takahashi H. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol. 2007;113:535–542. doi: 10.1007/s00401-007-0206-9. [DOI] [PubMed] [Google Scholar]
- Tanji K, Zhang HX, Mori F, Kakita A, Takahashi H, Wakabayashi K. p62/sequestosome 1 binds to TDP-43 in brains with frontotemporal lobar degeneration with TDP-43 inclusions. J Neurosci Res. 2012;90:2034–2042. doi: 10.1002/jnr.23081. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuling E, Ahmed S, Haasdijk E, Demmers J, Steinmetz MO, Akhmanova A, Jaarsma D, Hoogenraad CC. Motor neuron disease-associated mutant vesicle-associated membrane protein-associated protein (VAP) B recruits wild-type VAPs into endoplasmic reticulum-derived tubular aggregates. J Neurosci. 2007;27:9801–9815. doi: 10.1523/JNEUROSCI.2661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssou E, Takeda T, Lebon V, Boillee S, Doukouré B, Bataillon G, Sazdovitch V, Cazeneuve C, Meininger V, LeGuern E, et al. Mutations in SQSTM1 encoding p62 in amyotrophic lateral sclerosis: genetics and neuropathology. Acta Neuropathol. 2013;125:511–522. doi: 10.1007/s00401-013-1090-0. [DOI] [PubMed] [Google Scholar]
- Ticozzi N, Vance C, Leclerc AL, Keagle P, Glass JD, McKenna-Yasek D, Sapp PC, Silani V, Bosco DA, Shaw CE, et al. Mutational analysis reveals the FUS homolog TAF15 as a candidate gene for familial amyotrophic lateral sclerosis. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:285–290. doi: 10.1002/ajmg.b.31158. [DOI] [PubMed] [Google Scholar]
- Timchenko LT, Miller JW, Timchenko NA, DeVore DR, Datar KV, Lin L, Roberts R, Caskey CT, Swanson MS. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–4414. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, König J, Hortobágyi T, Nishimura AL, Župunski V, et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Huang C, Bi F, Wu Q, Huang B, Liu X, Li F, Zhou H, Xia XG. Expression of ALS-linked TDP-43 mutant in astrocytes causes non-cell-autonomous motor neuron death in rats. EMBO J. 2013;32:1917–1926. doi: 10.1038/emboj.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresse E, Salomons FA, Vesa J, Bott LC, Kimonis V, Yao TP, Dantuma NP, Taylor JP. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy. 2010;6:217–227. doi: 10.4161/auto.6.2.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troakes C, Maekawa S, Wijesekera L, Rogelj B, Siklós L, Bell C, Smith B, Newhouse S, Vance C, Johnson L, et al. An MND/ALS phenotype associated with C9orf72 repeat expansion: Abundant p62-positive, TDP-43-negative inclusions in cerebral cortex, hippocampus and cerebellum but without associated cognitive decline. Neuropathology. 2011;32:505–514. doi: 10.1111/j.1440-1789.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Han SM, Yang Y, Tong C, Lin YQ, Mohan K, Haueter C, Zoghbi A, Harati Y, Kwan J, et al. The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. 2008;133:963–977. doi: 10.1016/j.cell.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuiji H, Iguchi Y, Furuya A, Kataoka A, Hatsuta H, Atsuta N, Tanaka F, Hashizume Y, Akatsu H, Murayama S, et al. Spliceosome Integrity is Defective in the Motor Neuron Diseases ALS and SMA. EMBO Mol Med. 2012;5:221–234. doi: 10.1002/emmm.201202303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor EL, Galtrey CM, Perkinton MS, Lau KF, De Vos KJ, Mitchell JC, Ackerley S, Hortobágyi T, Vámos E, Leigh PN, et al. Amyotrophic lateral sclerosis mutant vesicle-associated membrane protein-associated protein-B transgenic mice develop TAR-DNA-binding protein-43 pathology. Neuroscience. 2010;167:774–785. doi: 10.1016/j.neuroscience.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Urushitani M, Sato T, Bamba H, Hisa Y, Tooyama I. Synergistic effect between proteasome and autophagosome in the clearance of polyubiquitinated TDP-43. J Neurosci Res. 2010;88:784–797. doi: 10.1002/jnr.22243. [DOI] [PubMed] [Google Scholar]
