Abstract
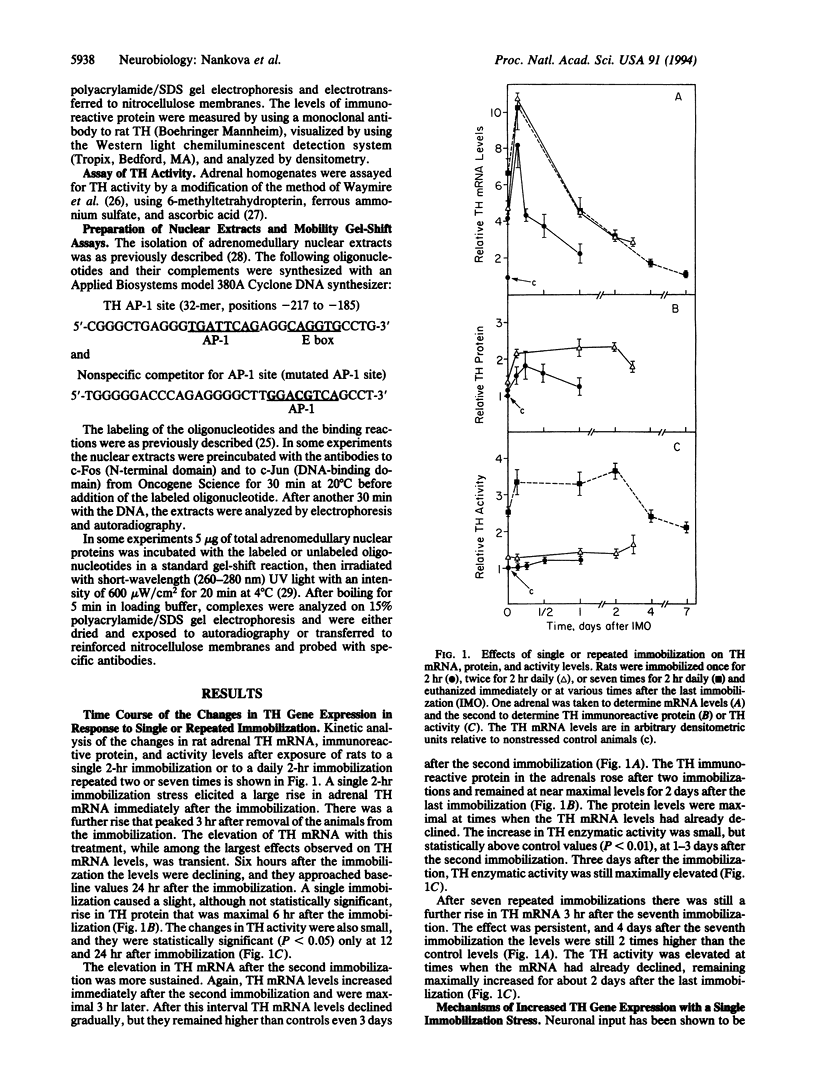
Stress stimulates the sympathoadrenal system, causing activation of the catecholamine biosynthetic enzymes. Here we examine the changes of gene expression of tyrosine hydroxylase (TH; EC 1.14.16.2), the initial enzyme of catecholamine biosynthesis, with stress. A single immobilization of rats led to a large transient elevation in TH mRNA and a small elevation in TH immunoreactive protein and activity. Repeated daily immobilizations triggered more sustained changes in TH mRNA levels. After two immobilizations, the levels remained elevated even 3 days later. The rise in TH mRNA was followed by increased immunoreactive protein but only a small elevation in activity. With seven repeated immobilizations, the animals did not appear to adapt and still manifested a further rise in TH mRNA. TH activity was markedly elevated and returned to control levels 7 days after the immobilization. The rise in TH mRNA with a single immobilization occurred even in adrenals of hypophysectomized rats that underwent splanchnic nerve section. Immobilization for 30 min was sufficient to increase TH mRNA. The effect was abolished by the transcriptional inhibitor actinomycin D. Mobility gel-shift assays revealed increased binding of c-Fos and c-Jun to the AP-1 transcription factor site after a single immobilization, and the binding was not further elevated with repeated stress. This study shows that a single immobilization can activate TH gene expression by a nonneuronal nonpituitary-mediated pathway associated with increased binding of AP-1 transcription factors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baruchin A., Vollmer R. R., Miner L. L., Sell S. L., Stricker E. M., Kaplan B. B. Cold-induced increases in phenylethanolamine N-methyltransferase (PNMT) mRNA are mediated by non-cholinergic mechanisms in the rat adrenal gland. Neurochem Res. 1993 Jul;18(7):759–766. doi: 10.1007/BF00966770. [DOI] [PubMed] [Google Scholar]
- Baruchin A., Weisberg E. P., Miner L. L., Ennis D., Nisenbaum L. K., Naylor E., Stricker E. M., Zigmond M. J., Kaplan B. B. Effects of cold exposure on rat adrenal tyrosine hydroxylase: an analysis of RNA, protein, enzyme activity, and cofactor levels. J Neurochem. 1990 May;54(5):1769–1775. doi: 10.1111/j.1471-4159.1990.tb01232.x. [DOI] [PubMed] [Google Scholar]
- Cambi F., Fung B., Chikaraishi D. 5' flanking DNA sequences direct cell-specific expression of rat tyrosine hydroxylase. J Neurochem. 1989 Nov;53(5):1656–1659. doi: 10.1111/j.1471-4159.1989.tb08567.x. [DOI] [PubMed] [Google Scholar]
- D'Mello S. R., Turzai L. M., Gioio A. E., Kaplan B. B. Isolation and structural characterization of the bovine tyrosine hydroxylase gene. J Neurosci Res. 1989 May;23(1):31–40. doi: 10.1002/jnr.490230105. [DOI] [PubMed] [Google Scholar]
- Fader D., Lewis E. J. Interaction of cyclic AMP and cell-cell contact in the control of tyrosine hydroxylase RNA. Brain Res Mol Brain Res. 1990 Jun;8(1):25–29. doi: 10.1016/0169-328x(90)90005-x. [DOI] [PubMed] [Google Scholar]
- Fossom L. H., Carlson C. D., Tank A. W. Stimulation of tyrosine hydroxylase gene transcription rate by nicotine in rat adrenal medulla. Mol Pharmacol. 1991 Aug;40(2):193–202. [PubMed] [Google Scholar]
- Fossom L. H., Sterling C. R., Tank A. W. Regulation of tyrosine hydroxylase gene transcription rate and tyrosine hydroxylase mRNA stability by cyclic AMP and glucocorticoid. Mol Pharmacol. 1992 Nov;42(5):898–908. [PubMed] [Google Scholar]
- Fung B. P., Yoon S. O., Chikaraishi D. M. Sequences that direct rat tyrosine hydroxylase gene expression. J Neurochem. 1992 Jun;58(6):2044–2052. doi: 10.1111/j.1471-4159.1992.tb10945.x. [DOI] [PubMed] [Google Scholar]
- Gizang-Ginsberg E., Ziff E. B. Nerve growth factor regulates tyrosine hydroxylase gene transcription through a nucleoprotein complex that contains c-Fos. Genes Dev. 1990 Apr;4(4):477–491. doi: 10.1101/gad.4.4.477. [DOI] [PubMed] [Google Scholar]
- Harrington C. A., Lewis E. J., Krzemien D., Chikaraishi D. M. Identification and cell type specificity of the tyrosine hydroxylase gene promoter. Nucleic Acids Res. 1987 Mar 11;15(5):2363–2384. doi: 10.1093/nar/15.5.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillel Z., Wu C. W. Photochemical cross-linking studies on the interaction of Escherichia coli RNA polymerase with T7 DNA. Biochemistry. 1978 Jul 25;17(15):2954–2961. doi: 10.1021/bi00608a003. [DOI] [PubMed] [Google Scholar]
- Hiremagalur B., Nankova B., Nitahara J., Zeman R., Sabban E. L. Nicotine increases expression of tyrosine hydroxylase gene. Involvement of protein kinase A-mediated pathway. J Biol Chem. 1993 Nov 5;268(31):23704–23711. [PubMed] [Google Scholar]
- Hoeldtke R., Lloyd T., Kaufman S. An immunochemical study of the induction of tyrosine hydroxylase in rat adrenal glands. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1045–1053. doi: 10.1016/0006-291x(74)90802-x. [DOI] [PubMed] [Google Scholar]
- Icard-Liepkalns C., Biguet N. F., Vyas S., Robert J. J., Sassone-Corsi P., Mallet J. AP-1 complex and c-fos transcription are involved in TPA provoked and trans-synaptic inductions of the tyrosine hydroxylase gene: insights into long-term regulatory mechanisms. J Neurosci Res. 1992 Jun;32(2):290–298. doi: 10.1002/jnr.490320219. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Spiegelman B. M., Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992 Nov 13;71(4):577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. J., Nankova B. B., Lewis E. J., McMahon A., Osaka H., Sabban D. B., Sabban E. L. Regulated expression of the tyrosine hydroxylase gene by membrane depolarization. Identification of the responsive element and possible second messengers. J Biol Chem. 1992 Apr 15;267(11):7563–7569. [PubMed] [Google Scholar]