- Urwin H, Authier A, Nielsen JE, Metcalf D, Powell C, Froud K, Malcolm DS, Holm I, Johannsen P, Brown J, et al. Disruption of endocytic trafficking in frontotemporal dementia with CHMP2B mutations. Hum Mol Genet. 2010;19:2228–2238. doi: 10.1093/hmg/ddq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk M, van Vught PWJ, van Es MA, Schelhaas HJ, van der Kooi AJ, de Visser M, Veldink JH, van den Berg LH. Novel optineurin mutations in sporadic amyotrophic lateral sclerosis patients. Neurobiol Aging. 2012;33:1016, e1011–1017. doi: 10.1016/j.neurobiolaging.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deerlin VM, Sleiman PMA, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M, et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42:234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee J, Urwin H, Engelborghs S, Bruyland M, Vandenberghe R, Dermaut B, De Pooter T, Peeters K, Santens P, De Deyn PP, et al. CHMP2B C-truncating mutations in frontotemporal lobar degeneration are associated with an aberrant endosomal phenotype in vitro. Hum Mol Genet. 2008;17:313–322. doi: 10.1093/hmg/ddm309. [DOI] [PubMed] [Google Scholar]
- van Es MA, Veldink JH, Saris CGJ, Blauw HM, van Vught PWJ, Birve A, Lemmens R, Schelhaas HJ, Groen EJN, Huisman MHB, et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat Genet. 2009;41:1083–1087. doi: 10.1038/ng.442. [DOI] [PubMed] [Google Scholar]
- Van Hoecke A, Schoonaert L, Lemmens R, Timmers M, Staats KA, Laird AS, Peeters E, Philips T, Goris A, Dubois B, et al. EPHA4 is a disease modifier of amyotrophic lateral sclerosis in animal models and in humans. Nat Med. 2012;18:1418–1422. doi: 10.1038/nm.2901. [DOI] [PubMed] [Google Scholar]
- Van Langenhove T, van der Zee J, Van Broeckhoven C. The molecular basis of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum. Ann Med. 2012;44:817–828. doi: 10.3109/07853890.2012.665471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IF, Wu LS, Chang HY, Shen CKJ. TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. J Neurochem. 2008;105:797–806. doi: 10.1111/j.1471-4159.2007.05190.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Sharma K, Grisotti G, Roos RP. The effect of mutant SOD1 dismutase activity on non-cell autonomous degeneration in familial amyotrophic lateral sclerosis. Neurobiol Dis. 2009;35:234–240. doi: 10.1016/j.nbd.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fan H, Ying Z, Li B, Wang H, Wang G. Degradation of TDP-43 and its pathogenic form by autophagy and the ubiquitin-proteasome system. Neuroscience Letters. 2010;469:112–116. doi: 10.1016/j.neulet.2009.11.055. [DOI] [PubMed] [Google Scholar]
- Watts GDJ, Thomasova D, Ramdeen SK, Fulchiero EC, Mehta SG, Drachman DA, Weihl CC, Jamrozik Z, Kwiecinski H, Kaminska A, et al. Novel VCP mutations in inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Clin Genet. 2007;72:420–426. doi: 10.1111/j.1399-0004.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- Watts GDJ, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton MW, Salamone AR, Mosnik DM, McDonald RO, Appel SH, Schmolck HI, Ringholz GM, Schulz PE. Cognitive impairment in familial ALS. Neurology. 2007;69:1411–1417. doi: 10.1212/01.wnl.0000277422.11236.2c. [DOI] [PubMed] [Google Scholar]
- Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Warraich ST, Yang S, Solski JA, Fernando R, Rouleau GA, Nicholson GA, Blair IP. UBQLN2/ubiquilin 2 mutation and pathology in familial amyotrophic lateral sclerosis. Neurobiol Aging. 2012;33:2527. doi: 10.1016/j.neurobiolaging.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Wils H, Kleinberger G, Janssens J, Pereson S, Joris G, Cuijt I, Smits V, Ceuterick-de Groote C, Van Broeckhoven C, Kumar-Singh S. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA. 2010;107:3858–3863. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton MJ, Igaz LM, Wong MM, Kwong LK, Trojanowski JQ, Lee VMY. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J Biol Chem. 2008;283:13302–13309. doi: 10.1074/jbc.M800342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LS, Cheng WC, Hou SC, Yan YT, Jiang ST, Shen CKJ. TDP-43, a neuro-pathosignature factor, is essential for early mouse embryogenesis. Genesis. 2010;48:56–62. doi: 10.1002/dvg.20584. [DOI] [PubMed] [Google Scholar]
- Wu LS, Cheng WC, Shen CK. Targeted Depletion of TDP-43 Expression in the Spinal Cord Motor Neurons Leads to the Development of Amyotrophic Lateral Sclerosis (ALS)-like Phenotypes in Mice. J Biol Chem. 2012;287:27335–27344. doi: 10.1074/jbc.M112.359000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Sanelli T, Dib S, Sheps D, Findlater J, Bilbao J, Keith J, Zinman L, Rogaeva E, Robertson J. RNA targets of TDP-43 identified by UV-CLIP are deregulated in ALS. Mol Cell Neurosci. 2011;47:167–180. doi: 10.1016/j.mcn.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Xu Z, Poidevin M, Li X, Li Y, Shu L, Nelson DL, Li H, Hales CM, Gearing M, Wingo TS, et al. Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc Natl Acad Sci U S A. 2013;110:7778–7783. doi: 10.1073/pnas.1219643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Mori A, Sakaguchi H, Suga T, Ishihara D, Ueda A, Yamashita T, Maeda Y, Uchino M, Hirano T. Sporadic juvenile amyotrophic lateral sclerosis caused by mutant FUS/TLS: possible association of mental retardation with this mutation. J Neurol. 2011;259:1039–1044. doi: 10.1007/s00415-011-6292-6. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Chen S, Yu Y, Yan B, Haertlein TC, Carrasco MA, Tapia JC, Zhai B, Das R, Lalancette-Hébert M, et al. FUS-SMN Protein Interactions Link the Motor Neuron Diseases ALS and SMA. Cell Rep. 2012;2:799–806. doi: 10.1016/j.celrep.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Iyer LM, He F, Aravind L. Discovery of Novel DENN Proteins: Implications for the Evolution of Eukaryotic Intracellular Membrane Structures and Human Disease. Front Genet. 2012;3:283. doi: 10.3389/fgene.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Huang C, Chen H, Wang D, Landel CP, Xia PY, Bowser R, Liu YJ, Xia XG. Transgenic rat model of neurodegeneration caused by mutation in the TDP gene. PLoS Genet. 2010;6:e1000887. doi: 10.1371/journal.pgen.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Licklider LJ, Gygi SP, Reed R. Comprehensive proteomic analysis of the human spliceosome. Nature. 2002;419:182–185. doi: 10.1038/nature01031. [DOI] [PubMed] [Google Scholar]
- Zinman L, Cudkowicz M. Emerging targets and treatments in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10:481–490. doi: 10.1016/S1474-4422(11)70024-2. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Sok J, Immanuel D, Yin Y, Ron D. TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. J Cell Sci. 1997;110(Pt 15):1741–1750. doi: 10.1242/jcs.110.15.1741. [DOI] [PubMed] [Google Scholar]
- Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MAC, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci USA. 2011;108:260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.