- Kvetnansky R., Mikulaj L. Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology. 1970 Oct;87(4):738–743. doi: 10.1210/endo-87-4-738. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R., Sun C. L., Lake C. R., Thoa N., Torda T., Kopin I. J. Effect of handling and forced immobilization on rat plasma levels of epinephrine, norepinephrine, and dopamine-beta-hydroxylase. Endocrinology. 1978 Nov;103(5):1868–1874. doi: 10.1210/endo-103-5-1868. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R., Weise V. K., Kopin I. J. Elevation of adrenal tyrosine hydroxylase and phenylethanolamine-N-methyl transferase by repeated immobilization of rats. Endocrinology. 1970 Oct;87(4):744–749. doi: 10.1210/endo-87-4-744. [DOI] [PubMed] [Google Scholar]
- Kvetnanský R., Fukuhara K., Pacák K., Cizza G., Goldstein D. S., Kopin I. J. Endogenous glucocorticoids restrain catecholamine synthesis and release at rest and during immobilization stress in rats. Endocrinology. 1993 Sep;133(3):1411–1419. doi: 10.1210/endo.133.3.8396019. [DOI] [PubMed] [Google Scholar]
- Kvetnanský R., Goldstein D. S., Weise V. K., Holmes C., Szemeredi K., Bagdy G., Kopin I. J. Effects of handling or immobilization on plasma levels of 3,4-dihydroxyphenylalanine, catecholamines, and metabolites in rats. J Neurochem. 1992 Jun;58(6):2296–2302. doi: 10.1111/j.1471-4159.1992.tb10977.x. [DOI] [PubMed] [Google Scholar]
- Kvetñanský R., Weise V. K., Gewirtz G. P., Kopin I. J. Synthesis of adrenal catecholamines in rats during and after immobilization stress. Endocrinology. 1971 Jul;89(1):46–49. doi: 10.1210/endo-89-1-46. [DOI] [PubMed] [Google Scholar]
- Lerner P., Nosé P., Ames M. M., Lovenberg W. Modification of the tyrosine hydroxylase assay. Increased enzyme activity in the presence of ascorbic acid. Neurochem Res. 1978 Oct;3(5):641–651. doi: 10.1007/BF00963765. [DOI] [PubMed] [Google Scholar]
- Lewis E. J., Harrington C. A., Chikaraishi D. M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A., Kvetnanský R., Fukuhara K., Weise V. K., Kopin I. J., Sabban E. L. Regulation of tyrosine hydroxylase and dopamine beta-hydroxylase mRNA levels in rat adrenals by a single and repeated immobilization stress. J Neurochem. 1992 Jun;58(6):2124–2130. doi: 10.1111/j.1471-4159.1992.tb10954.x. [DOI] [PubMed] [Google Scholar]
- Miner L. L., Pandalai S. P., Weisberg E. P., Sell S. L., Kovacs D. M., Kaplan B. B. Cold-induced alterations in the binding of adrenomedullary nuclear proteins to the promoter region of the tyrosine hydroxylase gene. J Neurosci Res. 1992 Sep;33(1):10–18. doi: 10.1002/jnr.490330103. [DOI] [PubMed] [Google Scholar]
- Nankova B., Devlin D., Kvetnanský R., Kopin I. J., Sabban E. L. Repeated immobilization stress increases the binding of c-Fos-like proteins to a rat dopamine beta-hydroxylase promoter enhancer sequence. J Neurochem. 1993 Aug;61(2):776–779. doi: 10.1111/j.1471-4159.1993.tb02188.x. [DOI] [PubMed] [Google Scholar]
- Pennypacker K. R., Hong J. S., Douglass J., McMillian M. K. Constitutive expression of AP-1 transcription factors in the rat adrenal. Effects of nicotine. J Biol Chem. 1992 Oct 5;267(28):20148–20152. [PubMed] [Google Scholar]
- Safer B., Cohen R. B., Garfinkel S., Thompson J. A. DNA affinity labeling of adenovirus type 2 upstream promoter sequence-binding factors identifies two distinct proteins. Mol Cell Biol. 1988 Jan;8(1):105–113. doi: 10.1128/mcb.8.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak M. K., Fluharty S. J., Stricker E. M., Zigmond M. J., Kaplan B. B. Molecular adaptations in catecholamine biosynthesis induced by cold stress and sympathectomy. J Neurosci Res. 1986;16(1):13–24. doi: 10.1002/jnr.490160104. [DOI] [PubMed] [Google Scholar]
- Stachowiak M. K., Jiang H. K., Poisner A. M., Tuominen R. K., Hong J. S. Short and long term regulation of catecholamine biosynthetic enzymes by angiotensin in cultured adrenal medullary cells. Molecular mechanisms and nature of second messenger systems. J Biol Chem. 1990 Mar 15;265(8):4694–4702. [PubMed] [Google Scholar]
- Stachowiak M., Sebbane R., Stricker E. M., Zigmond M. J., Kaplan B. B. Effect of chronic cold exposure on tyrosine hydroxylase mRNA in rat adrenal gland. Brain Res. 1985 Dec 16;359(1-2):356–359. doi: 10.1016/0006-8993(85)91450-7. [DOI] [PubMed] [Google Scholar]
- Tank A. W., Lewis E. J., Chikaraishi D. M., Weiner N. Elevation of RNA coding for tyrosine hydroxylase in rat adrenal gland by reserpine treatment and exposure to cold. J Neurochem. 1985 Oct;45(4):1030–1033. doi: 10.1111/j.1471-4159.1985.tb05519.x. [DOI] [PubMed] [Google Scholar]
- Waymire J. C., Bjur R., Weiner N. Assay of tyrosine hydroxylase by coupled decarboxylation of DOPA formed from 1- 14 C-L-tyrosine. Anal Biochem. 1971 Oct;43(2):588–600. doi: 10.1016/0003-2697(71)90291-0. [DOI] [PubMed] [Google Scholar]
- Yoon S. O., Chikaraishi D. M. Tissue-specific transcription of the rat tyrosine hydroxylase gene requires synergy between an AP-1 motif and an overlapping E box-containing dyad. Neuron. 1992 Jul;9(1):55–67. doi: 10.1016/0896-6273(92)90220-8. [DOI] [PubMed] [Google Scholar]